Abstract
Recently, it has been suggested that hepcidin, a peptide involved in iron homeostasis, is regulated by bone morphogenetic proteins (BMPs), apparently by binding to hemojuvelin (Hjv) as a coreceptor and signaling through Smad4. We investigate the role of Hfe, Tfr2 (transferrin receptor 2), and IL-6 in BMP2-, BMP4-, and BMP9-stimulated up-regulation of murine hepcidin, because these molecules, like Hjv, are known to be involved in hepcidin signaling. We show that the BMP signaling pathway acts independently of Hfe, Tfr2, and IL-6: The response to BMP2, BMP4, and BMP9 is similar in isolated hepatocytes of wild-type, Hfe−/−, IL-6−/−, and Tfr2m mutant mice. The potency of different human BMPs in stimulating hepcidin transcription by murine primary hepatocytes is BMP9 > BMP4 > BMP2. However, in human HepG2 cells, BMP4 and BMP9 are equally potent, whereas BMP2 requires a higher dose to become an effective hepcidin activator. Moreover, all of the tested BMPs are more potent regulators of hepcidin than IL-6 and thus are the most potent known stimulators of hepcidin transcription.
Keywords: HepG2, inflammation, iron
Bone morphogenetic proteins (BMPs) are cytokines belonging to the TGF-β superfamily. They play a crucial role in regulating cell proliferation, cell differentiation, and apoptosis and in the development of tissues (1, 2). There are 20 different human BMPs, with various expression profiles and tissue distribution. BMPs function by binding to specific receptors, which are divided into two separate groups: BMP receptor type I and type II. Binding to receptor homodimers is very weak, and high-affinity binding is accomplished by forming heterodimers of type I/type II receptors (3, 4).
Formation of the BMP–BMP receptor I/II type complex places these receptors in close proximity, leading to phosphorylation of the BMP type I subunit by the constitutively active serine/threonine kinase type II receptor. Phosphorylated receptor I is an active kinase that subsequently phosphorylates intracellular messengers of BMP signaling, including the Smad proteins and mitogen-activated protein kinase (5).
Smad proteins can be divided into three groups: receptor-mediated Smads (R-Smads) (Smad1, -2, -3, -5, and -8), the common mediator Smads (Co-Smads) (Smad4), and inhibitory Smads (Smad6 and -7). R-Smads are associated with the receptor complex and, upon ligand binding, become phosphorylated and form heterodimers with Co-Smad. The R-Smad/Co-Smad complexes translocate to the nucleus, where they bind directly or through specific transcriptional partners to promoter sequences of the target, regulated genes that are responsible for the transcriptional response to BMPs (6).
Recently, BMPs have been found to have a previously unexpected role in iron metabolism. Babitt et al. (7) demonstrated that RGMa, a homolog closely related to the protein associated with juvenile hemochromatosis (hemojuvelin, Hjv), was a coreceptor for BMP2 and BMP4. Babitt et al. (8) subsequently showed that Hjv was also a coreceptor for BMPs and suggested that the Hjv/BMP complex regulated hepcidin expression through a BMP signal transduction pathway. The importance of BMP signaling in iron homeostasis was confirmed in liver-specific Smad4-deficient mice, which showed a drastic decrease in hepcidin expression and an impaired hepcidin response to iron overload and IL-6. In addition, these mice exhibited abnormal iron accumulation and altered expression of several other proteins that are involved in iron uptake and metabolism, such as divalent metal transporter 1 (DMT1 or Nramp2), duodenal cytochrome b ferric reductase (DcytB), and ferroportin. Furthermore, hepcidin transcription was not stimulated by BMP4 treatment in hepatic cell lines derived from liver-specific Smad4-deficient mice, in contrast to cell lines derived from wild-type mice. The Smad4 responsive element was localized to a fragment of the murine Hepc1 promoter that spans the region 800 bp upstream of the start of transcription (9).
Hepcidin, a small, cysteine-rich cationic peptide with antibacterial properties, plays a key role in iron homeostasis. Expression of hepcidin mRNA is stimulated by iron excess and inflammation (10–12), whereas the expression of hepcidin mRNA is decreased by hypoxia and anemia (13, 14). Hepcidin binds to the iron exporter ferroportin and blocks its function by targeting ferroportin for internalization and degradation. As a result, iron uptake by the intestine is reduced, and iron release from stores in hepatocytes and macrophages is also diminished. It may also contribute to the anemia of chronic inflammation by diminishing the responsiveness of erythroid cells in the marrow to low levels of erythropoietin (15).
The signaling pathway that regulates hepcidin involves membrane-bound proteins such as Hjv, Hfe, and transferrin receptor 2 (Tfr2) (16, 17). Impairment of Hjv, Hfe, or Tfr2 leads to marked decrease in hepcidin expression and, subsequently, to abnormal tissue iron loading, particularly in hepatocytes, the phenotype of hereditary hemochromatosis (16, 18–21). The fact that Hjv acts as a coreceptor for BMPs raises the question of where Hfe and Tfr2 fit into the regulatory pathway. Could Hfe or Tfr2 also act as coreceptors? In this study, we examined whether Hfe or Tfr2 was required for BMP-induced hepcidin expression. Furthermore, we examined the question of whether the predominantly liver-specific BMP9 (22) might regulate hepcidin expression in the same manner as BMP2 and BMP4. Moreover, because the liver-specific Smad4-deficient mice have an impaired response to IL-6, we also investigated the role of IL-6 in BMP signaling. Using knockout Hfe−/− mice, IL-6−/− mice, and a Tfr2 mutant mouse, we demonstrate that Hfe, Tfr2, and IL-6 do not play a role in hepcidin stimulation by BMP2, BMP4, and BMP9 and thus are not in the pathway of BMP to hepcidin. Furthermore, we demonstrate that BMP9 is the most potent inducer of hepcidin expression currently known.
Results
Because the Hfe−/−, Tfr2m, and IL-6−/− mice were in different genetic backgrounds, separate experiments were performed, comparing the mutant mice with their own congenic strain in each case.
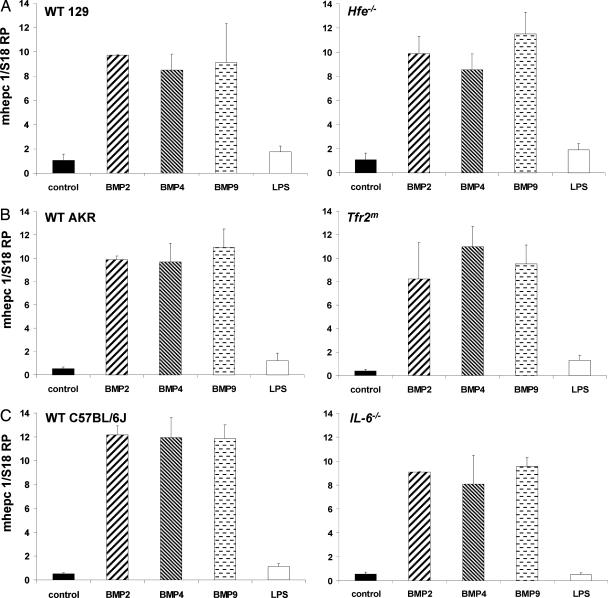
Hepcidin Is Up-Regulated by BMP2, BMP4, BMP9, and LPS in Wild-Type 129 and Hfe−/− Primary Murine Hepatocytes.
Treatment with BMP2 (150 ng/ml), BMP4 (10 ng/ml), and BMP9 (1 ng/ml) for 12–16 h resulted in an ≈10-fold increase in the expression of hepcidin in both wild-type and Hfe−/− primary hepatocytes. Treatment with 200 ng/ml LPS was used as a positive control because of its documented ability to regulate hepcidin expression and resulted in an ≈2-fold increase in the hepcidin mRNA levels (Fig. 1). The basal level of hepcidin expression was slightly decreased in the Hfe−/− knockout primary hepatocytes, in agreement with most (23, 24), but not all (16), previous reports.
Fig. 1.
Effect of BMP2, BMP4, BMP9, and LPS treatment on hepcidin expression in hepatocytes isolated from Hfe−/−, IL-6−/−, and Tfr2m mutant mice. Primary hepatocytes were isolated from perfused livers of Hfe−/− or wild-type 129 mice (A), Tfr2m mutant or AKR wild-type mice (B), and IL-6−/− or C57BL/6J mice (C), seeded at 1.5 × 105 cells per well, and allowed to attach to collagen-coated 12-well plates for 2 h, and BMP2 (150 ng/ml), BMP4 (10 ng/ml), BMP9 (1 ng/ml), or LPS (200 ng/ml) was applied for 12–16 h. Hepcidin and S18 ribosomal protein (S18 RP) (a normalization gene) expression was assayed by quantitative real-time RT-PCR using TaqMan probes. All samples were processed in triplicate, and a graph of experimental values ± SEM from at least two independent experiments is shown.
Hepcidin Is Up-Regulated by BMP2, BMP4, BMP9, and LPS in Wild-Type AKR and Tfr2m Mutant Primary Hepatocytes.
Treatment with BMP2 (150 ng/ml), BMP4 (10 ng/ml), and BMP9 (1 ng/ml) for 12–16 h resulted in an ≈10-fold or greater increase in the expression of hepcidin in both wild-type and Tfr2m primary hepatocytes. In contrast, treatment with 200 ng/ml LPS resulted in an ≈2-fold increase in hepcidin mRNA levels (Fig. 1). The basal level of hepcidin expression was decreased in the Tfr2m mutant primary hepatocytes, in agreement with previous reports (18).
Hepcidin Is Up-Regulated by BMP2, BMP4, and BMP9 in Wild-Type C57BL/6J and IL-6−/− Primary Hepatocytes.
Treatment with BMP2 (150 ng/ml), BMP4 (10 ng/ml), and BMP9 (1 ng/ml) for 12–16 h resulted in an ≈10-fold or greater increase in the expression of hepcidin in both wild-type and IL-6−/− primary hepatocytes. However, treatment with 200 ng/ml LPS resulted in an ≈2-fold increase in hepcidin mRNA levels in the wild-type mice and a significantly reduced response in IL-6−/− primary hepatocytes (Fig. 1).
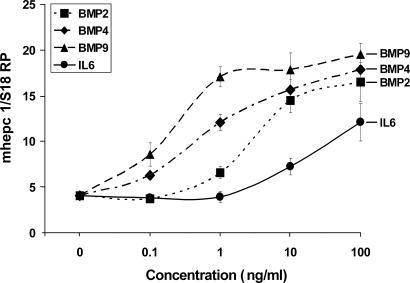
Dose–Response Curves for BMP2, BMP4, BMP9, and IL-6 in Murine Hepatocytes.
Isolated murine hepatocytes from the C57BL/6J strain were treated with 10-fold serial dilutions of BMP2, BMP4, BMP9, and IL-6, ranging from 0.1 to 100 ng/ml. As shown in Fig. 2, the most potent stimulator was BMP9, attaining its half maximal effect at ≈0.3 ng/ml. Approximately 1 ng/ml BMP4 and ≈3 ng/ml BMP2 were required to achieve the same effect. Interestingly, the known hepcidin stimulatory cytokine IL-6 was the least potent agent in this assay, and the maximal concentration of 100 ng/ml tested resulted in only approximately one-half of the stimulatory effect of BMPs.
Fig. 2.
Dose–response curves of BMP2, BMP4, BMP9, and murine IL-6. Primary hepatocytes were isolated from perfused livers of C57BL/6J mice, seeded at 1.5 × 105 cells per well, allowed to attach to the collagen-coated 12-well plates, and treated with BMP2, BMP4, BMP9, and murine IL-6 at concentrations ranging from 0.1 to 100 ng/ml for 12–16 h. Hepcidin and 18S ribosomal protein (S18 RP) (a normalization gene) expression was assayed by quantitative real-time RT-PCR using TaqMan probes. All samples were processed in triplicate, and the mean and SEM are shown.
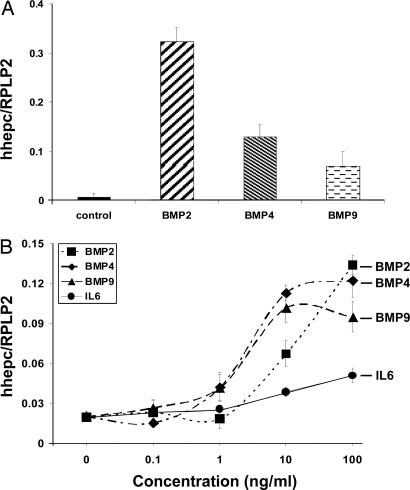
Hepcidin Is Up-Regulated by BMP2, BMP4, and BMP9 in Human HepG2 Cells.
Similar to our findings in murine primary hepatocytes, treatment with BMP2 (150 ng/ml), BMP4 (10 ng/ml), and BMP9 (1 ng/ml) for 12–16 h resulted in an ≈30-fold increase in the expression of hepcidin after stimulation by BMP2, a 10-fold increase after stimulation by BMP4, and an ≈5-fold increase after stimulation by BMP9 (Fig. 3A).
Fig. 3.
Effect of BMP2, BMP4, and BMP9 treatment on hepcidin expression in human HepG2 cells. (A) Human HepG2 hepatoma cells were seeded at 1.5 × 105 cells per well and allowed to attach to 12-well plates, and BMP2 (150 ng/ml), BMP4 (10 ng/ml), or BMP9 (1 ng/ml) was added. After 12–16 h of incubation, human hepcidin and human RPLP2 (a normalization gene) expression was assayed by quantitative real-time RT-PCR using TaqMan probes. All samples were processed in triplicate, and a graph of experimental values ± SEM from three independent experiments is shown. (B) Dose–response curves of BMP2, BMP4, BMP9, and human IL-6. Human hepatoma cells (HepG2) were seeded at 1.5 × 105 cells per well, allowed to attach to 12-well plates, and treated with BMP2, BMP4, BMP9, and human IL-6 at concentrations ranging from 0.1 to 100 ng/ml for 12–16 h. Human hepcidin and RPLP2 expression was assayed by quantitative real-time RT-PCR using TaqMan probes. All samples were processed in triplicate, and the mean and SEM are shown.
Dose–Response Curves for BMP2, BMP4, and BMP9 in Human HepG2.
HepG2 cells were treated with 10-fold serial dilutions of BMP2, BMP4, BMP9, and human IL-6, ranging from 0.1 to 100 ng/ml. As shown in Fig. 3B, BMP4 and BMP9 were equally effective, attaining half-maximal effect at ≈3–5 ng/ml, whereas BMP2 required ≈10 ng to achieve the same effect. Moreover, it appears that the induction of hepcidin transcription by BMP2 was not maximal even at a concentration of 100 ng/ml. As shown in Fig. 3A, BMP2 is able to increase hepcidin levels up to 30-fold compared with control at a concentration of 150 ng/ml. Interestingly, IL-6, a known hepcidin activator, was the least potent agent in this assay, and the maximal concentration of 100 ng/ml resulted in approximately doubling the hepcidin mRNA levels, whereas all BMPs at this concentration stimulated hepcidin ≈10-fold.
Discussion
Hepcidin is a key regulatory molecule of iron metabolism, but the mechanisms that regulate its expression are poorly understood. It has been shown that LPS; inflammatory cytokines such as IL-6, IL-1α, and IL-1β; and iron result in increased levels of hepcidin mRNA and protein (10–12) and that the expression of the hepcidin message is decreased by hypoxia and anemia (13, 14). More recently, the role of other classes of cytokines such as BMP and TGF-β has emerged, although the pathway and physiological relevance of these stimuli remain undefined (8, 9). Interestingly, the role of hepcidin processing and secretion has not yet been investigated as a regulatory mechanism, even though it may function as another level in the complex regulation of hepcidin expression and iron homeostasis.
A different scheme of hepcidin regulation recently has been suggested that depicts Hjv as a coreceptor of BMPs (8). The signal transduction pathway involves the Smad4 transcription factor; however, it is not clear which, if any, of the BMP receptor-associated Smads (Smad1, -5, or -8) is also required. Moreover, the nature of the stimulus that up-regulates BMP expression remains elusive, as well as the actual components and structure of the membrane-bound signaling complex.
To define this pathway more precisely, we have focused on other molecules that are involved in hepcidin regulation and signaling: in particular, Hfe and Tfr2. We hypothesized that if Hjv can act as a coreceptor of BMPs, it is possible that Hfe or Tfr2 is also a part of the signaling complex and/or it may modulate the response to BMPs. Furthermore, we tested whether IL-6−/− mice had impaired or modified BMP signaling because liver-specific Smad4-deficient mice do not respond to IL-6 treatment (9).
Our results show that human recombinant BMP2, BMP4, and BMP9 markedly up-regulated hepcidin mRNA in primary murine hepatocytes isolated from wild-type mice as well as Hfe−/− mice, IL-6−/− mice, and Tfr2m mutant mice. The observed increase in hepcidin expression was similar to that observed by Babitt et al. (8) and Wang et al. (9). However, we now show that BMP9 also up-regulate hepcidin. Moreover, we have demonstrated that BMP9 is the most potent inducer of murine hepcidin expression, followed by BMP4 and BMP2. Interestingly, all of the tested BMPs were more efficient in up-regulating murine hepcidin than the known hepcidin regulator, inflammatory cytokine IL-6.
Furthermore, the fact that human BMP2, BMP4, and BMP9 efficiently up-regulated hepcidin in primary murine hepatocytes as well as human hepatoma cell line HepG2 suggest that this signaling pathway is conserved in evolution. The dose of BMPs required to obtain a similar up-regulation of hepcidin was generally higher in HepG2 cells than in primary hepatocytes, and BMP4 and BMP9 were equipotent. However, the observed differences between murine primary hepatocytes and human HepG2 cells might be due to species differences or, more likely, to the transformed nature of the HepG2 cell line. The high potency and liver specificity of BMP9 suggest that it might be a previously unrecognized target for therapeutic intervention in iron overload disease and/or anemia of chronic inflammation.
We conclude that the BMP signaling pathway acts independently of Hfe, Tfr2, and IL-6, which suggests that either the BMP pathway is not involved in the iron sensing or, if it is, it functions downstream of Hfe and Tfr2. The studies of Wang et al. (9) indicate that liver cells in which the Smad4 gene is disrupted do not respond to IL-6 by up-regulating hepcidin transcription. We show, moreover, that IL-6 is not required for BMP-mediated (and therefore, presumably, Smad4-mediated) up-regulation of hepcidin transcription.
Materials and Methods
Materials.
Human recombinant BMP2, BMP4, BMP9, and IL-6 and murine recombinant IL-6 were obtained from R & D Systems. LPS and collagenase were from Sigma. Hepatocyte wash medium, minimal essential medium, Williams’ medium E, and liver digest medium were from Invitrogen.
Mice.
IL-6−/− mice were on a background of C57BL/6J (002650) and were obtained from The Jackson Laboratory. Hfe−/− mice and Tfr2 mutant (Tfr2m) mice were kind gifts from William Sly and Robert Fleming (both of Saint Louis University, St. Louis), respectively. We backcrossed the Hfe−/− mice into the 129 strain for 10 generations. The Tfr2m mutant mice, transgenic mice with a hypomorphic mutation that is found in humans, were on an AKR background.
Cells.
Human hepatoma cell line HepG2 was obtained from the American Type Culture Collection and cultivated in minimal essential medium supplemented with 5% FBS/100 units/ml penicillin/100 μg/ml streptomycin/1 mM sodium pyruvate/2 mM l-glutamine.
Isolation of Murine Primary Hepatocytes.
Each mouse was anesthetized and placed on a warm surface to keep the body temperature at 37°C. The abdominal and chest cavities were opened, and the inferior vena cava, portal vein, and heart were exposed. The inferior vena cava was cannulated with a 21-gauge catheter attached to a perfusion line, the heart was clamped, and the portal vein was cut to route retrograde perfusion primarily through the liver. A peristaltic pump was used to perfuse the liver with solutions placed in a 48°C water bath. Travel through the tubing lowered the temperature of the solutions entering the mouse to 37°C. The livers were perfused consecutively with 0.5 mM EGTA at a rate of 5 ml/min for 5 min, PBS at 5 ml/min for 2 min, and liver digest medium containing an additional 0.15 mg/ml collagenase at 2 ml/min for 10 min. The digested liver was excised and disaggregated by gentle rubbing with a cell scraper in a Petri dish with hepatocyte wash medium. The mixture was passed through a 70-μm nylon cell strainer (BD Biosciences) to obtain single cells, and the cells were washed twice with hepatocyte wash medium with centrifugation at 50 × g. To enrich the cells in hepatocytes, the cell pellet was resuspended in 25 ml of Williams’ medium E, layered over 20 ml of Percoll (Amersham Pharmacia Biosciences):Hanks’ balanced salt solution (9:1), and centrifuged at 250 × g for 10 min. Pelleted hepatocytes were resuspended in 12 ml of Williams’ medium E and centrifuged at 50 × g for 5 min. The hepatocyte pellet was resuspended in 12 ml of Williams’ medium E supplemented with 5% FBS, counted in a hemocytometer after staining with Trypan blue, and seeded at a density of 1.5 × 105 hepatocytes per well in a 12-well plate coated with collagen (BD Biosciences). Williams’ medium E and hepatocyte wash medium were supplemented with 100 units/ml penicillin/100 μg/ml streptomycin/2 mM l-glutamine before use.
BMP2, BMP4, BMP9, IL-6, and LPS Treatment of Primary Hepatocytes or HepG2 Cells.
BMP2, BMP4, and BMP9 and murine and human IL-6 were reconstituted according to the manufacturer’s instructions by dissolving in either a sterile aqueous solution containing 4 mM HCl and 0.1% BSA or a sterile aqueous PBS solution containing 0.1% BSA. Two to four hours after plating on collagen-coated 12-well plates, adherent murine hepatocytes were treated for 12–16 h with BMP2 (0.1–150 ng/ml), BMP4 (0.1–100 ng/ml), BMP9 (0.1–100 ng/ml), murine IL-6 (0.1–100 ng/ml), or LPS (200 ng/ml). HepG2 cells were plated into 12-well plates and treated for 12–16 h with BMP2 (0.1–150 ng/ml), BMP4 (0.1–100 ng/ml), BMP9 (0.1–100 ng/ml), and human IL-6 (0.1–100 ng/ml). The concentrations of BMPs used in this study were based on the concentrations described by Nakamura et al. (25) to activate the alkaline phosphatase gene.
RNA Isolation, cDNA Synthesis, and Quantitative Real-Time RT-PCR.
Total RNA was isolated from primary hepatocytes or HepG2 cells by using the Versagene RNA Purification Kit (Gentra Systems), including the DNase treatment step to avoid contamination with genomic DNA. cDNA first-strand synthesis was performed with 5–10 μg of RNA, Moloney murine leukemia virus reverse transcriptase (Invitrogen), and oligo(dT) primer (IDT) in a 20-μl volume. Quantitative PCR was carried out by using the Bio-Rad iCycler, using primers specific for murine Hepc1 (GenBank accession no. AF503444), with S18 ribosomal protein mRNA (GenBank accession no. AK050626) serving as a normalization gene. The hepcidin primers were designed to amplify only murine hepcidin 1 (Hepc1) and not hepcidin 2 (Hepc2) and are listed in Table 1. Amplification of human hepcidin (HEPC) (GenBank accession no. NC_0000019) and human ribosomal protein large P2 (RPLP2) (GenBank accession no. NM_001004) was performed with the primers listed in Table 1.
Table 1.
Quantitative real-time RT-PCR primers and probes
| Gene | Sense primer, 5′-3′ | Antisense primer, 5′-3′ | 5′-6-FAM/3BHQ 1-3′ probe |
|---|---|---|---|
| mhepcidin 1 | TTGCGATACCAATGCAGAAGA | GATGTGGCTCTAGGCTATGTT | AGAGACACCAACTTCCCCATCTGC |
| mS18 ribosomal protein | ACTTTTGGGGCCTTCGTGTC | GCCCAGAGACTCATTTCTTCTTG | ACACCAAGACCACTGGCCGCAG |
| hhepcidin | CACAACAGACGGGACAACTTG | CTCGCCTCCTTCGCCTCTGG | CCAGGACAGAGCTGGAGCCA |
| hRPLP2 | CGTCGCCTCCTACCTGCT | CCATTCAGCTCACTGATAACCTTG | TCGTCGTCCGCCTCGATACCCAC |
6-FAM, 6-carboxyfluorescein; 3BHQ 1, 3-black hole quencher-1.
The iCycler amplification was performed according to the manufacturer’s instructions in a buffer containing 20 mM Tris·HCl (pH 8.4), 50 mM KCl, 4.0 mM MgCl2, 200 μM dNTPs, 0.625 units of iTaq DNA polymerase (Bio-Rad), 300 nM sense primer, 300 nM antisense primer, and 300 nM 5′ 6-carboxyfluorescein-labeled TaqMan probe. One microliter of a 1:5 dilution of synthesized cDNA or standard was used in a 25-μl reaction, and all samples were analyzed in triplicate. The amplification protocol was as follows: 5 min of initial denaturation at 95°C followed by 40 cycles of 20-s denaturation at 95°C, 20 s of annealing at 59°C, and 20 s of extension at 72°C. A murine hepc1 standard curve was generated by using a murine hepc1 PCR product ranging from 0.068 to 680 amol per reaction. A murine S18 ribosomal protein standard curve was generated by using a PCR product ranging from 0.065 to 650 amol per reaction. A human hepcidin standard curve was generated by using a human hepcidin PCR product ranging from 0.069 to 690 amol per reaction. A human RPLP2 standard curve was generated by using a PCR product ranging from 0.014 to 140 amol per reaction. After 40 amplification cycles, threshold cycle values were automatically calculated, attomoles of target cDNA were deduced from the standard curve, and expression levels of hepc1 were expressed as the murine hepc 1/S18 ribosomal protein cDNA ratio in experiments with murine primary hepatocytes or the human hepc/RPLP2 cDNA ratio in experiments with HepG2 cells.
Acknowledgments
This work was supported by National Institutes of Health Grant DK53505 and the Stein Endowment Fund. This manuscript is no. 18141-MEM.
Abbreviations
- BMP
bone morphogenetic protein
- Tfr2
transferrin receptor 2
- Hjv
hemojuvelin
- RPLP2
ribosomal protein large P2.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Varga A. C., Wrana J. L. Oncogene. 2005;24:5713–5721. doi: 10.1038/sj.onc.1208919. [DOI] [PubMed] [Google Scholar]
- 2.Gambaro K., Aberdam E., Virolle T., Aberdam D., Rouleau M. Cell Death Differ. 2006;13:1075–1087. doi: 10.1038/sj.cdd.4401799. [DOI] [PubMed] [Google Scholar]
- 3.Ten D. P., Korchynskyi O., Valdimarsdottir G., Goumans M. J. Mol. Cell. Endocrinol. 2003;211:105–113. doi: 10.1016/j.mce.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 4.Ten D. P., Hill C. S. Trends Biochem. Sci. 2004;29:265–273. doi: 10.1016/j.tibs.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Liu F. Front. Biosci. 2003;8:s1280–s1303. doi: 10.2741/1149. [DOI] [PubMed] [Google Scholar]
- 6.Massague J., Seoane J., Wotton D. Genes Dev. 2005;19:2783–2810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- 7.Babitt J. L., Zhang Y., Samad T. A., Xia Y., Tang J., Campagna J. A., Schneyer A. L., Woolf C. J., Lin H. Y. J. Biol. Chem. 2005;280:29820–29827. doi: 10.1074/jbc.M503511200. [DOI] [PubMed] [Google Scholar]
- 8.Babitt J. L., Huang F. W., Wrighting D. M., Xia Y., Sidis Y., Samad T. A., Campagna J. A., Chung R. T., Schneyer A. L., Woolf C. J., et al. Nat. Genet. 2006;38:531–539. doi: 10.1038/ng1777. [DOI] [PubMed] [Google Scholar]
- 9.Wang R. H., Li C. L., Xu X. L., Zheng Y., Xiao C. Y., Zerfas P., Cooperman S., Eckhaus M., Rouault T., Mishra L., et al. Cell Metab. 2005;2:399–409. doi: 10.1016/j.cmet.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Lee P., Peng H., Gelbart T., Wang L., Beutler E. Proc. Natl. Acad. Sci. USA. 2005;102:1906–1910. doi: 10.1073/pnas.0409808102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee P., Peng H., Gelbart T., Beutler E. Proc. Natl. Acad. Sci. USA. 2006;101:9263–9265. doi: 10.1073/pnas.0403108101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ganz T. Curr. Opin. Hematol. 2004;11:251–254. doi: 10.1097/00062752-200407000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Means R. T. Blood Rev. 2004;18:219–225. doi: 10.1016/S0268-960X(03)00066-3. [DOI] [PubMed] [Google Scholar]
- 14.Nicolas G., Chauvet C., Viatte L., Danan J. L., Bigard X., Devaux I., Beaumont C., Kahn A., Vaulont S. J. Clin. Invest. 2002;110:1037–1044. doi: 10.1172/JCI15686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dallalio G., Law E., Means R. T. Blood. 2006;107:2702–2704. doi: 10.1182/blood-2005-07-2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmad K. A., Ahmann J. R., Migas M. C., Waheed A., Britton R. S., Bacon B. R., Sly W. S., Fleming R. E. Blood Cells Mol. Dis. 2002;29:361–366. doi: 10.1006/bcmd.2002.0575. [DOI] [PubMed] [Google Scholar]
- 17.Biasiotto G., Belloli S., Ruggeri G., Zanella I., Gerardi G., Corrado M., Gobbi E., Albertini A., Arosio P. Clin. Chem. 2003;49:1981–1988. doi: 10.1373/clinchem.2003.023440. [DOI] [PubMed] [Google Scholar]
- 18.Kawabata H., Fleming R. E., Gui D., Moon S. Y., Saitoh T., O’Kelly J., Umehara Y., Wano Y., Said J. W., Koeffler H. P. Blood. 2005;105:376–381. doi: 10.1182/blood-2004-04-1416. [DOI] [PubMed] [Google Scholar]
- 19.Nemeth E., Roetto A., Garozzo G., Ganz T., Camaschella C. Blood. 2005;105:1803–1806. doi: 10.1182/blood-2004-08-3042. [DOI] [PubMed] [Google Scholar]
- 20.Niederkofler V., Salie R., Arber S. J. Clin. Invest. 2005;115:2180–2186. doi: 10.1172/JCI25683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee P. L., Beutler E., Rao S. V., Barton J. C. Blood. 2004;103:4669–4671. doi: 10.1182/blood-2004-01-0072. [DOI] [PubMed] [Google Scholar]
- 22.Miller A. F., Harvey S. A., Thies R. S., Olson M. S. J. Biol. Chem. 2000;275:17937–17945. doi: 10.1074/jbc.275.24.17937. [DOI] [PubMed] [Google Scholar]
- 23.Frazer D. M., Wilkins S. J., Millard K. N., McKie A. T., Vulpe C. D., Anderson G. J. Br. J. Haematol. 2004;126:434–436. doi: 10.1111/j.1365-2141.2004.05044.x. [DOI] [PubMed] [Google Scholar]
- 24.Lee P. L., Peng H., Gelbart T., Beutler E. Proc. Natl. Acad. Sci. USA. 2004;101:9263–9265. doi: 10.1073/pnas.0403108101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakamura K., Shirai T., Morishita S., Uchida S., Saeki-Miura K., Makishima F. Exp. Cell Res. 1999;250:351–363. doi: 10.1006/excr.1999.4535. [DOI] [PubMed] [Google Scholar]