Abstract
Mice lacking the visual cycle enzymes RPE65 or lecithin-retinol acyl transferase (Lrat) have pupillary light responses (PLR) that are less sensitive than those of mice with outer retinal degeneration (rd/rd or rdta). Inner retinal photoresponses are mediated by melanopsin-expressing, intrinsically photosensitive retinal ganglion cells (ipRGCs), suggesting that the melanopsin-dependent photocycle utilizes RPE65 and Lrat. To test this hypothesis, we generated rpe65−/−; rdta and lrat−/−; rd/rd mutant mice. Unexpectedly, both rpe65−/−; rdta and lrat−/−; rd/rd mice demonstrate paradoxically increased PLR photosensitivity compared with mice mutant in visual cycle enzymes alone. Acute pharmacologic inhibition of the visual cycle of melanopsin-deficient mice with all-trans-retinylamine results in a near-total loss of PLR sensitivity, whereas treatment of rd/rd mice has no effect, demonstrating that the inner retina does not require the visual cycle. Treatment of rpe65−/−; rdta with 9-cis-retinal partially restores PLR sensitivity. Photic sensitivity in P8 rpe65−/− and lrat−/− ipRGCs is intact as measured by ex vivo multielectrode array recording. These results demonstrate that the melanopsin-dependent ipRGC photocycle is independent of the visual retinoid cycle.
Keywords: melanopsin, pupillary light response, retinal degeneration, retinal ganglion cell, visual photocycle
Mice blind from outer retinal degeneration retain the ability to entrain their circadian rhythms to external light-dark cycles, have active pupillary light responses (PLRs), and exhibit photically induced melatonin suppression (1–4). These responses depend on the opsin family member melanopsin (5), which is expressed exclusively in a subset of intrinsically photosensitive retinal ganglion cells (ipRGCs) (6–8). Mice with nonfunctional or degenerated rods and cones that also lack melanopsin show no photically influenced behavior or physiology (9, 10), and intrinsic photosensitivity of retinal ganglion cells is lost in the absence of melanopsin (9, 11, 12). Melanopsin forms a functional photopigment when heterologously expressed in COS cells (13), Xenopus oocytes (14), Neuro-2A cells (15), or HEK-293 cells (16). Melanopsin is thus necessary for ipRGC photoreception, and is sufficient to confer photosensitivity on intrinsically insensitive cell types. Taken together, these results strongly suggest that melanopsin is the photopigment of inner retinal photoreception.
All opsin photopigments use a retinoid chromophore that undergoes isomerization upon absorption of an appropriate wavelength photon. In rod photoreceptors, 11-cis-retinal chromophore is photoisomerized to all-trans-retinal which dissociates from the opsin protein (17). This all-trans-retinal photoproduct is recycled to the active (11-cis) form by enzymatic conversion in the adjacent retinal pigment epithelium (RPE) (18). The chromophore used by melanopsin in situ is not presently known. ipRGCs are physically distant from the enzymatic chromophore regeneration machinery of the RPE. It is presently unknown whether ipRGCs in situ use visual cycle chromophore regeneration mechanisms in the pigment epithelium, use components of this pathway in the inner retina, or use a novel pigment regeneration mechanism.
Mice mutant in several steps of visual photopigment regeneration have been generated by reverse genetics. Lecithin-retinol acyl transferase (Lrat) acylates all-trans-retinol with a fatty acid ester tail, trapping retinol in the RPE. This is an essential step in reconstitution of the 11-cis-retinal chromophore of visual photoreceptors. RPE65 is an abundant protein expressed primarily in the RPE; recent evidence has demonstrated that this enzyme is a critical component of the isomerisation reaction that converts fatty acid acylated all-trans-retinyl esters to 11-cis-retinol (19–21). Mice lacking RPE65 or Lrat show markedly reduced electroretinograms and PLRs (22, 23). Previous studies have demonstrated that the PLRs of mice lacking RPE65 or Lrat are ≈1,000-fold less sensitive than wild-type animals (23); furthermore, these animals show ≈100-fold lower pupillary response sensitivity than animals blind from outer retinal degeneration caused by the rodless–coneless (rdta;cl) and outer retinal degeneration (rd/rd) mutations (4, 24). Fu et al. (25) compounded mutations causing loss of outer retinal function [rod transducin (gnat1) and cone nucleotide gated channel (cnga3)] with mutations in rpe65 and found less sensitive PLRs in the gnat1−/−;cnga3−/−;rpe65−/− mice than in the gnat1−/−; cnga3−/− mice.
Two hypotheses may be invoked to explain these findings. First, the RPE65 and Lrat enzymes required for the visual photocycle might directly participate in melanopsin chromophore recycling. Alternatively, mutations in the lrat or rpe65 genes might have indirect effects on inner retinal photoreception through developmental or physiological alterations of retinal function. Developmentally, for instance, a “non-seeing” but anatomically intact outer retina (as in an rpe65−/− or lrat−/− mouse) might still influence ipRGC function either through synaptic modulation of ipRGC function or through compensatory retinal circuitry changes. Physiologically, the loss of visual cycle enzymes might alter chromophore availability to the inner retina without directly affecting the inner retinal photocycle.
Here we demonstrate that (i) paradoxically, outer retinal degeneration partially rescues the PLR phenotype of rpe65−/− and lrat−/− mice, (ii) acute pharmacologic inhibition of the visual retinoid cycle largely eliminates outer retinal photoreception, but does not affect inner retinal photoreception, (iii) treatment of rpe65−/− mice with outer retinal degeneration with oral retinal partially restores PLR sensitivity to these mice, and (iv) ex vivo, inner retinal photosensitivity is preserved in mice lacking visual retinoid recycling function. Taken together, these results suggest that ipRGC photoreception can function independently of outer retinal pigment recycling machinery, and suggest that chromophore depletion of the outer retina indirectly inhibits inner retinal function.
Results
PLRs of rpe65−/− and lrat−/− Mice Are Partially Rescued by Outer Retinal Degeneration.
Previous studies have demonstrated profound loss of pupillary light responsiveness in lrat−/− and rpe65−/− mice, to levels ≈100-fold lower than seen in rd/rd mice tested under identical conditions (4, 23, 24). To test whether this relative insensitivity of lrat−/− and rpe65−/− mice is due to a direct effect of these mutations on the inner retinal chromophore regeneration mechanism, we generated two compound mutations, combining outer retinal mutants rdta (in which diphtheria toxin is driven under a rod-specific promoter, resulting in early and severe outer retinal degeneration; ref. 26) and rd/rd (a null mutation in Pde6b resulting in total loss of rod and near-total loss of cone function; refs. 27–29) with rpe65−/− (22) and lrat−/− (23), respectively. If ipRGCs require the visual pigment recycling pathway in vivo, we would predict that rpe65−/−; rdta and lrat−/−; rd/rd would show additive epistasis for PLRs, and should have equivalent or lower PLR sensitivity than rpe65−/− or lrat−/− alone.
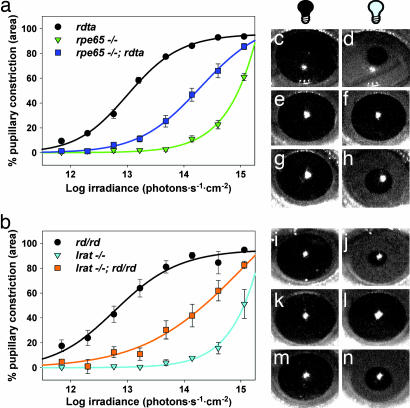
Histological examination of eyes from these mice revealed that rpe65 and lrat mutations did not attenuate or enhance the severe outer retinal degeneration caused by rdta and rd/rd (data not shown). To assay inner retinal photoreception, PLRs to narrow bandpass 470-nm light were measured (30) (Fig. 1). Consistent with previous reports (23, 25, 31), mice with mutations in visual cycle enzymes showed PLRs substantially less sensitive than those of mice with outer retinal degeneration. Lrat−/− and rpe65−/− mice showed half-maximal constriction of pupils at ≈8.9 × 1014 photons·cm−2·s−1 compared with half-maximal constriction at 6.3 × 1012 photons·cm−2·s−1 for rd/rd and rdta, and 1.5 × 1011 photons·cm−2·s−1 for wild-type C57BL/6 mice. Surprisingly, both rpe65−/−; rdta and lrat−/−; rd/rd mice were more photosensitive than mice lacking rpe65 or lrat only: each showed half-maximal constriction at ≈1.3 × 1014 photons·cm−2·s−1. Thus, rpe65−/−; rdta and lrat−/−; rd/rd PLRs are ≈7-fold more sensitive than those of rpe65−/− and lrat−/− mice. In turn, rpe65−/−; rdta and lrat−/−; rd/rd mice are ≈20-fold less sensitive than rdta or rd/rd mice.
Fig. 1.
PLRs. Irradiance–response relations for PLRs of rdta, rpe65−/−, rpe65−/−;rdta (a) and rd/rd, lrat−/−, lrat−/−;rd/rd (b) mice stimulated by 470-nm narrow bandpass filtered light (n = 6–9, mean ± SEM). Pupillary constriction (c–n) was stimulated by 470 nm light (3.98 × 1014 photons·sec−1·cm−2). An rdta pupil before (c) and after (d) 30 s of light exposure is shown. An rpe65−/− pupil before (e) and after (f) 30 s of light exposure is shown. An rpe65−/−; rdta pupil before (g) and after (h) 30 s of light exposure is shown. An rd/rd pupil before (i) and after (j) 30 s of light exposure is shown. An lrat−/− pupil before (k) and after (l) 30 s of light exposure is shown. An lrat−/−; rd/rd pupil before (m) and after (n) 30 s of light exposure is shown.
Pharmacologic Inhibition of Visual Retinoid Cycle Causes Loss of PLR Sensitivity in Wild-Type and Melanopsin Mutant Mice, but Not in rd/rd Mice.
In addition to direct effects on photoreceptors, the rd/rd mutation also results in alterations in inner retinal circuitry (32–34). Similar changes have also been reported in retinas of mice with mutations in the Leber’s congenital amaurosis-causing gene Crx (35) and after simple visual deprivation (36, 37). Recently, acute outer retinal degeneration has been reported to alter melanopsin expression levels in the inner retina of the rat (38, 39). Thus, the combined effects of visual retinoid cycle mutations and retinal degeneration mutations could, in part, be due to secondary changes in retinal circuitry from either mutation affecting ipRGC function. Alternatively, loss of visual cycle enzymes might alter chromophore availability to the inner retina, without directly affecting the inner retinal photocycle itself. Such a mechanism is consistent with the observations of Fu et al. (25) that pupillary sensitivity of gnat1−/−;cnga3−/−;rpe65−/− could be increased after treatment with 9-cis-retinal. Thus, it becomes important to distinguish acute from chronic effects of visual cycle enzyme loss.
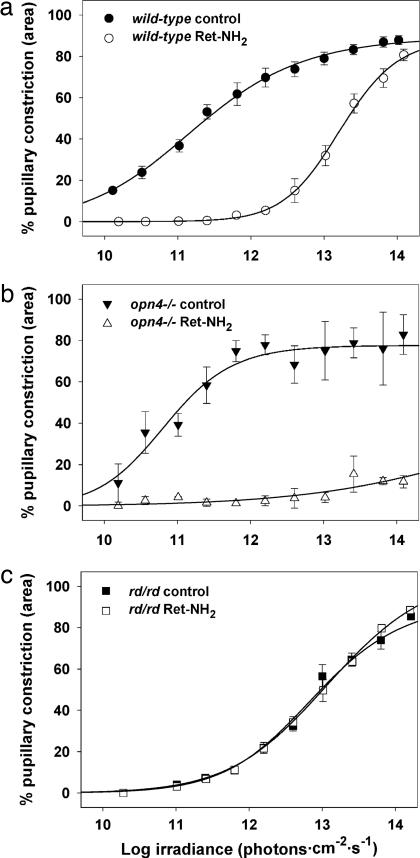
To test for the acute effect of loss of retinoid processing on inner retinal photoreception in adult animals, we used the potent and specific visual cycle inhibitor all-trans-retinylamine (Ret-NH2) (40, 41). Mice gavaged with 1 mg of Ret-NH2 show substantial loss of electroretinogram within one day (41). Wild-type mice treated with Ret-NH2 by gastric gavage showed a ≈100-fold decrement in pupillary light responsiveness 24 h after gavage (Fig. 2a). This effect persisted for 1 week after gavage but was fully reversed 2 weeks after treatment (data not shown). The sensitivity of PLRs in Ret-NH2-treated mice was greater than that seen in rpe65−/− mice, suggesting either incomplete blockage of the visual photocycle by Ret-NH2 or the presence of compensatory developmental changes in rpe65−/− mice. To determine the extent of blockade of visual photocycle activity, we treated melanopsin-deficient mice (opn4−/−) with Ret-NH2 (Fig. 2b). Melanopsin-deficient mice treated with retinylamine showed <15% constriction at maximal irradiance stimulation, suggesting near-total blockade of outer retinal photoreception by Ret-NH2. To determine the acute effect of visual photocycle inhibition on inner retinal photoreception, we treated rd/rd mice with Ret-NH2 (Fig. 2c). Ret-NH2 treatment had no effect on the sensitivity of PLRs of rd/rd mice. Similarly, treatment of rpe65−/−; rdta and lrat−/−; rd/rd with Ret-NH2 had no effect on PLR sensitivity (Fig. 6, which is published as supporting information on the PNAS web site). These results strongly support the hypothesis that visual photocycle function is not acutely required for inner retinal photoreceptive function in vivo.
Fig. 2.
All-trans-retinylamine inhibits outer but not inner retinal photosensitivity. Irradiance–response relations for PLRs of C57BL/6 (a), opn4−/− (b), and rd/rd (c) mice before (control) and 24 h after (Ret-NH2) oral gavage of 1 mg Ret-NH2. n = 3, 3, and 5 for C57BL/6, opn4−/−, and rd/rd, respectively; mean ± SEM.
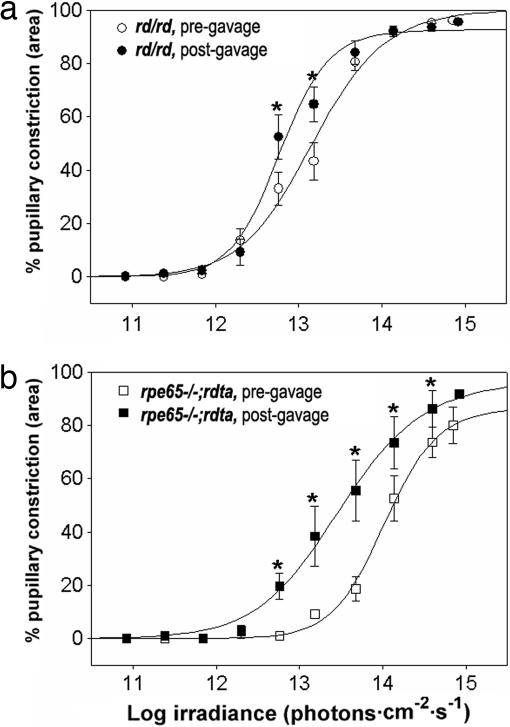
The effects of visual cycle enzyme loss in rpe65−/− and lrat−/− on inner retinal photoreception would therefore appear to be due to chronic rather than acute changes. To determine what proportion of these changes are due to reduced chronic chromophore availability, we compared the effects of oral gavage of 9-cis-retinal of rpe65−/−; rdta mice to gavage of outer retinal degenerate mice (Fig. 3). Consistent with the previous report of Fu et al. (25), treatment of rpe65−/−; rdta mice with 9-cis-retinal resulted in a ≈0.7 log increase in PLR sensitivity, indicating that the inner retinas of these mice have chronic, reversible chromophore depletion. Interestingly, we saw a small but statistically significant increase in the PLR sensitivity of rd/rd mice treated with 9-cis-retinal, suggesting that in the rd/rd animal, melanopsin may not be saturated with chromophore. Importantly, the posttreatment PLR sensitivity of rpe65−/−; rdta remained nearly 1 log less sensitive than that of treated outer retinal degenerate mice. Thus, loss of visual cycle enzyme function leads to both chromophore-dependent and chromophore-independent reduction in inner retinal photosensitivity.
Fig. 3.
9-cis-retinal partially rescues PLR sensitivity of rpe65−/−;rdta mice. Irradiance–response relations for PLRs of rd/rd (a) and rpe65−/−;rdta (b) mice before and 24 h after oral gavage of 1-mg 9-cis-retinal. n = 5 for rpe65−/−;rdta, n = 6 for rd/rd; mean ± SEM; asterisks indicate significance P < 0.05 by Wilcoxon ranked sums test.
Effects of RPE65 and Lrat Deficiency on ipRGC Light Responses.
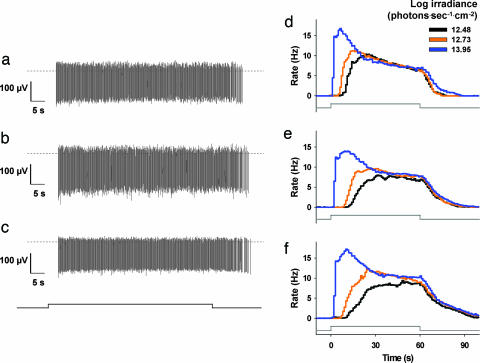
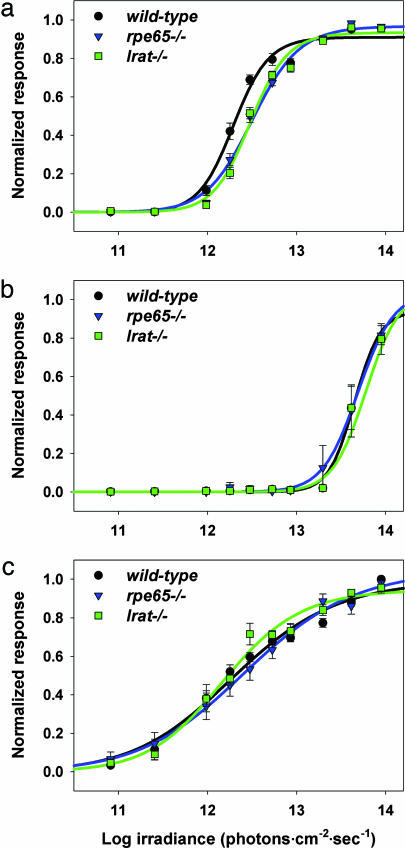
To determine the direct effect of mutations in retinoid processing machinery on intrinsic photosensitivity of ipRGCs, we tested postnatal day 8 (P8) wild-type, rpe65−/− and lrat−/− mice via multielectrode array (MEA) recording of inner retinal photoreponses (11) (Figs. 4 and 5). Light-induced signaling from rod and cone photoreceptors to retinal ganglion cells is not yet developed at this age, so ipRGC function can be assessed directly. Because of very high spontaneous ganglion cell firing activity, it is not possible at present to record ipRGCs of wild-type adults by MEA (11). Retinas were recorded under conditions of glutamatergic and cholinergic blockade to eliminate intrinsic retinal wave activity. Using MEA recordings, we have previously shown that all inner retinal light responses in P8 postnatal mice require melanopsin expression, and that these cells have spectral response characteristics identical to those of ipRGCs identified by retrograde tracing and recorded by patch-clamp technique (6, 11). Light-induced ipRGC action potentials were observed in both rpe65−/− and lrat−/− mice (Fig. 4). In P8–P10 retinas, three ipRGC response types have been described (11): type I cells (≈70%) have high photosensitivity but long latency to activity onset in response to dim subsaturating illumination; type II cells (≈15%) have low sensitivity and long latency; and type III cells (≈15%) have high sensitivity and short latency. All three response types were observed in lrat−/− and rpe65−/− retinas with frequency statistically indistinguishable from wild-type (Table 1). Individual type I ipRGC light responses from wild-type, rpe65−/−, and lrat−/− retinas were qualitatively indistinguishable (Fig. 4), as were type II and III ipRGC light responses (data not shown). Irradiance–response analysis of postnatal types I, II, and III ipRGCs to 480-nm light revealed that wild-type, rpe65−/−, and lrat−/− ipRGCs have very similar photosensitivity (Fig. 5), although the type I cells of rpe65−/− and lrat−/− mice displayed slightly decreased responses to subsaturating light stimulation (≈0.15 log lower than wild type).
Fig. 4.
ipRGC light responses of rpe65−/− and lrat−/− mice. Light-induced action potentials recorded from individual ipRGCs from C57BL/6 (a), rpe65−/− (b), and lrat−/− (c) P8 mouse retinas via multielectrode array. Timing of light stimulus (60 s, 480 nm, 4.11 × 1013 photons·sec−1·cm−2) is indicated by step in horizontal line below recordings. Zero voltage is indicated by dashed line. Cumulative average time course of type I ipRGC activity from C57BL/6 (d), rpe65−/− (e), and lrat−/− (f) retinas in response to subsaturating and saturating light stimulation (60 s, 480 nm light pulse is indicated by step in horizontal line below histograms). n = 27, 45, and 43 cells for d, e, and f, respectively; mean, 1-s bins.
Fig. 5.
Irradiance–response relations of rpe65−/− and lrat−/− ipRGCs. Irradiance–response relationships for P8 type I (a), II (b), and III (c) ipRGCs from C57BL/6, rpe65−/−, and lrat−/− mice. For C57BL/6, rpe65−/−, and lrat−/−, respectively: n = 27, 45, and 43 cells in a, n = 6, 3, and 8 cells in b, and n = 4, 5, and 8 cells in c; mean ± SEM.
Table 1.
Distribution of ipRGC types in wild-type, rpe65−/−, and lrat−/− mice
| Retinas | Number of ipRGCs recorded (% of total) |
||||
|---|---|---|---|---|---|
| Total | T1 | T2 | T3 | ||
| Wild type | 2 | 37 | 27 (72.9%) | 6 (16.2%) | 4 (10.8%) |
| rpe65−/− | 3 | 53 | 45 (84.9%) | 3 (5.6%) | 5 (9.4%) |
| lrat−/− | 3 | 59 | 43 (72.8%) | 8 (13.6%) | 8 (13.6%) |
T1, T2, and T3 refer to type I, II, and III cells (see text). No significant difference in distribution of cell types among genotypes was discerned (ANOVA).
Discussion
Several lines of evidence strongly suggest that melanopsin functions as the photopigment in intrinsically photosensitive retinal ganglion cells. Mice with outer retinal dysfunction or degeneration that also lack melanopsin show no nonvisual photoreceptive function (9, 10); no light responses of ipRGCs can be recorded from opn4−/− animals (9, 11, 12); and heterologously expressed melanopsin confers photosensitivity to nonphotoreceptive cells including Xenopus oocytes and immortalized mammalian cell lines (13–16). Although the requirement for exogenous retinal differs in different heterologous expression systems (e.g., HEK293-TrpC3 cells do not require exogenous retinal; ref. 16), it is presumed that melanopsin, like all opsins, utilizes a retinal-based chromophore for its photopigment function.
The chromophore used by melanopsin in situ and the means by which melanopsin chromophore is recycled are presently unknown. In Neuro-2a culture, cells transfected with a human melanopsin cDNA, 11-cis- and 9-cis-retinal were both able to serve as chromophore (15). Interestingly, in these cells, all-trans-retinal could function as chromophore if cells were pretreated with long-wavelength light (15). This finding suggests the possibility that melanopsin serves as both pigment and isomerase via sequential photon absorptions. Such bistability has been observed in amphioxus melanopsin (42) as well as a lamprey pineal UV-sensitive opsin (43). In addition to the bistability hypothesis, there are two other possible hypotheses explaining the reconstitution of melanopsin photopigment. Melanopsin might use existing enzymatic pathways for visual photopigment regeneration, namely the Lrat-RPE65-dependent visual cycle. Alternatively, melanopsin could be recycled via a cell-autonomous or inner retina-localized enzymatic regeneration mechanism.
The finding that PLRs of rpe65−/− and lrat−/− mice are both less sensitive than those of mice with outer retinal degeneration is consistent with a model in which ipRGCs use Lrat and RPE65 for their chromophore regeneration. However, such a model predicts that the effects of outer retinal degeneration should be additive with those of rpe65−/− and lrat−/−. Fu et al. (25) reported that combining two mutations, rod transducin (gnat1) and cone nucleotide gated channel (cnga3), with rpe65−/− resulted in mice that had significantly lower PLRs than gnat1−/−; cnga3−/− mice alone; however, comparison of the gnat1−/−; cnga3−/−; rpe65−/− mutant mice to rpe65−/− was not reported. In the present experiments, we similarly found that compounding outer retinal degeneration mutations rdta and rd/rd with rpe65−/− and lrat−/−, respectively, resulted in mice with less sensitive PLRs than those with outer retinal degeneration mutations alone. However, we observed that (paradoxically) outer retinal degeneration partially restored PLR sensitivity to rpe65−/− and lrat−/− mice. This result is inconsistent with a model in which ipRGCs require visual cycle enzymatic machinery for pigment regeneration.
We can posit two hypotheses for the finding that outer retinal degeneration mutations partially restore PLR sensitivity in rpe65−/− and lrat−/− mice. First, due to loss of ≈70% of the retinal thickness in rd/rd and rdta mice, the ipRGCs may be physically closer to the RPE and thus retinoid stores. Small amounts of retinal are recycled in rpe65−/− mice, possibly through a RGR opsin-dependent pathway (44), and low-level rod function (<0.1%) persists in these mice via 9-cis-retinal (45, 46). ipRGCs may have better access to these stores in rpe65−/− or lrat−/− retinas with outer degeneration. Alternatively, the intact but nonfunctional outer retinas of rpe65−/− and lrat−/− mice may suppress light-dependent ipRGC activity, either by direct synaptically mediated inhibition or by developmental alterations in intrinsic retinal circuitry (38). The results of our experiments using pharmacologic blockade of the visual cycle support both aspects of this latter model. Whereas administration of the visual retinoid cycle inhibitor Ret-NH2 caused a ≈100-fold decrement in photosensitivity of wild-type mouse PLR, administration of Ret-NH2 to rd/rd mice resulted in no loss of PLR sensitivity, demonstrating that the inner retina does not acutely require visual cycle enzyme function. As acute treatment with Ret-NH2 functionally reduces available outer retinal chromophore, were ipRGCs dependent on outer retina retinoid stores, we would have expected a loss in sensitivity in rd/rd mice treated with Ret-NH2. However, inner and outer retinal photoreception are not completely independent. The PLR sensitivity of rd/rd mice treated with Ret-NH2 was greater than that of wild-type mice treated with this compound, suggesting that the nonfunctional outer retina of treated wild-type mice exerted a net inhibitory effect on ipRGC sensitivity. Outer retinal inhibition of ipRGC function has been previously suggested by the finding that light-induced c-fos immunoreactivity is increased in the suprachiasmatic nuclei of mice with outer retinal degeneration (47, 48).
The present experiments confirm earlier findings that the PLR of mice lacking visual cycle enzymes is substantially lower than that of mice with outer retinal degeneration (23, 31). As suggested by Fu et al. (25), a component of this decreased sensitivity appears to be due to chromophore depletion, as PLR sensitivity can be partially rescued by systemic administration of cis-retinoid. These chromophore depletion effects appear to be due to chronic alterations in visual pigment processing because rd/rd mice show no decrease in sensitivity after acute blockade of the visual cycle. However, cis-retinoid treatment only partially restores visual sensitivity to rpe65−/−;rdta mice; such mice still have ≈10-fold lower sensitivity than rd/rd mice treated with cis-retinal. This finding suggests that mutations in rpe65 and lrat have non-chromophore-dependent developmental or physiological effects on retinal circuitry causing long-term changes in inner retinal sensitivity.
Previous work by Fu et al. (25) suggested that ipRGCs of rpe65−/− mice are ≈20-fold less sensitive (as measured by single cell patch clamp recording) than those of wild-type animals. We did not observe a significant decrease in sensitivity of ipRGCs of rpe65−/− or lrat−/− mice in multielectrode array extracellular recordings. This may have been due to differences in age of the animals: we recorded from P8 retinas, a time when developmental or chromophore-depletion effects of visual cycle retinoid mutations may not have been manifest. Alternatively, the observed decrement in rpe65−/− membrane currents seen in patch clamp recording may not have been sufficient to alter action potential firing as measured by multielectrode array.
The persistence of ipRGC light-dependent activity in rpe65−/− and lrat−/− retinas demonstrates that inner retinal photoreception can function without these enzymes ex vivo. The full preservation of PLR sensitivity in rd/rd mice treated with Ret-NH2 demonstrates that the inner retina does not acutely use visual cycle machinery in vivo. Because all inner retinal photoreception is melanopsin-dependent, this strongly suggests that melanopsin utilizes a visual cycle-independent mechanism for chromophore regeneration.
Materials and Methods
Mice.
Melanopsin-deficient (opn4−/−) mice were obtained from S. Panda (49). rpe65−/− were obtained from T. M. Redmond (22). rpe65−/−, rdta (26), lrat−/− (23), and C3H/HeJ (rd/rd) mice were crossed to generate rpe65−/−; rdta and lrat−/−; rd/rd mice. Mice were genotyped by using established protocols from tail-snip DNA (22, 23, 26, 48). Sibling mixed-strain mice were used for PLR measurements. C57BL/6J mice were used as wild-type. For multielectrode array experiments, rpe65−/− and lrat−/− mice were produced via homozygous mating pairs. Mice were maintained in 12-h light/12-h dark conditions with food and water available ad libitum. All studies were carried out under approved institutional animal protocol, in accordance with ARVO guidelines for animal studies.
Histology.
Eyes enucleated from killed 7- to 8-month-old mice were immediately fixed in 10% formalin at room temperature overnight. Fixed globes were embedded in glycol methacrylate, sectioned (3 μm), and stained with toluidine blue.
Pupillometry.
Pupillometry was performed as described (24, 30). Briefly, mice were dark-adapted for ≥1 h before recordings and tested without anesthesia at fixed circadian time. PLRs were recorded under infrared conditions using a CCD video camera fitted with IR filter and macro lenses. A halogen source was used for light stimuli; wavelength and intensity were manipulated via neutral density and narrow bandwidth (10 nm) interference filters (Newport Corp-Oriel, Stratford, CT). Irradiance measurements (W·m−2) were made by using a calibrated radiometer (Advanced Photonics International, White Plains, NY). Irradiance–response relations were fit with a Michaelis–Menten equation (50–52).
Retinoid Treatment.
Ret-NH2 was synthesized as described (41). Mice were fed 1 mg of Ret-NH2 suspended in vegetable oil via oral gavage. For 9-cis-retinal treatment, 1 mg (Sigma) was resuspended in 1 ml vegetable oil, and fed to mice via oral gavage. For Ret-NH2 treatment, 24 h after gavage, mice were exposed to 30 min of light (≈2,500 lux, white fluorescent bulb) and then dark-adapted for 2 h before pupillometry. For 9-cis-retinal treatment, mice were tested after dark adaptation. In each case, pre- and posttreatment pupillary responses were compared as paired data from individual animals.
Multielectrode Array Recordings.
Retinas from postnatal (P8) rpe65−/−, lrat−/−, and wild-type mice were tested for ipRGC light responses via multielectrode array recordings, and data were analyzed as described (11). Briefly, each dissected retina (without RPE) was positioned with its inner retinal surface in contact with a planar array of 60 extracellular electrodes (Multi Channel Systems, Reutlingen, Germany). Recordings were carried out in the presence of glutamatergic and cholinergic blockade as described (11). Data were acquired and analyzed via custom software (53). Single units (with distinct interspike refractory periods) were isolated via cluster analysis based on differences in spike waveform. The light responses (total number of light-induced action potentials) of individual cells were normalized to each cell’s maximal response to 480 nm light. Light stimuli were calibrated and delivered as described (11).
Supplementary Material
Acknowledgments
We thank T. Michael Redmond (National Eye Institute, Bethesda) for generously providing rpe65−/− mice and S. Panda and J. Hogenesch (Genomics Institute of the Novartis Research Foundation, San Diego) for providing opn4−/− mice. This research was supported in part by National Institutes of Health Grants EY14988 (to R.N.V.G.) and EY09339 (to K.P.), the Medical Scientist Training Program of Washington University (D.C.T.), the Culpepper Medical Scholars Program of the Rockefeller Brothers Foundation (R.N.V.G.), the McDonnell Systems Neuroscience Award (to R.N.V.G.), Research to Prevent Blindness (R.N.V.G.), and awards to the Washington University Department of Ophthalmology and Visual Sciences from an unrestricted grant from Research to Prevent Blindness, Inc., and National Institutes of Health CORE Grants P30 EY 02687 and P30 EY11373.
Abbreviations
- PLR
pupillary light response
- ipRGC
intrinsically photosensitive retinal ganglion cell
- RPE
retinal pigment epithelium
- Lrat
lecithin-retinol acyl transferase.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
See Commentary on page 10153.
References
- 1.Ebihara S., Tsuji K. Physiol. Behav. 1980;24:523–527. doi: 10.1016/0031-9384(80)90246-2. [DOI] [PubMed] [Google Scholar]
- 2.Freedman M. S., Lucas R. J., Soni B., von Schantz M., Munoz M., David-Gray Z., Foster R. Science. 1999;284:502–504. doi: 10.1126/science.284.5413.502. [DOI] [PubMed] [Google Scholar]
- 3.Lucas R. J., Freedman M. S., Munoz M., Garcia-Fernandez J. M., Foster R. G. Science. 1999;284:505–507. doi: 10.1126/science.284.5413.505. [DOI] [PubMed] [Google Scholar]
- 4.Lucas R. J., Douglas R. H., Foster R. G. Nat. Neurosci. 2001;4:621–626. doi: 10.1038/88443. [DOI] [PubMed] [Google Scholar]
- 5.Provencio I., Rodriguez I. R., Jiang G., Hayes W. P., Moreira E. F., Rollag M. D. J. Neurosci. 2000;20:600–605. doi: 10.1523/JNEUROSCI.20-02-00600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berson D. M., Dunn F. A., Takao M. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 7.Hattar S., Liao H. W., Takao M., Berson D. M., Yau K. W. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Provencio I., Rollag M. D., Castrucci A. M. Nature. 2002;415:493. doi: 10.1038/415493a. [DOI] [PubMed] [Google Scholar]
- 9.Hattar S., Lucas R. J., Mrosovsky N., Thompson S., Douglas R. H., Hankins M. W., Lem J., Biel M., Hofmann F., Foster R. G., Yau K. W. Nature. 2003;424:75–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panda S., Provencio I., Tu D. C., Pires S. S., Rollag M. D., Castrucci A. M., Pletcher M. T., Sato T. K., Wiltshire T., Andahazy M., et al. Science. 2003;301:525–527. doi: 10.1126/science.1086179. [DOI] [PubMed] [Google Scholar]
- 11.Tu D. C., Zhang D., Demas J., Slutsky E. B., Provencio I., Holy T. E., Van Gelder R. N. Neuron. 2005;48:987–999. doi: 10.1016/j.neuron.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 12.Sekaran S., Lupi D., Jones S. L., Sheely C. J., Hattar S., Yau K. W., Lucas R. J., Foster R. G., Hankins M. W. Curr. Biol. 2005;15:1099–1107. doi: 10.1016/j.cub.2005.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newman L. A., Walker M. T., Brown R. L., Cronin T. W., Robinson P. R. Biochemistry. 2003;42:12734–12738. doi: 10.1021/bi035418z. [DOI] [PubMed] [Google Scholar]
- 14.Panda S., Nayak S. K., Campo B., Walker J. R., Hogenesch J. B., Jegla T. Science. 2005;307:600–604. doi: 10.1126/science.1105121. [DOI] [PubMed] [Google Scholar]
- 15.Melyan Z., Tarttelin E. E., Bellingham J., Lucas R. J., Hankins M. W. Nature. 2005;433:741–745. doi: 10.1038/nature03344. [DOI] [PubMed] [Google Scholar]
- 16.Qiu X., Kumbalasiri T., Carlson S. M., Wong K. Y., Krishna V., Provencio I., Berson D. M. Nature. 2005;433:745–749. doi: 10.1038/nature03345. [DOI] [PubMed] [Google Scholar]
- 17.Filipek S., Stenkamp R. E., Teller D. C., Palczewski K. Annu. Rev. Physiol. 2003;65:851–879. doi: 10.1146/annurev.physiol.65.092101.142611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McBee J. K., Palczewski K., Baehr W., Pepperberg D. R. Prog. Retin. Eye Res. 2001;20:469–529. doi: 10.1016/s1350-9462(01)00002-7. [DOI] [PubMed] [Google Scholar]
- 19.Jin M., Li S., Moghrabi W. N., Sun H., Travis G. H. Cell. 2005;122:449–459. doi: 10.1016/j.cell.2005.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moiseyev G., Chen Y., Takahashi Y., Wu B. X., Ma J. X. Proc. Natl. Acad. Sci. USA. 2005;102:12413–12418. doi: 10.1073/pnas.0503460102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Redmond T. M., Poliakov E., Yu S., Tsai J. Y., Lu Z., Gentleman S. Proc. Natl. Acad. Sci. USA. 2005;102:13658–13663. doi: 10.1073/pnas.0504167102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Redmond T. M., Yu S., Lee E., Bok D., Hamasaki D., Chen N., Goletz P., Ma J. X., Crouch R. K., Pfeifer K. Nat. Genet. 1998;20:344–351. doi: 10.1038/3813. [DOI] [PubMed] [Google Scholar]
- 23.Batten M. L., Imanishi Y., Maeda T., Tu D. C., Moise A. R., Bronson D., Possin D., Van Gelder R. N., Baehr W., Palczewski K. J. Biol. Chem. 2004;279:10422–10432. doi: 10.1074/jbc.M312410200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Gelder R. N., Wee R., Lee J. A., Tu D. C. Science. 2003;299:222. doi: 10.1126/science.1079536. [DOI] [PubMed] [Google Scholar]
- 25.Fu Y., Zhong H., Wang M. H., Luo D. G., Liao H. W., Maeda H., Hattar S., Frishman L. J., Yau K. W. Proc. Natl. Acad. Sci. USA. 2005;102:10339–10344. doi: 10.1073/pnas.0501866102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCall M. A., Gregg R. G., Merriman K., Goto Y., Peachey N. S., Stanford L. R. Exp. Eye Res. 1996;63:35–50. doi: 10.1006/exer.1996.0089. [DOI] [PubMed] [Google Scholar]
- 27.Pittler S. J., Baehr W. Proc. Natl. Acad. Sci. USA. 1991;88:8322–8326. doi: 10.1073/pnas.88.19.8322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bowes C., Li T., Frankel W. N., Danciger M., Coffin J. M., Applebury M. L., Farber D. B. Proc. Natl. Acad. Sci. USA. 1993;90:2955–2959. doi: 10.1073/pnas.90.7.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carter-Dawson L. D., LaVail M. M., Sidman R. L. Invest. Opthalmol. Vis. Sci. 1978;17:489–498. [PubMed] [Google Scholar]
- 30.Van Gelder R. N. Methods Enzymol. 2005;393:746–755. doi: 10.1016/S0076-6879(05)93039-5. [DOI] [PubMed] [Google Scholar]
- 31.Batten M. L., Imanishi Y., Tu D. C., Doan T., Zhu L., Pang J., Glushakova L., Moise A. R., Baehr W., Van Gelder R. N., et al. PLoS Med. 2005;2:e333. doi: 10.1371/journal.pmed.0020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marc R. E., Jones B. W., Watt C. B., Strettoi E. Prog. Retin. Eye Res. 2003;22:607–655. doi: 10.1016/s1350-9462(03)00039-9. [DOI] [PubMed] [Google Scholar]
- 33.Strettoi E., Pignatelli V., Rossi C., Porciatti V., Falsini B. Vision Res. 2003;43:867–877. doi: 10.1016/s0042-6989(02)00594-1. [DOI] [PubMed] [Google Scholar]
- 34.Strettoi E., Porciatti V., Falsini B., Pignatelli V., Rossi C. J. Neurosci. 2002;22:5492–5504. doi: 10.1523/JNEUROSCI.22-13-05492.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pignatelli V., Cepko C. L., Strettoi E. J. Comp. Neurol. 2004;469:351–359. doi: 10.1002/cne.11019. [DOI] [PubMed] [Google Scholar]
- 36.Tian N., Copenhagen D. R. Neuron. 2003;39:85–96. doi: 10.1016/s0896-6273(03)00389-1. [DOI] [PubMed] [Google Scholar]
- 37.Tian N., Copenhagen D. R. Neuron. 2001;32:439–449. doi: 10.1016/s0896-6273(01)00470-6. [DOI] [PubMed] [Google Scholar]
- 38.Wan J., Zheng H., Hu B. Y., Xiao H. L., She Z. J., Chen Z. L., Zhou G. M. Neurosci. Lett. 2006;400:48–52. doi: 10.1016/j.neulet.2006.02.084. [DOI] [PubMed] [Google Scholar]
- 39.Hannibal J., Georg B., Hindersson P., Fahrenkrug J. J. Mol. Neurosci. 2005;27:147–155. doi: 10.1385/JMN:27:2:147. [DOI] [PubMed] [Google Scholar]
- 40.Golczak M., Imanishi Y., Kuksa V., Maeda T., Kubota R., Palczewski K. J. Biol. Chem. 2005;280:42263–42273. doi: 10.1074/jbc.M509351200. [DOI] [PubMed] [Google Scholar]
- 41.Golczak M., Kuksa V., Maeda T., Moise A. R., Palczewski K. Proc. Natl. Acad. Sci. USA. 2005;102:8162–8167. doi: 10.1073/pnas.0503318102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koyanagi M., Kubokawa K., Tsukamoto H., Shichida Y., Terakita A. Curr. Biol. 2005;15:1065–1069. doi: 10.1016/j.cub.2005.04.063. [DOI] [PubMed] [Google Scholar]
- 43.Koyanagi M., Kawano E., Kinugawa Y., Oishi T., Shichida Y., Tamotsu S., Terakita A. Proc. Natl. Acad. Sci. USA. 2004;101:6687–6691. doi: 10.1073/pnas.0400819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen P., Hao W., Rife L., Wang X. P., Shen D., Chen J., Ogden T., Van Boemel G. B., Wu L., Yang M., Fong H. K. Nat. Genet. 2001;28:256–260. doi: 10.1038/90089. [DOI] [PubMed] [Google Scholar]
- 45.Fan J., Woodruff M. L., Cilluffo M. C., Crouch R. K., Fain G. L. J. Physiol. 2005;568:83–95. doi: 10.1113/jphysiol.2005.091942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fan J., Rohrer B., Moiseyev G., Ma J. X., Crouch R. K. Proc. Natl. Acad. Sci. USA. 2003;100:13662–13667. doi: 10.1073/pnas.2234461100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Foster R. G., Argamaso S., Coleman S., Colwell C. S., Lederman A., Provencio I. J. Biol. Rhythms. 1993;8(Suppl.):S17–S23. [PubMed] [Google Scholar]
- 48.Selby C. P., Thompson C., Schmitz T. M., Van Gelder R. N., Sancar A. Proc. Natl. Acad. Sci. USA. 2000;97:14697–14702. doi: 10.1073/pnas.260498597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Panda S., Sato T. K., Castrucci A. M., Rollag M. D., DeGrip W. J., Hogenesch J. B., Provencio I., Kay S. A. Science. 2002;298:2213–2216. doi: 10.1126/science.1076848. [DOI] [PubMed] [Google Scholar]
- 50.Naka K. I., Rushton W. A. H. J. Physiol. 1966;185:587–599. doi: 10.1113/jphysiol.1966.sp008003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baylor D. A., Hodgkin A. L., Lamb T. D. J. Physiol. 1974;242:685–727. doi: 10.1113/jphysiol.1974.sp010731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dacey D. M., Liao H. W., Peterson B. B., Robinson F. R., Smith V. C., Pokorny J., Yau K. W., Gamlin P. D. Nature. 2005;433:749–754. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- 53.Holy T. E., Dulac C., Meister M. Science. 2000;289:1569–1572. doi: 10.1126/science.289.5484.1569. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.