Among the most surprising findings in visual science of the last decade has been that photoreception in the mammalian retina is not restricted to the rods and cones, but extends to a small number of cells in the inner retina. These new photoreceptors, which comprise a small subset of retinal ganglion cells (1), are capable of driving a number of “non-image-forming” light responses including circadian photoentrainment and the regulation of pupil size even in the absence of functional rods and cones (2, 3). The so-called intrinsically photosensitive retinal ganglion cells (ipRGCs) absorb light through an opsin/retinaldehyde-based photopigment called melanopsin (4–8). Although the details of melanopsin’s photochemistry in mammals remain only partially defined, the evidence to date supports the hypothesis that, just like other opsin photopigments, the critical first event in melanopsin activation is photoisomerization of the retinaldehyde chromophore from a cis to an all-trans conformation (6–8). An important implication of such a mechanism is that to attain photosensitivity melanopsin requires a steady supply of cis-retinaldehyde. The primary source of chromophore in the vertebrate eye is a multistep enzymatic pathway, known as the retinoid or visual cycle, by which 11-cis retinaldehyde is regenerated from bleached all-trans originating in photoreceptor outer segments (Fig. 1A). Critical elements of this pathway occur in the retinal pigment epithelium (RPE). As melanopsin is found in the more superficial layers of the retina, distant from the RPE, it would seem poorly placed to obtain cis-retinaldehyde from this source. This raises the question of how melanopsin recovers from bleach. An attractive hypothesis is that it uses an independent, more local, source of cis-retinaldehdye rather than relying on the RPE-based visual cycle. Two articles in this issue of PNAS (9, 10) test this hypothesis and provide the most compelling evidence to date that melanopsin does indeed use its own separate regeneration mechanism in vivo.
Fig. 1.
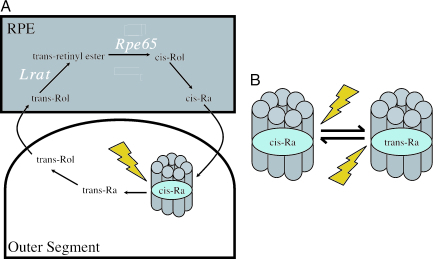
Chromophore regeneration in vertebrate and invertebrate photoreceptors. (A) Simplified version of the vertebrate visual cycle by which 11-cis retinaldehyde (Ra) is regenerated after bleach. The enzymes Lrat and Rpe65 (in white) are critical components of this pathway, which reside in the RPE. (B) In invertebrate rhabdomeric photoreceptors, opsin protein binds both cis- and trans-retinaldehyde, allowing photoregeneration of bleached photopigment. Rol, retinol. Opsin protein is represented in cartoon form by seven transmembrane α-helices.
Doyle et al. (9) and Tu et al. (10) set out to test whether the activity of ipRGCs is altered by lesions of the classical visual cycle. To this end, they assessed non-image-forming light responses (photoentrainment and the pupillary light reflex) and, in the case of Tu et al., ipRGC activity directly, in mice lacking Rpe65 or lecithin-retinol acyl transferase (Lrat), critical components of the visual cycle (Fig. 1A and ref. 11). Rpe65−/− and Lrat−/− mice experience substantial deficits in chromophore availability and a consequent near-total collapse of rod/cone activity (12, 13). At first blush these animals seem to disprove the hypothesis that ipRGCs are independent of the visual cycle. Pupillary responses (13, 14) and circadian photoentrainment (9) both are impaired beyond the level predicted by rod/cone loss, and direct recording of ipRGCs confirms that a decrease in their photosensitivity underlies these effects (14). How then to explain these surprising findings? Does melanopsin in fact use the visual cycle? The most important contribution of these two articles (9, 10) is to reveal that the decrease in ipRGC activity in these animals is in fact secondary to the profound effects of visual-cycle impairment on the activity of outer retinal photoreceptors.
The researchers reach this conclusion from experiments in which they introduce visual-cycle deficiency to mice either homozygous for the rd mutation or bearing the rdta transgene. Both rd/rd and rdta mice are established models of outer retinal degeneration. rd is a null mutation of the gene encoding the β-subunit of the rod-specific phosphodiesterase, which abolishes rod phototransduction and induces a gradual degeneration of rod cells (15). The rdta transgene also induces rod degeneration, but in this case, through targeted expression of diphtheria toxin (16). Both models experience substantial secondary effects on cone viability, but, importantly, ipRGCs seem to be largely unaffected. Surprisingly, despite reducing the theoretical photoreceptive capacity compared with Rpe65−/− or Lrat−/− animals, Rpe65−/−;rdta and Lrat−/−rd/rd mice show a paradoxical increase in photosensitivity. This observation reveals that the reduced ipRGC activity of animals lacking visual cycle is largely attributable to an inhibitory influence of the inactive outer retina and suggests that ipRGCs are in fact resistant to the loss of Rpe65 or Lrat. Importantly, Tu et al. (10) continue to confirm this by using all-trans-retinylamine to block the visual cycle acutely. ipRGC activity is not impaired by this treatment. Together, these data (9, 10) provide the most comprehensive in vivo evidence yet that melanopsin acts largely independently of the visual cycle.
If the most important implication of these articles relates to the visual cycle, it is worth digressing to ask why an intact outer retina should render ipRGCs particularly sensitive to the effects of visual-cycle lesions. Sadly, there is no simple answer to this question. The most parsimonious explanation is that although melanopsin does not directly rely on the visual cycle, the intact rods/cones of Rpe65−/− and Lrat−/− mice impair retinaldehyde availability for ipRGCs. Perhaps the large pool of chromophore-hungry rod and cone opsin alters retinoid balance in the retina to the detriment of melanopsin. In support of this possibility, previous work suggests that exogenous retinaldehyde can reverse the effects of Rpe65 loss on non-image-forming responses even if any effects of this treatment on rod/cone activity are excluded (14).
However, simple chromophore depletion need not account for all of the effects of visual-cycle loss on ipRGCs, and the authors of refs. 9 and 10 raise two other potential consequences. The first is that the inactive outer retina may provide a physiological inhibition of ipRGC activity in Rpe65−/− and Lrat−/− mice. The issue of rod/cone input to ipRGCs is currently one of great interest. There is anatomical evidence for a convergence of rod/cone pathways onto ipRGCs in the rodent retina (17) and, in the primate, ipRGCs receive a physiological “on” signal from rods and L/M cones, but an “off” signal from S cones (18). The precise nature of rod/cone input to mouse ipRGCs remains unknown, although an excitatory input is suggested by the observation that classical photoreceptors can drive non-image-forming light responses in the absence of melanopsin (19, 20). It may be that the influence of the largely inactive photoreceptors of Rpe65−/− and Lrat−/− mice is primarily inhibitory. However, previous work showing that ipRGC activity survives unimpaired in mice lacking rod/cone phototransduction (Cnga3−/− Gnat1−/−) but that additional loss of Rpe65 does impair ipRGC function would argue against this explanation for the Rpe65−/− phenotype (14).
The final possibility proposed is that the inactive outer retina of Rpe65−/− and Lrat−/− mice may impair the development and/or viability of ipRGCs. Doyle et al. (9) provide some intriguing evidence in support of this possibility. They show that the number of cells immunoreactive for melanopsin is reduced in Rpe65−/− mice compared with either WT or Rpe65−/−; rdta animals. There is also a suggestion of reorganization of the remaining cells, with melanopsin staining particularly reduced in the outer inner plexiform layer. Interestingly, alterations in melanopsin expression have also been reported after some other types of retinal dystrophy (21, 22), suggesting that the outer retina may indeed play a role in the development and maintenance of ipRGCs.
Elucidating rod/cone input to ipRGCs and identifying the factors regulating melanopsin expression and ipRGC development are important areas for future research. However, undoubtedly the most important implication of these articles (9, 10) relates to the nature of melanopsin’s local chromophore supply. If melanopsin does not use the visual cycle to obtain cis-retinaldehyde, what does it use? The most elegant potential solution comes from the world of invertebrate vision. The rhabdomeric photoreceptors of invertebrates also use opsin/retinaldehyde-based photopigments. However, a critical difference in their photochemistry is that rather than releasing bleached all-trans retinaldehyde for recycling elsewhere, these opsins retain it and, through absorption of a further photon, regenerate 11-cis (Fig. 1B). These so-called bistable pigments therefore have an intrinsic regeneration mechanism and are resistant to bleach.
Support for the theory that melanopsin functions as such a bistable pigment comes from heterologous expression studies. In the most comprehensive analysis of this subject to date, Koyanagi et al. (23) showed that melanopsin from the protochordate Amphioxus could be regenerated with 11-cis retinaldehyde and subsequently bleached to form a stable, light-sensitive product. By using appropriate wavelengths, they were able to drive photointerconversion between these two states, supporting the hypothesis that they represent melanopsin binding retinaldehyde in all-trans and 11-cis conformations. We await a comparable data set for mammalian melanopsin. Nonetheless, the evidence available to date supports the conclusion that mammalian melanopsin also is a bistable pigment because, under heterologous expression, both human and mouse melanopsins can drive G protein signaling cascades in a light-dependent manner when provided with either cis- or all-trans isoforms of retinaldehyde (6, 7).
The hypothesis that mammalian melanopsins are bistable awaits direct demonstration, and, even if proved, would not preclude the possibility that ipRGCs use additional, supplementary regeneration mechanisms. The mammalian retina has two putative photoisomerases, retinal G protein-coupled receptor (RGR) and peropsin, thought to have the ability to regenerate 11-cis from all-trans retinaldehyde in a light-dependent manner (24, 25). Both photoisomerases are found in the RPE, whereas RGR is also localized to the Müller cells that span the retina. In theory, either or both photoisomerases could form the basis of a visual cycle-independent regeneration of melanopsin. There is also evidence of a regeneration cycle based in Müller cells used by cones (25), which melanopsin could also co-opt. Consequently, although the clever money is going on the possibility that melanopsin acts as its own photoisomerase, it may be some time before we can approach a full understanding of the regeneration pathways supporting inner retinal photoreception.
Footnotes
References
- 1.Berson D. M., Dunn F. A., Takao M. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 2.Lucas R. J., Freedman M. S., Munoz M., Garcia-Fernandez J. M., Foster R. G. Science. 1999;284:505–507. doi: 10.1126/science.284.5413.505. [DOI] [PubMed] [Google Scholar]
- 3.Lucas R., Douglas R., Foster R. Nat. Neurosci. 2001;4:621–626. doi: 10.1038/88443. [DOI] [PubMed] [Google Scholar]
- 4.Provencio I., Rodriguez I. R., Jiang G., Hayes W. P., Moreira E. F., Rollag M. D. J. Neurosci. 2000;20:600–605. doi: 10.1523/JNEUROSCI.20-02-00600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lucas R. J., Hattar S., Takao M., Berson D. M., Foster R. G., Yau K. W. Science. 2003;299:245–247. doi: 10.1126/science.1077293. [DOI] [PubMed] [Google Scholar]
- 6.Panda S., Nayak S. K., Campo B., Walker J. R., Hogenesch J. B., Jegla T. Science. 2005;307:600–604. doi: 10.1126/science.1105121. [DOI] [PubMed] [Google Scholar]
- 7.Melyan Z., Tarttelin E. E., Bellingham J., Lucas R. J., Hankins M. W. Nature. 2005;433:741–745. doi: 10.1038/nature03344. [DOI] [PubMed] [Google Scholar]
- 8.Qiu X., Kumbalasiri T., Carlson S. M., Wong K. Y., Krishna V., Provencio I., Berson D. M. Nature. 2005;433:745–749. doi: 10.1038/nature03345. [DOI] [PubMed] [Google Scholar]
- 9.Doyle S. E., Castrucci A. M., McCall M., Provencio I., Menaker M. Proc. Natl. Acad. Sci. USA. 2006;103:10432–10437. doi: 10.1073/pnas.0600934103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tu D. C., Owens L. A., Anderson L., Golczak M., Doyle S. E., McCall M., Menaker M., Palczewski K., Van Gelder R. N. Proc. Natl. Acad. Sci. USA. 2006;103:10426–10431. doi: 10.1073/pnas.0600917103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin M. H., Li S. H., Moghrabi W. N., Sun H., Travis G. H. Cell. 2005;122:449–459. doi: 10.1016/j.cell.2005.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Redmond T. M., Yu S., Lee E., Bok D., Hamasaki D., Chen N., Goletz P., Ma J. X., Crouch R. K., Pfeifer K. Nat. Genet. 1998;20:344–351. doi: 10.1038/3813. [DOI] [PubMed] [Google Scholar]
- 13.Batten M. L., Imanishi Y., Maeda T., Tu D. C., Moise A. R., Bronson D., Possin D., Van Gelder R. N., Baehr W., Palczewski K. J. Biol. Chem. 2004;279:10422–10432. doi: 10.1074/jbc.M312410200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu Y., Zhong H., Wang M. H., Luo D. G., Liao H. W., Maeda H., Hattar S., Frishman L. J., Yau K. W. Proc. Natl. Acad. Sci. USA. 2005;102:10339–10344. doi: 10.1073/pnas.0501866102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carter-Dawson L. D., LaVail M. M., Sidman R. L. Invest. Ophthalmol. Visual Sci. 1978;17:489–498. [PubMed] [Google Scholar]
- 16.McCall M. A., Gregg R. G., Merriman K., Goto N. S., Peachey N. S., Stanford L. R. Exp. Eye Res. 1996;63:35–50. doi: 10.1006/exer.1996.0089. [DOI] [PubMed] [Google Scholar]
- 17.Sollars P. J., Smeraski C. A., Kaufman J. D., Ogilvie M. D., Provencio I., Pickard G. E. Visual Neurosci. 2003;20:601–610. doi: 10.1017/s0952523803206027. [DOI] [PubMed] [Google Scholar]
- 18.Dacey D. M., Liao H. W., Peterson B. B., Robinson F. R., Smith V. C., Pokorny J., Yau K. W., Gamlin P. D. Nature. 2005;433:749–754. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- 19.Panda S., Provencio I., Tu D. C., Pires S. S., Rollag M. D., Castrucci A. M., Pletcher M. T., Sato T. K., Wiltshire T., Andahazy M., et al. Science. 2003;301:525–527. doi: 10.1126/science.1086179. [DOI] [PubMed] [Google Scholar]
- 20.Hattar S., Lucas R. J., Mrosovsky N., Thompson S., Douglas R. H., Hankins M. W., Lem J., Biel M., Hofmann F., Foster R. G., Yau K. W. Nature. 2003;424:75–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakamoto K., Liu C. M., Tosini G. J. Neurosci. 2004;24:9693–9697. doi: 10.1523/JNEUROSCI.2556-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wan J., Zheng H., Xiao H. L., She Z. J., Chen Z. L., Zhou G. M. Neurosci. Lett. 2006;400:48–52. doi: 10.1016/j.neulet.2006.02.084. [DOI] [PubMed] [Google Scholar]
- 23.Koyanagi M., Kubokawa K., Tsukamoto H., Shichida Y., Terakita A. Curr. Biol. 2005;15:1065–1069. doi: 10.1016/j.cub.2005.04.063. [DOI] [PubMed] [Google Scholar]
- 24.Koyanagi M., Terakita A., Kubokawa K., Shichida Y. FEBS Lett. 2002;531:525–528. doi: 10.1016/s0014-5793(02)03616-5. [DOI] [PubMed] [Google Scholar]
- 25.Chen P., Hao W. S., Rife L., Wang X. P., Shen D. W., Chen J., Ogden T., Van Boemel G. B., Wu L. Y., Yang M., Fong H. K. W. Nat. Genet. 2001;28:256–260. doi: 10.1038/90089. [DOI] [PubMed] [Google Scholar]