Abstract
Moderate physical exercise (PE) combined with metabolic treatment (MT) (antioxidants and l-arginine) are well known to reduce atherosclerotic lesion formation in hypercholesterolemic mice. However, the long-term beneficial effects on unstable atheroma remain poorly understood. We started early PE training in large groups of 6-week-old hypercholesterolemic mice (by graduated swimming) alone or in combination with nutritional supplementation (1.0% vitamin E added to the chow and 0.05% vitamin C and 6% l-arginine added to the drinking water). Inactive controls did not receive PE. The spontaneous development of atherosclerotic plaque rupture (associated with advanced atherosclerosis) and survival rates were evaluated. Moderate PE elicited an increase in plasma levels of nitric oxide. Early combined treatment with PE and MT in the hypercholesterolemic mice significantly reduced lesions (also detected noninvasively at 10 months) and spontaneous atherosclerotic plaque rupture and prolonged survival more effectively than each intervention alone. Thus, early concerted actions of MT and PE improve the natural history of atherosclerotic lesions and reduce the plaque instability in hypercholesterolemic mice.
Keywords: antioxidants, nitric oxide, physical exercise, l-arginine
There is a plethora of experimental and clinical studies supporting the evidence that moderate physical exercise (PE) is a deterrent of cardiovascular diseases and atherosclerosis (1–5). In a recent study, we also provided clear evidence for the beneficial effects of graduated PE training (swimming) and metabolic treatment (MT) (antioxidants and l-arginine) on atherosclerotic lesion formation in hypercholesterolemic mice (6). These protective mechanisms could include increased antioxidant defenses and nitric oxide (NO) bioactivity, reduced basal production of oxidants (reduced oxidative stress), and reduction of radical leak during oxidative phosphorylation (6–10). Basically, early oxidation-sensitive mechanisms are associated with early stages of human atherogenesis (10–12), and these observations suggest that novel strategies could be developed in the primary prevention of atherosclerotic-related diseases. Another study (13) also provided direct evidence that inactivity enhances vascular oxygen radical production, endothelial dysfunction, and atherosclerosis in hypercholesterolemic mice. Thus, graduated PE can increase NO bioavailability and convey benefits in vasculoprotection (6, 14, 15). NO generated in this way may even scavenge overwhelming radicals, such as superoxide anion, thereby preventing tissue damage. In contrast, prolonged strenuous PE and high blood pressure reduce the cyclic pulsations (physiological shear stress), thereby limiting NO production (14, 15). NO bioavailability can be restored by antioxidants and l-arginine, the natural precursor of NO (16).
Several small-scale studies have demonstrated that i.v. l-arginine augments endothelial function and improves exercise ability in patients with cardiovascular disease by enhancing vasodilation and reducing monocyte adhesion (reviewed in ref. 17). Administration of l-arginine normalizes aerobic capacity (18) and reduces fat mass in Zucker diabetic fatty rats (19). The lack of appropriate generation of endogenous NO is an important progression factor of atherosclerosis in mice (20) and l-arginine may reduce atherogenesis in hypercholesterolemic mice (21). Although there is convincing evidence that moderate PE and MT have beneficial effects on atherosclerotic lesions, the long-term effects of this therapeutic approach on lesion progression remain poorly understood. The present study provides evidence for the reduction of spontaneous atherosclerotic plaque instability and rupture coupled to prolonged survival in hypercholesterolemic mice achieved by the early program administered to young mice and consisting of graduated swim training together with MT with antioxidants (vitamins E and C) and l-arginine.
Results
General Effects Afforded by Early Preventive Treatment in Hypercholesterolemic Mice.
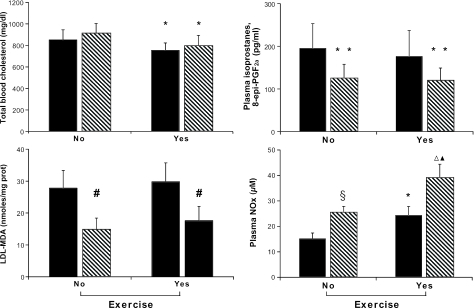
Cumulative data on cholesterol levels and oxidative stress in the various groups are reported in Fig. 1. As expected, indicators of systemic oxidative stress decreased in mice receiving PE training and MT. Indeed, plasma isoprostanes and parameters of low-density lipoprotein (LDL) oxidizability (lag time and LDL-malondialdehyde) were significantly decreased after MT, with or without the PE program (Fig. 1). In contrast, plasma nitrite and nitrate (NOx) levels, an index of NO bioactivity, were significantly increased by concurrent graduated PE and MT. Consistent with previous findings (6), plasma cholesterol decreased in the PE groups. This decrease in cholesterol is unlikely to be due to an activation of the LDL receptor pathway because the animals used in the present study lack the receptor. PE-induced oxidative stress could promote plasma oxidation of circulating LDL and its clearance by the liver. Because a liver pathway for the catabolism of oxidized LDL has been proposed, many studies have implicated hepatic scavenger receptors in the clearance of oxidized LDL and cholesterol from plasma (7, 10).
Fig. 1.
Bar graph depicting the characteristics of hypercholesterolemic mice and evaluation of oxidative stress and NOx production in the study groups. Results are cumulative of all mice at the time of death. Data are expressed as mean ± SD. Malondialdehyde (MDA) was generated at 12 h after exposure to 1 μM copper sulfate. Lag-time represents an index of LDL oxidizability; increased values of lag-time reflect increased resistance of LDL to oxidative modification (see Methods). Solid bar graphs indicate HFD, and hatched bar graphs indicate HFD+MT. ∗, P < 0.05 vs. respective group (no PE); ∗∗, P < 0.05 vs. respective HFD; #, P < 0.01 vs. respective HFD; §, P < 0.05 vs. HFD (no PE); ▵, P < 0.001 vs. HFD (no PE); ▴, P < 0.05 vs. HFD+MT (no PE).
Effects of PE and MT on Atherosclerosis Lesion Progression and Plaque Instability.
To establish lesion progression in the various study groups, we first noninvasively evaluated the atherosclerotic lesion progression (Table 1). The thoracic aorta could be visualized by MRI in great detail in all mice studied. Both mean aortic wall thickness and maximal wall thickness in mice receiving high-fat diet alone (HFD) were significantly increased compared with treated mice (Table 1). Indeed, early graduated PE and MT improved luminal aortic and vessel wall areas (Table 1).
Table 1.
Computer-assisted morphometric analysis of MRI results in thoracic aorta among the study groups
| Mouse group | VWA, mm2 | LVA, mm2 | TVA, mm2 | VWTmean, mm | VWTmax, mm | VWTmin, mm |
|---|---|---|---|---|---|---|
| HFD | 1.28 ± 0.21 | 1.44 ± 0.23 | 2.55 ± 0.25 | 0.152 ± 0.032 | 0.256 ± 0.034 | 0.075 ± 0.015 |
| HFD + MT | 1.33 ± 0.15 | 1.95 ± 0.21* | 2.75 ± 0.26* | 0.130 ± 0.21* | 0.233 ± 0.025 | 0.073 ± 0.009 |
| HFD + PE | 1.35 ± 0.16 | 2.06 ± 0.22* | 2.85 ± 0.25* | 0.124 ± 0.030* | 0.195 ± 0.031* | 0.068 ± 0.016 |
| HFD + PE + MT | 1.49 ± 0.18† | 2.12 ± 0.21† | 3.00 ± 0.31† | 0.106 ± 0.018†‡§ | 0.151 ± 0.025†‡§ | 0.062 ± 0.010†‡ |
Shown are the vessel wall area (VWA), luminal vessel area (LVA), total vessel area (TVA), and the mean, maximal, and minimal values for vessel wall thickness (VWT). Data represent means ± SD (n = 8 mice per group; 10 months of age), and results are given as means ± SE.
∗, P < 0.05 vs. HFD;
†, P < 0.01 vs. HFD;
‡, P < 0.05 vs. HFD + MT;
§, P < 0.05 vs. HFD + PE.
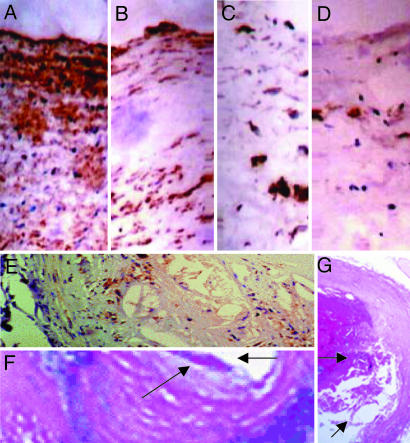
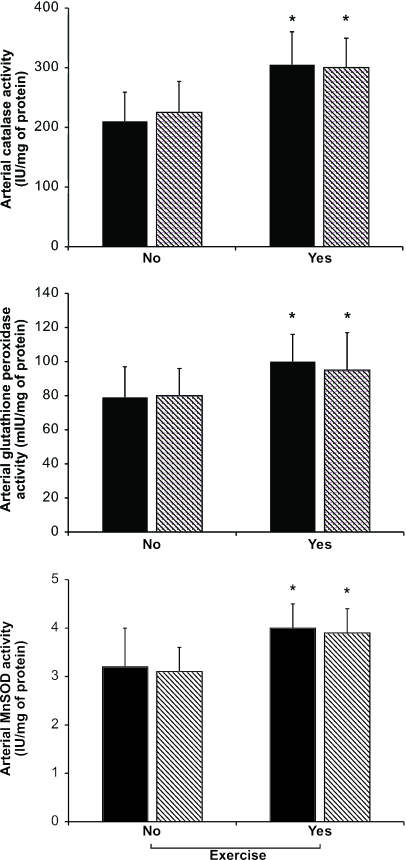
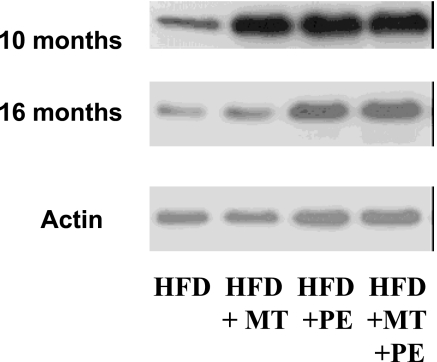
After this preliminary evaluation of lesion progression, we considered the computer-assisted imaging analysis of atherosclerotic lesions at the time of death. Under the present experimental conditions, determination of atherosclerotic lesion areas confirmed the working hypothesis that graduated PE significantly decreased the progression of atherosclerotic lesions over the natural life span of the mice (Table 2). This effect was further improved with MT (Table 2). Interestingly, the decrease in plasma isoprostane levels correlated well with the reduction in atherosclerotic lesion area in the group of mice receiving PE training and MT (r = 0.71, P < 0.004) as did the increase in plasma NOx levels (r = 0.65, P < 0.01). More importantly, the occurrence of spontaneous plaque rupture and organizing thrombi on atherosclerotic plaques was significantly reduced in treated mice compared with untreated mice (Table 2). The group of mice receiving graduated PE and MT showed pronounced protection against unstable atheroma. These events occurred primarily in abdominal aorta and coronary arteries and were associated with thin fibrous caps and high levels of plaque lipid content (Table 2 and Fig. 2E–G). As we have previously observed (6), the number of macrophage-derived foam cells (immunostained with the F4/80 monoclonal antibody) decreased significantly in mice receiving PE training, especially in mice also receiving MT (Fig. 2 A–D); control immunohistochemistry without the primary antibody yielded no staining. The decrease of F4/80 immunostaining correlated significantly with the reduction in atherosclerotic lesion area in the group of mice receiving PE training and MT (r = 0.61, P < 0.01), whereas the decrease in total plasma cholesterol in groups of mice receiving PE was only poorly correlated with atherosclerotic lesion size detected at the time of death (r = 0.22, P value not significant), suggesting that the decreased vascular inflammation was related to the improvement in the treated mice. As previously reported (6), early graduated PE also stimulated arterial enzymatic activities of catalase, glutathione peroxidase, and manganese superoxide dismutase (Fig. 3). Moreover, PE increased arterial endothelial NO synthase (eNOS) expression over time, especially in the group receiving MT with antioxidants and l-arginine (Fig. 4). At 10 months, band density was 2.4 ± 0.7-fold increased in the group receiving a HFD and MT (HFD+MT group), 2.1 ± 0.5-fold increased in the group receiving a HFD and PE (HFD+PE group), and 2.9 ± 0.6-fold increased in the group receiving a HFD, PE, and MT (HFD+PE+MT group) when compared to the group receiving HFD alone (P < 0.01 and P < 0.005 vs. HFD). This effect also was maintained at 16 months [band density was increased 1.7 ± 0.5-fold in the HFD+MT group, 2.4 ± 0.5-fold in the HFD+PE group, and 2.7 ± 0.5-fold in the HFD+PE+MT group when compared with the group receiving HFD alone (P < 0.05 and P < 0.01 vs. HFD)]. The increase in eNOS expression correlated with NOx levels and, importantly, with the reduction in atherosclerotic lesion area in the HFD+PE+MT group (r = 0.55 and P < 0.02 and r = 0.62 and P < 0.01, respectively).
Table 2.
Cumulative parameters of unstable atheroma and plaque morphology among groups
| Mouse group | Aortic plaque rupture | Atherosclerosis | Severity |
Coronary plaque rupture | Thrombus | Caps per animal | Cap thickness | Plaque lipid | Plaque area | |
|---|---|---|---|---|---|---|---|---|---|---|
| 50–75% | >75% | |||||||||
| HFD | 14/80 | 56/80 | 34/56 | 22/56 | 10/56 | 8/56 | 2.97 ± 0.55 | 2.31 ± 0.40 | 47.7 ± 3.4 | 254.4 ± 34.5 |
| HFD + MT | 11*/80 | 49/80 | 31/49 | 18/49 | 9/49 | 5/49 | 2.23 ± 0.62* | 7.18 ± 0.70* | 30.6 ± 2.8* | 195.3 ± 28.8* |
| HFD + PE | 7*/80 | 45†/80 | 40/45 | 5†/45 | 6*/45 | 3†/45 | 1.83 ± 0.41† | 8.20 ± 0.67† | 25.5 ± 3.6† | 165.3 ± 38.5† |
| HFD + PE + MT | 4†‡/80 | 39†/80 | 33/39 | 6†/39 | 3†‡/3 | 1†‡/39 | 1.55 ± 0.40†‡ | 10.99 ± 1.05† | 23.7 ± 2.9† | 149.3 ± 29.8†‡ |
Results reflect the cumulative of all mice at the time of death. Results are given as the number per total number of mice for the following parameters: aortic plaque rupture associated with death; atherosclerosis in two or more coronary arteries; mice with lesions with severity between 50% and 75% and more severe than 75%; coronary plaque rupture; and intraluminal coronary thrombus. Also shown are the number of buried fibrous caps per animal, the fibrous cap thickness in micrometers, the percentage of plaque liipid content, and the plaque area in squared micrometers ×103.
∗, P < 0.05 vs. HFD;
†, P < 0.001 vs. HFD;
‡, P< 0.05 vs. HFD + PE.
Fig. 2.
Representative immunostaining with the F4/80 antibody in the respective group of mice receiving PE training. (A–D) Lesions from the group receiving the HFD (A) showed extensive staining for macrophage-derived foam cells, but the degree of staining was progressively decreased with MT (B), graduated PE (C), and PE coupled with MT (D). (Magnification, ×400.) (E–G) An example of complex vulnerable aortic plaque (E), higher magnification of a plaque erosion (F) (arrows), and the plaque rupture site (arrow) with an occlusive thrombus (G) in aging mice (16–18 months) receiving HFD. (Magnifications: E, ×400; F, ×1,000; G, ×240.)
Fig. 3.
Radical scavenger enzyme activities in the various study groups. Results are cumulative of all mice at the time of death. Solid bar graphs indicate HFD, and hatched bar graphs indicate HFD+MT. Data are expressed as mean ± SD. ∗, P < 0.05 vs. no PE.
Fig. 4.
Representative Western blots of eNOS expression of aortic protein extracts of hypercholesterolemic mice from different study groups at 10 or 16 months. eNOS protein expression was estimated in aortic extracts with the use of eNOS and actin antibodies.
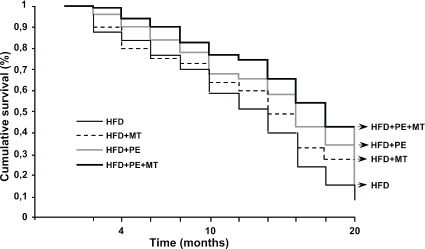
Effects of PE and MT on Survival of Mice.
Taken together, the beneficial effects of the early combined program with graduated PE and MT prolonged survival of mice compared with untreated mice (Fig. 5).
Fig. 5.
Survival curves among different groups of the study population.
Discussion
In the present study, we tested the hypothesis that early administration of a graduated PE program together with MT achieved with antioxidants and l-arginine could be beneficial against long-term effects induced by atherosclerosis. By using male hypercholesterolemic mice on a HFD, we have shown that this combined treatment reduced unstable atheroma and plaque rupture and, more importantly, that this vasculoprotective effect was coupled to prolonged survival of treated animals. Moreover, oxidative stress was reduced, and eNOS expression was increased in the aorta of these animals.
Despite increased understanding of risk factors and pathogenic mechanisms for atherosclerosis-related diseases, such diseases remain nearly endemic in Western society (22, 23). Nevertheless, despite the high prevalence, only a fraction of those with the disease progress to develop a frank myocardial infarction (22, 23), possibly because of other factors that contribute to atherogenesis. Over the past decade, it has become clear that vascular inflammation plays an important role in the pathogenesis of coronary heart disease (22–24). Inflammatory arterial disease therefore may be a more appropriate term for the subset of patients that develop the serious adverse clinical consequences related to the rupture of the intracoronary plaque. Accordingly, it is important to note that several autoptic studies have shown that a substantial number of victims of sudden coronary death show superficial plaque erosion without plaque rupture (22, 23). In addition to rupture or erosion of an atherosclerotic plaque, the occurrence, composition and size of a thrombus are regulated by mechanical hemodynamic effects, the thrombogenicity of the arterial surface, the relative concentration of cellular blood components, and the efficiency of fibrinolysis (10, 22, 23). Thus, attention needs to be focused on effective treatments able to reduce plaque instability. The loss of integrity of the vascular wall in plaque thrombosis may depend on an immunoinflammatory response directed against it (22–24). In other words, plaque thrombosis would be the result of a failure in the regulation of the blood–vessel interface. The key to such regulatory mechanisms is intercellular communication (10, 22–24).
By developing animal models related to plaque vulnerability, particular emphasis is set on the atherosclerotic mouse model (25–29). Overall, this model shows spontaneous plaque thrombosis and external interventions, such as chronic HFD have been used to increase the rate of plaque rupture/thrombosis. With increased aging, spontaneous plaque thrombosis occurs in a substantial number of mice (25–29). Usually, plaques with a thrombus showed reduced collagen content but increased lipid pool and enhanced inflammation as compared with “stable” nonthrombosed plaques, thus supporting the commonly used definition of “vulnerable plaques” in hypercholesterolemic mice. The atherosclerotic process in the mouse model is not histologically identical to that in humans. Therefore, animals and humans might not respond exactly the same to therapeutic intervention. Nevertheless, in a recent study (28), the use of pravastatin, a hypocholesterolemic drug able to reduce cardiovascular events in humans (reviewed in ref. 29), reduced unstable atheroma and death in hypercholesterolemic mice as well, suggesting similar disease mechanisms.
The benefits of early graduated PE on the survival of mice observed in the present study can be attributed to an induction of aortic antioxidant defenses and eNOS expression and the reduction in atherosclerotic lesion progression at 10 months, detected both noninvasively by MRI and by histology at the time of death. Moreover, the present study also indicates that concurrent MT with antioxidants and l-arginine can elicit additional beneficial effects in mice receiving graduated PE training by further inhibiting vulnerable plaque and thrombosis. Progressive adaptation to graduated PE may therefore represent an economical therapy to preserve health and diminish the rate of decline of the physiological processes associated with aging. Interestingly, caloric restriction, an approach able to reduce cardiovascular aging (30, 31), also promotes the expression of eNOS (32).
Clinical studies have shown that severe PE can lead to the generation of more free radicals than the endogenous antioxidant systems can scavenge, whereas moderate intensity aerobic PE improves endothelial function and reduces cardiovascular risk (8, 9). Several highly plausible protective mechanisms have been postulated, including decreased myocardial oxygen demand, increased myocardial oxygen supply, reduced propensity toward ventricular arrhythmias, reduced platelet aggregation, improved lipid profile, and increased plasma fibrinolytic activity (8, 9). Despite the substantial body of literature, pathogenic mechanisms at the cellular and molecular level by which PE might benefit vascular diseases are poorly understood. However, it is conceivable that arterial cells can be affected by multiple signal transduction events promoted by early graduated PE. Short-term oral administration of l-arginine improved hemodynamics and PE capacity in patients with precapillary pulmonary hypertension (33) and enhanced myocardial perfusion in coronary heart disease patients (34–39). In addition, oral l-arginine supplementation enhanced the beneficial effect of PE training on endothelial dysfunction in patients with chronic heart failure (40). To date, we need to establish whether protective effects by dietary supplementation can reverse the natural history of atherogenesis, endothelial dysfunction, vascular inflammation, and oxidative stress in humans (41–43). Consistently, in a recent study (44), experimental data obtained in a large animal model of atherosclerosis (i.e., rabbit) l-citrulline and l-arginine supplementation retarded the progression of high-cholesterol-diet-induced atherosclerosis. However, the proatherogenic network represented by vascular inflammation and oxidative stress should be prevented clinically earlier than previously assumed given that the development of atherogenesis starts during fetal development (11, 12, 45). A large number of studies have provided data suggesting that consumption of dietary antioxidants is associated with reduced risk for cardiovascular disease, but prospective studies provided conflicting results in terms of beneficial effects of antioxidants on the reduction of cardiovascular events (reviewed in refs. 7, 29, and 43). Unfortunately, most of these clinical studies were conducted in patients with advanced atherosclerotic lesions, and, under these conditions, antioxidants may have reduced protective effects. Similarly, we need to evaluate the impact of early moderate PE on the long-term development of atherosclerosis and unstable atheroma in humans. The present study suggests that the primary prevention of atherosclerosis-related diseases in humans can be affected therapeutically through an early implementation of a balanced program of moderate PE and appropriate MT with antioxidants plus l-arginine. To this regard, a very recent study (46) shows the feasibility of optical coherence tomography to identify the components of vulnerable plaques in a murine model of human atherosclerosis. This protocol holds promise for the identification of features defining vulnerable plaque including fibrous cap thickness, lipid core size, and the percentage of lipid content.
Methods
Mice, Protocol of the Study, and Noninvasive Measurements.
The experiments conformed to the Guide for the Care and Use of Laboratory Animals (National Institutes of Health publication No. 85-23, revised 1996) and were carried out on four large groups (n = 80) of 6-week-old male LDL receptor-deficient hypercholesterolemic mice (mean weight, 32.4 ± 10.1 g). Quality standards of laboratories at the University of Naples are in accordance with rules established by the Italian Ministry of Health and the European College of Laboratory Animal Medicine, and the laboratories of the University of California at Los Angeles, the Mayo Clinic College of Medicine, and Whitaker Cardiovascular Institute at Boston University are all in accordance with standards of the Association for Assessment and Accreditation of Laboratory Animal Care. We selected only male mice to avoid gender-related differences (6, 47, 48). Based on a previous study (6), mice were randomized to one of two dietary interventions: (i) HFD, containing (by weight) 21% fat, 0.15% cholesterol, and 19.5% casein (Harlan Teklad, Madison, WI) (6, 47, 48) or (ii) HFD+MT, with 1.0% vitamin E added to the chow and 0.05% vitamin C added to the drinking water and with 6% l-arginine added to the drinking water. In each dietary regimen, mice were assigned randomly to either PE or continued standard activity. Graduated training consisted of a progressive swimming program (as described in ref. 6). The study protocol was administered until death, and mice that had experienced sudden death were immediately removed and processed for histological examination. In a subset of mice (n = 8, at 10 months of age) for each group, morphology of thoracic aorta was estimated noninvasively by magnetic resonance imaging (MRI) according to the method developed by Wiesmann et al. (49), in which comparison of MRI measurements with corresponding cross-sectional histopathology showed excellent agreement of aortic vessel wall area (49). Briefly, MRI experiments were performed during isoflurane anesthesia [1.5–2.0% (vol/vol) with 1 liter of oxygen flow] on a 7-T horizontal bore MRI scanner equipped with a microscopic system allowing for maximal gradient strength of 870 mT/m and a rise time of 280 μs at complete switching (49). MRI parameters were optimized for visualization of the thoracic aorta. Black blood image acquisition was gated to midsystole, allowing for suppression of blood signals, and sets of eight slices of 300-μm thickness were acquired in a total time of 15 min (49). Vessel wall cross-sectional area in each image slice was calculated as total vessel area minus luminal vessel area (area measurements were given in squared millimeters).
Preparation of Arterial Samples, Western Blot Analysis, Immunohistochemistry, and Enzyme Measurements.
The aorta was continuously soaked with PBS containing 10 μg/ml aprotinin and 0.1 mmol/liter PMSF from the time of the dissection until the determination of the lesion area was completed (as described refs. 6, 47, and 48). A 2-mm cross section was saved for immunohistochemical analysis. Tissue sections (5 μm) were blocked for endogenous peroxidase activity by 15-min incubation with 10% H2O2 in methanol. Slides were incubated with 3% BSA for 30 min, and then with the F4/80 antibody against macrophage-derived foam cells (1 h at room temperature; 1:100 dilution) (47, 48). The primary antibody was detected with horseradish peroxidase-conjugated goat antibody to rabbit IgG (1:100 dilution in 3% BSA–PBS) as described in refs. 6, 47, and 48. Slices (250 μm thick) were used for quantification of collagen content by picrosirius red staining and polarized light microscopy analysis and for evaluation of cellular/extracellular components of plaques by Masson’s trichrome staining and platelet immunohistochemistry (DAKO), as described in ref. 50. Tissue activities of the oxygen-radical scavengers glutathione peroxidase, catalase, and manganese superoxide dismutase were determined spectrophotometrically (6). All enzymatic activities were normalized for protein content. The remainder of the aorta was homogenized for further determination of eNOS protein expression (Western blot) by using a rabbit polyclonal eNOS antibody (1:500 dilution for 1.5 h at room temperature) (6, 47, 48).
Evaluation of Oxidative Stress and NOx Levels.
Blood was collected at death in Eppendorf tubes containing 1 mM Na2EDTA. Plasma cholesterol was determined enzymatically (6). Vitamins E and C concentrations in plasma were determined by HPLC (6). Plasma LDL (d = 1.006–1.063 g/ml) were isolated by sequential-density ultracentrifugation (6, 48). The formation of thiobarbituric acid reactive substances was determined by thiobarbituric acid (6), and isoprostane 8-epi-PGF2α purified from plasma samples was measured by immunoassay (Cayman Chemical, Ann Arbor, MI) (6, 48). Finally, plasma NOx levels were measured with Griess reagent (Calbiochem).
Statistical Analysis.
Results are expressed as mean ± SD. The difference among groups was evaluated by a one- or two-factor ANOVA by two independent investigators in a blinded fashion regarding treatment of mice. Statistical significance was accepted at P < 0.05. Comparison of survival curves was made by the Kaplan and Meier calculation procedures.
Acknowledgments
We thank Joseph Loscalzo (Boston University) and Amir Lerman (Mayo Clinic College of Medicine) for helpful discussions in the field, Arlene Lising for technical and secretarial assistance, and the external reviewers for helpful comments and suggestions. This work was supported by National Research Funds from the University of Naples (Ministries of Health and University, and Regione Campania) and by the Mayo Foundation.
Abbreviations
- LDL
low-density lipoprotein
- NOx
nitrite and nitrate
- HFD
high-fat (cholesterol) diet
- MT
metabolic treatment
- PE
physical exercise
- eNOS
endothelial NO synthase.
Footnotes
Conflict of interest statement: L.J.I. helped develop and has a financial interest in a commercially available dietary supplement that contains some of the amino acids and antioxidants studied in this article.
References
- 1.Blumenthal J. A., Emery C. F., Madden D. J., George L. K., Coleman R. E., Riddle M. W., McKee D. C., Reasoner J., Williams R. S. J. Gerontol. 1989;44:M147–M157. doi: 10.1093/geronj/44.5.m147. [DOI] [PubMed] [Google Scholar]
- 2.Kramsch D. M., Aspen A. J., Abramowitz B. M., Kreimendahl T., Hood W. B., Jr N. Engl. J. Med. 1981;305:1483–1489. doi: 10.1056/NEJM198112173052501. [DOI] [PubMed] [Google Scholar]
- 3.Shephard R. J., Balady G. J. Circulation. 1999;99:963–972. doi: 10.1161/01.cir.99.7.963. [DOI] [PubMed] [Google Scholar]
- 4.Thompson P. D., Buchner D., Pina I. L., Balady G. J., Williams M. A., Marcus B. H., Berra K., Blair S. N., Costa F., Franklin B., et al. Circulation. 2003;107:3109–3116. doi: 10.1161/01.CIR.0000075572.40158.77. [DOI] [PubMed] [Google Scholar]
- 5.Lee I. M., Sesso H. D., Oguma Y., Paffenbarger R. S., Jr Circulation. 2003;107:1110–1116. doi: 10.1161/01.cir.0000052626.63602.58. [DOI] [PubMed] [Google Scholar]
- 6.Napoli C., Williams-Ignarro S., de Nigris F., Lerman L. O., Rossi L., Guarino C., Mansueto G., Di Tuoro F., Pignalosa O., De Rosa G., et al. Proc. Natl. Acad. Sci. USA. 2004;101:8797–8802. doi: 10.1073/pnas.0402734101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steinberg D., Witztum J. L. Circulation. 2002;105:2107–2111. doi: 10.1161/01.cir.0000014762.06201.06. [DOI] [PubMed] [Google Scholar]
- 8.Leaf D. A., Kleinman M. T., Hamilton M., Deitrick R. W. Am. J. Med. Sci. 1999;317:295–300. doi: 10.1097/00000441-199905000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Leeuwenburgh C., Heinecke J. W. Curr. Med. Chem. 2001;8:829–838. doi: 10.2174/0929867013372896. [DOI] [PubMed] [Google Scholar]
- 10.de Nigris F., Lerman A., Ignarro L. J., Williams-Ignarro S., Sica V., Baker A. H., Lerman L. O., Geng Y. J., Napoli C. Trends Mol. Med. 2003;9:351–359. doi: 10.1016/s1471-4914(03)00139-4. [DOI] [PubMed] [Google Scholar]
- 11.Napoli C., D’Armiento F. P., Mancini F. P., Postiglione A., Witztum J. L., Palumbo G., Palinski W. J. Clin. Invest. 1997;100:2680–2690. doi: 10.1172/JCI119813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Napoli C., Glass C. K., Witztum J. L., Deutsch R., D’Armiento F. P., Palinski W. Lancet. 1999;354:1234–1241. doi: 10.1016/S0140-6736(99)02131-5. [DOI] [PubMed] [Google Scholar]
- 13.Laufs U., Wassmann S., Czech T., Munzel T., Eisenhauer M., Bohm M., Nickenig G. Arterioscler. Thromb. Vasc. Biol. 2005;25:809–814. doi: 10.1161/01.ATV.0000158311.24443.af. [DOI] [PubMed] [Google Scholar]
- 14.Kingwell B. A. FASEB J. 2000;14:1685–1696. doi: 10.1096/fj.99-0896rev. [DOI] [PubMed] [Google Scholar]
- 15.Stefano G. B., Prevot V., Cadet P., Dardik I. Int. J. Mol. Med. 2001;7:119–129. doi: 10.3892/ijmm.7.2.119. [DOI] [PubMed] [Google Scholar]
- 16.de Nigris F., Lerman L. O., Ignarro S. W., Sica G., Lerman A., Palinski W., Ignarro L. J., Napoli C. Proc. Natl. Acad. Sci. USA. 2003;100:1420–1425. doi: 10.1073/pnas.0237367100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boger R. H., Bode-Boger S. M. Annu. Rev. Pharmacol. Toxicol. 2001;41:79–99. doi: 10.1146/annurev.pharmtox.41.1.79. [DOI] [PubMed] [Google Scholar]
- 18.Maxwell A. J., Ho H. V., Le C. Q., Lin P. S., Bernstein D., Cooke J.P. J. Appl. Physiol. 2001;90:933–938. doi: 10.1152/jappl.2001.90.3.933. [DOI] [PubMed] [Google Scholar]
- 19.Fu W. J., Haynes T. E., Kohli R., Hu J., Shi W., Spencer T. E., Carroll R. J., Meininger C. J., Wu G. J. Nutr. 2005;135:714–721. doi: 10.1093/jn/135.4.714. [DOI] [PubMed] [Google Scholar]
- 20.Kauser K., da Cunha V., Fitch R., Mallari C., Rubanyi G. M. Am. J. Physiol. 2000;278:H1679–H1685. doi: 10.1152/ajpheart.2000.278.5.H1679. [DOI] [PubMed] [Google Scholar]
- 21.Aji W., Ravalli S., Szabolcs M., Jiang X. C., Sciacca R. R., Michler R. E., Cannon P. J. Circulation. 1997;95:430–437. doi: 10.1161/01.cir.95.2.430. [DOI] [PubMed] [Google Scholar]
- 22.Jefferson B. K., Topol E. J. Curr. Probl. Cardiol. 2005;30:333–374. doi: 10.1016/j.cpcardiol.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Ruberg F. L., Leopold J. A., Loscalzo J. Prog. Cardiovasc. Dis. 2002;44:381–394. doi: 10.1053/pcad.2002.123469. [DOI] [PubMed] [Google Scholar]
- 24.Ross R. N. Engl. J. Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 25.Lowe H. C., Jang I. K., Khachigian L. M. Thromb. Haemostasis. 2003;90:774–780. doi: 10.1160/TH03-06-0374. [DOI] [PubMed] [Google Scholar]
- 26.Virmani R., Kolodgie F. D., Burke A. P., Finn A.V., Gold H. K., Tulenko T. N., Wrenn S. P., Narula J. Arterioscler. Thromb. Vasc. Biol. 2005;25:2054–2061. doi: 10.1161/01.ATV.0000178991.71605.18. [DOI] [PubMed] [Google Scholar]
- 27.Johnson J. L., Jackson C. L. Atherosclerosis. 2001;154:399–406. doi: 10.1016/s0021-9150(00)00515-3. [DOI] [PubMed] [Google Scholar]
- 28.Johnson J. L., Carson K., Williams H., Karanam S., Newby A., Angelini G., George S., Jackson C. L. Circulation. 2005;111:1422–1430. doi: 10.1161/01.CIR.0000158435.98035.8D. [DOI] [PubMed] [Google Scholar]
- 29.Napoli C., Sica V., Pignalosa O., de Nigris F. Curr. Med. Chem. 2005;12:1755–1772. doi: 10.2174/0929867054367257. [DOI] [PubMed] [Google Scholar]
- 30.Abete P., Cacciatore F., Ferrara N., Calabrese C., de Sanctis D., Testa G., Galizia G., Del Vecchio S., Leosco D., Napoli C., et al. Am. J. Clin. Nutr. 2003;78:796–801. doi: 10.1093/ajcn/78.4.796. [DOI] [PubMed] [Google Scholar]
- 31.Abete P., Testa G., Galizia G., Gazzella F., Della Morte D., de Santis D., Calabrese C., Cacciatore F., Ferrara N., Rengo G., et al. Exp. Gerontol. 2005;40:43–50. doi: 10.1016/j.exger.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 32.Nisoli E., Tonello C., Cardile A., Cozzi V., Bracale R., Tedesco L., Falcone S., Valerio A., Cantoni O., Clementi E., et al. Science. 2005;310:314–317. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- 33.Nagaya N., Uematsu M., Oya H., Sato N., Sakamaki F., Kyotani S., Ueno K., Nakanishi N., Yamagishi M., Miyatake K. Am. J. Respir. Crit. Care. Med. 2001;163:887–891. doi: 10.1164/ajrccm.163.4.2007116. [DOI] [PubMed] [Google Scholar]
- 34.Bednarz B., Wolk R., Chamiec T., Herbaczynska-Cedro K., Winek D., Ceremuzynski L. Int. J. Cardiol. 2000;75:205–210. doi: 10.1016/s0167-5273(00)00324-7. [DOI] [PubMed] [Google Scholar]
- 35.Fujita H., Yamabe H., Yokoyama M. J. Nucl. Cardiol. 2000;7:97–102. doi: 10.1016/s1071-3581(00)90028-x. [DOI] [PubMed] [Google Scholar]
- 36.Napoli C., Ignarro L. J. Annu. Rev. Pharmacol. Toxicol. 2003;43:97–123. doi: 10.1146/annurev.pharmtox.43.100901.140226. [DOI] [PubMed] [Google Scholar]
- 37.Piatti P., Fracasso G., Monti L. D., Setola E., Lucotti P., Fermo I., Paroni R., Galluccio E., Pozza G., Chierchia S., et al. Circulation. 2003;107:429–436. doi: 10.1161/01.cir.0000046489.24563.79. [DOI] [PubMed] [Google Scholar]
- 38.Maxwell A. J., Zapien M. P., Pearce G. L., MacCallum G., Stone P. H. J. Am. Coll. Cardiol. 2002;39:37–45. doi: 10.1016/s0735-1097(01)01708-9. [DOI] [PubMed] [Google Scholar]
- 39.Iannuzzi A., Iannuzzo G., Sapio C., Pauciullo P., Iorio D., Spampanato N., Mancini M., Rubba P. J. Cardiovasc. Pharmacol. Ther. 2001;6:121–127. doi: 10.1177/107424840100600203. [DOI] [PubMed] [Google Scholar]
- 40.Hambrecht R., Hilbrich L., Erbs S., Gielen S., Fiehn E., Schoene N., Schuler G. J. Am. Coll. Cardiol. 2000;35:706–713. doi: 10.1016/s0735-1097(99)00602-6. [DOI] [PubMed] [Google Scholar]
- 41.Boak L., Chin-Dusting J. P. Curr. Vasc. Pharmacol. 2004;2:45–52. doi: 10.2174/1570161043476546. [DOI] [PubMed] [Google Scholar]
- 42.Cooke J. P., Oka R. K. Curr. Atheroscler. Rep. 2001;3:252–259. doi: 10.1007/s11883-001-0068-x. [DOI] [PubMed] [Google Scholar]
- 43.Napoli C., Ignarro L. J. Nitric Oxide. 2001;5:88–97. doi: 10.1006/niox.2001.0337. [DOI] [PubMed] [Google Scholar]
- 44.Hayashi T., Juliet P. A., Matsui-Hirai H., Miyazaki A., Fukatsu A., Funami J., Iguchi A., Ignarro L. J. Proc. Natl. Acad. Sci. USA. 2005;102:13681–13686. doi: 10.1073/pnas.0506595102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Napoli C., Palinski W. Eur. Heart J. 2001;22:4–9. doi: 10.1053/euhj.2000.2147. [DOI] [PubMed] [Google Scholar]
- 46.Cilingiroglu M., Oh J. H., Sugunan B., Kemp N. J., Kim J., Lee S., Zaatari H. N., Escobedo D., Thomsen S., Milner T. E., Feldman M. D. Catheter Cardiovasc. Interv. 2006 Apr 6; doi: 10.1002/ccd.20717. 10.1002/ccd.20717. [DOI] [PubMed] [Google Scholar]
- 47.Napoli C., de Nigris F., Welch J. S., Calara F. B., Stuart R. O., Glass C. K., Palinski W. Circulation. 2002;105:1360–1367. doi: 10.1161/hc1102.106792. [DOI] [PubMed] [Google Scholar]
- 48.Napoli C., Ackah E., De Nigris F., Del Soldato P., D’Armiento F. P., Crimi E., Condorelli M., Sessa W. C. Proc. Natl. Acad. Sci. USA. 2002;99:12467–12470. doi: 10.1073/pnas.192244499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wiesmann F., Szimtenings M., Frydrychowicz A., Illinger R., Hunecke A., Rommel E., Neubauer S., Haase A. Magn. Reson. Med. 2003;50:69–74. doi: 10.1002/mrm.10500. [DOI] [PubMed] [Google Scholar]
- 50.Calara F., Silvestre M., Casanada F., Yuan N., Napoli C., Palinski W. J. Pathol. 2001;195:257–263. doi: 10.1002/path.915. [DOI] [PubMed] [Google Scholar]