Abstract
The human telomerase reverse transcriptase (hTERT) is an essential component of the holoenzyme complex that adds telomeric repeats to the ends of chromosomes. The hTERT transcript has been shown to have two deletion type alternative splicing sites. One deletion site induces the α-deletion variant, lacking 36 bp from exon 6, and the other induces the β-deletion variant, lacking 182 bp from exons 7 and 8. Here, we identified a novel deletion variant of the hTERT transcript in hepatocellular carcinoma cell lines. The deleted transcript was characterized by an in-frame deletion of 189 bp, spanning nucleotides 2710 to 2898, corresponding to the complete loss of exon 11 (γ-deletion). The region lacking in the γ-deletion lies within RT motifs D and E, suggesting that it is missing conserved residues from the catalytic core of the protein. Both γ- and α-deletion variants were occasionally detected, but the β-deletion variant was frequently observed. Our results may provide important information for more detailed studies on the regulation of telomerase activity.
Keywords: telomerase, hTERT, alternative splicing, γ-deleton ASV, telomere
Introduction
Pre-mRNA splicing is a fundamental biological process involved in the expression of genes, and up to a third of human genes are thought to be alternatively spliced [1,2]. General alternative splicing takes place in variant proteins and expression patterns as products of different genes. Recently, Serine-Arginine (SR) proteins have been shown to play a role in constitutive and alternative splicing, and the characters and functions of SR proteins have now been extensively investigated [3,4]. Some alternatively spliced variants (ASVs) have been associated with a subset of human diseases [5–8]; however, the pathological importance of ASVs in most human diseases is still uncertain.
Human telomerase reverse transcriptase (hTERT) [9–11], which has two deletion-type ASVs [12,13], is the protein component for telomerase. Telomerase adds DNA repeats to the ends of chromosomes [14], and the enzyme telomerase plays a crucial role in cellular proliferation, development, and tumorigenesis [15,16]. The hTERT gene consists of 16 exons and the transcript is about 4.0 kb long [17]. The protein has a molecular mass of 127 kDa and contains a telomerase-specific motif and seven reverse transcriptase (RT) motifs [17,18]. The deletion-type ASVs are α- and β-deletion types [19–21]. The α-deletion ASV lacks 36 nucleotides from exon 6 including motif A, and the β-deletion ASV lacks 182 nucleotides from exons 7 and 8, including motif B′. The α-deletion ASV mRNA has occasionally been detected, whereas the β-deletion mRNA is frequently observed irrespective of the telomerase activity [19–21].
We have now identified a novel ASV of hTERT mRNA. The ASV identified lacks the entire exon 11, and the protein encoded by this ASV was conserved with no frameshift mutation. Although ASVs are transcripts from a single gene, it is important to discriminate the full-length isoform from ASVs. The aim of the present investigation was to demonstrate evidence of a novel ASV of hTERT gene expression in hepatocellular carcinoma (HCC) cell lines. Here, we developed a polymerase chain reaction (PCR)-based specific assay for the quantification of alternatively spliced hTERT expression.
Materials and Methods
Total RNA was extracted from three cell lines [Japan Health Science Foundation (HSRRB), Osaka, Japan] derived from HCC, Huh 7, HLE, and Huh 6-clone 5, and from two normal cell lines (HSRRB) LI 90 (derived from normal Ito cells) and HUVEC-C (derived from normal endothelial cells) (Table 1) using the RNAzolB reagent (Sawady, Shinjuku, Japan) following the manufacturer's instructions. The final RNA preparations were resuspended in diethylpyrocarbonate-treated water and quantified by absorbance analysis at 260 nm. Complementary DNA (cDNA) was prepared by incubating DNasetreated total RNA (1.0 µg) with M-MLV RT (Invitrogen, Carlsbad, CA, USA) in the presence of random primers.
Table 1.
Description of Cell Lines in this Study.
| Cell lines | Source | JCRB number | Establisher |
| Huh 6 - clone 5 | Well-differentiated hepatoma | JCRB0401 | I. Doi, J. Sato |
| Huh 7 | Differentiated hepatoma | JCRB0403 | H. Nakabayashi, J. Sato |
| HLE | Nondifferentiated hepatoma | JCBR0404 | I. Doi, J. Sato |
| LI 90 | Normal Ito cell | JCBR0160 | K. Murakami, et al. |
| HUV-EC-C | Normal endothelial cell | IFO50271 | H. Hoshi |
ASV Identification
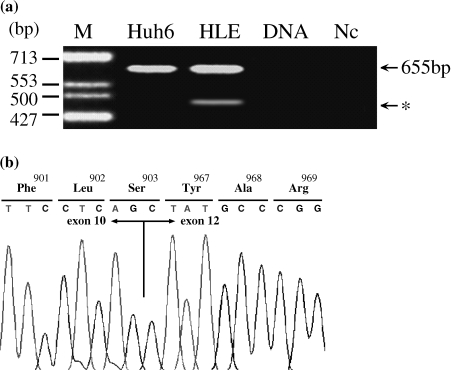
The primer set for the amplification of an hTERT mRNA was designed according to GenBank AF128894, using forward primers at the exon 10 region (5′-CTG CAG GAG ACC AGC CCG C-3′) and reverse primers at the exon 13 region (5′-ACA CCG TCT GGA GGC TGT TCA-3′). The reaction parameters were 95°C for 30 seconds, 60°C for 40 seconds, and 72°C for 30 seconds for 45 cycles, followed by a 10-minute extension at 72°C using AmpliTaq Gold DNA polymerase (PE Applied Biosystems, Foster City, CA). Half of the PCR product was separated by electrophoresis in TBE buffer in a 3.0% agarose gel, stained with ethidium bromide, and then detected with ultraviolet light (Figure 1a).
Figure 1.
Alternative splicing of hTERT mRNA. (a) RT-PCR products of the hTERT gene spanning exons 10 to 13. An obvious shorter signal (*arrow) was observed in the HLE sample. Huh 6, Huh 6 - clone 5 cell line; HLE, HLE cell line; DNA, genomic DNA; Nc, negative control (RNase-free water); M, molecular weight markers ϕX174 DNA/HinfI digest. (b) Sequence analysis of the transcript bearing the deletion of exon 11 of the hTERT gene. The sequence in (a) shows the inframe deletion spanning codons 904 to 966, corresponding to the complete loss of exon 11. An arginine-to-serine (Arg→Ser) transition is revealed at codon 903.
The PCR products of hTERT were purified using a High Pure PCR Product Purification Kit (Roche Molecular Biochemicals Diagnostics, Indianapolis, IN), cloned into a pCR2.1 vector (Invitrogen) and then sequenced using a BigDye Terminator Cycle Sequencing Ready Reaction Kit (PE Applied Biosystems) with an ABI PRISM 3100 Genetic Analyzer (PE Applied Biosystems). Finally, the sequence was compared with the full-length hTERT mRNA sequence (Figure 1b).
Specific Quantitative System (RT Real-Time PCR)
The real-time PCR reaction mixture was prepared using a TaqMan PCR Core Reagents Kit (PE Applied Biosystems). The primers and probes to amplify the mRNA of the fulllength isoform and ASVs mRNA are shown in Table 2. The real-time PCR reaction was performed for 55 cycles (95°C for 30 seconds, 60°C for 45 seconds, 72°C for 30 seconds) using a real-time PCR system (ABI PRISM 7700 Sequence Detection System; PE Applied Biosystems).
Table 2.
Primers and Probes for Quantification of hTERT mRNA.
| Primers and probes | Positions | |
| ex6-F primer | 5′-ATG TGA CGG GCG CGT ACG A-3′ | Exon 6, nt 2135–2154 |
| Adel-F primer | 5′-CTG AGC TGT ACT TTG TCA AGG ACA GG-3′ | Exon 5, nt 2111–2130; exon 6, nt 2167–2172 |
| ex11-R primer | 5′-GGA AGT TCA CCA CTG TCT TCC GC-3′ | Exon 11, nt 2699–2721 |
| Gdel-R primer | 5′-CTT CCT CAG CTA TGC CCGGAC CT-3′ | Exon 10, nt 2646–2654; exon 12, nt 2843–2856 |
| ex7 probe | 5′-CCT GCA GGA GAC CAG CCC GCT GAG G-3′ | Exon 7, nt 2337–2361 |
| Bdel probe | 5′-CTT CAA GAG CCA CGT CCT ACG TCC AGT GCC-3′ | Exon 6, nt 2274–2286; exon 9, nt 2369–2385 |
To prepare standard RNA for the quantification, PCR products were cloned into a pBluescript vector (Stratagene, La Jolla, CA) and standard RNA was synthesized using T7 RNA polymerase and purified by RNAzolB and DNase I (TaKaRa, Shiga, Japan) treatment.
Quantification of the mRNA samples was carried out by relating the PCR threshold cycle obtained from the cell line samples to the amplicon-specific standard curves. Serial dilution of standard RNA was carried out in duplicate from 107 copies to 102 copies and used in triplicate RT real-time PCR reactions. The mRNA expression levels are presented as the mRNA copy number per microgram of total RNA [22].
Telomerase Activity
The telomerase activities of all cells were assessed using a TRAP-eze Kit (Intergen, New York, NY) with a Cy-5-labeled TS primer as described elsewhere [23,24].
Results
Identification of a Novel ASV of hTERT
The PCR mixture presented two bands on electrophoresis (Figure 1a). The sizes of these PCR products were 655 bp (no-deletion isoform) and 466 bp. The sequence of the 466-bp product lacked 189 bp from the entire exon 11 (γ-deletion; Figure 1b). For the γ-deletion ASV, longer cDNA were sequenced based on 5′ and 3′ rapid amplification of cDNA ends (RACE) with long-distance PCR. The protein encoded by the novel γ-deletion ASV mRNA was 63aa shorter than the full-length isoform. Amino acid sequences without the missing region were conserved because no frameshift mutation was observed.
To verify whether the exon deletion observed in our samples was caused by genomic hTERT alteration, we amplified the region of the hTERT genomic DNA that included the splicing site of exon 11. Single-strand conformation polymorphism (SSPC) analysis of exon 11 did not demonstrate germline mutations responsible for the deletion (data not shown). The possibility that the deleted transcript could have resulted from PCR false priming or other artifacts was excluded because triplicate experiments based on independent RNA extractions and cDNA preparations yielded consistent results.
Here, we have identified a novel ASV, γ-deletion ASV. The sequence was compared with nonredundant sequences in GenBank, and no identical sequences were found. The sequence of the γ-deletion ASV was submitted to GenBank (access no. AB085628).
Estimation of hTERT Expression
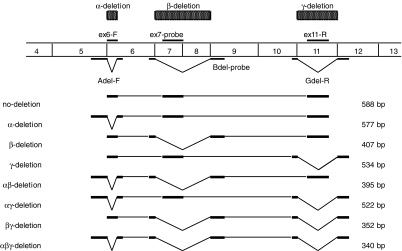
Theoretically, alternative splicing of the hTERT transcript may lead to eight isoforms, as illustrated in Figure 2. The α-deletion ASV, γ-deletion ASV, and (an ASV combination of these) the αγ deletion ASV were characterized by in-frame deletion, whereas the β-deletion ASV, αβ deletion ASV, βγ deletion ASV, and αβγ deletion ASV were characterized by out-of-frame deletion, followed by premature termination. All the ASVs were observed in this study.
Figure 2.
Theoretically, alternative splicing of the hTERT transcript may lead to many isoforms. The α site causes a 36-base deletion resulting in a no-frameshift mutation, and the β splice site results in a 182-base deletion resulting in a nonsense mutation. The γ splice site induces a 189-base deletion resulting in a no-frameshift mutation. The size of the PCR products produced by each primer set depends upon the alternative splicing of the hTERT transcript in the samples. The Adel-F and ex6-F primers were forward primers, and ex11-R and Gdel-R were reverse primers.
The results of the relative levels are shown in Table 3. No hTERT mRNA were detected in LI 90 and HUV-EC-C cells. In the three cell lines with hTERT signals, the average intensities of the no-deletion isoform, α-deletion, β-deletion, γ-deletion, αβ deletion, αγ deletion, βγ deletion, and αβγ deletion ASVs were 22%, 4%, 64%, 1%, 7%, < 1%, 2%, and < 1%, respectively. The total hTERT level was more than 104 copies in the Huh 7 and HLE cell lines, and about 103 copies in the Huh 6-clone 5 cell line. The β-deletion ASV showed the highest expression of all the hTERT isoforms. The γ-deletion ASV and the combination ASVs of the αγ, βγ, and αβγ deletion were of very low intensities.
Table 3.
Expression and Relative Levels of the hTERT and Alternatively Spliced Isoforms.
| Cell lines | hTERT mRNA* | Telomerase† activity | |||||||||
| No deletion | α | β | γ | αβ | αγ | βγ | αβγ | No deletion + β | Total hTERT | ||
| Huh 6 | 2.77 | 2.23 | 2.95 | 0.00 | 1.98 | 0.00 | 1.77 | 0.00 | 3.17 | 3.26 | 44 |
| (32.5%) | (9.4%) | (49.5%) | (0.0%) | (5.3%) | (0.0%) | (3.3%) | (0.0%) | (82.0%) | (100%) | ||
| Huh 7 | 3.28 | 1.93 | 4.03 | 1.79 | 3.08 | 1.57 | 2.06 | 0.00 | 4.10 | 4.15 | 76 |
| (13.4%) | (0.6%) | (75.9%) | (0.4%) | (8.6%) | (0.3%) | (0.8%) | (0.0%) | (89.3%) | (100%) | ||
| HLE | 3.29 | 2.30 | 3.84 | 2.32 | 2.89 | 0.00 | 2.04 | 1.93 | 3.95 | 4.01 | 69 |
| (18.9%) | (1.9%) | (67.7%) | (2.0%) | (7.5%) | (0.0%) | (1.1%) | (0.8%) | (86.6%) | (100%) | ||
| LI 90 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0 |
| (0.0%) | (0.0%) | (0.0%) | (0.0%) | (0.0%) | (0.0%) | (0.0%) | (0.0%) | (0.0%) | (0.0%) | ||
| HUV-EC-C | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0 |
| (0.0%) | (0.0%) | (0.0%) | (0.0%) | (0.0%) | (0.0%) | (0.0%) | (0.0%) | (0.0%) | (0.0%) | ||
Upper row: Each mRNA was calculate as log copies per microgram of total RNA; lower row: relative level of each mRNA was calculated.
The means of triplicate determinations are shown.
The means of duplicate determinations are shown (TPG units).
The average telomerase activities in the Huh 6-clone 5, Huh 7, and HLE cells were 44, 76, and 69 units, respectively.
Discussion
Telomerase is activated in a variety of malignant tumors [25]. Thus, regulation of telomerase activity could be an important mechanism to limit the growth of cancer cells. Telomerase activity has been shown to correlate well with the expression level of hTERT [26–28]. Furthermore, it has been reported that alternative splicing plays an important role in hTERT regulation [29,30]. The β-deletion ASV causes premature translation termination, whereas the α-deletion ASV is 36 bp and lies within RT motif A, suggesting that it may be a candidate as a dominant-negative inhibitor of telomerase [20,21]. The ASVs of the hTERT gene have been reported not only as deletion types, but also as insertion types. The insertion types of ASVs are the 38-nucleotide insertion of intron 4, the partial insertion of intron 11, the 159-nucleotide insertion of intron 14, and the replacement of the complete exon 15 and the 5′ part of exon 16 with the first 600 nucleotides of intron 14 [12]. Both the α- and γ-deletion ASVs were in-frame deletions, whereas the others were out-offrame mutations. The novel γ-deletion ASV will cause serious defects because the γ-deletion causes the loss of RT motifs D and E. Although the authors investigated only a limited number of hTERT-expressing cell lines, it is possible that the γ-deletion ASV may be a candidate as a dominant-negative inhibitor of telomerase, along with the α-deletion ASV.
It was clear that the intensity of telomerase activity in HCC nodules was closely associated with tumor differentiation [31]. The telomerase activity in well-differentiated HCC tissues was lower than that in poorly differentiated HCC tissues [32]. The total hTERT mRNA level in Huh 6-clone 5, a welldifferentiated cell line, was one sixth of that in Huh 7, a differentiated cell line, and one eighth of that in HLE, a nondifferentiated cell line. In this study, the hTERT expression in the well-differentiated HCC cell line was also lower than that in the not well-differentiated HCC cell lines.
In the HCC cell lines, our results complement those of Yi et al. [33] who described that the β-deletion ASV showed the highest expression of all the hTERT isoforms. In this study, the amount of both the β-deletion ASV and the no-deletion isoform also accounted for more than 80% of the total hTERT levels. If the γ-deletion ASV was a dominant-negative inhibitor, the hTERT mRNA that introduced telomerase activity were both the β-deletion ASV and the no-deletion isoforms. In this study, there was a statistically significant difference between the amount of the no-deletion isoform as well as the β-deletion ASV expression and the telomerase activity (r2 = 0.983). Thus, researchers who utilize a genetic marker instead of the telomerase activity [34,35] should measure both hTERT levels.
Although the γ-deletion ASV showed a low expression in the cell lines with high telomerase activity, this ASV may occasionally be detected in samples with low telomerase activity, such as HCC nodules. This ASV was not necessarily specific for tumor cell lines; however, it may have some roles as a dominant-negative inhibitor, along with the α-deletion ASV.
While there are still concerns, our results might provide the basis for more detailed studies on the regulation of telomerase activity, and may lead to the development of new cancer therapies. Because this study on the associations between these ASVs and the clinicopathological features have only been developing recently, it will provide useful information not only for developing prevention strategies for HCC but also for clarifying the biological mechanism of ASVs in human diseases.
Acknowledgements
The authors are indebted to J. Patrick Barron of the International Medical Communication Center of the Tokyo Medical University for his review of this manuscript. We are also grateful to Akiko Haraki (SRL Inc., Kobe, Japan) for her kind assistance in this study.
Abbreviations
- hTERT
human telomerase reverse transcriptase
- ASV
alternatively spliced variant
- HCC
hepatocellular carcinoma
- RT real-time PCR
reverse transcriptase real-time polymerase chain reaction
- TRAP
telomeric repeat amplification protocol
Footnotes
The sequences reported in this paper have been deposited in the GenBank database (accession nos. AB085628).
References
- 1.Hanke J, Brett D, Zastrow I, Aydin A, Delbruck S, Lehmann G, Luft F, Reich J, Bork P. Alternative splicing of human genes more the rule than the exception. Trends Genet. 1999;15:389–390. doi: 10.1016/s0168-9525(99)01830-2. [DOI] [PubMed] [Google Scholar]
- 2.Mironov AA, Fickett JW, Gelfland MS. Frequent alternative splicing of human genes. Genome Res. 1999;9:1288–1293. doi: 10.1101/gr.9.12.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Der Houven Van Oordt W, Newton K, Screaton GR, Caceres JF. Role of SR protein modular domains in alternative splicing specificity in vivo. Nucleic Acids Res. 2000;28:4822–4831. doi: 10.1093/nar/28.24.4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pilch B, Allemand E, Facompre M, Bailly C, Riou JF, Soret J, Tazi J. Specific inhibition of serine- and arginine-rich splicing factors phosphorylation, spliceosome assembly, and splicing by the antitumor drug NB-506. Cancer Res. 2001;61:6876–6884. [PubMed] [Google Scholar]
- 5.Stallings-Mann ML, Ludwiczak RL, Klinger KW, Rottman F. Alternative splicing of exon 3 of the human growth hormone receptor is the result of an unusual genetic polymorphism. Proc Natl Acad Sci USA. 1996;93:12394–12399. doi: 10.1073/pnas.93.22.12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu W, Qian C, Francke U. Silent mutation induces exon skipping of fibrillin-1 gene in Marfan syndrome. Nat Genet. 1997;16:328–329. doi: 10.1038/ng0897-328. [DOI] [PubMed] [Google Scholar]
- 7.Siffert W, Posskopf D, Siffert G, Busch S, Moritz A, Erbel R, Sharma AM, Ritz E, Wichmann HE, Jakobs KH, Horsthemke B. Association of human G-protein beta3 subunit variant hypertension. Nat Genet. 1998;18:45–48. doi: 10.1038/ng0198-45. [DOI] [PubMed] [Google Scholar]
- 8.Hisatomi H, Kohno N, Wakita K, Nagao K, Hirata H, Hikiji K, Harada S. A novel alternatively spliced variant with a deletion of 52 bp in exon 6 of the progesterone receptor gene is frequently observed in breast cancer. Int J Cancer. 2003 doi: 10.1002/ijc.11050. (in press) [DOI] [PubMed] [Google Scholar]
- 9.Nakamura TM, Morin GB, Chapman KB, Weinrich SL, Andrews WH, Linger J, Harley CB. Telomerase catalytic subunit homologs from fission yeast and human. Science. 1997;277:955–959. doi: 10.1126/science.277.5328.955. [DOI] [PubMed] [Google Scholar]
- 10.Meyerson M, Counter CM, Eaton EN, Ellisen LW, Steiner P, Caddle SD, Ziaugra L, Beijersbergen RL, Davidoff MJ, Liu Q, Bacchetti S, Haber DA, Weinberg RA. hEST2, putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell. 1997;90:785–795. doi: 10.1016/s0092-8674(00)80538-3. [DOI] [PubMed] [Google Scholar]
- 11.Harrington L, Zhou W, McPhail T, Oulton R, Yeung DS, Mar V, Bass MB, Robinson MO. Human telomerase contains evolutionarily conserved catalytic and structural subunits. Genes Dev. 1997;11:3109–3115. doi: 10.1101/gad.11.23.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kilian A, Bowtell DD, Abud HE, Hime GR, Venter DJ, Keese PK, Duncan EL, Reddel RR, Jefferson RA. Isolation of a candidate human telomerase catalytic subunit gene, which complex splicing patterns in different cell types. Hum Mol Genet. 1997;6:2011–2019. doi: 10.1093/hmg/6.12.2011. [DOI] [PubMed] [Google Scholar]
- 13.Ulaner GA, Hu JF, Vu TH, Gludine LC, Hoffman AR. Telomerase activity in human development is regulated by human telomerase reverse transcriptase (hTERT) transcription and by alternate splicing of hTERT transcripts. Cancer Res. 1998;58:4168–4172. [PubMed] [Google Scholar]
- 14.Shay JW, Zou Y, Hiyama E, Wright WE. Telomerase and cancer. Hum Mol Genet. 2001;10:677–685. doi: 10.1093/hmg/10.7.677. [DOI] [PubMed] [Google Scholar]
- 15.Shay JW. Telomerase in human development and cancer. J Cell Physiol. 1997;173:266–270. doi: 10.1002/(SICI)1097-4652(199711)173:2<266::AID-JCP33>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 16.Holt SE, Shay JW. Role of telomerase in cellular proliferation and cancer. J Cell Physiol. 1999;180:10–18. doi: 10.1002/(SICI)1097-4652(199907)180:1<10::AID-JCP2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 17.Wick M, Zubov D, Hagen G. Genomic organization and promoter characterization of the gene encoding the human telomerase reverse transcriptase (hTERT) Gene. 1999;232:97–106. doi: 10.1016/s0378-1119(99)00108-0. [DOI] [PubMed] [Google Scholar]
- 18.Lingner J, Hughes TR, Shevchenko A, Mann M, Lundblad V, Cech TR. Reverse transcriptase motif in the catalytic subunit of telomerase. Science. 1997;276:561–567. doi: 10.1126/science.276.5312.561. [DOI] [PubMed] [Google Scholar]
- 19.Yokoyama Y, Wan X, Takahashi Y, Shinohara A, Tamaya T. Alternatively spliced variant deleting exon 7 and 8 of the human telomerase reverse transcriptase gene is dominantly expressed in the uterus. Mol Hum Reprod. 2001;7:853–857. doi: 10.1093/molehr/7.9.853. [DOI] [PubMed] [Google Scholar]
- 20.Colgin LM, Wilkinson C, Englezou A, Kilian A, Robinson MO, Reddel RR. The hTERT alpha splice variant is a dominant negative inhibitor of telomerase activity. Neoplasia. 2000;2:426–432. doi: 10.1038/sj.neo.7900112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yi X, White DM, Aisner DL, Baur JA, Wright WE, Shay JW. An alternate splicing variant of the human telomerase catalytic subunit inhibits telomerase activity. Neoplasia. 2000;2:433–440. doi: 10.1038/sj.neo.7900113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;25:169–173. doi: 10.1677/jme.0.0250169. [DOI] [PubMed] [Google Scholar]
- 23.Ohyashiki JH, Ohyashiki K, Toyama K, Shay JW. A non radioactive fluorescence-based telomeric repeat amplification protocol to detect and quantitate telomerase activity. Trends Genet. 1996;12:395–396. doi: 10.1016/s0168-9525(96)90097-9. [DOI] [PubMed] [Google Scholar]
- 24.Hisatomi H, Nagao K, Komatsu H. Quantification of telomerase activity in human liver tissues by fluorescence-based TRAP analysis. Hepatol Res. 1997;7:35–42. [Google Scholar]
- 25.Hiyama E, Hiyama K. Clinical utility of telomerase in cancer. Oncogene. 2002;21:643–649. doi: 10.1038/sj.onc.1205070. [DOI] [PubMed] [Google Scholar]
- 26.Cong YS, Wright WE, Shay JW. Human telomerase and its regulation. Microbiol Mol Biol Rev. 2002;66:407–425. doi: 10.1128/MMBR.66.3.407-425.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toshikuni N, Nouso K, Higashi T, Nakatsukasa H, Onishi T, Kaneyoshi T, Kobayashi Y, Kariyama K, Yamamoto K, Tsuji T. Expression of telomerase-associated protein 1 and telomerase reverse transcriptase in hepatocellular carcinoma. Br J Cancer. 2000;82:833–837. doi: 10.1054/bjoc.1999.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ito H, Kyo S, Kanaya T, Takakura M, Inoue M, Namiki M. Expression of human telomerase subunits and correlation with telomerase activity in urothelial cancer. Clin Cancer Res. 1998;4:1603–1608. [PubMed] [Google Scholar]
- 29.Tomoda R, Seto M, Tsumuki H, Iida K, Yamazaki T, Sonoda J, Matsumine A, Uchida A. Telomerase activity and human telomerase reverse transcriptase mRNA expression are correlated with clinical aggressiveness in soft tissue tumors. Cancer. 2002;95:1127–1133. doi: 10.1002/cncr.10793. [DOI] [PubMed] [Google Scholar]
- 30.Ulaner GA, Hu JF, Vu TH, Oruganti H, Giudice LC, Hoffman AR. Regulation of telomerase by alternative splicing of human telomerase reverse transcriptase (hTERT) in normal and neoplastic ovary, endometrium and myometrium. Int J Cancer. 2000;85:330–335. [PubMed] [Google Scholar]
- 31.Kanamaru T, Yamamoto M, Morita Y, Ito T, Kuroda Y, Hisatomi H. Clinical implications of telomerase activity in resected hepatocellular carcinoma. Int J Mol Med. 1999;4:267–271. [PubMed] [Google Scholar]
- 32.Ide T, Tahara H, Nakashio R, Kitamoto M, Nakanishi T, Kajiyama G. Telomerase in hepatocellular carcinogenesis. Hum Cell. 1996;9:283–286. [PubMed] [Google Scholar]
- 33.Yi X, Shay JW, Wright WE. Quantitation of telomerase components and hTERT mRNA splicing patterns in immortal human cells. Nucleic Acids Res. 2001;29:4818–4825. doi: 10.1093/nar/29.23.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gertler R, Rosenberg R, Stricker D, Werner M, Lassmann S, Ulm K, Nekarda H, Siewert JR. Prognostic potential of the telomerase subunit human telomerase reverse transcriptase in tumor tissue and nontumorous mucosa from patients with colorectal carcinoma. Cancer. 2002;95:2103–2111. doi: 10.1002/cncr.10939. [DOI] [PubMed] [Google Scholar]
- 35.Kawakami Y, Kitamoto M, Nakanishi T, Yasui W, Tahara E, Nakayama J, Ishikawa F, Tahara H, Ide T, Kajiyama G. Immunohistochemical detection of human telomerase reverse transcriptase in human liver tissues. Oncogene. 2000;19:3888–3893. doi: 10.1038/sj.onc.1203733. [DOI] [PubMed] [Google Scholar]