Abstract
Medulloblastomas are malignant brain tumors that arise in the cerebella of children. The presumed cells-of-origin are undifferentiated precursors of granule neurons that occupy the external granule layer (EGL) of the developing cerebellum. The overexpression of proteins that normally stimulate proliferation of neural progenitor cells may initiate medulloblastoma formation. Two known mitogens for neural progenitors are the c-Myc oncoprotein and Sonic hedgehog (Shh), a crucial determinant of embryonic pattern formation in the central nervous system. We modeled the ability of c-Myc and Shh to induce medulloblastoma in mice using the RCAS/tv-a system, which allows postnatal gene transfer and expression in a cell type-specific manner. We targeted the expression of Shh and c-Myc to nestin-expressing neural progenitor cells by injecting replication-competent ALV splice acceptor (RCAS) vectors into the cerebella of newborn mice. Following injection with RCAS-Shh alone, 3/32 (9%) mice developed medulloblastomas and 5/32 showed multifocal hyperproliferation of the EGL, possibly a precursor stage of medulloblastoma. Following injection with RCAS-Shh plus RCAS-Myc, 9/39 (23%) mice developed medulloblastomas. We conclude that nestin-expressing neural progenitors, present in the cerebellum at birth, can act as the cells-of-origin for medulloblastoma, and that c-Myc cooperates with Shh to enhance tumorigenicity.
Keywords: medulloblastoma, RCAS/tv-a, Sonic hedgehog, Myc, neural progenitors
Introduction
Primitive neuroectodermal tumors (PNETs) are the most common malignant brain tumors in children. Most often, these tumors arise in the cerebellum where they are called medulloblastomas. Although the precise histogenesis of medulloblastoma remains uncertain, one hypothesis maintains that the cells-of-origin are undifferentiated precursors of granule cell neurons (reviewed in Ref. [1]). Granule neuron precursors (GNPs) occupy the external granule layer (EGL), a germinal zone on the cortical surface of the developing cerebellum. In mice, rapid proliferation of GNPs after birth expands the EGL to a maximal thickness on postnatal day 6. Afterwards, GNPs migrate inward where they differentiate to generate the millions of neurons that make up the internal granule layer of the cerebellum (reviewed in Ref. [2]).
Overexpression of proteins that normally stimulate proliferation and/or block differentiation of neural progenitor cells may initiate medulloblastoma formation. One known mitogen for neural progenitor cells is Sonic hedgehog (Shh), a secreted protein that is a crucial determinant of embryonic pattern formation in the central nervous system. Shh binds transmembrane protein, Patched (Ptc), at the cell surface. This interaction activates a signal transduction pathway, leading to the transcription of genes that govern various aspects of neural development (reviewed in Ref. [3]). In the developing cerebellum, Shh stimulates the proliferation of GNPs and blocks their differentiation into neurons [4–6].
Mutations that activate the Ptc signaling pathway are oncogenic. Transgenic mice expressing mutant Smoothened in the skin develop basal cell carcinomas [7]. Inherited mutations in the human PTC gene segregate in families with Gorlin's Syndrome, a condition wherein affected individuals develop neural tube defects, craniofacial abnormalities, and predisposition to various neoplasms, including medulloblastomas [8]. PTC gene mutations occur in 3% to 14% of sporadic medulloblastomas [9–11]. Mice that are heterozygous defective for Ptc develop medulloblastomas spontaneously [12,13]. Expression of the wild-type Ptc allele in tumors from Ptc+/- mice suggests that haploinsufficiency, not a two-hit mechanism of Ptc gene inactivation, promotes medulloblastoma growth [14,15]. Intrauterine injection of a Shh-expressing retrovirus into the cerebellum of mice on embryonic day 13.5 induces medulloblastoma formation [16].
Another mitogen for neural progenitors is the oncoprotein, c-Myc. We have shown that c-Myc promotes the proliferation of neural progenitor cells in mice [17]. The c-Myc gene is amplified in 5% to 8% of human medulloblastomas and overexpressed in 42% to 90% of cases [18,19]. Moreover, the accumulation of c-Myc mRNA is an unfavorable prognostic indicator for medulloblastoma patients [20,21]. Although these clinical correlations support a significant role for c-Myc in medulloblastoma, we do not know whether c-Myc expression is an early event critical for tumor initiation, or a late event involved in tumor progression.
We modeled the ability of c-Myc and Shh to induce medulloblastoma in mice using the RCAS/tv-a system, which allows postnatal gene transfer in a cell type-specific manner [22,23]. This system utilizes replication-competent ALV splice acceptor (RCAS) vectors, derived from the avian retrovirus, ALV (subgroup A), and a transgenic mouse line (Ntv-a) that produces TVA (the receptor for ALV-A) under the control of the Nestin gene promoter. Nestin is an intermediate filament protein expressed by neuronal and glial progenitors [24]. After infection, RCAS sequences integrate into the host cell genome where the exogenous gene is expressed from a spliced message under control of the constitutive retroviral promoter, LTR. Combinations of genes can be transferred simultaneously to individual cells by infecting them with multiple RCAS vectors carrying different genes. We transferred genes encoding Shh and c-Myc to nestin-expressing neural progenitor cells in the cerebella of newborn mice. We show here that nestin-expressing neural progenitors, present in the cerebellum at birth, can act as the cells-of-origin for medulloblastoma and that c-Myc cooperates with Shh to enhance tumorigenicity.
Materials and Methods
Mice
The production of the Ntv-a transgenic mouse line has been described previously [25]. Ntv-a/Ptc+/- mice were created by breeding Ntv-a mice with mice containing a mutant allele (Ptcneo67), wherein a neomycin resistance cassette replaces exons 6 and 7 of the Ptc gene [13]. Therefore, the mice used in these experiments are mixtures of the following strains: C57BL/6, BALB/C, FVB/N, and CD1.
Vector Constructs
RCAS-Shh was constructed by ligating full-length, chicken Shh cDNA 3′ of the retroviral env gene into parent retroviral vector, RCASBP(A) [22]. The Shh insert contained an in-frame, carboxy-terminal epitope tag containing six repeats of the influenza virus hemagglutinin epitope (HA tag). RCAS-Myc contained full-length, human c-Myc cDNA as described previously [17].
Cell Culture
To produce live virus, we used DF-1 cells, an immortalized line of chicken fibroblasts. DF-1 cells were cultivated in DMEM supplemented with 5% fetal bovine serum, 5% calf serum, 1% chicken serum, and 0.2% tryptose phosphate broth. We transfected plasmid versions of RCAS vectors into DF-1 cells and allowed them to replicate as viral vectors in culture.
In Vivo Infection of Transgenic Mice
DF-1 virus producer cells were harvested by trypsin digestion, collected by centrifugation, and resuspended in phosphate-buffered saline. Injections of 1 to 2 µl (105 cells) were made with a gas-tight Hamilton syringe into the lateral cerebellum just posterior to the bregma of the skull, or into the right frontal lobe just anterior to the coronal suture within 48 hours after birth. The mice were sacrificed 12 weeks after injection, or sooner if they showed signs of increased intracranial pressure or became debilitated. The brains were removed and fixed in phosphate-buffered formalin (10%). After fixation, each brain was divided into quarters by parallel incisions in the coronal plane and embedded in paraffin. Tissue sections (4 µm) were mounted on glass slides for histochemical and immunocytochemical analysis.
Immunocytochemistry
To analyze protein expression in tissue sections, we used an immunoperoxidase staining method described previously [26]. We used the following monoclonal antibodies (and dilutions) from the indicated commercial sources: 9E10 (1:50)—human c-Myc (Santa Cruz Biotechnology; Santa Cruz, CA); C19 (1:200)—mouse c-Myc (Santa Cruz Biotechnology); F7 (1:50)—HA (Santa Cruz Biotechnology); 2F11 (1:100)—70-kDa neurofilament protein (Dako, Carpinteria, CA); TuJ1 (1:400)—βIII tubulin (Research Diagnostics, Flanders, NJ); 401 (1:50)—nestin (Becton Dickinson PharMingen, San Diego, CA); Mab377 (1:100)—NeuN (Chemicon, Temecula, CA). To detect GFAP expression, we used a rabbit, anticow polyclonal antibody (1:1000; Dako). For double immunofluorescence staining, we incubated formalin-fixed tissue sections with mouse monoclonal antibody 9E10 (anti-Myc), and rabbit polyclonal antibody Y11 (anti-HA; Santa Cruz Biotechnology), followed by secondary antibodies Cy5-conjugated antimouse IgG and FITC-conjugated antirabbit IgG. Cell nuclei were counterstained with propidium iodide. Digital images were generated using confocal microscopy.
Western Blot Analysis of Primary Brain Cultures Infected with RCAS-Shh
We prepared primary brain cultures from newborn Ntv-a mice, infected them with RCAS-Shh viral vector, and prepared whole cell lysates of the cultured cells 48 hours after infection as described previously [17]. Protein samples (60 µg) were resolved by SDS-PAGE using 10% gels, transferred to nitrocellulose filters, and probed with monoclonal antibody F7, directed against the HA epitope tag (85 amino acids) located on the carboxy terminus of the recombinant Shh protein. Detection was carried out using alkaline phosphatase-conjugated secondary antibody (goat antimouse IgG) and bromochloroindoyl phosphate/nitro blue tetrazolium substrate.
Results
Overexpression of Shh in Neural Progenitors Induces EGL Hyperproliferation and Medulloblastoma Formation
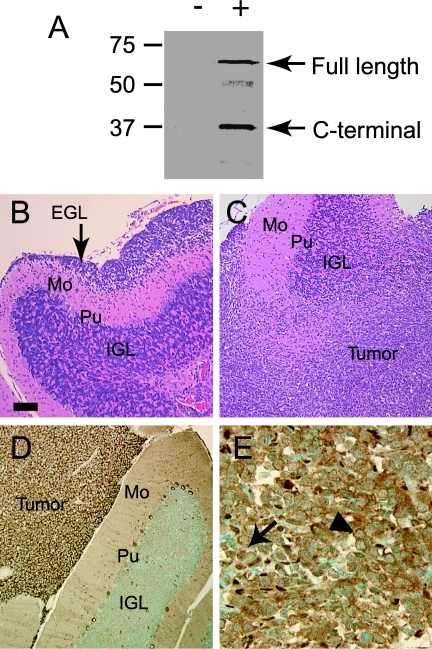
We modeled the ability of Shh and c-Myc, alone and in combination, to induce medulloblastoma formation by injecting RCAS vectors into the cerebella of newborn Ntv-a mice. To study the effect of Ptc, we injected multiple, different litters of mice born of Ntv-a/Ptc+/- parents. We reported previously that RCAS-Myc produces an intact c-Myc protein that stimulates proliferation of neural progenitor cells [17]. To show that our RCAS-Shh vector was capable of producing an intact Shh protein, we performed Western blot analysis of primary brain cultures from Ntv-a mice before and after infection with RCAS-Shh. Two major proteins, 60 and 37 kDa in size, were detected in infected cells with an antibody directed against the carboxy-terminal HA epitope tag of RCAS-Shh (Figure 1A). These two proteins correspond to full-length Shh and a carboxy-terminal fragment resulting from Shh autoproteolysis [27].
Figure 1.
Histopathology of cerebellar lesions induced by Shh. (A) Western blot analysis of Ntv-a mouse brain cultures infected with RCAS-Shh. Two major bands (60 and 37 kDa) corresponding to full-length Shh and carboxy-terminal peptide derived from autoproteolysis were detected with anti-HA antibody in infected cells (+) but not uninfected cells (-). (B) Focal hyperproliferation of EGL induced by Shh (Mo—molecular layer; Pu—Purkinje cell layer; IGL—internal granule layer). (C) Shh-induced medulloblastoma infiltrating cerebellar cortex. (D) Cytoplasmic expression of endogenous c-Myc in tumor cells in Shh-induced medulloblastoma. (E) Human medulloblastoma specimen showing expression of c-Myc in nuclei (arrow) and cytoplasm (arrowhead) of tumor cells. Scale bar = 110 µm (B,C), 55 µm (D), 27 µm (E).
In 5/32 mice (16%) injected with RCAS-Shh alone, the cerebella contained multiple foci where the EGL was expanded to 10 to 20 cell layers (Figure 1B). Normally, the EGL contains only none to two layers at the developmental stages of mice we examined. The cells in the thickened EGL expressed neuronal markers, βIII tubulin, and NeuN, characteristic of proliferating GNPs. In 3/32 mice (9%), RCAS-Shh produced infiltrating tumors that closely resembled human medulloblastomas (Figure 1C).
c-Myc Enhances Shh-Induced Medulloblastoma Formation
Immunoperoxidase staining with an antibody against endogenous c-Myc showed abundant immunoreactivity in the cytoplasm of cells within the EGL hyperproliferations and the tumors (Figure 1D). c-Myc is normally a nuclear protein, but it localizes to the cytoplasm in cerebellar Purkinje neurons and in various tumors [28]. To determine whether the cytoplasmic localization of c-Myc in mouse tumors accurately modeled human medulloblastomas, we mapped the subcellular localization of c-Myc in 16 human medulloblastoma specimens by immunocytochemistry. Consistent with previous reports [19], c-Myc localized to the nucleus in 11 tumors. c-Myc immunoreactivity was restricted to the cytoplasm in four tumors and present in both compartments in one tumor (Figure 1E).
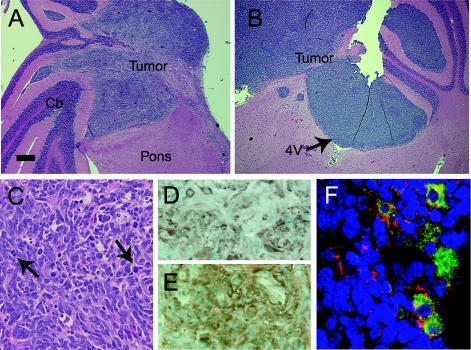
Immunoperoxidase staining of normal mouse cerebellum at postnatal day 3 showed expression of c-Myc throughout the EGL (not shown). Previous experiments indicate that c-Myc helps maintain neural progenitor cells in an undifferentiated and proliferating state [17]. Therefore, we hypothesized that additional c-Myc might cooperate with Shh to enhance tumorigenicity. To test this hypothesis, we injected Ntv-a mice with RCAS-Shh plus RCAS-Myc. With this combination, tumors developed in 9/39 mice (23%), a higher percentage than with RCAS-Shh alone (Table 1). The tumors arose from the lateral aspect of the cerebellar hemisphere and infiltrated the adjacent brainstem (Figure 2A). In one case, the tumor filled the fourth ventricle, a growth pattern mimicking human medulloblastomas (Figure 2B). Like human medulloblastomas, these murine tumors were composed of densely packed sheets of cells with hyperchromatic nuclei and scant cytoplasm, overlying a delicate vascular network (Figure 2C). Nuclear pleomorphism and mitotic figures were present, indicating a rapid proliferative rate. To verify that cells within the observed brain lesions expressed the genes transferred through RCAS vectors, we demonstrated specific staining of most cells within tumors and EGL hyperproliferations with antibodies against epitope tags within RCAS-Shh and RCAS-Myc (Figure 2, D and E). Double immunofluorescence staining showed that 5% to 10% of cells coexpressed Shh and c-Myc (Figure 2F). All tumors showed expression of synaptophysin, a reliable histopathological marker for medulloblastomas [29] (Figure 3A). We did not see tumors or EGL hyperproliferation in mice injected with RCAS-Myc alone.
Table 1.
Medulloblastoma Formation and EGL Hyperproliferation Induced by Shh and/or c-Myc.
| RCAS vector | |||
| Shh | Myc + Shh | Myc | |
| Medulloblastoma | 3/32 | 9/39 | 0/43 |
| EGL hyperproliferation | 5/32 | 0/39 | 0/43 |
Figure 2.
Histopathology of medulloblastomas induced by c-Myc plus Shh. (A,B) Medulloblastomas infiltrating the lateral pons and cerebellum (Cb) and filling the fourth ventricle (4V). (C) Cytoarchitecture of medulloblastoma induced by c-Myc plus Shh. Arrows indicate mitotic figures. (D,E) Immunoperoxidase staining of virally transduced Shh and c-Myc in tumor cells with antibodies against HA (D) and Myc (E) epitopes. (F) Double immunofluorescence staining of Shh (green) and c-Myc (red) shows colocalization (yellow) of both proteins in the cytoplasm of a subpopulation of medulloblastoma cells. Nuclei are counterstained with propidium iodide (blue). Scale bar = 474 µm (A,B), 27 µm (C–E), 27 µm (F).
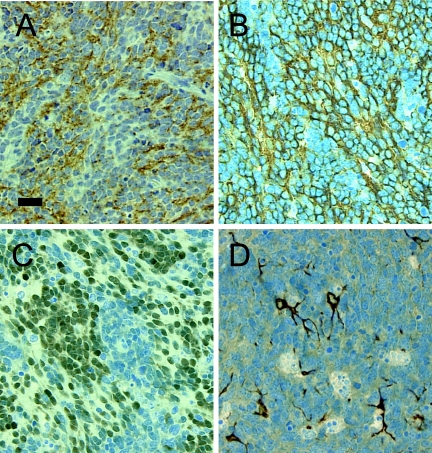
Figure 3.
Expression of neural antigens in medulloblastomas induced by c-Myc plus Shh. Expression of synaptophysin (A), βIII tubulin (B), and NeuN (C) in tumor cells and GFAP (D) in processes of entrapped astrocytes. Scale bar = 27 µm.
Some human medulloblastomas contain subpopulations of cells expressing antigens characteristic of differentiated neurons or glia, whereas others are composed entirely of undifferentiated cells. To assess the differentiation status of the mouse tumors, we performed an immunocytochemical analysis of seven tumors induced by Myc + Shh and two by Shh using antibodies specific for nestin, glial fibrillary acidic protein (GFAP), neurofilament protein, NeuN, and hIII tubulin. Nestin was detected in 4/9 tumors where the percentage of nestin-expressing cells ranged from 5% to 20%. This low percentage indicates the lack of selective pressure for infected cells to maintain nestin expression in vivo. The cell type specificity of the RCAS/tv-a system is limited to the original gene transfer event, after which the transferred gene is expressed from the retroviral promoter, LTR.
All tumors showed abundant expression of βIII tubulin in the cytoplasm and NeuN in the nucleus (Figure 3, B and C). The expression of βIII tubulin by central nervous system progenitor cells is an early marker of neuronal differentiation [30]. NeuN is a nuclear protein expressed by many types of mature neurons [31]. Like βIII tubulin, NeuN first appears at developmental time points corresponding to the initiation of neuronal differentiation. We did not detect the expression of neurofilament protein, a marker of terminally differentiated neurons, in any tumor. GFAP immunoreactivity was visible only in processes of entrapped astrocytes interdigitated among unstained tumor cells (Figure 3D). These immunocytochemistry results support an origin of the induced tumors from neuronal precursors.
Effect of Ptc Genotype and Gene Transfer Site on Tumor Formation
Ptc+/- mice develop rhabdomyosarcomas and medulloblastomas spontaneously [12,13], but tumor incidence is highly dependent upon genetic background. In point of fact, rhabdomyosarcomas arise in 15% of CD1 Ptc+/-, mice but no such tumors arise on a C57BL/6 background [32]. To determine whether medulloblastomas arise spontaneously on the mixed genetic background of our Ntv-a/Ptc+/- mice, we examined histological sections of brains from sixteen 1-year-old mice. We found no tumors in the forebrain, brainstem, or cerebellum. Furthermore, we genotyped the 71 mice injected into the cerebellum with RCAS-Shh alone or in combination with RCAS-Myc. In this group, 53 were Ptc+/- and 18 were Ptc+/+. These proportions reflect the distribution of alleles among the progeny of heterozygous parents (Ptc nullizygotes die early in embryonic development). Medulloblastomas occurred more frequently in Ptc+/+ mice (5/18 = 28%) than in Ptc+/- mice (7/53 = 13%) (Table 2). Similarly, EGL hyperproliferation occurred more frequently in Ptc+/+ mice (4/18 = 22%) than in Ptc+/- mice (1/53 = 2%). For these reasons, we cannot attribute the cerebellar lesions observed following targeted expression of Shh and/or c-Myc simply to spontaneous tumor formation.
Table 2.
Effect of Ptc Genotype on Medulloblastoma Formation and EGL Hyperproliferation Induced by Shh and c-Myc + Shh.
| Ptc genotype | ||
| Ptc+/+ | Ptc+/- | |
| Medulloblastoma | 5/18 | 7/53 |
| EGL hyperproliferation | 4/18 | 1/53 |
PNETs arise in the cerebral hemispheres in children, albeit much less frequently than in the cerebellum. Nestin-expressing neural progenitors that are susceptible to infection and gene transfer with RCAS vectors are present in the cerebral hemispheres. To determine whether these cells could produce PNETs when stimulated by Shh and c-Myc, we injected RCAS-Shh plus RCAS-Myc into the right frontal lobe of newborn Ntv-a mice. Microscopic examination of brain sections from 29 mice sacrificed after 13.7 weeks showed that no brain tumors had formed.
Discussion
Using the RCAS/tv-a system, we have recapitulated in mice some of the molecular events that mediate medulloblastoma formation in humans. Expression profile analysis of human medulloblastomas using oligonucleotide microarrays has shown that transcriptional targets of Shh (Gli transcription factors and Ptc) are frequently upregulated [33]. This profile was found predominantly in a histological variant known as desmoplastic medulloblastoma, which is characterized by dense fibrous stroma surrounding aggregates of tumor cells. The fact that our mouse tumors did not resemble desmoplastic medulloblastomas indicates that Shh/Ptc pathway activation is not unique to the desmoplastic variant and may be a more general molecular derangement in medulloblastoma.
Overexpression of the c-Myc oncogene occurs frequently in human medulloblastomas and correlates with poor patient prognosis [20,21]. We showed previously that c-Myc stimulates the proliferation of undifferentiated neural progenitor cells in the forebrain of mice, and we suggested that, through this mechanism, c-Myc might promote PNET formation [17]. In the present study, c-Myc was not sufficient to induce medulloblastoma formation. The enhanced tumorigenicity in mice injected with two RCAS vectors expressing c-Myc and Shh suggests that c-Myc cooperates with Shh to drive proliferating neural progenitors into malignant transformation. The fact that a minority of tumor cells coexpressed Shh and c-Myc suggests that the tumorigenic effect of Shh (a secreted protein) is mediated predominantly through a paracrine, rather than an autocrine (cell-automatous), mechanism.
Other mouse models of medulloblastoma have shown that these tumors can arise by subversion of cell growth pathways other than the Shh/Ptc pathway. Medulloblastomas develop in p53-null mice after conditional inactivation of the Rb gene [34], or in combination with homozygous loss of Lig4, which encodes a nuclear ligase critical for DNA repair [35]. Loss of p53 accelerates medulloblastoma formation in Ptc+/- mice [36]. Transgenic mice expressing SV40 virus large T antigen, which functionally inactivates both Rb and p53, develop brainstem PNETs [37]. Considering the fact that loss-of-function mutations in either RB or P53 rarely occur in human medulloblastomas, our model has the advantage of more closely paralleling the molecular pathways relevant to human tumors.
Other investigators recently reported that ectopic expression of Shh in prenatal mouse cerebellum induces EGL thickening and medulloblastomas [16]. They utilized a murine leukemia virus vector, injected in utero, to infect a broad population of dividing cells. Our results confirm their observations and extend them by showing that c-Myc cooperates with Shh to enhance tumorigenicity. Furthermore, our results in Ntv-a mice define a more narrow population of candidate cells-of-origin for medulloblastomas, namely, nestin-expressing neural progenitors.
We cannot conclude that the tumors originated from GNPs within the EGL because we did not specifically target these precursor cells. Nevertheless, several features of our model are consistent with a GNP origin for medulloblastoma. First, the EGL contains the highest concentration of mitotically active, nestin-expressing cells susceptible to infection with RCAS vectors. Second, significant numbers of cells within the EGL thickenings and in the tumors expressed βIII tubulin and NeuN—markers for early neuronal differentiation. Finally, the coexistence of EGL thickening and medulloblastoma in cerebella injected with RCAS-Shh implicates hyperproliferation of GNPs as a precursor stage in the genesis of medulloblastoma.
We could not produce tumors in the forebrain by injecting RCAS-Shh, alone or in combination with RCAS-Myc, even though nestin-expressing progenitors are present in that part of the brain in postnatal mice. These cells are known to give rise to glioblastomas in vivo with specific activation of the Ras and Akt signaling pathways [38], and to oligodendrogliomas with overexpression of platelet-derived growth factor [39]. Our observation that cerebral progenitor cells are less responsive to the same molecules that are potent mitogens for cerebellar progenitors could explain why cerebral hemisphere PNETs are rare compared with cerebellar medulloblastomas.
Acknowledgements
The authors thank Ken Hill and John Rose (Veterans Administration Medical Center, Salt Lake City, UT) for assistance with confocal microscopy.
Abbreviations
- PNET
primitive neuroectodermal tumor
- GNP
granule neuron precursor
- EGL
external granule layer
Footnotes
This work was supported by grants from the Pediatric Brain Tumor Foundation of the US and The Brain Tumor Society.
References
- 1.Wechsler-Reya R, Scott MP. The developmental biology of brain tumors. Annu Rev Neurosci. 2000;24:385–428. doi: 10.1146/annurev.neuro.24.1.385. [DOI] [PubMed] [Google Scholar]
- 2.Hatten ME, Heintz N. Mechanisms of neural patterning and specification in the developing cerebellum. Annu Rev Neurosci. 1995;18:385–408. doi: 10.1146/annurev.ne.18.030195.002125. [DOI] [PubMed] [Google Scholar]
- 3.Ho KS, Scott MP. Sonic hedgehog in the nervous system: functions, modifications and mechanisms. Curr Opin Neurobiol. 2002;12:57–63. doi: 10.1016/s0959-4388(02)00290-8. [DOI] [PubMed] [Google Scholar]
- 4.Wechsler-Reya RJ, Scott MP. Control of neuronal precursor proliferation in the cerebellum by sonic hedgehog. Neuron. 1999;22:103–114. doi: 10.1016/s0896-6273(00)80682-0. [DOI] [PubMed] [Google Scholar]
- 5.Wallace VA. Purkinje-cell-derived Sonic hedgehog regulates granule neuron precursor cell proliferation in the developing mouse cerebellum. Curr Biol. 1999;9:445–448. doi: 10.1016/s0960-9822(99)80195-x. [DOI] [PubMed] [Google Scholar]
- 6.Dahmane N, Ruiz-i-Altaba A. Sonic hedgehog regulates the growth and patterning of the cerebellum. Development. 1999;126:3089–3100. doi: 10.1242/dev.126.14.3089. [DOI] [PubMed] [Google Scholar]
- 7.Xie J, Murone M, Luoh S, Ryan A, Gu W, Zhang C, Bonifas JM, Lam C, Hynes M, Goddard A, Rosenthal A, Epstein EH, de Sauvage SJ. Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature. 1998;391:90–92. doi: 10.1038/34201. [DOI] [PubMed] [Google Scholar]
- 8.Johnson RL, Rothman AO, Xie J, Goodrich LV, Bare JW, Bonifas JM, Quinn AG, Myers RM, Cox DR, Epstein EH, Scott MP. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272:1668–1671. doi: 10.1126/science.272.5268.1668. [DOI] [PubMed] [Google Scholar]
- 9.Raffel C, Jenkins RB, Frederick L, Hebrink D, Aldrete B, Fults DW, James CD. Sporadic medulloblastomas contain PTCH mutations. Cancer Res. 1997;57:842–845. [PubMed] [Google Scholar]
- 10.Pietsch T, Waha A, Koch A, Kraus J, Albrecht S, Tonn J, Sorensen N, Berthold N, Henk B, Schmandt N, Wolf HK, von Deimling A, Wainwright G, Chenevix-Trench G, Wiestler OD, Wicking C. Medulloblastomas of the desmoplastic variant carry mutations of the human homologue of Drosophila patched. Cancer Res. 1997;57:2085–2088. [PubMed] [Google Scholar]
- 11.Wolter M, Reifenberger J, Sommer C, Ruzicka T, Reifenberger G. Mutations in the human homologue of the Drosophila segment polarity gene patched (PTCH) in sporadic basal cell carcinomas of the skin and primitive neuroectodermal tumors of the central nervous system. Cancer Res. 1997;57:2581–2585. [PubMed] [Google Scholar]
- 12.Goodrich LV, Milenkovic L, Huggins KM, Scott MP. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277:1109–1113. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- 13.Hahn H, Wojnowski L, Zimmer AM, Hall J, Miller G, Zimmer A. Rhabdomyosarcomas and radiation hypersensitivity in a mouse model of Gorlin syndrome. Nat Med. 1998;4:619–622. doi: 10.1038/nm0598-619. [DOI] [PubMed] [Google Scholar]
- 14.Zurawel RH, Allen C, Wechsler-Reya R, Scott MP, Raffel C. Evidence that haploinsufficiency of Ptch leads to medulloblastoma in mice. Genes Chromosomes Cancer. 2000;28:77–81. [PubMed] [Google Scholar]
- 15.Wetmore C, Eberhart DE, Curran T. The normal patched allele is expressed in medulloblastomas from mice with heterozygous germ-line mutation of patched. Cancer Res. 2000;60:2239–2246. [PubMed] [Google Scholar]
- 16.Weiner HL, Bakst R, Hurlbert MS, Ruggiero J, Ahn E, Lee WS, Stephen D, Zagzag D, Joyner AL, Turnbull DH. Induction of medulloblastomas in mice by Sonic hedgehog, independent of Gli1. Cancer Res. 2002;62:6385–6389. [PubMed] [Google Scholar]
- 17.Fults D, Pedone C, Dai C, Holland EC. MYC expression promotes the proliferation of neural progenitor cells in culture and in vivo. Neoplasia. 2002;4:32–99. doi: 10.1038/sj.neo.7900200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aldosari N, Bigner SH, Burger PC, Becker L, Kepner JL, Friedman HS, McLendon RE. MYCC and MYCN oncogene amplification in medulloblastoma. A fluorescence in situ hybridization study on paraffin sections from the Children's Oncology Group. Arch Pathol Lab Med. 2002;126:540–544. doi: 10.5858/2002-126-0540-MAMOAI. [DOI] [PubMed] [Google Scholar]
- 19.Bruggers CS, Tai KF, Murdock T, Sivak L, Le K, Perkins SL, Coffin CM, Carroll WL. Expression of the c-Myc protein in childhood medulloblastoma. J Pediatr Hematol Oncol. 1998;20:18–25. doi: 10.1097/00043426-199801000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Herms J, Neidt I, Luscher B, Sommer A, Schurmann P, Schroder T, Bergmann M, Wilken B, Probst-Cousin S, Hernaiz-Driever P, Behnke J, Hanefeld F, Pietsch T, Kretzschmar HA. C-MYC expression in medulloblastoma and its prognostic value. Int J Cancer. 2000;89:395–402. [PubMed] [Google Scholar]
- 21.Grotzer MA, Hogarty MD, Janss AJ, Liu X, Zhao H, Eggert A, Sutton LB, Rorke LB, Brodeur GM, Phillips PC. MYC messenger RNA expression predicts survival outcome in childhood primitive neuroectodermal tumor/medulloblastoma. Clin Cancer Res. 2001;7:2425–2433. [PubMed] [Google Scholar]
- 22.Federspiel MJ, Bates P, Young JAT, Varmus HE, Hughes SH. A system for tissue-specific gene targeting: transgenic mice susceptible to subgroup A avian leukosis virus-based retroviral vectors. Proc Natl Acad Sci USA. 1994;91:11241–11245. doi: 10.1073/pnas.91.23.11241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holland EC, Varmus HE. Basic fibroblast growth factor induces cell migration and proliferation after glia-specific gene transfer in mice. Proc Natl Acad Sci USA. 1998;95:1218–1223. doi: 10.1073/pnas.95.3.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lendahl U, Zimmerman LB, McKay RDG. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- 25.Holland EC, Hively WP, DePinho RA, Varmus HE. A constitutively active epidermal growth factor receptor cooperates with disruption of G1 cell-cycle arrest pathways to induce glioma-like lesions in mice. Genes Dev. 1998;12:3675–3685. doi: 10.1101/gad.12.23.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fults D, Pedone C. Immunocytochemical mapping of the phosphatase and tensin homolog (PTEN/MMAC1) tumor suppressor protein in human gliomas. Neuro-Oncology. 2000;2:71–79. doi: 10.1093/neuonc/2.2.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bumcrot DA, Takada R, McMahon AP. Proteolytic processing yields two secreted forms of Sonic hedgehog. Mol Cell Biol. 1995;15:2294–2303. doi: 10.1128/mcb.15.4.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okanao HJ, Park W, Corradi JP, Darnell RB. The cytoplasmic Purkinje onconeural antigen cdr2 down-regulates c-Myc function: implications for neuronal and tumor cell survival. Genes Dev. 1999;13:2087–2097. doi: 10.1101/gad.13.16.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coffin CM, Wick MR, Braun JT, Dehner LP. A clinicopathological and immunohistochemical analysis of 53 cases of medulloblastoma with emphasis on synaptophysin expression. Mod Pathol. 1990;3:164–170. [PubMed] [Google Scholar]
- 30.Easter SS, Ross LS, Frankfurter A. Initial tract formation in the mouse brain. J Neurosci. 1993;13:285–299. doi: 10.1523/JNEUROSCI.13-01-00285.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- 32.Calzada-Wack J, Kappler R, Schnitzbauer TR, Nathrath M, Rosemann M, Wagner SN, Hein R, Hahn H. Unbalanced overexpression of the mutant allele in murine Patched mutants. Carcinogenesis. 2002;23:727–733. doi: 10.1093/carcin/23.5.727. [DOI] [PubMed] [Google Scholar]
- 33.Pomeroy SL, Tamayo P, Gaasenbeek M, Sturla LM, Angelo M, McLaughlin ME, Kim JYH, Goumnerova LC, Black PM, Lau C, Allen JC, Zagzag D, Olson JM, Curran T, Wetmore C, Biegel JA, Poggio T, Mukherjee S, Rifkin R, Califano A, Stolovitsky G, Louis DN, Mesirov JP, Lander ES, Golub TR. Prediction of central nervous system embryonal tumour outcome based on gene expression. Nature. 2002;415:436–442. doi: 10.1038/415436a. [DOI] [PubMed] [Google Scholar]
- 34.Marino S, Vooijs M, van der Gulden H, Jonkers J, Berns A. Induction of medulloblastomas in p53-null mutant mice by somatic inactivation of Rb in the external granular layer cells of the cerebellum. Genes Dev. 2000;14:994–1004. [PMC free article] [PubMed] [Google Scholar]
- 35.Lee Y, McKinnon PJ. DNA ligase IV suppresses medulloblastoma growth. Cancer Res. 2002;62:6395–6399. [PubMed] [Google Scholar]
- 36.Wetmore C, Eberhart DE, Curran T. Loss of p53 but not ARF accelerates medulloblastoma in mice heterozygous for patched. Cancer Res. 2001;61:513–516. [PubMed] [Google Scholar]
- 37.Fung KM, Trojanowski JQ. Animal models of medulloblastomas and related primitive neuroectodermal tumors: a review. J Neuropathol Exp Neurol. 1995;54:285–296. doi: 10.1097/00005072-199505000-00001. [DOI] [PubMed] [Google Scholar]
- 38.Holland EC, Celestino J, Dai C, Schaefer L, Sawaya RE, Fuller GN. Combined activation of Ras and Akt in neural progenitors induces glioblastoma formation in mice. Nat Genet. 2000;25:55–57. doi: 10.1038/75596. [DOI] [PubMed] [Google Scholar]
- 39.Dai C, Celestino JC, Okada Y, Louis DN, Fuller GN, Holland EC. PDGF autocrine stimulation dedifferentiates cultured astrocytes and induces oligodendrogliomas and oligoastrocytomas from neural progenitors and astrocytes in vivo. Genes Dev. 2001;15:1913–1925. doi: 10.1101/gad.903001. [DOI] [PMC free article] [PubMed] [Google Scholar]