Abstract
A growing proportion of the U.S. workforce will have been raised in disadvantaged environments that are associated with relatively high proportions of individuals with diminished cognitive and social skills. A cross-disciplinary examination of research in economics, developmental psychology, and neurobiology reveals a striking convergence on a set of common principles that account for the potent effects of early environment on the capacity for human skill development. Central to these principles are the findings that early experiences have a uniquely powerful influence on the development of cognitive and social skills and on brain architecture and neurochemistry, that both skill development and brain maturation are hierarchical processes in which higher level functions depend on, and build on, lower level functions, and that the capacity for change in the foundations of human skill development and neural circuitry is highest earlier in life and decreases over time. These findings lead to the conclusion that the most efficient strategy for strengthening the future workforce, both economically and neurobiologically, and improving its quality of life is to invest in the environments of disadvantaged children during the early childhood years.
Keywords: child development, early experience, economic productivity, critical and sensitive periods, brain development
The future success of the U.S. economy will depend in part on well educated and highly resourceful workers who are capable of learning new skills so that they remain competitive in a continually changing global market. That success is in jeopardy because a growing fraction of the nation’s workforce will consist of adults who were raised in disadvantaged environments, a segment of the population that has historically been less likely to attain high levels of education and skill development than the general population (1, 2).
Research in child development over the past several decades has led to an increasingly refined understanding of the characteristics of disadvantaged environments (3). Central to this concept is the statistical association between a select number of “risk factors” and the increased probability of adverse outcomes in the domains of cognitive, emotional, and social development, leading to diminished economic success and decreased quality of life in adulthood. The most extensively studied risk factor is poverty, but others include limited parent education, parental mental health problems, significant social deprivation or neglect, and exposure to interpersonal violence (3–7).
Behavioral research confirms that the early years are foundational for a full range of human competencies and are a period of heightened sensitivity to the effects of both positive and negative experiences (3, 8). In a parallel fashion, studies of human capital formation indicate that the quality of the early childhood environment is a strong predictor of adult productivity (9) and that early enrichment for disadvantaged children increases the probability of later economic success (10). Although explanatory mechanisms for interpreting these correlations still are being developed, recent advances in neuroscience are illuminating, because they demonstrate the extent to which early experience influences the development of neural circuits that mediate cognitive, linguistic, emotional, and social capacities (11, 12).
This paper focuses on the striking convergence of four core concepts that have emerged from decades of mutually independent research in economics, neuroscience, and developmental psychology. First, the architecture of the brain and the process of skill formation are both influenced by an inextricable interaction between genetics and individual experience. Second, both the mastery of skills that are essential for economic success and the development of their underlying neural pathways follow hierarchical rules in a bottom-up sequence such that later attainments build on foundations that are laid down earlier. Third, cognitive, linguistic, social, and emotional competencies are interdependent, all are shaped powerfully by the experiences of the developing child, and all contribute to success in the workplace. Fourth, although adaptation continues throughout life, human abilities are formed in a predictable sequence of sensitive periods, during which the development of specific neural circuits and the behaviors they mediate are most plastic and, therefore, optimally receptive to environmental influences.
Discussion
Early Experience Shapes the Foundation for Adult Productivity.
A landmark study commissioned by the Institute of Medicine and the National Research Council concluded that “virtually every aspect of early human development, from the brain’s evolving circuitry to the child’s capacity for empathy, is affected by the environments and experiences that are encountered in a cumulative fashion, beginning in the prenatal period and extending throughout the early childhood years.” (ref. 3, p. 6).
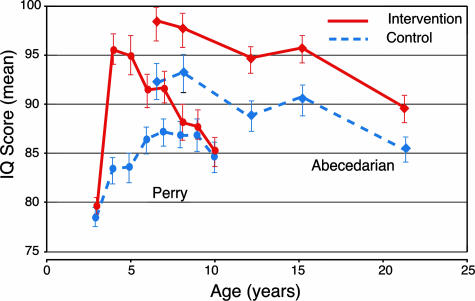
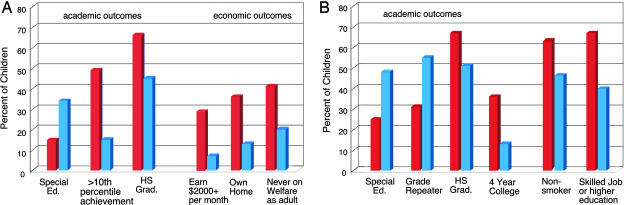
Independent econometric studies have reached similar conclusions. Extensive evidence indicates that cognitive, social, and emotional capacities play important roles in the attainment of adult economic productivity, and all are shaped by early life experiences. The most reliable data come from experiments that provided substantial enrichment of the early environments of children living in low-income families. Two of these investigations, the Perry Preschool Program and the Abecedarian Program (see Box 1), are the most informative for the purposes of this discussion because they used a random assignment design and collected long-term followup data (10). Both of these longitudinal studies demonstrated substantial, positive effects of early environmental enrichment on a range of cognitive (Fig. 1) and “noncognitive” skills, school achievement, job performance, and social behaviors (Fig. 2), long after the intervention ended (13, 14). Data from noncontrolled assessments of Head Start and the Chicago Child-Parent Centers programs suggest similar conclusions, although the data from Head Start represent only short-term effects.
Box 1: Early Intervention Programs for Disadvantaged Children.
Two different intervention programs, the Perry Preschool Program and the Abecedarian Program, have used randomized child assignment and long-term follow up to study the effects of early interventions on social behaviors of severely disadvantaged children (19, 80). The Perry Program was an intensive preschool program that was administered to 64 disadvantaged, black children in Ypsilanti, MI, between 1962 and 1967 (see Supporting Materials, which is published as supporting information on the PNAS web site, for details). The treatment consisted of a daily 2.5-h classroom session on weekday mornings and a weekly 90-min home visit by the teacher on weekday afternoons. The length of each preschool year was 30 weeks. The control and treatment groups have been followed through age 40. The Abecedarian Program involved 111 disadvantaged children, born between 1972 and 1977, whose families scored high on a risk index (see Supporting Materials for details). The mean age at entry was 4.4 months. The program was a year-round, full-day intervention that continued through age 8. The children were followed up until age 21, and the project is ongoing.
In both the Perry and Abecedarian Programs, there was a consistent pattern of successful outcomes for treatment group members compared with control group members. For the Perry Program, an initial increase in IQ (Fig. 1, red circles) disappeared gradually over 4 years after the intervention, as has been observed in other studies. However, in the more intense Abecedarian Program, which intervened earlier (starting at age 4 months) and lasted longer (until age 8), the gain in IQ (Fig. 1, red diamonds) persisted into adulthood (21 years old). This early and persistent increase in IQ is important because IQ is a strong predictor of socioeconomic success.
Positive effects of these interventions also were documented for a wide range of social behaviors (Fig. 2). At the oldest ages tested (Perry, 40 yrs; Abecedarian, 21 yrs), individuals scored higher on achievement tests, reached higher levels of education, required less special education, earned higher wages, were more likely to own a home, and were less likely to go on welfare or be incarcerated than individuals from the control groups. Many studies have shown that these aspects of behavior translate directly or indirectly into high economic return. An estimated rate of return (the return per dollar of cost) to the Perry Program is in excess of 17% (19). This high rate of return is much higher than standard returns on stock market equity and suggests that society at large can benefit substantially from these kinds of interventions.
Fig. 1.
Mean IQ scores as a function of age for intervention and control groups in the Perry Preschool and Abecedarian Programs. Perry, circles; Abecedarian, diamonds; intervention group, red symbols; matched control group, blue symbols. Bars indicate SEs. Data from High/Scope and from the Carolina Abecedarian Project and the Carolina Approach to Responsive Education, 1972–1992.
Fig. 2.
Academic, economic, and social outcomes for the Perry Preschool and Abecedarian Programs. (A) Data from the Perry Program collected when the individuals were 27 years old (High/Scope). >10th percentile achievement, children who scored above the lowest 10% on the California Achievement Test (1970) at age 14; HS Grad, number of children who graduated high school on time. (B) Data from the Abecedarian Program collected when the individuals were 21 years old (Carolina Abecedarian Project and the Carolina Approach to Responsive Education, 1972–1992). Red bars, intervention group; blue bars, control group.
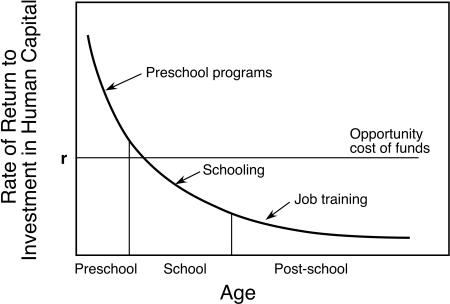
Several observations regarding the evidence from these intervention studies are relevant to this paper (Fig. 3). First, skills beget skills. That is, all capabilities are built on a foundation of capacities that are developed earlier. This principle stems from two characteristics that are intrinsic to the nature of learning: (i) early learning confers value on acquired skills, which leads to self-reinforcing motivation to learn more, and (ii) early mastery of a range of cognitive, social, and emotional competencies makes learning at later ages more efficient and, therefore, easier and more likely to continue.
Fig. 3.
Rates of return to investment in human capital as function of age when the investment was initiated. The data were derived from a life cycle model of dynamic human capital accumulation with multiple periods and credit constraints. Investments were initially set to be equal across all ages. r represents the cost of the funds. Data are from Cunha et al. (19).
Second, early intervention lowers the cost of later investment. For example, young children at risk for school failure who participate in early childhood programs are less likely to repeat grades or to require special education services (Fig. 2), thereby resulting in lower costs to the education system over time.
A more refined analysis of the intervention literature reveals significant increases in achievement across a broad range of outcomes (e.g., academic achievement tests, years of schooling completed, adult wages, and home ownership) among disadvantaged individuals as a result of exposure to an enriched preschool environment (Fig. 2). In most studies, these results are independent of IQ effects and are hypothesized to be related to differences in motivation, perseverance, temperament, and other dimensions of social competence as a result of the influence of enriched early experiences (10, 15–18). Some investigators have speculated that the positive effects of the early intervention programs are due to improvements in the social skills and emotional well-being of the children and that these effects, in turn, underlie the positive outcomes in school performance and wages earned later in adult life (19).
In contrast to the documentation of significant long-term effects from model preschool interventions, later remediation efforts have been shown to be considerably less effective (Fig. 3). School-age remedial programs for children and youth with cognitive limitations, for example, generally have had a poor record of success. Similarly, public job training programs, adult literacy services, prisoner rehabilitation programs, and education programs for disadvantaged adults have yielded low economic returns, with the returns for males often being negative (19). Moreover, for several studies in which later intervention showed benefits, the performance of these children still was behind the performances of children who experienced earlier interventions in the preschool years (19).
Although investments in older individuals realize relatively less return overall, such investments are still clearly beneficial. Indeed, the advantages gained from effective early interventions are sustained best when they are followed by continued high quality learning experiences (19). The technology of skill formation shows that the returns on school investment are higher for persons with higher ability, where ability is formed in the early years. Stated simply, early investments must be followed by later investments if maximum value is to be realized.
The studies cited above support the conclusion that early childhood experience has a powerful influence on the development of the cognitive, social, and emotional capacities that are prerequisites for strong economic productivity in adulthood. It is important to note, however, that the most convincing data for this assertion come from high quality intervention programs, which are not representative of the effectiveness of a wide range of services typically available to children from disadvantaged environments.
Ethical, practical, and cost considerations impose stringent limitations on how far research on humans can be pursued in rigorously controlled studies. Given these constraints, we turn to the research literature on other species to assess what has been learned regarding the fundamental principles of developmental neurobiology that might explain how early experience shapes social, emotional, and cognitive capacities in a way that has a lasting impact into the adult years.
Early Experience Shapes Temperament and Social Development.
Many of the emotional and social behaviors that are exhibited by humans also are observed in other species. Experiments with monkeys and rats, for example, have demonstrated that certain fundamental emotional and social behaviors are shaped dramatically by early experience (20–25). One of the most salient examples is the powerful influence of early interactions between an infant and its mother in shaping the temperament and social behavior of the developing animal. Much of this work has focused on the effects of early disruption of close affiliative bonds (8, 26–28).
In monkeys, considerable experimental work has demonstrated the extent to which the disruption of an early affiliative bond has long-term effects on the nature of an animal’s interactions with other monkeys, leading to a decrease in affiliative behaviors and an increase in aggressive interactions later in life (26, 28, 29). Although much of the evidence for these effects originally came from studies of monkeys reared in abnormal environments, such as in isolation or only with other young animals (21, 28–30), recent investigations have shown significant effects, even when infants were reared in more normal, complex, social environments (see Box 2).
Box 2: Consequences of Early Affiliative Bond Disruption in Monkeys.
Numerous studies have documented the effects of removing the mother on the development of social and emotional behaviors in infant monkeys (23, 26). In a recent study, long-lasting behavioral effects were observed even when the infant monkeys remained in an otherwise complex social environment, i.e., the infants remained in a rich home environment, but their mothers were removed (26, ‖).
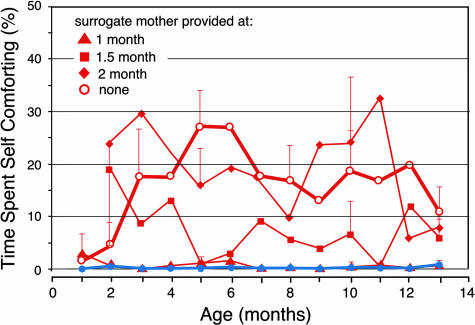
Infants deprived of their mothers at 1 week of age developed normally in many respects. However, compared with infants reared with their mothers, they exhibited a striking decrease in social interactions with other monkeys and a sharp increase in self-comforting behaviors, such as thumb-sucking (Fig. 4, red open circles). In addition, they rarely sought social comfort when placed in anxiety-provoking situations, such as a novel playroom, tending instead to vocalize loudly and to engage in self-comforting behaviors. As they matured, they continued to exhibit fewer social interactions, such as touching and sitting in proximity to other monkeys. When placed in new social groups, they displayed other atypical social behaviors. For example, they exhibited a strong propensity to try social interactions with unfamiliar monkeys, including unusual levels of both affiliative and aggressive behaviors.
The nature and severity of the effects of removing the mother changed with the age of the infants at the time of separation. Once the infants had reached 6 months old, removal of the mother from the group had no apparent impact on the infant (Fig. 4, blue filled circles). In contrast, infants who had their mothers removed at 1 month of age exhibited acute withdrawal and depression, followed by increased seeking of social comfort from other monkeys and a variety of atypical social behaviors, many of which persisted into adulthood. Attempts to remediate the social and emotional consequences of early affiliative bond disruption generally had limited impact. Placing a nurturing surrogate mother into a social group with an infant deprived of its mother at 1 week of age was capable of normalizing many aspects of the infant’s behavior (increasing time spent in social contact and decreasing the display of self-comforting behaviors) but only when the surrogate mother was introduced within the first month of the infant’s life (Fig. 4, red triangles). Progressively later placement of the surrogate mother with the infant was progressively less effective in remediating the adverse behavioral consequences of early affiliative bond disruption (Fig. 4, red squares and diamonds).
These results demonstrate that, for monkeys, there is sensitive period when an early environment that lacks a close, nurturing relationship with a primary caregiver (mother or surrogate mother) results in adult monkeys who respond aberrantly to social signals and do not integrate well into social groups. The deleterious consequences of this impoverished early experience on these social and emotional behaviors become extremely difficult (and, therefore, more costly) to remediate at a later age.
Studies in rodents also demonstrate that differences in affiliative behavior experienced early in life can have long-term effects on social behaviors and anxiety in adulthood (see Box 3). These findings, that both differences in, and disruptions of, close affiliative bonds early in life can have lifelong effects on the development of social behaviors, raise important concerns regarding the extent to which analogous early life experiences influence human development.
Box 3: A Sensitive Period for Shaping the Temperament of Rodents.
Certain aspects of the temperament of individual rats can be altered profoundly by early social experience (20, 24, 71). For example, rat pups that are cared for during the first week after birth by a mother who grooms them extensively (high-grooming) and nurses them in a way that facilitates their access to milk (arched-back nursing) become more adventurous, less fearful, less anxious, and less reactive to stress than rat pups raised by a mother who does not act in this manner. These emotional traits, shaped by experience during this sensitive period, have positive effects on the development of the individual’s social and cognitive behaviors that persist in adulthood.
Cross-fostering experiments show that the transmission of these traits is dominated by early experience, not by genetics (71, 81, 82). Rats born to low-grooming mothers (nonattentive, little grooming, and no arched-back nursing), but raised by high-grooming mothers, become themselves calm, adventurous adults and high-grooming parents. Conversely, rats born to high-grooming mothers, but reared by low-grooming mothers, become anxious adults and low-grooming parents. Thus, the transmission of these emotional and social traits is nongenetic, although without the intervention, the traits would have seemed entirely genetically based. Experiments such as these demonstrate that although genetics constrains the ranges of social and emotional characteristics that an individual rat can express, early experience can modify these characteristics over remarkably large extents. The genetic and molecular mechanisms that underlie this particular influence of early social experience on temperament (62, 67) are described in Supporting Materials.
Extensive animal research also demonstrates the existence of sensitive periods, usually early in life, when the systems underlying the development of social skills are particularly plastic, followed by a period during which this plasticity decreases with age (see Boxes 2 and 3; refs. 3, 20, 30, and 31). Together these findings argue strongly for early intervention programs to counteract adverse environmental circumstances that jeopardize the long-term development of social skills that, in turn, are likely to affect an individual’s long-term economic productivity. In a parallel fashion, developmental research in humans indicates that there may be sensitive periods in early childhood when greater responsiveness to therapeutic interventions might enhance lifelong outcomes and decrease the probability of later mental health problems, such as anxiety or depression (32–36).
Early Experience Shapes Perceptual and Cognitive Abilities.
The effects of early experience on perceptual and cognitive skills have been studied extensively by neuroscientists, and the same principles discussed above for social and emotional development hold true. Complex cognitive capacities, which mature and change throughout our lifetimes, depend on the analytic, synthetic, and recognition capabilities of specific neural circuits (37). The properties of many of these brain circuits have been shown to be particularly sensitive to the shaping influences of experience during early life (12, 31, 38–40).
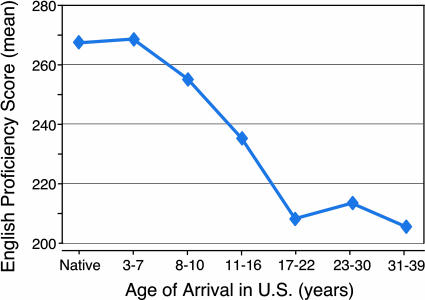
Language acquisition in humans is a well studied example of a complex cognitive ability that is shaped by early experience (see Box 4). All children at birth are capable of learning any of the world’s languages. As they experience a particular language, they become expert in analyzing, interpreting, and producing its distinctive sounds, and individuals who are exposed to multiple languages during the early years of life learn to speak each with equal facility (41, 42). Social factors play an important role in regulating this early learning process, because both the production and the perception of speech are learned substantially faster when a child learns from a human tutor rather than from taped or video speech (41). Learning a second language as an adult requires far greater effort than learning it as a child, and the result is never as complete (Fig. 5). Thus, language acquisition demonstrates both the hierarchical nature of learning, i.e., early skills influence the ability to master later skills (44), and the phenomenon of sensitive periods in development, i.e., times early in life when specific abilities can be mastered and shaped more readily than later (42).
Box 4: Sensitive Periods in the Acquisition of Language.
Language is an example of a cognitive skill that is acquired readily in early life but that requires great effort and is never learned as thoroughly as an adult (42, 83). The dependence of language learning on age holds for first languages and second languages (Fig. 5) and for spoken languages and sign languages. For most people, a thorough command of language is attained when learning occurs before ≈7 years of age. Statistically, language proficiency decreases progressively as language learning is delayed beyond 7 years and reaches adult levels by the end of adolescence. People who have never experienced language throughout their childhood are apparently incapable of acquiring a facility with language at a later age, despite intense training. Not all aspects of language learning are subject to sensitive periods. For example, proficiency with phonetic comprehension and production, grammar, and syntax is learned most effectively early in life, whereas semantics and vocabulary are learned with similar facility throughout life (41, 42, 84).
For language, as for many cognitive skills, early learning begets later learning (43). In the first stage of language learning, young children learn to discriminate among acoustically similar sounds (phonemes) that convey different meaning (41). This learning is critical to the next stage of language acquisition, which is to learn to segment phonemes into words. Sound segmentation is critical, in turn, to attaching meaning to words and finally to deriving meaning from grammar and syntax. As predicted by this hierarchy of information processing, the ability of children to discriminate phonemes at 6 months of age predicts their ability to understand words and phrases at later ages, and an inability to discriminate phonemes leads to pervasive language disabilities later in life (85).
Fig. 5.
Sensitive period for second language acquisition. English language proficiency scores as a function of age of arrival in the United States for a group of Chinese and Korean adult immigrants (n = 46). All subjects were students or faculty at the University of Illinois and had been in the United States for at least 10 years before testing. The test measured a variety of grammatic judgments. Data are from Johnson and Newport (43).
Early Experience Shapes Brain Architecture.
To understand why early experience exerts such a powerful influence on skill development, we must understand how experience shapes the neural circuits that underlie all behavior. Although some capacity for plasticity persists in virtually all neural circuits throughout our lifetimes, many circuits are particularly susceptible to the influence of experience during sensitive periods as they are maturing (ref. 12; see Boxes 3 and 5).
Box 5: A Sensitive Period for Changing Brain Architecture in Owls.
The ability of early experience to instruct the functional properties and architecture of neural circuits has been studied in the central auditory system of barn owls. Sound localization is a critical auditory function that allows animals to find mates, avoid danger, and target prey based on the sounds they hear. For barn owls, as nocturnal predators, sound localization is vital. To localize sounds, the central auditory system measures a variety of acoustic cues, such as interaural time differences (ITDs) and interaural level differences, and associates particular cue values with the location in space that produces them. Behavioral studies on barn owls have shown that these cue–location associations are shaped by experience most dramatically during a sensitive period that lasts until the individual approaches adulthood (86).
Neural circuits responsible for establishing cue–location associations in the owl’s central auditory system have been identified (87). One pathway in the midbrain transforms a neural representation of ITD and interaural level difference values into a topographic map of space, in a structure called the external nucleus of the inferior colliculus (ICX). This auditory space map then is sent to another structure, the optic tectum, where it merges with a visual map of space.
The representation of cues in the auditory space map is customized for the individual owl based on experience (88, 89). Manipulations of experience that alter auditory orienting behavior also alter the functional properties of neurons in this circuit. The magnitudes of the changes that are induced by experience depend greatly on the age of the animal (Fig. 6A). Large changes in neuronal response properties occur readily in juvenile owls and are accompanied by anatomical changes in the pattern of projections of axons into the space map (52). The change in circuit architecture that results from learning is due to the elaboration of axons and synapses in atypical portions of the ICX, as instructed by experience (Fig. 6B, learned axons). Similar structural changes have been observed in the visual cortex of kittens deprived of vision in one eye during a sensitive period (46). Once this additional circuitry is acquired and stabilized as a consequence of learning, it becomes highly resistant to elimination and can persist into adulthood (54). Owls that have learned an alternative map of auditory cues as juveniles can reexpress that alternative map as adults should it become adaptive once again (Fig. 4C), much like adult humans can readily relearn a language that they have learned as children. Thus, for this circuit, early learning establishes a brain architecture in juveniles that enables increased functional plasticity in adulthood.
A classic example of a neural circuit whose architecture is shaped by early experience is the circuit that conveys visual signals from the thalamus to the primary visual cortex in mammals (45, 46). When the quality of vision in one eye is substantially worse than that in the other, the axons conveying information from the disadvantaged eye disconnect from neurons in the visual cortex and withdraw most of their branches. Conversely, axons conveying information from the advantaged eye elaborate branches profusely and establish an abnormally high number of connections with cortical neurons (47, 48). This change in architecture is associated with a fundamental change in the function of the visual cortex, which becomes dominated by input from the advantaged eye. This effect of experience on brain architecture occurs only during a limited sensitive period in the development of this neural circuit, and once the circuit has matured, the major effects are irreversible (49, 50).
The response of the brain to poor input from one eye is adaptive in that the nervous system alters its circuitry so that it differentially processes input from the superior eye. However, the inability of this circuit to recover normal architecture and function after the end of the sensitive period, even when input to the disadvantaged eye is restored, has no apparent adaptive advantage. This characteristic underscores the critical importance of normative early experience for the development of this neural circuit. As with skill development, an impoverished early environment results in a reduced capacity that is difficult or impossible to remediate at a later age.
In a different neural circuit, early experience has been shown to be capable of altering brain architecture in a way that greatly expands the capacity of the adult brain to process information adaptively (Box 5). In this case, early experience takes advantage of the increased capacity for neural plasticity in young animals to create new architectures that then persist into adulthood and support alternative ways of processing information (Fig. 6). As with the previous example, the capacity of the brain to make fundamental architectural changes guided by experience appears to be limited to a sensitive period in early life, in this case, during the juvenile period (52).
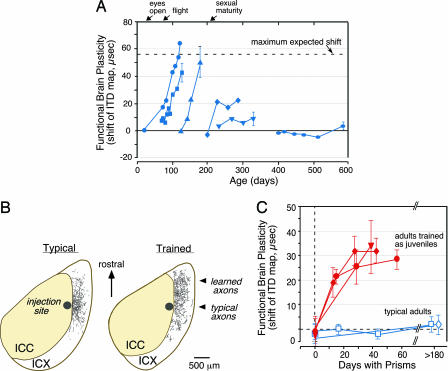
Fig. 6.
Functional and structural brain plasticity in the central auditory system of the barn owl. (A) Functional plasticity. Sensitive period for the visual calibration of the auditory system’s map of interaural time difference (ITD) in the midbrain. The data indicate mean adaptive shifts in the tuning of neurons in the optic tectum to ITD in 6 owls (different symbols) that resulted from experience with chronic displacement of the visual field with prismatic spectacles, beginning at different ages. Data are from Brainard and Knudsen (51). (B) Structural plasticity. Adaptive elaboration of axons and synapses in the brains of juvenile owls in response to experience with prism spectacles. Axon and synapse labeling in the external nucleus (ICX) after a tracer injection into the central nucleus (ICC) in a normal adult (typical) and in an owl that had acquired a learned ITD map as a juvenile (trained). Data are from DeBello et al. (52). (C) Increased adult plasticity. Early training leaves a memory trace that increases the capacity for functional plasticity in the adult brain. The data compare shifts in the ITD map in two typically reared adults (blue open symbols) with shifts in the ITD maps in three adults that had learned the alternative ITD map previously as juveniles (red filled symbols). Data are from Knudsen (53). Recent experiments indicate that the increased functional plasticity in previously trained adults is due to the persistence of altered architecture (see B) acquired during juvenile learning (54).
In the hierarchies of neural circuits that support complex behavior, sensitive periods for circuits at lower levels in the hierarchy, which perform more fundamental computations, tend to close before those for circuits at higher levels (38, 55, 56). For example, the sensitive period for circuits responsible for combining visual inputs from the two eyes ends long before the sensitive period for circuits responsible for recognizing biologically important objects (38, 57). This sequencing of sensitive periods is logical, because higher levels in a hierarchy depend on precise and reliable information from lower levels to accomplish their functions (i.e., early learning begets later learning, and skills beget skills). Thus, experience-dependent shaping of high-level circuits depends on the quality of the information provided by lower-level circuits, and the shaping of high-level circuits cannot be completed until the computations carried out by lower-level circuits are stable and reliable. The sensitive periods for most lower-level circuits end relatively early in life (12). In contrast, sensitive periods for some high-level circuits remain open until the individual approaches adulthood (e.g., Boxes 4 and 5).
Early Experience Shapes Gene Expression and Neurochemistry.
The activation of neural circuits by experience also can cause dramatic changes in the genes that are expressed (“turned on”) in specific circuits (58–60). The protein products of these genes can have far reaching effects on the chemistry of neurons and, therefore, on their excitability and architecture. For example, induced gene products can regulate the formation or elimination of synaptic connections or the responsiveness of neurons to neural activity or to specific hormones, neuromodulators, or neurotransmitters (61–66). These changes can have an enormous impact on the properties of a neural circuit and on the behaviors that it mediates. Most importantly, some genes are turned on or off, or can have their expression levels adjusted by experience, only during a limited sensitive period in a circuit’s maturation (27, 58, 59, 67–70).
A salient example of the effects of early experience on brain biochemistry and gene expression is the influence of early mothering of young rats on the release of “stress hormones” (glucocorticoids) and the subsequent lifelong change in the expression of genes for glucocorticoid receptors in key regions of the brain (Box 3). In this example, early social interactions modify gene expression in a way that changes a critical set point in a circuit that influences the animal’s temperament throughout life (20, 71). Beyond changing homeostatic set points in maturing neural circuits, experience-induced alterations in gene expression can lead to changes in patterns of connectivity, excitability, and biochemistry that alter how a circuit processes information and how it responds to circulating hormones, neuromodulators, and neurotransmitters well into adulthood (45, 64, 71).
Why Experience During Circuit Maturation Is So Effective.
The strong shaping influence of experience on neural circuits during their maturation results primarily from two factors. First, the molecular and cellular mechanisms that mediate neural plasticity during a sensitive period are highly active, enabling circuits to undergo substantial changes in architecture, chemistry, and gene expression in response to experiential influences (12, 31, 45, 72, 73). After a sensitive period has passed, one or more of these critical mechanisms no longer operate or operate less effectively.
A second factor is that it is far easier to form a pattern of connections in a neural circuit that does not already have an established configuration. When a circuit first develops, patterns and strengths of connections form according to genetically encoded mechanisms, but these patterns tend to be relatively imprecise, and the strengths tend to be relatively weak. Impulse activity that results from experience sharpens and strengthens these innate patterns of connections so that the circuit processes information in a certain way (12, 74, 75). This shaping and strengthening process engages cellular and circuit level mechanisms that stabilize the instructed pattern of connectivity (such as synapse consolidation and lateral inhibition). Concurrently, these induced changes antagonize the formation of alternative patterns of connectivity, making it more difficult for subsequent experience to change the initial configuration (12, 76, 77). Thus, the earliest experience is particularly influential because it has the unique advantage of instructing a pattern of connectivity in a circuit without interference from an already-established pattern.
Both of these factors, the unique availability of highly effective plasticity mechanisms and the relative ease of forming the first strong pattern of connections, contribute to the powerful influence of early experience on the development of neural circuits. The first factor, however, is the most critical. Thus, as long as the appropriate mechanisms required for mediating change continue to operate effectively, experience that comes later in a sensitive period will overcome or add to the effects of earlier experience. However, later experience requires relatively more intensity and tends to be less efficacious (12).
Conclusions and Implications
Decades of research in developmental psychology have documented the highly interactive process through which children develop the cognitive, social, and emotional capacities that are foundational for school achievement and adult economic productivity (3). In addition, extensive evidence from early intervention studies with disadvantaged children indicates that experience during early childhood can have a significant and lasting impact on a range of important adult outcomes (13, 14, 33)—positive early experiences enable individuals to become more fit cognitively and emotionally. These findings are complemented by extensive literature on human capital formation, indicating that later remediation of disadvantaged environments is much less effective than the provision of growth-promoting experiences early in life (19).
Supporting evidence for these conclusions comes from numerous studies of animal behavior, demonstrating that the early environments in which animals are reared exert powerful influences (both positive and negative) on their temperament, social behavior and cognitive skills, and that experiences later in life are substantially less effective in shaping many behaviors. It is important to note that the relevance of animal research to human circumstances rests not on the direct applicability of specific results to humans, but on the elucidation of underlying developmental and neurobiological principles. For example, the empirical findings of the positive effects of living in complex cages on learning capacity and brain architecture in young rats (78, 79) should not be equated with the relative impacts of a more or less stimulating home environment on the development of young humans. Rather, the value of such research lies in its demonstration of basic principles of neurobiology that apply across species, the extent to which brain architecture is influenced by both experience and genetics, the hierarchical nature of brain and behavioral development, and the concepts of sensitive periods and decreasing neuroplasticity over time.
Our understanding of the mechanisms that underlie the far-reaching effects of early experience on the development of the brain and behavior is advancing at an accelerating pace. The development of the brain is driven by two interacting forces: genetics and experience. In recent years, neuroscientists have made considerable progress in elucidating how different experiences affect the architecture, biochemistry, and gene expression exhibited by neural circuits that mediate cognitive, emotional, and social behaviors. These shaping influences are particularly powerful during sensitive periods of circuit maturation, when specific circuit functions can be altered in fundamental ways that customize their information processing capabilities according to the demands of the experience. Across species, experience is essential to the unfolding of brain development, the more adaptable the species, the more experience plays a role. Later in life, equivalent experiences induce far more subtle changes.
The implications of this rapidly evolving science for human capital formation are striking. The workplace of the 21st century will favor individuals with intellectual flexibility, strong problem-solving skills, emotional resilience, and the capacity to work well with others in a continuously changing and highly competitive economic environment. In this context, the personal and societal burdens of diminished capacity will be formidable, and the need to maximize human potential will be greater than ever before.
The evidence presented in this paper indicates that the most cost-effective strategy for strengthening the future American workforce is to invest greater human and financial resources in the social and cognitive environments of children who are disadvantaged, beginning as early as possible. The greatest return derives from investing in disadvantaged children because their home environments are impoverished. Therefore, for them, the difference between the stimulating intervention environment and the environment they would otherwise experience is extremely large. In contrast, for typical children, the difference between the intervention environment and the home environment is small or nothing. Among disadvantaged children, the greatest return derives from investing in the earliest years because early experiences exert particularly powerful influences at a time when foundational skills and behavioral patterns are being established and when underlying neural circuits are most plastic and optimally receptive to alteration at fundamental levels of architecture, chemistry, and gene expression. As the brain matures, experience continues to shape the neurobiology and behavioral manifestations of cognitive, emotional, and social capacities that, in turn, facilitate the subsequent development of a wide range of adult capabilities, including those that affect performance in the workplace.
The cognitively stimulating experiences in early childhood that are most important for the promotion of healthy development are provided through attentive, nurturing, and stable relationships with invested adults. When development is jeopardized by impaired relationships or other sources of environmental disadvantage, the biological and financial costs increase with age. Thus, although adaptation generally remains possible well into adult life, the decreasing plasticity of the maturing brain indicates that early intervention to mitigate the effects of disadvantaged environments is more efficient (in both energy costs to the nervous system and program costs to society) than later remediation for individuals with limited skills and problematic behavior. Stated simply, skills beget skills, success breeds success, and the provision of positive experiences early in life is considerably less expensive and more effective than the cost and effectiveness of corrective intervention at a later age. The issue of what the optimal strategies might be for maximizing the productivity of the future U.S. workforce is beyond the scope of the current discussion. However, as we confront the human capital needs of the future, the findings of neuroscience, behavioral research, and economics all agree on the following conclusion: Prevention is more effective and less costly than remediation, and earlier is far better than later.
Supplementary Material
Fig. 4.
Sensitive period for remediation of aberrations in macaque social behavior caused by early life affiliative bond disruption. Young monkeys whose mothers were present in the social group for the first 6 months of life (filled blue circles; n = 6) spent very little time engaged in self-comforting behaviors (thumb sucking and rocking) during the first year of life. In contrast, young monkeys who experienced removal of their mother from their social group at 1 week of age (open red circles; n = 6) spent significantly more time displaying self-comforting behaviors throughout the first year of life (ANOVA; P < 0.05). Introduction of a surrogate, nurturing mother at 1 month of age (red triangles; n = 3) was effective in preventing the development of self-comforting behaviors. In contrast, introduction of a surrogate mother at 1.5 months of age was less effective in ameliorating self-comforting behaviors (filled squares; n = 3), and introduction of a surrogate mother at 2–2.5 months of age (filled diamonds; n = 3) was ineffective in ameliorating self-comforting behaviors. Bars indicate SEs. Data are from McCormick et al. (‖).
Acknowledgments
We thank P. Knudsen for design of the figures. This paper was conceived and written under the auspices of the National Scientific Council on the Developing Child, with support from The John D. and Catherine T. MacArthur Foundation, The Susan A. Buffett Foundation, The Buffett Early Childhood Fund, and The Johnson & Johnson Pediatric Institute. We acknowledge support by grants from the MacArthur Research Network on Early Experience and Brain Development, the National Institutes of Deafness and Other Communication Disorders (to E.I.K.), and the Pew Foundation through the Committee on Economic Development and from National Institute of Child Health and Human Development (to J.J.H.).
Abbreviation
- ITD
interaural time difference
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
‖McCormick, K., Kerr, D., Rockcastle, N., Bytheway, J., Colosimo, D., Singer, L. & Cameron, J. L. (2004) Abstr. Soc. Neurosci. 426, 20 (abstr.).
References
- 1.Ellwood D. The Sputtering Labor Force of the 21st Century: Can Social Policy Help? New York: Russell Sage Found.; 2001. [Google Scholar]
- 2.DeLong J. B., Goldin C., Katz L. Sustaining U.S. Economic Growth. Washington, DC: The Brookings Inst.; 2003. [Google Scholar]
- 3.Shonkoff J., Phillips D. From Neurons to Neighborhoods: The Science of Early Childhood Development. Washington, DC: Natl. Acad. Press; 2000. [PubMed] [Google Scholar]
- 4.Rutter M. L., Kreppner J. M., O’Connor T. G. Br. J. Psychiatry. 2001;179:97–103. doi: 10.1192/bjp.179.2.97. [DOI] [PubMed] [Google Scholar]
- 5.Tennant C. Arch. Gen. Psychiatry. 1988;45:1045–1050. doi: 10.1001/archpsyc.1988.01800350079012. [DOI] [PubMed] [Google Scholar]
- 6.Goodman S. H., Gotlib I. H. Psychol. Rev. 1999;106:458–490. doi: 10.1037/0033-295x.106.3.458. [DOI] [PubMed] [Google Scholar]
- 7.Cicchetti D., Toth S. L. J. Am. Acad. Child Adolesc. Psychiatry. 1995;34:541–565. doi: 10.1097/00004583-199505000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Heim C., Nemeroff C. B. Biol. Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- 9.Carneiro P., Heckman J. J. Inequality in America: What Role for Human Capital Policy? Cambridge, MA: MIT Press; 2003. [Google Scholar]
- 10.Currie J., Blau D. Preschool, Day Care, and Afterschool Care: Who’s Minding the Kids? Amsterdam: North–Holland; 2005. [Google Scholar]
- 11.Katz L. C., Shatz C. J. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 12.Knudsen E. I. J. Cognit. Neurosci. 2004;16:1412–1425. doi: 10.1162/0898929042304796. [DOI] [PubMed] [Google Scholar]
- 13.Campbell F., Ramey C. Child Dev. 1994;65:684–698. [PubMed] [Google Scholar]
- 14.Schweinhart L. J., Montie J., Xiang Z., Barnett W. S., Belfield C. R., Nores M. Lifetime Effects: The High/Scope Perry Preschool Study Through Age 40. Ypsilanti, MI: High/Scope Found.; 2005. [Google Scholar]
- 15.Barnett W. S. Future Child. 1995;5:25–50. [Google Scholar]
- 16.Yoshikawa H. Future Child. 1995;5:51–75. [PubMed] [Google Scholar]
- 17.Olds D. L., Kitzman H., Cole R., Robinson J., Sidora K., Luckey D. W., Henderson C. R., Jr., Hanks C., Bondy J., Holmberg J. Pediatrics. 2004;114:1550–1559. doi: 10.1542/peds.2004-0962. [DOI] [PubMed] [Google Scholar]
- 18.Love J. M., Kisker E. E., Ross C., Raikes H., Constantine J., Boller K., Brooks-Gunn J., Chazan-Cohen R., Tarullo L. B., Brady-Smith C., et al. Dev. Psychol. 2005;41:885–901. doi: 10.1037/0012-1649.41.6.88. [DOI] [PubMed] [Google Scholar]
- 19.Cunha F., Heckman J., Lochner L., Masterov D. Interpreting the Evidence on Life Cycle Skill Formation. Amsterdam: North–Holland; 2005. [Google Scholar]
- 20.Meaney M. J. Annu. Rev. Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- 21.Suomi S. J. Genetic, Maternal, and Environmental Influences on Social Development in Rhesus Monkeys. Berlin: Springer; 1981. [Google Scholar]
- 22.Liu D., Diorio J., Day D. C., Francis D. D., Meaney M. J. Nat. Neurosci. 2000;3:799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- 23.Coplan J. D., Rosenblum L. A., Gorman J. M. Psychiatr. Clin. North Am. 1995;18:727–743. [PubMed] [Google Scholar]
- 24.Caldji C., Tannenbaum B., Sharma S., Francis D., Plotsky P. M., Meaney M. J. Proc. Natl. Acad. Sci. USA. 1998;95:5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanchez M. M., Ladd C. O., Plotsky P. M. Dev. Psychopathol. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- 26.Nelson C. A., Bloom F. E., Cameron J. L., Amaral D., Dahl R. E., Pine D. Dev. Psychopathol. 2002;14:499–520. doi: 10.1017/s0954579402003061. [DOI] [PubMed] [Google Scholar]
- 27.Meaney M. J., Diorio J., Francis D., Widdowson J., LaPlante P., Caldji C., Sharma S., Seckl J. R., Plotsky P. M. Dev. Neurosci. 1996;18:49–72. doi: 10.1159/000111395. [DOI] [PubMed] [Google Scholar]
- 28.Suomi S. J., Collins M. L., Harlow H. F., Ruppenthal G. C. J. Child Psychol. Psychiatry. 1976;17:101–112. doi: 10.1111/j.1469-7610.1976.tb00382.x. [DOI] [PubMed] [Google Scholar]
- 29.Kraemer G., Ebert M., Schmidt D., McKinney W. Child Dev. 1991;62:548–566. [PubMed] [Google Scholar]
- 30.Harlow H. F., Harlow M. K. Sci. Am. 1962;207:136–146. doi: 10.1038/scientificamerican1162-136. (5) [DOI] [PubMed] [Google Scholar]
- 31.Horn G. Nat. Rev. Neurosci. 2004;5:108–120. doi: 10.1038/nrn1324. [DOI] [PubMed] [Google Scholar]
- 32.Luby J. In: in Handbook of Infant Mental Health. Zeanah C, editor. New York: Guilford; 2000. [Google Scholar]
- 33.Shonkoff J., Meisels S. Handbook of Early Childhood Intervention. New York: Cambridge Univ. Press; 2000. [Google Scholar]
- 34.Mifsud C., Rapee R. M. J. Am. Acad. Child Adolesc. Psychiatry. 2005;44:996–1004. doi: 10.1097/01.chi.0000173294.13441.87. [DOI] [PubMed] [Google Scholar]
- 35.Ramey S. L., Ramey C. T. Ment. Retard. Dev. Disabil. Res. Rev. 1999;5:1–10. [Google Scholar]
- 36.Belsky J. Dev. Psychol. 2006;42:38–58. doi: 10.1037/0012-1649.42.1.38. [DOI] [PubMed] [Google Scholar]
- 37.Shaw P., Greenstein D., Lerch J., Clasen L., Lenroot R., Gogtay N., Evans A., Rapoport J., Giedd J. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- 38.Pascalis O., Scott L. S., Kelly D. J., Shannon R. W., Nicholson E., Coleman M., Nelson C. A. Proc. Natl. Acad. Sci. USA. 2005;102:5297–5300. doi: 10.1073/pnas.0406627102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brunson K. L., Kramar E., Lin B., Chen Y., Colgin L. L., Yanagihara T. K., Lynch G., Baram T. Z. J. Neurosci. 2005;25:9328–9338. doi: 10.1523/JNEUROSCI.2281-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schorr E. A., Fox N. A., van Wassenhove V., Knudsen E. I. Proc. Natl. Acad. Sci. USA. 2005;102:18748–18750. doi: 10.1073/pnas.0508862102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuhl P. K. Nat. Rev. Neurosci. 2004;5:831–843. doi: 10.1038/nrn1533. [DOI] [PubMed] [Google Scholar]
- 42.Newport E. L., Bavelier D., Neville H. J. In: Language, Brain and Cognitive Development: Essays in Honor of Jacques Mehler. Doupoux E., editor. Cambridge, MA: MIT Press; 2001. pp. 481–502. [Google Scholar]
- 43.Johnson J. S., Newport E. L. Cognit. Psychol. 1989;21:60–99. doi: 10.1016/0010-0285(89)90003-0. [DOI] [PubMed] [Google Scholar]
- 44.Morgan J. L., Demuth K. Signal to Syntax: Bootstrapping from Speech to Grammar in Early Acquisition. Hillsdale, NJ: Erlbaum; 1996. [Google Scholar]
- 45.Hubel D. H., Wiesel T., LeVay S. Philos. Trans. R. Soc. London B. 1977;278:377–409. doi: 10.1098/rstb.1977.0050. [DOI] [PubMed] [Google Scholar]
- 46.Hensch T. K. Curr. Top. Dev. Biol. 2005;69:215–237. doi: 10.1016/S0070-2153(05)69008-4. [DOI] [PubMed] [Google Scholar]
- 47.Antonini A., Stryker M. P. J. Comp. Neurol. 1996;369:64–82. doi: 10.1002/(SICI)1096-9861(19960520)369:1<64::AID-CNE5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 48.Antonini A., Fagiolini M., Stryker M. P. J. Neurosci. 1999;19:4388–4406. doi: 10.1523/JNEUROSCI.19-11-04388.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wiesel T. N., Hubel D. H. J. Neurophysiol. 1965;28:1060–1072. doi: 10.1152/jn.1965.28.6.1060. [DOI] [PubMed] [Google Scholar]
- 50.Jampolsky A. Symposium on Strabismus, Trans. New Orleans Acad. Ophthal. St. Louis: Mosby; 1978. pp. 358–492. Chapter 26. [Google Scholar]
- 51.Brainard M. S., Knudsen E. I. J. Neurosci. 1998;18:3929–3942. doi: 10.1523/JNEUROSCI.18-10-03929.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DeBello W. M., Feldman D. E., Knudsen E. I. J. Neurosci. 2001;21:3161–3174. doi: 10.1523/JNEUROSCI.21-09-03161.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Knudsen E. I. Science. 1998;279:1531–1533. doi: 10.1126/science.279.5356.1531. [DOI] [PubMed] [Google Scholar]
- 54.Linkenhoker B. A., von der Ohe C. G., Knudsen E. I. Nat. Neurosci. 2005;8:93–98. doi: 10.1038/nn1367. [DOI] [PubMed] [Google Scholar]
- 55.Jones K. R., Spear P. D., Tong L. J. Neurosci. 1984;4:2543–2552. doi: 10.1523/JNEUROSCI.04-10-02543.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.DeBello W. M., Knudsen E. I. J. Neurosci. 2004;24:6853–6861. doi: 10.1523/JNEUROSCI.0480-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Daw N. W. Optom. Vis. Sci. 1997;74:690–694. doi: 10.1097/00006324-199709000-00002. [DOI] [PubMed] [Google Scholar]
- 58.Tagawa Y., Kanold P. O., Majdan M., Shatz C. J. Nat. Neurosci. 2005;8:380–388. doi: 10.1038/nn1410. [DOI] [PubMed] [Google Scholar]
- 59.Corriveau R. A., Huh G. S., Shatz C. J. Neuron. 1998;21:505–520. doi: 10.1016/s0896-6273(00)80562-0. [DOI] [PubMed] [Google Scholar]
- 60.Kaczmarek L., Chaudhuri A. Brain Res. Brain Res. Rev. 1997;23:237–256. doi: 10.1016/s0165-0173(97)00005-2. [DOI] [PubMed] [Google Scholar]
- 61.Kelly M. P., Deadwyler S. A. J. Neurosci. 2003;23:6443–6451. doi: 10.1523/JNEUROSCI.23-16-06443.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weaver I. C., Champagne F. A., Brown S. E., Dymov S., Sharma S., Meaney M. J., Szyf M. J. Neurosci. 2005;25:11045–11054. doi: 10.1523/JNEUROSCI.3652-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang H. G., Lu F. M., Jin I., Udo H., Kandel E. R., de Vente J., Walter U., Lohmann S. M., Hawkins R. D., Antonova I. Neuron. 2005;45:389–403. doi: 10.1016/j.neuron.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 64.Kandel E. R. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- 65.Cabelli R. J., Shelton D. L., Segal R. A., Shatz C. J. Neuron. 1997;19:63–76. doi: 10.1016/s0896-6273(00)80348-7. [DOI] [PubMed] [Google Scholar]
- 66.Huh G. S., Boulanger L. M., Du H., Riquelme P. A., Brotz T. M., Shatz C. J. Science. 2000;290:2155–2159. doi: 10.1126/science.290.5499.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weaver I. C., Diorio J., Seckl J. R., Szyf M., Meaney M. J. Ann. N.Y. Acad. Sci. 2004;1024:182–212. doi: 10.1196/annals.1321.099. [DOI] [PubMed] [Google Scholar]
- 68.Philpot B. D., Sekhar A. K., Shouval H. Z., Bear M. F. Neuron. 2001;29:157–169. doi: 10.1016/s0896-6273(01)00187-8. [DOI] [PubMed] [Google Scholar]
- 69.Huang Z. J., Kirkwood A., Pizzorusso T., Porciatti V., Morales B., Bear M. F., Maffei L., Tonegawa S. Cell. 1999;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- 70.Corriveau R. A., Shatz C. J., Nedivi E. J. Neurosci. 1999;19:7999–8008. doi: 10.1523/JNEUROSCI.19-18-07999.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weaver I. C., Cervoni N., Champagne F. A., D’Alessio A. C., Sharma S., Seckl J. R., Dymov S., Szyf M., Meaney M. J. Nat. Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 72.Berardi N., Pizzorusso T., Maffei L. Curr. Opin. Neurobiol. 2000;10:138–145. doi: 10.1016/s0959-4388(99)00047-1. [DOI] [PubMed] [Google Scholar]
- 73.Daw N. W. Invest. Ophthalmol. Vis. Sci. 1994;35:4168–4179. [PubMed] [Google Scholar]
- 74.Engert F., Tao H. W., Zhang L. I., Poo M. M. Nature. 2002;419:470–475. doi: 10.1038/nature00988. [DOI] [PubMed] [Google Scholar]
- 75.Lu W., Constantine-Paton M. Neuron. 2004;43:237–249. doi: 10.1016/j.neuron.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 76.Hensch T. K. Nat. Rev. Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- 77.Zheng W., Knudsen E. I. J. Neurosci. 2001;21:4356–4365. doi: 10.1523/JNEUROSCI.21-12-04356.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grossman A. W., Churchill J. D., McKinney B. C., Kodish I. M., Otte S. L., Greenough W. T. J. Child Psychol. Psychiatry. 2003;44:33–63. doi: 10.1111/1469-7610.t01-1-00102. [DOI] [PubMed] [Google Scholar]
- 79.Cancedda L., Putignano E., Sale A., Viegi A., Berardi N., Maffei L. J. Neurosci. 2004;24:4840–4848. doi: 10.1523/JNEUROSCI.0845-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Barnett S., Masse L. N. Benefit Cost Analysis of the Abecedarian Early Childhood Intervention. New Brunswick, NJ: Natl. Inst. Early Educ. Res.; 2002. [Google Scholar]
- 81.Liu D., Diorio J., Tannenbaum B., Caldji C., Francis D., Freedman A., Sharma S., Pearson D., Plotsky P. M., Meaney M. J. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 82.Francis D., Diorio J., Liu D., Meaney M. J. Science. 1999;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- 83.Doupe A. J., Kuhl P. K. Annu. Rev. Neurosci. 1999;22:567–631. doi: 10.1146/annurev.neuro.22.1.567. [DOI] [PubMed] [Google Scholar]
- 84.Neville H. J., Mills D. L., Lawson D. S. Cereb. Cortex. 1992;2:244–258. doi: 10.1093/cercor/2.3.244. [DOI] [PubMed] [Google Scholar]
- 85.Tsao F. M., Liu H. M., Kuhl P. K. Child Dev. 2004;75:1067–1084. doi: 10.1111/j.1467-8624.2004.00726.x. [DOI] [PubMed] [Google Scholar]
- 86.Knudsen E. I. J. Comp. Physiol. 1999;185:305–321. doi: 10.1007/s003590050391. [DOI] [PubMed] [Google Scholar]
- 87.Knudsen E. I. Nature. 2002;417:322–328. doi: 10.1038/417322a. [DOI] [PubMed] [Google Scholar]
- 88.Brainard M. S., Knudsen E. I. J. Neurosci. 1993;13:4589–4608. doi: 10.1523/JNEUROSCI.13-11-04589.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gold J. I., Knudsen E. I. J. Neurosci. 2000;20:862–877. doi: 10.1523/JNEUROSCI.20-02-00862.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.