Abstract
We show that southern elephant seal (Mirounga leonina) colonies existed proximate to the Ross Ice Shelf during the Holocene, well south of their core sub-Antarctic breeding and molting grounds. We propose that this was due to warming (including a previously unrecognized period from ≈1,100 to 2,300 14C yr B.P.) that decreased coastal sea ice and allowed penetration of warmer-than-present climate conditions into the Ross Embayment. If, as proposed in the literature, the ice shelf survived this period, it would have been exposed to environments substantially warmer than present.
Keywords: Antarctica, southern elephant seals
With the warming climate of the past decades, rapid break-ups (caused by pooling of surface melt, seepage into crevasses, and wedging through the ice shelf) have destroyed several small, thin ice shelves fringing the Antarctic Peninsula (1, 2). Removal of ice-shelf back pressure has resulted in a marked increase in the seaward flow of glaciers discharging into the now abandoned embayments (i.e., ref. 3). With continued warming, this mechanism potentially could spread southward and affect the huge Ross and Ronne–Filchner Ice Shelves, the disintegration of which could result in accelerated ice-stream flow, catastrophic thinning of the West Antarctic Ice Sheet (WAIS), and global sea-level rise (4, 5). Several studies have suggested a time frame that could lead to disintegration of the Ross Ice Shelf over decades within this century (1, 4, 5).
We present Holocene climate data, which result from our discovery of abandoned southern elephant seal colonies along the Victoria Land Coast (VLC) (Fig. 1), that may bear on the question of Ross Ice Shelf susceptibility to warmer-than-present climate. The southern elephant seal has a circumpolar distribution today, typically breeding in sub-Antarctic latitudes on isolated islands. It is the largest of the phocid seals. They parturate and breed annually (after delayed implantation of the embryo) and spend the rest of the year foraging. Relatively few elephant seals have been observed in Antarctica, and these consist primarily of seals on foraging excursions or isolated molting groups (6), although some breeding takes place at Palmer Station and in the Windmill Islands (7, 8). The closest extant rookery to the VLC occurs in the sub-Antarctic 3,400 km to the north on Macquarie Island. Although a few southern elephant seals forage in the Ross Sea today, there are no breeding colonies or molting sites in the region and less than a dozen seals have been noted on the beaches since the discovery of Victoria Land in 1841 (9).
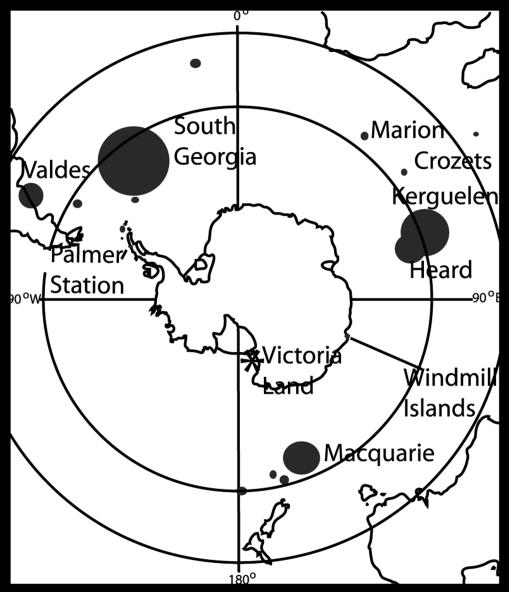
Fig. 1.
Map showing the present distribution of M. leonina (circles) and the former VLC colonies (asterisk) (6).
Results
We collected seal skin and hair from Holocene raised beaches (Fig. 2). Small pieces were found in nearly every excavation and, in places, were in roughly equal proportions to sand and gravel. Both visual inspection and DNA analysis confirm that the material is from southern elephant seals. We identified nine individuals from five sites, ranging in age from 266 to 2,864 14C yr B.P., as M. leonina, based on mtDNA amplification and sequence comparison with modern samples and all closely related local species. There were eight haplotypes among the nine ancient samples and all fit into the southern elephant seal lineage (Fig. 3). All but one of the ancient haplotypes was new [compared against modern haplotypes (10)]; therefore, they could not be the result of contamination. In addition to molted skin, mummified southern elephant seal tissue encasing essentially complete skeletal remains were found, totaling six adults and two pups.
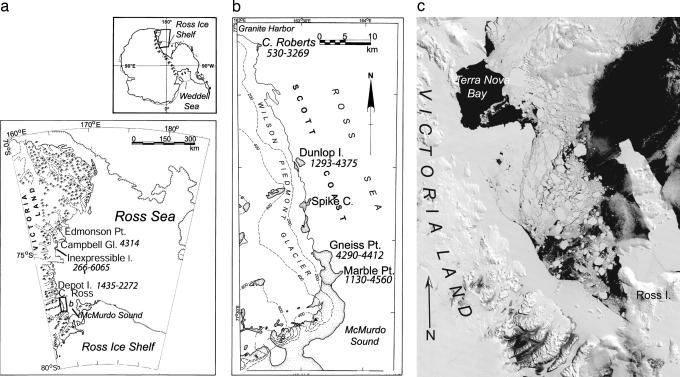
Fig. 2.
Index map of the western Ross Sea, giving the time range for seal habitation at each site. At the right is a MODIS satellite image, 29 January 2003. Fast ice is visible along the entire VLC, with the exception of Terra Nova Bay, a persistent polynya. Conditions along the VLC contrast with those in eastern McMurdo Sound, where warm ocean currents and strong katabatic winds prevent the formation of extensive land-fast ice. Existing penguin colonies are confined to areas of open water and pack ice.
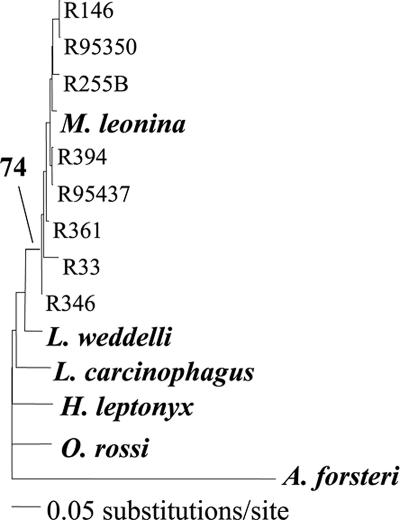
Fig. 3.
Neighbor-joining phylogeny including all Antarctic phocid and one otariid species (root), showing the clustering of mtDNA control region haplotypes from the abandoned VLC sites (all start with “R”) with the M. leonina sample. The bootstrap value for the node defining the Mirounga lineage is shown.
Fifty radiocarbon dates of the seal remains ranged from 266 to 6,065 14C yr B.P., after correction for the marine reservoir effect (see Table 1, which is published as supporting information on the PNAS web site, and Figs. 2 and 4). Initial colonization began ≈6,000 14C yr B.P. at Inexpressible Island. More southerly sites (Marble and Gneiss Points, and Dunlop Island) were occupied by ≈4,500 14C yr B.P. Seals were present at Gneiss Point, Cape Ross, and Campbell Glacier only briefly, probably because suitable access was absent after glacio-isostatic rebound. In addition, the record at Depot Island (Peninsula) also is short because of only limited reconnaissance. We exclude these sites from the analysis but note that existing data are consistent with our conclusions.
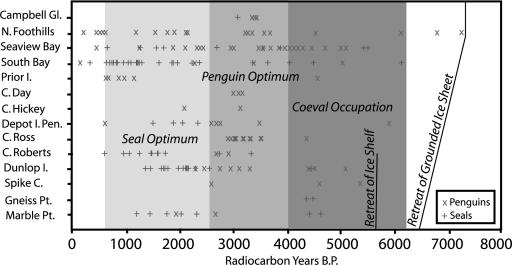
Fig. 4.
Diagram showing the distribution of radiocarbon dates for elephant seal (“+”) and Adélie penguin (“×”) samples from each site listed from south (bottom) to north (top).
The presence and distribution of elephant seals varied through time (Fig. 4). Both Marble Point and Dunlop Island apparently were unoccupied at ≈2,500–4,200 14C yr B.P., and only one sample from this time was found at Cape Roberts. Inexpressible Island shows small gaps in the record (2,300–3,300 14C yr B.P. South Bay; 2,600–3,500 14C yr B.P. Seaview Bay) coeval with those seen farther south. The period 2,500–4,000 14C yr B.P. probably represents an overall decline in seal numbers, along with abandonment of less favorable southern sites. In contrast, all analyzed locations show the presence of seals at ≈1,100–2,200 14C yr B.P. Seals declined thereafter, apparently from south to north, with site-abandonment beginning ≈1,100 yr ago at Marble Point and Dunlop Island and later at Cape Roberts (530 14C yr B.P.) and Inexpressible Island (≈260–500 14C yr B.P.). The disappearance of the population before sub-Antarctic sealing indicates that abandonment of the VLC was not human-induced but rather the result of environmental change.
Discussion
Foraging excursions into the Ross Sea are unlikely to explain the concentrated deposits of skin because elephant seals forage alone. The large amount of molted hair/skin suggests that the region was used for molting. Mummified pup remains suggest that the region also was used for breeding. In the sub-Antarctic, breeding takes place from September through November and molting from December through April (6). Mummified remains of adult males (which typically molt later in the season) may indicate their presence in cooler months: in spring to breed and autumn to molt. Male elephant seals molt from January through April in the Windmill Islands (11). Laws (12) reasoned that the distribution of southern elephant seals is related to climate, with pack-ice extent being the main factor limiting the establishment of southern rookeries. Breeding colonies south of the spring pack-ice limit have a precarious existence as has been evident for small rookeries in the South Orkney and South Shetland Islands (12).
The reconstructed seal colonization patterns are consistent with sea-ice control of haul-out access. The longest overall record comes from Inexpressible Island, which, because of its northern location and adjacent polynya, may be the most favorable site in the study area. In contrast, southern locations (Marble Point and Dunlop Island) with little open-water access today have the shortest habitation records of any of the major sites (Figs. 2 and 4). Because elephant seals have a predominantly sub-Antarctic distribution and a known propensity to haul out adjacent to open water, we conclude that expansion of land-fast, perennial sea ice, probably coupled with increased pack ice, may have prevented access to haul-out sites and resulted in colony demise. Moreover, the former presence of seal colonies indicates that unlike today at least seasonally open water must have been present along the VLC in the middle to late Holocene. If these sites were used for both breeding and molting, as we have suggested, open water would have had to be present from early spring through to the autumn.
If the seals had only been found near the Terra Nova Bay polynya or on Ross Island (Fig. 1), a viable alternative explanation would be that katabatic winds controlled the sea-ice extent rather than temperature. However, seals were found over the entire length of the Scott Coast, including areas that are relatively sheltered from katabatic winds. In general, the areas where we found the seals are almost always locked in fast ice today despite the occasional presence of open water a few tens of kilometers to the east. Icebergs today sometimes locally increase the thickness and duration of sea ice. However, the widespread distribution of seal remains reduces substantially the likelihood that it was icebergs rather than temperature controlling sea ice that affected the seals' use of the area. Past seal distribution also could have been affected by prey distribution, but because elephant seals feed in this region today, and often breed and molt far from where they feed, we do not think prey access is likely the factor that controlled their historical use of the area for breeding and/or molting. Taken together the data best support the hypothesis that sea-ice distribution controlled by temperature changes is what affected elephant seal use of the area.
Relict Adélie penguin rookeries along the VLC also have been used as sea-ice indictors (13). Comparison of the seals and penguins indicates that the timing of occupation at southern sites and possibly at southern Inexpressible Island appears to have been mutually exclusive, with little overlap between the species (Fig. 4). This relationship is not surprising, because today the habitats of these two species overlap only rarely. During the climate warming (and sea-ice decrease) of the last three decades, Adélie penguins have declined 70% in the vicinity of Palmer Station (thought to be related to reduced sea ice; Fig. 1), whereas elephant seals increased 300% (14, 15) (W. Fraser, personal communication). Whereas southern elephant seals favor open water, pack ice is important to the breeding success of Adélie penguins, offering one of the few reliable zones of prey concentration during spring (16, 17). Thus, these penguins tend to live south of the pack-ice limit year round. However, excessive, intact sea ice negatively impacts a colony by increasing distance to open water and delaying arrival at the nest sites (18). Adélie penguins, therefore, occupy a narrow zonal habitat optimum between insufficient and excessive sea ice (16, 19).
Between ≈4,000 and 6,000 14C yr B.P., the two species coexisted along the VLC, indicating less sea ice than at present (a requirement for elephant seal colonies) but still sufficient regional pack ice for Adélie penguins. Land-fast ice must have been at a minimum, and seasonally open water must have persisted right up to the shore. Between 2,300 and 4,000 14C yr B.P., a decline and even possible disappearance of elephant seals suggest that sea ice returned. The penguin colonies spread, perhaps because an increase in pack ice resulted in more favorable foraging ecology. However, ice still must have been less severe than at present along the VLC (where penguins cannot survive today) and may have resembled conditions seen today adjacent to northern Ross Island (Fig. 2).
The period between 1,100 and 2,300 14C yr B.P., marked by significant expansion of elephant seal colonies and a disappearance of Adélie penguins, represents the greatest sea-ice decline (and probably the warmest ocean and air temperatures) in the Ross Sea in the last 6,000 yr. This was followed by an increase in sea ice and the development of land-fast ice ≈1,000 yr ago on the VLC, which we propose led to the abandonment of seal colonies. The ice regime remains too severe for either elephant seals or penguins to occupy the southern VLC today. Integration of southern elephant seal and Adélie penguin data affords a distinctly different record of Holocene sea-ice change than that previously derived from penguin data alone. For example, the disappearance of penguins from the southern VLC (≈2,500 14C yr B.P.), originally thought to reflect severe ice (13), is now interpreted as indicating a period of sea-ice reduction so great that Adélie penguins no longer were a viable population. Although our new reconstruction differs from some previous interpretations (13, 20, 21), it is consistent with late Holocene atmospheric circulation intensity records from Siple Dome (22) and with methanesulfonic acid data from nearby Newall Glacier that suggest expanded sea ice between 300 and 1,250 yr B.P (23). In addition, our “seal optimum” is coeval with a significant accumulation rate increase at Taylor Dome, which may have resulted from sea-ice reduction and greater moisture availability (24). The disappearance of elephant seals from the VLC is broadly contemporaneous with the onset of Little Ice Age climatic conditions in the Northern Hemisphere and with glacier advance near Terra Nova Bay (25).
Our reconstruction implies that during parts of the middle and late Holocene, sea ice was less extensive, the warm season was likely longer, and the environment was more like the sub-Antarctic than at present in the Ross Embayment. Our data on seal distribution cannot inform us about the fate of the Ross Ice Shelf during these periods. However, the lack of coastal landforms, such as beaches south of the present calving front (unpublished observation), and the dates of marine organisms on the adjacent McMurdo Ice Shelf both suggest that the Ross Ice Shelf has been continuously present over the last 7,000 yr (26). Domack et al. (27) recently showed that the Larsen B ice shelf survived warm conditions in the mid-Holocene, even though it recently collapsed catastrophically. They infer from their data (based on oxygen isotope measurements in planktonic foraminifera) that the Larsen B ice shelf has been thinning throughout the Holocene and suggest that this, together with recent warming, led to the collapse. If the Ross Ice Shelf did survive, it too may have been thinning throughout the middle and late Holocene in response to grounding-line retreat (28). However, the critical parameter for recent ice-shelf collapse is thought to be an increase in the intensity and length of the summer melt season with ice shelves on the Antarctic Peninsula becoming susceptible to rapid breakup when January temperatures have exceeded −1.5°C (1). The presence of southern elephant seal colonies (which today exclusively occupy areas where the mean January temperature exceeds 0°C, usually by considerable margins), the disappearance of ice-obligate penguins, and the inferred significant reduction in sea ice, both in intensity and seasonal duration, suggest that the front of the Ross Ice Shelf could have been subject to January temperatures that surpassed the −1.5°C threshold during two long periods at ≈1,000–2,300 and 4,000–6,000 14C yr B.P. It may be that the environment, although warmer than present, did not reach the critical temperature over a sufficiently large portion of the ice shelf necessary to initiate rapid collapse, or that a steep climate gradient left much of the shelf in a stable zone. In addition, pinning points, such as Ross Island, may have contributed to its stability.
Materials and Methods
Field Methods.
We collected samples (see Table 1) from hand-dug excavations within raised beaches from sea level to ≈30 m elevation, the marine limit. All raised beaches in the study area were examined. Samples were obtained by careful examination of excavated sediment and extraction with trowels and tweezers. Seal skin and hair were abundant in most locations, although collection required careful searching. Mummified remains were identified as elephant seals based on skeletal morphology and pelage. Adult males (five of the six adults) could be identified by their distinctive canine teeth and body length. Two adult mummies were from Marble Point, one was from Cape Ross, and three adults and two pups were from Inexpressible Island.
Sample Preparation and DNA Extraction.
Ancient DNA extractions were carried out in a laboratory physically separated from the main building and exclusively dedicated to ancient DNA manipulation. Seven of the nine samples amplified are indicated in Table 1 (the samples that were dated). PCR and post-PCR analyses were carried out in the main laboratory. In addition, a one-way (from the ancient laboratory to the PCR laboratory) procedure was always followed to avoid the imperceptible carrying of DNA aerosols on clothes or skin into the ancient DNA laboratory (29). All plastic-ware was double autoclaved, and the PCR setup was done in a laminar flow hood. To detect possible contamination, extraction, PCR, and carrier negative controls were undertaken every 10 samples. No southern elephant seal DNA had ever been in the building housing the ancient laboratory prior to this study. Replications were done from a different piece of the same skin sample.
Dried skin pieces (from which the hairs had been plucked) were incubated overnight at 55°C with 3 ml of lysis buffer (0.5 M EDTA, pH 8.0/0.5% SDS) and 50 μl of proteinase K (20 mg/ml). Samples were centrifuged for 3 min at high speed, and aliquots of 500 μl of supernatants were transferred to clean tubes. Five volumes of QIAquick PB buffer (Qiagen) were added and thoroughly mixed. A 700-μl aliquot of this solution was loaded into a QIAquick PCR purification column (Qiagen) and centrifuged at high speed for 45 sec. The flow-through was discarded, and the process repeated until all of the extract was passed through the column. DNA binding to the silica filter was washed by adding 750 μl of QIAquick PE buffer and centrifuged for 1 min at high speed. The flow-through was discarded, and the samples were centrifuged for another 2 min to eliminate any trace of ethanol. DNA was eluted in 35 μl of EB buffer (TE-based buffer; Qiagen). Samples were centrifuged at maximum speed for 30 sec, and the DNA solution was collected in a clean tube. The DNA solution was kept at 4°C for 3 days and then stored at −20°C until being used for the PCR.
Primer Design and PCR Amplification.
Primers were designed across a segment of mtDNA 5′ control region sequence that showed both indel and sequence variation among Antarctic phocids, including four fixed differences between the southern elephant seal and each of the other Antarctic phocid species (Leptonychotes weddellii, Ommatophoca rossii, Lobodon carcinophaga, and Hydrurga leptonyx). The sequence also included fixed differences between the southern and northern elephant seal (Mirounga angustirostris) species, and extensive differentiation between Antarctic phocids and otariids. The primer sites showed no differentiation among 41 haplotypes [from screening 128 seals (10)] for the southern elephant seal. To facilitate amplification from ancient DNA, the fragment was designed to be short (209 bp including primers), from which 144 bp could be reliably sequenced (omitting sequence near the forward primer used for sequencing but including all fixed difference sites). The following primers were used: SESanc3f, 5′-GCTGACATTCTACTTAAACT-3′, and SESanc3r, 5′-ATGTACATGCTTATATGCAT-3′. Each 25-μl PCR contained 0.2 pM/μl each primer, 0.2 mM each dNTP, Platinum 1X High Fidelity Buffer [60 mM Tris-SO4, pH 8.9/18 mM (NH4)2SO4 (Invitrogen)], 15 mM MgCl2, 0.08 μg/μl BSA, and 1 unit of Taq High Fidelity DNA polymerase (Invitrogen). PCR conditions were as follows: 95°C for 8 min (enzyme activation); 45 cycles of 94°C for 45 sec, 52°C for 1.5 min, and 72°C for 1.5 min; and a final extension at 72°C for 5 min. Results were confirmed by repeated amplification from independent samples.
DNA Sequencing and Phylogenetic Analysis.
PCR products were purified with Qiagen PCR purification columns and sequenced directly by using the ABI dye-terminator method on an ABI 377 automated sequencer (Applied Biosystems). DNA sequences were aligned by using clustalx (30) and saved as a NEXUS file for input into paup (31). A neighbor-joining tree was constructed by using Kimura two-parameter distances and 1,000 bootstrap replications with a 50% criterion for node retention. Sequence from the otariid Arctocephalus forsteri was used to root the tree. All unique haplotypes from the ancient samples were included together with one representative from each of the five phocid species found in the Antarctic (including the southern elephant seal).
Supplementary Material
Acknowledgments
N. Gardner, P. Marcotte, A. Roy, and C. Smith assisted in the field. We thank P. Broady for first identifying the skin; M. Bryden, J. Ling, D. Siniff, and R. Vadas for examining skin or skeletal remains; C. Davis for providing DNA sequences for Ross and Crabeater seals; W. Fraser for insight into modern species interactions on the Antarctic Peninsula; and C. Campagna and T. Scambos for reviewing the paper. This work was supported by the Office of Polar Programs of the National Science Foundation and the Italian Antarctic Research Program.
Abbreviation
- VLC
Victoria Land Coast.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Scambos T., Hulbe C., Fahnestock M. Antarct. Res. Ser. 2004;79:79–92. [Google Scholar]
- 2.Vaughan D., Doake C. Nature. 1996;379:328–331. [Google Scholar]
- 3.DeAngelis H., Skvarca P. Science. 2003;299:1560–1562. doi: 10.1126/science.1077987. [DOI] [PubMed] [Google Scholar]
- 4.Mercer J. Nature. 1978;271:321–325. [Google Scholar]
- 5.Oppenheimer M., Alley R. Clim. Change. 2004;64:1–10. [Google Scholar]
- 6.LeBoeuf B., Laws R. Elephant Seals: Population Ecology, Behavior, and Physiology. Berkeley: Univ. of California; 1994. [Google Scholar]
- 7.Murray M. Polar Rec. 1981;20:370–371. [Google Scholar]
- 8.Heimark R., Heimark G. J. Mammal. 1986;67:189–190. [Google Scholar]
- 9.Brownell R., Ainley D. Antarct. J. U.S. 1976;11:189–190. [Google Scholar]
- 10.Fabiani A., Hoelzel A. R., Galimberti F., Muelbert M. Science. 2003;299:676. doi: 10.1126/science.299.5607.676. [DOI] [PubMed] [Google Scholar]
- 11.Van den Hoff J., Davies R., Burton H. Wildl. Res. 2003;30:275–280. [Google Scholar]
- 12.Laws R. Nor. Hvalfangst Tid. 1960;49:446–476. [Google Scholar]
- 13.Baroni C., Orombelli G. Geology. 1994;22:23–26. [Google Scholar]
- 14.Fraser W., Patterson D. L. In: Antarctic Communities: Species, Structure, and Survival, Battaglia B., Valencia J., Walton D., editors. Cambridge, U.K.: Cambridge Univ. Press; 1997. pp. 445–452. [Google Scholar]
- 15.Smith R., Fraser W., Stammerjohn S., Vernet M. Antarct. Res. Ser. 2003;79:131–158. [Google Scholar]
- 16.Fraser W., Trivelpiece W. Antarct. Res. Ser. 1996;70:257–272. [Google Scholar]
- 17.Daly K., Macaulay M. Mar. Ecol. Prog. Ser. 1991;79:37–66. [Google Scholar]
- 18.Ainley D., LeResche R. Condor. 1973;75:235–239. [Google Scholar]
- 19.Fraser W., Trivelpiece W., Ainley D., Trivelpiece S. Polar Biol. 1992;11:525–531. [Google Scholar]
- 20.Emslie S., Berkman P., Ainley D., Coats L., Polito M. Mar. Ecol. Prog. Ser. 2003;262:19–25. [Google Scholar]
- 21.Leventer A., Dunbar R., DeMaster D. Paleoceanography. 1993;8:373–386. [Google Scholar]
- 22.Meyerson E. A., Mayewski P. A., Sneed S., Kurbatov A., Kreutz K., Zielinski G., Taylor K., Brook E., Steig E., Yan Y., et al. The Holocene, in press. [Google Scholar]
- 23.Mayewski P. A., Lyons W. B., Zielinski G., Twickler M., Whitlow S., Dibb J., Grootes P., Taylor K., Whung P.-Y., Fosberry L., et al. Antarct. Res. Ser. 1995;67:33–45. [Google Scholar]
- 24.Monnin E., Steig E., Siegenthaler U., Kawamura K., Schwander J., Stauffer B., Stocker T., Morse D., Barnola J.-M., Bellier B., et al. Earth Planet. Sci. Lett. 2004;224:45–54. [Google Scholar]
- 25.Baroni C., Orombelli G. Antarct. Sci. 1994;6:497–505. [Google Scholar]
- 26.Stuiver M., Denton G., Hughes T., Fastook J. In: The Last Great Ice Sheets, Denton G., Hughes T., editors. New York: Wiley; 1981. pp. 319–436. [Google Scholar]
- 27.Domack E., Duran D., Leventer A., Ishman S., Doane S., McCallum S., Amblas D., Ring J., Gilbert R., Prentice M. Nature. 2005;436:681–685. doi: 10.1038/nature03908. [DOI] [PubMed] [Google Scholar]
- 28.Conway H., Hall B., Denton G., Gades A., Waddington E. Science. 1999;286:280–283. doi: 10.1126/science.286.5438.280. [DOI] [PubMed] [Google Scholar]
- 29.MacHugh D., Edwards C., Bailey J., Bancroft D., Bradley D. Ancient Biomol. 2000;3:81. [Google Scholar]
- 30.Higgins D., Sharp P. Gene. 1988;73:237–244. doi: 10.1016/0378-1119(88)90330-7. [DOI] [PubMed] [Google Scholar]
- 31.Swofford D. paup* 4.0b10. Sunderland, MA: Sinauer; 1998. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.