Abstract
Background
During recent years, a correlation between the presence of antibodies in sera against p53 and survival has been reported. The aim of the present study was to analyze anti-p53 antibodies in sera from patients with non small cell lung cancer (NSCLC) prior to thoracic surgery and their correlation to survival, nodal involvement, and tumor volume.
Patients and Methods
Serum samples from 58 patients with NSCLC admitted to the Department of Pulmonary Medicine in Uppsala were collected between 1993 and 1995 and analyzed for the expression of anti-p53 antibodies.
Results
Antibodies against p53 were detected in 12 patients (21%). No association was found between increased levels of anti-p53 antibodies and tumor volume (P = .84). There was a numerical trend towards higher levels of anti-p53 antibodies in patients without nodal disease, when compared with patients with nodal involvement, although not statistically significant (P = .136). However, when patients with metastatic disease were included, statistically significantly lower levels of anti-p53 antibodies were demonstrated, in comparison to patients without any sign of nodal engagement or metastatic disease (P = .038). Anti-p53 antibodies and survival showed no correlation between increasing index levels of anti-p53 antibodies and survival (P = .18). Neither was a correlation found between using the cutoff (>1.1) described by the manufacturer and survival.
CONCLUSION
The presence of anti-p53 antibodies was correlated neither to survival nor to tumor volume in the present study. However, patients with either nodal or metastatic disease had lower levels of anti-p53 antibodies in comparison to patients without signs of either nodal or metastatic disease. These issues are discussed.
Keywords: non small cell lung cancer, p53 antibodies, sera and survival, tumor volume, nodal involvement
Introduction
Lung cancer causes approximately 1 million deaths each year [1]. Lung cancer is divided into non small cell lung cancer (NSCLC) and small cell lung cancer (SCLC) due to histological differences. Treatment of early limited NSCLC is based on surgery, that is, if the patient is willing to undergo surgery and is physically fit to withstand surgery. If not, the patient is offered radiotherapy and/or chemotherapy based on the staging of the tumor and the performance status of the individual patient.
The p53 suppressor gene is implicated in the control of the cell cycle, DNA synthesis, and DNA repair as well as apoptosis [2]. If a mutation occurs within the p53 gene, these functions might be disturbed. Antibodies against p53 can be detected in sera from cancer patients, and a correlation between the expression of anti-p53 antibodies and mutations within the p53 gene has been established [3]. Several studies have been conducted in which the presence of p53 antibodies has been correlated to various clinical parameters. In a previous study [4] by our group, we demonstrated that the presence of p53 antibodies in sera could predict increased survival if expressed before the start of radiotherapy.
Because tumor size is probably associated with a greater amount of p53 antigen in vivo, the immune system might be more efficient in inducing an immune response. Thus, a larger tumor size could be associated with increased antibody response to p53. In the present study, we have focused on the index levels of anti-p53 antibodies prior to thoracic surgery and their correlation to survival, and, further, on the issue if a correlation exists between increasing index levels of anti-p53 antibodies and increased tumor volume.
Patients and Methods
Serum samples from patients with NSCLC admitted to the Department of Pulmonary Medicine in Uppsala were collected between 1993 and 1995 prior to thoracic surgery. All patients gave informed consent prior to collection of blood samples and the samples were stored at -20°C until analyzed. The study was reviewed and approved by the research ethical committee (no. 02-010) of the Uppsala University (Uppsala, Sweden). The following clinical parameters were studied: age, gender, performance status, weight loss, histopathology, tumor volume, lymph node involvement according to pathology records or from reevaluation from CT scans in conjunction with thoracic surgery, relapse, and survival. The primary tumor volume was reevaluated and measured by an experienced radiologist. Estimations were reevaluated in 62% of the materials from CT scans whereas the other cases were measured from pulmonary X-rays. The calculations regarding tumor volume were performed using the following formula: 4πX radius x Y radius x Z radius/3. Unfortunately, in three cases, tumor volume measurements could not be performed due to missing data.
Anti-p53 Antibody Investigation
Blood was collected in 7-ml serum tubes without additive (367609; Becton Dickinson, Rutherford, NJ). A sandwich ELISA, commercially available from Dianova (Hamburg, Germany), was utilized to measure the presence of anti-p53 antibodies from the sera. The measurements were performed according to the manufacturer's instructions and the procedure has previously been reported [5]. Briefly, human recombinant p53 is bound to microtiter plates. Standards and samples were pipetted into the wells. After incubation and washing, a horseradish peroxidase-conjugated polyclonal goat antihuman IgG was added. After incubation and washing, a chromogenic substrate was added and the color intensity was measured at 450 nm in a Titertek Multiskan (Edinburgh, Scotland). A relative index for patients' sera was calculated, according to manufacturer's instructions, as follows: E450 (sample) - E450 (low control)/E450 (high control) - E450 (low control). The ELISA assays were performed without knowledge of clinical data.
Statistics
The survival functions were estimated with the Kaplan-Meier product-limit method and the median survival time was estimated with linear interpolation of the survival function. Cox regression analysis was applied to study if certain factors had an impact on survival. In cases where there was only one dichotomous explanatory factor, the results from the Cox analysis were the same as from the log-rank test. Kruskal-Wallis test was applied to study differences between groups. Throughout this study, a 5% significance level is used in the statistical tests.
Results
The median age of the patients was 64 years (39–80) and the majority of patients (63%) were men. Almost all patients were smokers or ex-smokers (84%). The majority of the patients were classified as having performance status 0-I (Table 1). Involuntary weight loss was found in 18% of the patients. In 54 patients (93%), surgical resection was microscopically radical. In three of those patients in which surgical resection was not microscopically radical, these patients received postoperative radiation.
Table 1.
Description of Patients and Their Correlation to Index Levels of Anti-p53 Antibodies.
| TNM Class | Number of Patients | Mean (Index Value of Anti-p53 Antibodies) | Median (Index Value of Anti-p53 Antibodies) | Squamous Cell Carcinoma | Adenocarcinoma | Other Histology | Weight Reduction (%) | Tumor Volume |
| I | 37 | 1.3 | 0.1 | 16 | 19 | 2 | 11.1 | 51.4 |
| II | 3 | 0.2 | 0.0 | 0 | 3 | 0 | 33.3 | 75.7 |
| IIIa | 10 | 1.9 | 0.0 | 5 | 5 | 0 | 44.4 | 32.7 |
| IIIb | 2 | 0.3 | 0.3 | 0 | 1 | 1 | 0 | 53.3 |
| IV | 2 | 0.1 | 0.1 | 0 | 1 | 1 | 50.0 | 50.9 |
| Not determined | 4 | 0.4 | 0.03 | 0 | 3 | 1 | 0 | 167.0 |
| Total | 58 | 1.2 | 0.04 | 21 | 32 | 5 | 17.8 | 57.5 |
Antibodies against p53 were detected in 12 of 58 (21%). Twenty-four percent of the men and 14% of the women expressed anti-p53 antibodies. Those patients expressing the autoantibodies had a median index value of 5.66 (1.56–8.07).
Minimal follow-up was performed up to 3 years. After 3 years, 23 patients (40%) were alive. Patient characteristics and their distribution of anti-p53 antibodies are shown in Table 1.
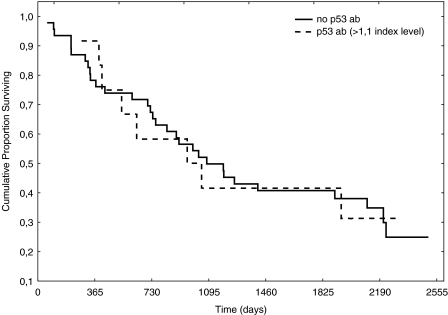
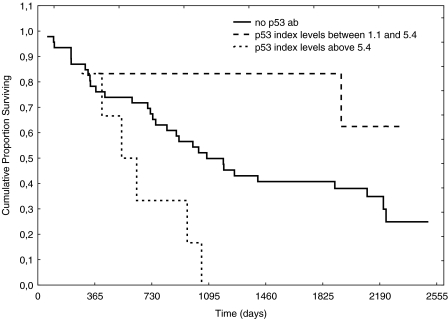
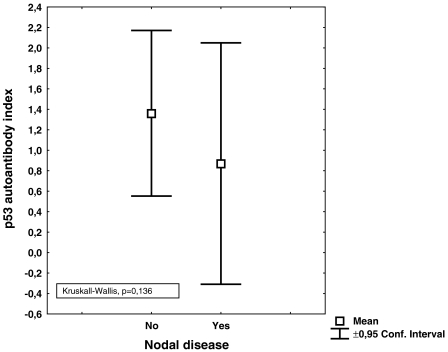
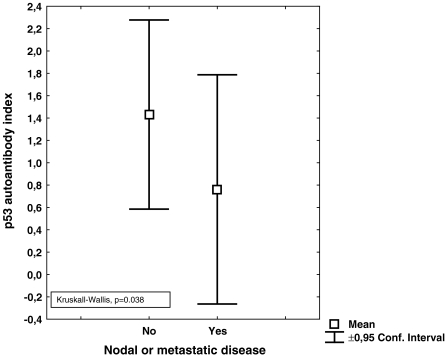
No correlation was found between the index values of anti-p53 antibodies and tumor volume; neither was any correlation found between relapse and index values of antip53 antibodies. The distribution according to histology is shown in Table 1. Using the manufacturer's cutoff, no survival difference was found between patients with index levels of anti-p53 >1.1 or <1.1 (Figure 1). Neither were increasing index values of anti-p53 antibodies correlated to survival (Figure 2). There was a numerical trend towards higher levels of anti-p53 antibodies in patients without nodal disease, when compared with patients with nodal involvement, although not statistically significant (P = .136) (Figure 3). However, when patients with metastatic disease were included, there were statistically significantly lower levels of anti-p53 antibodies in comparison with patients with TN0M0 (P = .038) (Figure 4).
Figure 1.
Anti-p53 antibodies and survival according to cutoff (>1.1).
Figure 2.
Survival according to index values of anti-p53 antibodies.
Figure 3.
Index values of anti-p53 antibodies in patients with or without nodal involvement.
Figure 4.
Index values of anti-p53 antibodies in patients with metastatic or nodal disease in comparison with patients without nodal involvement or metastatic disease.
Discussion
In the majority of cases, lung cancer is associated with a poor prognosis. After treatment, surveillance is founded on symptoms and chest X-rays. Today, no reliable serological tumor marker has been established, although a number of biological markers (TPA, NSE, SCC, Cyfra 21-1, and CEA) have been investigated regarding their potential as tumor markers [6].
Anti-p53 antibodies have been extensively studied because it has been shown that anti-p53 antibodies correlate to the mutational status of the p53 gene [3]. In a previous study [4] by our group, we found that patients with NSCLC expressing antibodies against p53 prior to radiation therapy had a prolonged survival in comparison with those patients who did not express anti-p53 antibodies. Studies regarding resectable NSCLC patients have focused on whether or not anti-p53 antibodies in sera are associated with survival. In a study by Mitsudomi et al. [7], tumor material and sera were collected in relation to thoracic surgery from 188 patients with NSCLC. They concluded that the incidence of antibodies against p53 was positively associated with tumor stage and p53 overexpression but not with survival. In another study, in which 84 patients with resectable NSCLC were studied in relation to thoracic surgery, the authors [8] found that patients with antibodies against p53 had a lower probability of overall and diseasefree survival. Similar data were presented by Mack et al. [9]. They investigated 99 NSCLC patients with different stages and found that anti-p53 antibodies mainly were found in patients with advanced disease and that anti-p53 antibodies were associated with a poorer prognosis. Further, Sangrajrang et al. [10] investigated 133 lung cancer patients and found that the incidence of anti-p53 antibodies was higher in patients with more advanced disease (stages III and IV). In the present study, serum samples were collected prior to thoracic surgery and the clinical courses of the patients were followed for a minimum of 3 years after surgery. The index levels ranged between 0.0 and 8.07 and, when using cutoffs recommended by the manufacturer, we found that 21% of the patients in our study overexpressed antibodies against p53 (Figure 1). We did not find a correlation between increasing anti-p53 antibody index values or survival. Neither did we find survival differences according to the defined cutoff (>1.1) (Figures 1 and 2). These data support the findings by Mitsudomi et al. [7]. Secondary aims of the study were to investigate if index levels of anti-p53 antibodies correlate with the primary tumor volume. Intuitively, an increased tumor volume should result in increased amounts of tumor antigens, which in turn would activate the immune system. Nevertheless, in the present study, tumor volume was not associated with index values of anti-p53 antibodies (P = .83). However, a reduction of index levels of anti-p53 antibody titers was demonstrated in patients with nodal and metastatic disease (P = .038) (Figures 3 and 4). One hypothetical explanation might be that some tumors might produce cytokines that induce expression of CD 95-L in the lymphocytes, resulting in initiation of apoptosis in these lymphocytes, whereby the tumor counteracts the response of the immune system [11]. Further, patients with large tumors generally have a suppressed immune system and thus might be incapable of producing significant amounts of antibodies to counteract the disease [12]. Another hypothetical explanation might be that because these serum samples are not collected from the immediate surroundings of the tumor, the index values of anti-p53 antibodies amounts measured might only partly reflect the immune response because there is a possibility that the majority of antibodies might have reacted with their antigen and thus are not detectable in sera [13].
In summary, data concerning the prognostic and predictive information regarding anti-p53 antibodies diverge and reflect rather the complex nature of NSCLC then a “true” tumor marker potential; however, further studies addressing the impact different therapies have on index levels are needed. In conclusion, the value of anti-p53 antibodies in patients with NSCLC, in conjunction with thoracic surgery, was not associated with either tumor volume or survival in the present study.
Acknowledgements
The authors are indebted to Gustav Ullenhag (Section of Oncology, Department of Oncology, Radiology, and Clinical Immunology, Uppsala University Hospital, Sweden) for reading and commenting on this manuscript. The authors are also indebted to Anita Klinga for skillful laboratory work.
Footnotes
This work was supported by the research fund of the Department of Oncology, Uppsala University Hospital and the Swedish Medical Research Council.
References
- 1.Minna JaZ-M S. Molecular changes in lung carcinogenesis American Society of Clinical Oncology Educational Book. In: Perry MC, editor. American Society of Clinical Oncology, Educational Book, 38th Annual Meeting. Baltimore, USA: Lippincott Williams & Wilkins; 2002. [Google Scholar]
- 2.May P, May E. Twenty years of p53 research: structural and functional aspects of the p53 protein. Oncogene. 1999;18:7621–7636. doi: 10.1038/sj.onc.1203285. [DOI] [PubMed] [Google Scholar]
- 3.Soussi T. p53 Antibodies in the sera of patients with various types of cancer: a review. Cancer Res. 2000;60:1777–1788. [PubMed] [Google Scholar]
- 4.Bergqvist M, Brattstrom D, Larsson A, Holmertz J, Hesselius P, Rosenberg L, Wagenius G, Brodin O. p53 Auto-antibodies in nonsmall cell lung cancer patients can predict increased life expectancy after radiotherapy. Anticancer Res. 1998;18:1999–2002. [PubMed] [Google Scholar]
- 5.Hofele C, Schwager-Schmitt M, Volkmann M. Prognostic value of antibodies against p53 in patients with oral squamous cell carcinoma—five years survival rate. Laryngo-, Rhino-, Otologie. 2002;81:342–345. doi: 10.1055/s-2002-28341. [DOI] [PubMed] [Google Scholar]
- 6.Foa P, Fornier M, Miceli R, Seregni E, Santambrogio L, Nosotti M, Massaron S, Cataldo I, Oldani S, Iurlo A, Caldiera S, Bombardieri E. Preoperative CEA, NSE, SCC, TPA and CYFRA 21.1 serum levels as prognostic indicators in resected non-small cell lung cancer. Int J Biol Markers. 1999;14:92–98. doi: 10.1177/172460089901400206. [DOI] [PubMed] [Google Scholar]
- 7.Mitsudomi T, Suzuki S, Yatabe Y, Nishio M, Kuwabara M, Gotoh K, Hatooka S, Shinoda M, Suyama M, Ogawa M, Takahashi T, Ariyoshi Y. Clinical implications of p53 autoantibodies in the sera of patients with non-small-cell lung cancer. J Natl Cancer Inst. 1998;90:1563–1568. doi: 10.1093/jnci/90.20.1563. [DOI] [PubMed] [Google Scholar]
- 8.Laudanski J, Burzykowski T, Niklinska W, Chyczewski K, Furman M, Niklinski J. Prognostic value of serum p53 antibodies in patients with resected non-small cell lung cancer. Lung Cancer. 1998;22:191–200. doi: 10.1016/s0169-5002(98)00088-9. [DOI] [PubMed] [Google Scholar]
- 9.Mack U, Ukena D, Montenarh M, Sybrecht GW. Serum anti-p53 antibodies in patients with lung cancer. Oncol Rep. 2000;7:669–674. doi: 10.3892/or.7.3.669. [DOI] [PubMed] [Google Scholar]
- 10.Sangrajrang S, Sornprom A, Chernrungroj G, Soussi T. Serum p53 antibodies in patients with lung cancer: correlation with clinicopathologic features and smoking. Lung Cancer. 2003;39:297–301. doi: 10.1016/s0169-5002(02)00509-3. [DOI] [PubMed] [Google Scholar]
- 11.Shiraki K, Tsuji N, Shioda T, Isselbacher KJ, Takahashi H. Expression of Fas ligand in liver metastases of human colonic adenocarcinomas. Proc Natl Acad Sci USA. 1997;94:6420–6425. doi: 10.1073/pnas.94.12.6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mocellin S, Wang E, Marincola FM. Cytokines and immune response in the tumor microenvironment. J Immunother. 2001;24:392–407. [PubMed] [Google Scholar]
- 13.Merimsky O, Baharav E, Shoenfeld Y, Chaitchik S, Tsigelman R, Cohen-Aloro D, Fishman P. Anti-tyrosinase antibodies in malignant melanoma. Cancer Immunol Immunother. 1996;42:297–302. doi: 10.1007/s002620050286. [DOI] [PMC free article] [PubMed] [Google Scholar]