Abstract
Estrogen receptor α (ERα) plays a major role in the regulation of neuroendocrine functions and behaviors by estrogens. Although the generation of ERα knockout mice advanced our knowledge of ERα functions, gene deletion using this method is global and potentially confounded by developmental consequences. To achieve a site-specific knockdown of ERα in the normally developed adult brain, we have generated an adeno-associated virus vector expressing a small hairpin RNA targeting ERα. After bilateral injection of this vector into the hypothalamic ventromedial nucleus in ovariectomized female mice, expression levels of ERα as well as the estrogen-inducible progesterone receptor were profoundly reduced despite the continued presence of this receptor elsewhere in the brain. Functionally, silencing of ERα in the ventromedial nucleus abolished female proceptive and receptive sexual behaviors while enhancing rejection behavior. These results provide evidence that adeno-associated virus-mediated long-term knockdown of genes can be used to delineate their effects on complex behaviors in discrete brain regions.
Keywords: adeno-associated virus, lordosis, RNA interference, viral vector
Estrogen elicits many important biochemical responses in the brain, yet the respective actions of the two estrogen receptors, ERα and ERβ, in mediating these effects are not completely understood (1–3). Generation of ERα knockout (ERKO) mice has shed light on some of the functions of ERα signaling. Of particular interest is estrogen action in the ventromedial nucleus of hypothalamus (VMN), which appears to be critical for several behaviors, including female reproductive behavior. VMN is a key player in a neural circuit controlling the execution of lordosis, a hormone-dependent reflexive posture exhibited by sexually receptive female rodents in response to male mounting (4). Analysis of homozygous ERKO females revealed that they completely lack lordosis behavior. Furthermore, these mice are also deficient in sexual behavioral interactions that precede the lordosis response. They are extremely aggressive and rejective toward attempted mounts by male mice, thus preventing copulation (5–7). In dramatic contrast, reproductive behaviors of ERβ knockout females are undistinguishable from those of wild-type mice (3).
Although these studies identified ERα as a gene essential for several mammalian behaviors, it remained unclear as to which of these effects are due to a mere absence of this receptor in the mature neurons and which deficits result from the lack of ERα expression during development or as a consequence of genetic compensation. Moreover, recent findings indicate a potential confound in the design of ERKO mice, because these animals express an abnormal splicing variant of ERα (8). This truncated form of the receptor retains both the DNA- and ligand-binding domains and is able to mediate, albeit far less efficiently, at least some estrogen effects. Therefore, somatic gene knockdown in individual nuclei of normally developed brain through RNA interference (RNAi) may provide a valuable alternative to conventional transgenic techniques.
Here we used small hairpin RNA (shRNA) delivered by a recombinant adeno-associated virus (AAV) to generate region-specific ERα knockdown mice. When gene silencing was restricted to a single ERα-positive nucleus, VMN, female animals displayed no sexual behavior and showed vigorous rejection toward males. In contrast to ERKO mice, estrogen responses in other parts of the brain and the uterus were unaffected. These findings unambiguously identify ERα signaling in the VMN as a key regulator of female sexual behaviors. Furthermore, these results demonstrate the utility of viral-mediated gene silencing to define the role of specific genes within particular neural networks in regulating complex behaviors.
Results
Design of Viral Vectors Expressing shRNAs.
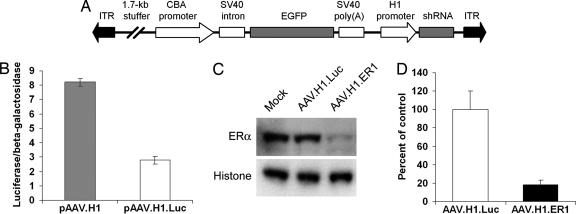
We have constructed a basic AAV vector, AAV.H1, containing the human PolIII H1 promoter for expression of shRNA as well as an independent EGFP expression cassette to detect transduced cells (Fig. 1A). First, we generated a control vector AAV.H1.Luc that encodes for a firefly luciferase-specific shRNA. This vector was functionally tested to suppress luciferase expression (Fig. 1B). To silence mouse ERα, several vectors for different shRNAs have been designed. A blast homology search predicted that these shRNAs would not affect ERβ or any other known mouse gene. Because of the limited number of mouse ERα-expressing cell lines that can be efficiently transduced with AAV, we developed a double-infection paradigm to test our viral vectors in vitro. First, human glioblastoma U87-MG cells were infected with an AAV vector encoding for a mouse ERα (AAV.mERα). These cells were chosen because they do not express ERα and are easily transduced by AAV at 100% efficiency. The cells were then superinfected with equal titers of different shRNA-expressing vectors, and the levels of ERα were analyzed. As evident from a Western blot in Fig. 1C, transduction with AAV.H1.ER1 resulted in ERα silencing, whereas AAV.H1.Luc had no effect. Given that small double-stranded RNAs might also work via the mRNA pathway by binding to mRNA and blocking translation without cleaving the messenger (9), we have analyzed ERα mRNA levels by quantitative PCR (Fig. 1D). The results revealed a reduction similar to that determined by Western blot (≈80%), indicating that AAV.H1.ER1 indeed directed RNAi.
Fig. 1.
AAV-mediated RNAi reduces ERα expression in vitro. (A) Schematic representation of the AAV used in this study. See Materials and Methods for details. ITR, inverted terminal repeat. (B) Functional assay of a control vector pAAV.H1.Luc. This vector was tested to silence the firefly luciferase as described in Materials and Methods. Data are presented as the mean ± SEM. (C) Western blot analysis of ERα protein levels in U87-MG cells infected with AAV.mERα and superinfected with different shRNA-expressing vectors. A nuclear protein histone H2b was used as a loading control. Note reduced ERα protein levels 48 h after transduction with AAV.H1.ER1 compared with AAV.H1.Luc and mock infection controls. (D) Quantitative PCR analysis of AAV-mediated silencing. Cells were treated as described in the previous experiment, and ERα mRNA levels were determined. Data represent the mean ± SEM of three independent infections. Note that the extent of mRNA reduction (≈80%) is comparable to the degree of silencing seen at the protein level.
Silencing of ERα Expression in the Brain.
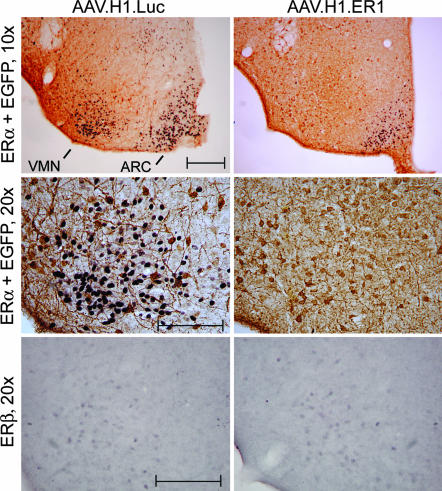
To generate conditional ERα knockdown mice, adult ovariectomized females were injected with AAV.H1.ER1 or AAV.H1.Luc bilaterally into the VMN. To test efficacy of shRNA-mediated ERα silencing in vivo before behavior studies, animals were killed 8 weeks after surgery, and ERα expression was analyzed by immunohistochemistry. As evident from Fig. 2, hypothalamic injections resulted in efficient transduction of VMN neurons. Double labeling for ERα (purple nuclear staining) and EGFP (brown cytoplasmic staining) revealed that virtually all EGFP-positive cells in the area were also ERα-negative in mice injected with AAV.H1.ER1 (Fig. 2, Top Right and Middle Right). No change in ERα staining was observed in control animals treated with AAV.H1.Luc, despite equivalent EGFP staining. The fact that AAV.H1.ER1-infected neurons produced EGFP, retained normal morphology, and were as abundant as AAV.H1.Luc-transduced cells suggested that the lack of ERα immunoreactivity was not due to cell loss caused by ERα shRNA toxicity but was rather a result of specific suppression of ERα expression. It is important to emphasize at this juncture that a precise needle placement on both sides of the brain was achieved in only approximately half of the animals. Mice that were injected outside of the VMN on at least one side were eliminated at the end of the experiment after immunohistochemical staining of the tissue.
Fig. 2.
AAV-mediated ERα silencing in vivo. Double-label immunostaining for EGFP (brown) and ERα (purple) in the ventromedial area of the hypothalamus of female mice 8 weeks after injections into the VMN with either AAV.H1.Luc or AAV.H1.ER1 (Top). Note a lack of ERα nuclear staining in the VMN but not in the ARC in mice injected with AAV.H1.ER1 compared with a control. Higher-magnification images of the VMN (Middle) reveal that although ERα is not detectable in mice treated with AAV.H1.ER1, the intensity of EGFP staining is comparable in both groups of animals. (Bottom) The number of ERβ-positive cells in the ventrolateral part of the VMH is similar between the two groups, suggesting that AAV.H1.ER1 did not suppress the expression of ERβ. (Scale bar: 200 μm.)
To confirm that ERα silencing is specific, we examined the expression of the ERα homologue, ERβ. This nuclear receptor is present in the ventral part of the VMN, albeit with a relatively low expression level compared with that in other brain regions, e.g., the paraventricular nucleus (data not shown). Both genes share overall 65% homology with 96% and 58% similarity in the DNA-binding domain and the ligand-binding domain, respectively (2). In fact, our ERα-specific shRNA sequence ER1 (GGCATGGAGCATCTCTACA) is 79% homologous to ERβ (GGCATGGAACATCTGCTCA, mismatched nucleotides are underlined). Although it has been demonstrated that a single base pair substitution in the antisense strand of the shRNA duplex would prevent RNAi in vitro (10), the fidelity of this process in vivo is not well characterized. The results established the specificity of ERα shRNA-mediated silencing, because transduction of VMN neurons with AAV.H1.ER1 had no effect on ERβ immunoreactivity when compared with controls (Fig. 2, Bottom Right).
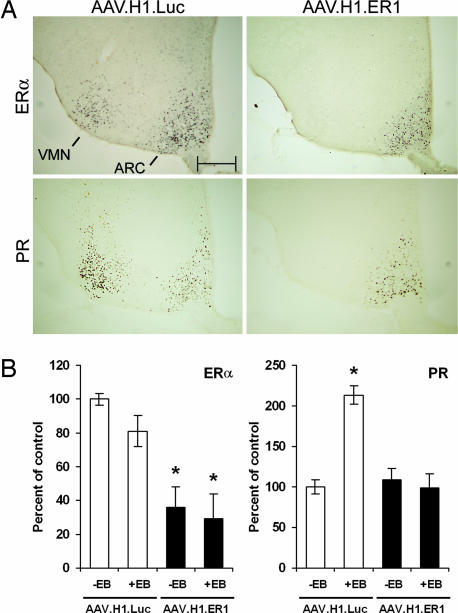
To characterize the consequences of ERα silencing in vivo, in a second set of experiments, AAV.H1.Luc or AAV.H1.ER1 was bilaterally injected into the VMN of ovariectomized mice, and 13 weeks after surgery, they were treated with 17β-estradiol-3-benzoate (EB) for 2 days. On the third day, animals were killed, and expression of different genes in the VMN as well as adjacent and distal nuclei was analyzed by immunohistochemistry. Consistent with the previous experiment, infection with AAV.H1.ER1 resulted in a significant reduction of ERα immunoreactivity in the VMN compared with AAV.H1.Luc-injected mice (Fig. 3A Upper). In addition, we did not observe any decrease in the ERα immunoreactivity in other ERα-positive brain regions such as the juxtaposed arcuate nucleus (ARC, Fig. 3A Upper) and the anteriorly located medial preoptic area. The latter contained EGFP-positive projections from the transduced neurons but no EGFP-immunoreactive cell bodies (see Fig. 5, which is published as supporting information on the PNAS web site).
Fig. 3.
ERα silencing in the brain blocks estrogen-induced up-regulation of PR expression. AAV.H1.Luc or AAV.H1.ER1 was injected into the VMN of the animals. Thirteen weeks after surgery, the mice were treated with sesame oil or EB (10 μg in 100 μl of sesame oil per day) for 2 consecutive days to induce PR and then killed to assess expression of ERα and PR. (A) Immunohistochemical staining of brain slices. Note silencing of ERα by AAV.H1.ER1 restricted to the VMN (Upper) and suppression of estrogen-induced PR expression in the VMN but not ARC (Lower). Images shown are adjacent sections from one representative animal from each treatment group. (Scale bar: 200 μm.) (B) Analysis of ERα and PR mRNA levels. In a separate experiment, animals were treated similarly with oil or EB, and mRNA extracted from VMN tissue punches was analyzed by using quantitative PCR. Data are presented as the mean ± SEM. Notice a reduction in ERα mRNA expression in AAV.H1-treated animals compared with control mice (Left) as well as a lack of PR response after EB administration (Right). ∗, P < 0.01.
One of the main downstream effects of ERα signaling in the brain after estrogen exposure is up-regulation of progesterone receptor (PR) transcription. We and others have previously shown that up-regulation of PR after an estrogen surge is critical for female reproductive behavior because inhibition of PR translation by antisense oligonucleotides significantly reduces female proceptive and receptive responses (11–13). We therefore set out to determine whether ERα silencing would suppress activation of PR expression after estrogen administration. No PR-immunopositive cells were detected in vehicle-treated animals (data not shown). As anticipated, in mice injected with AAV.H1.Luc, EB treatment resulted in a robust PR immunoreactivity in the VMN and ARC (Fig. 3A Lower) as well as in the medial preoptic area (Fig. 5) and other ERα-positive nuclei (data not shown). In contrast, in AAV.H1.ER1-treated mice, detectable PR expression in the VMN was almost completely eliminated (Fig. 3A Lower), yet it was unaffected in the ARC (Fig. 3A Lower), medial preoptic area (Fig. 5), and other brain areas (data not shown). To provide further evidence that a reduction in ERα immunoreactivity is in fact due to decreased ERα mRNA levels, we performed mRNA analysis from the VMN samples using quantitative PCR. As evident from Fig. 3B Left, ERα mRNA content in the VMN from AAV.H1.ER1-injected mice was significantly lower compared with control animals. In this experiment, ERα expression was not influenced significantly by EB treatment in either group. Nonetheless, when the same samples were analyzed for PR expression, an increase in PR levels was observed in EB-treated control mice, whereas in the animals injected with AAV.H1.ER1, estrogen treatment had no effect (Fig. 3B Right). These findings demonstrate that AAV-mediated shRNA delivery can be used to achieve a precise, region-specific silencing of ERα to a level sufficient to suppress the normal physiological signaling cascade of this nuclear receptor in neurons.
Behavioral Effects of ERα Knockdown.
Female sexual behavior was examined in two independent groups of mice (Experiments 1 and 2). Postmortem immunohistochemical analysis revealed that in either experiment, ≈50% of animals received symmetrical injections that resulted in ERα silencing restricted to VMN. In the remaining mice, the tip of the injection needle was found to be outside of the VMN on at least one side of the brain. Behavioral monitoring and subsequent data analysis of all animals were blinded. Only animals with bilateral knockdown of ERα were included, however, to eliminate potential confounding effects of altering ERα expression outside the VMN and an incomplete silencing within the VMN.
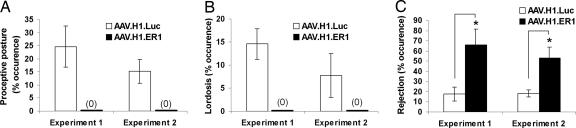
As expected, after priming with EB, mice in the control group injected with AAV.H1.Luc became sexually receptive, displaying proceptive still posture (Fig. 4A) and the lordosis response (Fig. 4B). In addition, they demonstrated very few rejections toward male mounting (Fig. 4C). In female mice treated with AAV.H1.ER1, however, sexual receptivity toward males was completely abolished (Fig. 4 A and B). Instead, these female mice showed vigorous rejection, such as kicking and defensive fight back toward male approach and attempted mounts (Fig. 4C). Because the female rejections were very strong, stud males could hardly show normal mounts or intromissions (see Movie 1, which is published as supporting information on the PNAS web site). The results were consistent in two independent experiments. It thus appears that silencing of ERα restricted to the VMN of normal adult mice confers a phenotype virtually undistinguishable from that of transgenic ERKO mice. Analysis of data from female mice treated with AAV.H1.ER1 but with injection sites located outside of the VMN showed receptivity comparable to that of control animals (data not shown), confirming the importance of ERα expression to this behavior specifically in the VMN.
Fig. 4.
Knockdown of ERα in the VMN inhibits estrogen-inducible female sexual behavior. Mice were primed with EB and tested with stud male mice 3 weeks (Experiment 1, n = 10 for each group) or 4 weeks (Experiment 2, n = 7 for AAV.H1.ER1 and n = 6 for AAV.H1.Luc) after stereotaxic virus injection. In both experiments, mice treated with AAV.H1.ER1 in the VMN did not show any receptive behaviors, i.e., proceptive postures (A) or lordosis (B) compared with the animals injected with AAV.H1.Luc. Instead, they showed vigorous rejection (such as kicking, fleeing, and upright posture) toward male approaches, attempted mounts, and mounts (C). ∗, P < 0.01. Data are presented as the mean ± SEM. Only the mice with bilateral VMN-specific knockdown of ERα were included in the analysis.
Discussion
In this study, we demonstrate that site-specifically silencing ERα expression in a single nucleus (VMN) of normally developed adult mice blocked estrogen-induced sexual behavior. These findings extend previous reports that document the importance of this brain region in female reproductive behavior. Local infusions of estrogen, lesion studies, and molecular neuroendocrine studies have identified the VMN as a critical region to mediate female sexual behavior in rodents (14). The neural network that governs lordosis, an estrogen-dependent female sexual posture induced after stimulation by a male, involves projections from VMN neurons to the aqueductal gray, followed by the medullary reticular formation, lumbar ventral horn, and finally the deep back muscles directly involved in the execution of the behavior (14). Thus, the VMN appears to function as a gate, allowing transmission of a sensory stimulatory signal to motor neurons only in the presence of steroid hormones. Furthermore, this process is contingent upon estrogen-mediated activation of transcription because VMN neurons cannot be immediately stimulated by estrogen but rather require priming with the hormone, and this effect can be blocked by transcription or translation inhibitors (14). We have previously shown that female mice lacking ERα but not ERβ demonstrate profoundly reduced proceptive and receptive behaviors and are very aggressive toward males (3, 6, 7). Extending the above findings with our methodology, disruption of neural circuitry in the VMN by silencing of ERα in our experiments abolished estrogen-induced female sexual behaviors and mimicked the ERKO phenotype.
One intriguing question that has raised debate in the literature is the role of ERα in mediating estrogen signaling in the brain and other organs. Although ovariectomized wild-type mice respond to estrogen by a robust increase in PR immunoreactivity in the VMH, medial preoptic area, and other brain regions, in ERKO mice, this effect is also observed, albeit to a considerably lesser degree and in a smaller area compared with wild-type animals (15). Furthermore, estrogens have been shown to trigger the mitogen-activated protein kinase (MAPK) cascade in the cerebral cortex of ERKO mice (16). Several hypotheses have been proposed to explain this paradox. First, this effect could be mediated by the other estrogen receptor, ERβ. However, analysis of double knockouts (ERα/βKO) generated by crossing ERKO mice with ERβ knockout mice has revealed that estrogen binding sites in the brain as well as PR induction by estrogen are still preserved, suggesting that ERβ cannot fully account for this effect (17). Shughrue et al. (17) have discovered the expression of a truncated form of ERα in ERKO mice. It appeared to result from a splicing event between a cryptic donor site in the neo cassette of the cloning vector and the acceptor site of exon 3 of ERα. Intriguingly, the ERα open reading frame was preserved, and the fusion protein contained both the DNA- and ligand-binding domains (17). Although the presence of this truncated receptor could certainly explain the continued estrogen response in ERKO mice, the presence of another, yet unknown, estrogen receptor (ERγ) cannot be ruled out. In fact, an existence of this putative receptor has been suggested by several research groups, and all of them have arrived at this conclusion using studies that involved ERKO mice (15–18). Nonetheless, the identification of a functional mutant receptor in these animals, combined with our data demonstrating concordance among silencing of ERα, loss of PR expression, and subsequent inhibition of sexual behavior, indicates that the presence of an unidentified estrogen receptor (ERγ) is no longer necessary to explain these effects.
The development of DNA-based vectors to express shRNA (10, 19) has opened the possibility of designing viral vectors for gene silencing in vivo. In fact, several recent reports have demonstrated the feasibility of such manipulations in the brain (20–23). Viral vector-mediated RNAi technology provides several advantages over existing transgenic techniques. First, it allows for stable suppression of gene expression in animals without a significant investment of time and resources. Gene silencing can also be restricted to a single brain nucleus and can be performed in a normally developed animal. This option is particularly valuable when developmental consequences of gene silencing might confound analysis of data generated in the resulting adult animals. These advantages were essential in our study given the known importance of estrogens in brain development (24). Our results demonstrate that site-specific knockdown of ERα in a single discrete brain region in a normally developed adult animal is sufficient to eliminate estrogen-induced female sexual behavior. These findings indicate that viral vector-mediated RNAi technology can greatly facilitate studies aimed at identifying genes and neural networks involved in complex brain functions and may provide novel therapeutic options for diseases in which gene silencing would be desirable.
Materials and Methods
Details regarding standard laboratory techniques such as quantitative PCR, Western blotting, and immunohistochemistry may be found in Supporting Text, which is published as supporting information on the PNAS web site.
Construction of AAV Vectors.
Human H1 promoter was amplified from genomic DNA essentially as described in ref. 10. The PCR product was cloned into an AAV cis-plasmid to generate pAAV.H1. This vector also contains an EGFP expression cassette under the control of a hybrid chicken β-actin/CMV promoter (CBA) as well as a 1.7-kb noncoding portion of the human placenta alkaline phosphatase used as a stuffer to increase the size of the vector to allow efficient packaging. The transgene is flanked by the AAV-2 inverted terminal repeats. The following oligos were annealed and cloned immediately downstream from the H1 promoter of pAAV.H1 into BglII and XbaI sites to generate pAAV.H1.Luc and pAAV.H1.ER1, respectively: Luc (5′- GATCCCCCCGCTGGAGAGCAACTGCATCTTCCTGTCAATGCAGTTGCTCTCCAGCGGTTTTTGGAA-3′ and 5′-CTAGTTCCAAAAACCGCTGGAGAGCAACTGCATTGACAGGAAGATGCAGTTGCTCTCCAGCGGGGG-3′), ER1 (5′-GATCCCCGGCATGGAGCATCTCTACACTTCCTGTCA TGTAGAGATGCTCCATGCCTTTTTTGGAAT-3′ and 5′-CTAGATTCCAAAAAA GGCATGGAGCATCTCTACATGACAGGAAGTGTAGAGATGCTCCATGCCGGG-3′). The nucleotides specific for luciferase or ERα are underlined, and the spacer derived from mouse miR-23 (25) is shown in bold. All shRNA expression cassettes were verified by sequencing. Virus stocks were prepared by packaging the vector plasmids into AAV serotype 2 particles using a helper-free plasmid transfection system. The vectors were purified by using heparin affinity chromatography as described in ref. 26 and dialyzed against PBS. AAV titers were determined by quantitative PCR using EGFP-specific primers and adjusted to 1012 genomic particles per ml.
In Vitro Assays.
To test pAAV.H1.Luc, 293 cells were cotransfected with an empty vector pAAV.H1 or pAAV.H1.Luc together with pGL2-control (Promega) and pCMVβ (Clontech) plasmids encoding for firefly luciferase and β-galactosidase, respectively. After 48 h, luciferase activity was measured and normalized to β-galactosidase levels. To test the shRNA-expressing vectors, human glioblastoma U87-MG cells were first infected with AAV.mERα encoding for the mouse ERα at a multiplicity of infection of 1,000 for 24 h. This experimental paradigm yields ≈100% transduction efficiency. Infected cells were then split into multiple wells and infected with shRNA-expressing viruses at a multiplicity of infection of 1,000. Forty-eight hours postinfection, the cells were lysed and ERα expression was analyzed by quantitative PCR and Western blotting.
Stereotaxic Surgery.
Ovariectomized C57BL/6J female mice (12–30 weeks old) were used in this study. Animals were group-housed in plastic cages and maintained on a 12-h light/12-h dark cycle. Food and water were available ad libitum. All surgical procedures were performed under sodium pentobarbital anesthesia. After each mouse was placed in a stereotaxic frame, 2 μl of each vector (2 × 109 packaged genomic particles total) in PBS was injected into VMN (anteroposterior −0.9, mediolateral ±0.7, dorsoventral −6.0) over 10 min using a 5-μl Hamilton syringe attached to a microinfusion pump (World Precision Instruments, Sarasota, FL). The needle was left for an additional 5 min and then slowly withdrawn. Animals received bilateral injections of ether AAV.H1.Luc or AAV.H1.ER1.
Behavior Tests.
A total of 23 mice were tested for sexual behavior 3–4 weeks after vector injection in two independent experiments (Experiment 1, n = 10 for each group; Experiment 2, n = 7 for AAV.H1.ER1 and n = 6 for AAV.H1.Luc). The animals were first primed with EB (two daily injections of 5 μg in 50 μl of sesame oil) followed by progesterone (10 μg in 50 μl of sesame oil 6 h before behavior studies). Mice were tested starting 48 h after the first EB injection in the stud males’ home cages (singly housed Swiss–Webster mice, Taconic Farms). To minimize the influence of behavioral differences in male mice, each female mouse treated with AAV.H1.ER1 was paired with a female mouse treated with in AAV.H1.Luc, and each pair of mice was tested against the same two male mice consecutively and in random order. Each test lasted until females received 10 mounts from each of two stimulus males. All behavioral tests were preformed during the dark phase under red light and videotaped for analysis by a blinded observer who was not aware of the treatment group of the mice. Female behavior against each of a total of 20 (10 from each of two stimulus males) male approachs/attempted mounts/mounts was rated as (i) rejection, (ii) proceptive posture, or (iii) lordosis response. Percent of a particular response per total mounts (20) was calculated for each female mouse.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Heath Grants MH62147 and MH67775 (to S.O.) and NS044978 (to M.G.K.).
Abbreviations
- ERα
estrogen receptor α
- ERKO
ERα knockout mice
- AAV
adeno-associated virus
- shRNA
small hairpin RNA
- VMN
ventromedial nucleus of hypothalamus
- RNAi
RNA interference
- EB
17β-estradiol-3-benzoate
- PR
progesterone receptor.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Ogawa S., Chester A. E., Hewitt S. C., Walker V. R., Gustafsson J. A., Smithies O., Korach K. S., Pfaff D. W. Proc. Natl. Acad. Sci. USA. 2000;97:14737–14741. doi: 10.1073/pnas.250473597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mosselman S., Polman J., Dijkema R. FEBS Lett. 1996;392:49–53. doi: 10.1016/0014-5793(96)00782-x. [DOI] [PubMed] [Google Scholar]
- 3.Ogawa S., Chan J., Chester A. E., Gustafsson J. A., Korach K. S., Pfaff D. W. Proc. Natl. Acad. Sci. USA. 1999;96:12887–12892. doi: 10.1073/pnas.96.22.12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pfaff D. W., Sakuma Y. J. Physiol. 1979;288:189–202. [PMC free article] [PubMed] [Google Scholar]
- 5.Rissman E. F., Early A. H., Taylor J. A., Korach K. S., Lubahn D. B. Endocrinology. 1997;138:507–510. doi: 10.1210/endo.138.1.4985. [DOI] [PubMed] [Google Scholar]
- 6.Ogawa S., Taylor J. A., Lubahn D. B., Korach K. S., Pfaff D. W. Neuroendocrinology. 1996;64:467–470. doi: 10.1159/000127154. [DOI] [PubMed] [Google Scholar]
- 7.Ogawa S., Eng V., Taylor J. A., Lubahn D. B., Korach K. S., Pfaff D. W. Endocrinology. 1998;139:5070–5081. doi: 10.1210/endo.139.12.6357. [DOI] [PubMed] [Google Scholar]
- 8.Moffatt C. A., Rissman E. F., Shupnik M. A., Blaustein J. D. J. Neurosci. 1998;18:9556–9563. doi: 10.1523/JNEUROSCI.18-22-09556.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doench J. G., Petersen C. P., Sharp P. A. Genes Dev. 2003;17:438–442. doi: 10.1101/gad.1064703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brummelkamp T. R., Bernards R., Agami R. Science. 2002;296:550–553. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- 11.Mani S. K., Blaustein J. D., Allen J. M. C., Law S. W., O’Malley B. W., Clark J. H. Endocrinology. 1994;135:1409–1414. doi: 10.1210/endo.135.4.7925102. [DOI] [PubMed] [Google Scholar]
- 12.Pollio G., Xue P., Zanisi M., Nicolin A., Maggi A. Mol. Brain Res. 1993;19:135–139. doi: 10.1016/0169-328x(93)90158-l. [DOI] [PubMed] [Google Scholar]
- 13.Ogawa S., Olazabal U. E., Parhar I. S., Pfaff D. W. J. Neurosci. 1994;14:1766–1774. doi: 10.1523/JNEUROSCI.14-03-01766.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfaff D. W. Drive. Cambridge, MA: MIT Press; 1999. [Google Scholar]
- 15.Shughrue P. J., Lubahn D. B., Negro-Vilar A., Korach K. S., Merchenthaler I. Proc. Natl. Acad. Sci. USA. 1997;94:11008–11012. doi: 10.1073/pnas.94.20.11008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh M., Setalo G., Jr, Guan X., Frail D. E., Toran-Allerand C. D. J. Neurosci. 2000;20:1694–1700. doi: 10.1523/JNEUROSCI.20-05-01694.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shughrue P. J., Askew G. R., Dellovade T. L., Merchenthaler I. Endocrinology. 2002;143:1643–1650. doi: 10.1210/endo.143.5.8772. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh D., Taylor J. A., Green J. A., Lubahn D. B. Endocrinology. 1999;140:3526–3533. doi: 10.1210/endo.140.8.6877. [DOI] [PubMed] [Google Scholar]
- 19.Miyagishi M., Taira K. Nat. Biotechnol. 2002;20:497–500. doi: 10.1038/nbt0502-497. [DOI] [PubMed] [Google Scholar]
- 20.Xia H., Mao Q., Paulson H. L., Davidson B. L. Nat. Biotechnol. 2002;20:1006–1010. doi: 10.1038/nbt739. [DOI] [PubMed] [Google Scholar]
- 21.Xia H., Mao Q., Eliason S. L., Harper S. Q., Martins I. H., Orr H. T., Paulson H. L., Yang L., Kotin R. M., Davidson B. L. Nat. Med. 2004;10:816–820. doi: 10.1038/nm1076. [DOI] [PubMed] [Google Scholar]
- 22.Hommel J. D., Sears R. M., Georgescu D., Simmons D. L., DiLeone R. J. Nat. Med. 2003;9:1539–1544. doi: 10.1038/nm964. [DOI] [PubMed] [Google Scholar]
- 23.Babcock A. M., Standing D., Bullshields K., Schwartz E., Paden C. M., Poulsen D. J. Mol. Ther. 2005;11:899–905. doi: 10.1016/j.ymthe.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 24.McEwen B. S. Int. Rev. Physiol. 1983;27:99–145. [PubMed] [Google Scholar]
- 25.Kawasaki H., Taira K. Nucleic Acids Res. 2003;31:700–707. doi: 10.1093/nar/gkg158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clark K. R., Liu X., McGrath J. P., Johnson P. R. Hum. Gene Ther. 1999;10:1031–1039. doi: 10.1089/10430349950018427. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.