Abstract
We evaluated the effects of a 0.5% cholesterol-enriched diet (HCD) on nitric-oxide synthase (NOS) and arginase expression and the modulating role of 17β-estradiol (E2) on this phenomenon. Thirty oopherectomized rabbits were divided into three groups and treated for 15 weeks. Group I received normal chow; group II, HCD; and group III, HCD plus E2 pellets. Animals in group II showed an increase in plasma lipids, and they demonstrated atheromatous lesions as well as expression of arginase I and II accompanied by a significant number of BrdU-positive cells in endothelial cells and intimal muscle cells, suggestive of an increase in cellular proliferation. There was significant expression of inducible NOS and increased staining of nitrotyrosine-positive areas. These were not observed in group I animals. In both groups, E2 levels were low. In group III animals, E2 supplementation led to a decrease in atheromatous lesions and BrdU-positive cells and reduced expression of both inducible NOS and arginase I and II accompanied by a decrease in nitrotyrosine staining. E2 levels were increased. Our results suggest that E2 was responsible for these effects, despite the animals being hyperlipidemic, similar to those in group II. Because arginase is responsible for cell proliferation by converting l-arginine to polyamines, our results indicate that expression of arginase may play an important role in cellular proliferation in atherosclerosis, and inhibition of arginase expression by E2 may be another potential mechanism in attenuating atherogenesis.
Keywords: arteriosclerosis, l-arginine, nitric oxide, endothelium, nitric, oxide synthase
Estrogens retard the development of atherosclerosis by attenuating the adhesion of circulating monocytes to endothelial cells and their subsequent migration to the subendothelial layer (1). Decreased expression of vascular cell adhesion molecule 1 (VCAM-1) and monocyte chemoattractant protein 1 (MCP-1) by estrogens may at least in part account for this effect (1–3). We (4, 5) and others (6) have demonstrated that estrogens increase nitric oxide (NO) production by endothelial cells, and it is now known that NO, either on its own (7) or produced after stimulation by estrogens (8), can attenuate the cytokine-induced expression of VCAM-1 (1) as well as MCP-1 (9, 10). Furthermore, the inhibition of NO production in animals administered an inhibitor of nitric-oxide synthase (NOS) results in potentiation of atherosclerotic lesions in high-cholesterol diet (HCD)-induced atherosclerosis (11) and also causes atherosclerotic coronary lesions, especially at microvascular levels in experimental animals (12). l-Arginine is a substrate for NOS, which catalyzes formation of Nω-hydroxy-l-arginine as an intermediate that subsequently forms NO (13).
l-Arginine can be a substrate for NOS, which catalyzes its breakdown to release NO in endothelial cells. l-Arginine is also converted by arginase to ornithine, the only source of synthesis in mammalian cells of the polyamines putrescine, spermidine, and spermine, which are essential for cell proliferation and regulation of the cell cycle (14, 15) and, which are, therefore, proatherosclerotic. The exact mechanism by which polyamines increase cell proliferation is not known. In vertebrates there are two isoforms of arginase, both of which catalyze the conversion of arginine to ornithine and urea. They differ with regard to subcellular localization, tissue distribution, and certain enzymatic properties, reflecting the fact that different genes encode them (14, 15). Arginase I is expressed almost exclusively in the cytosol of liver cells, whereas arginase II is located within the mitochondrial matrix and is expressed at low levels in many tissues (15). Furthermore, citrulline, the end product of the NOS-mediated reaction, is converted to l-arginine by arginosuccinate synthetase and arginosuccinate lyase (16). The present work was, therefore, undertaken to assess whether arginase expression is increased in atherosclerotic lesions and to determine the modulating role of estrogen, if any, on this phenomenon.
Results
Blood Chemistry.
All of the rabbits appeared to be healthy throughout the study. No significant differences in serum high-density lipoprotein (HDL)-cholesterol, total serum protein, or body weight existed among the three groups over the course of the study. In animals fed normal chow (group I), there was no difference in total cholesterol levels compared with basal values. The addition of 0.5% cholesterol to the diet (groups II and III) increased the total cholesterol levels significantly compared with the baseline value (Table 1).The treatment with E2 did not significantly affect the plasma lipid levels in this study; however, it increased the plasma E2 concentration up to physiological levels similar to that observed in ovary-intact rabbits (Table 1), as reported in ref. 2.
Table 1.
Plasma lipid (total cholesterol, triglycerides, and HDL-cholesterol), total protein, and E2 concentration in rabbits fed a standard diet (group I), HCD (group II), and HCD plus E2 (group III)
| Group | Total cholesterol, mg/dl | Triglycerides, mg/dl | HDL-cholesterol, mg/dl | Total protein, g/dl | E2, pg/ml |
|---|---|---|---|---|---|
| I | 80.2 ± 8.2 | 36.9 ± 5.1 | 30.4 ± 4.1 | 8.1 ± 1.0 | 15.8 ± 4.1 |
| II | 1,451.5 ± 120.5* | 82.4 ± 15.6* | 30.9 ± 5.2 | 7.8 ± 1.1 | 12.9 ± 2.5 |
| III | 1,298.8 ± 140.9* | 74.5 ± 11.5* | 35.2 ± 8.2 | 8.2 ± 1.1 | 40.0 ± 2† |
Rabbits were treated with each condition for 15 weeks. Results are the mean ± SEM of 10 rabbits. P was measured by an unpaired Student t test.
∗, P < 0.05 versus group I (control);
†, P < 0.05 versus group I (control) and group II (HCD).
Histological Examination of Atherosclerosis.
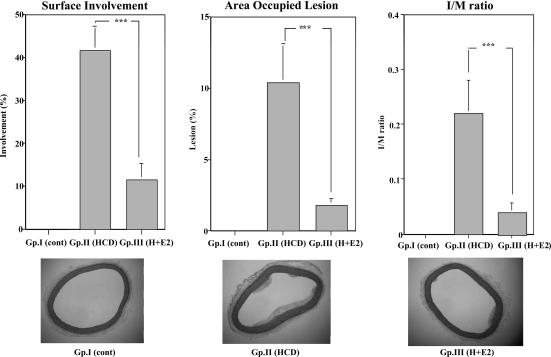
In animals fed normal chow (group I), no atheromatous lesions were observed. On the other hand, histological examination of the thoracic aortas of animals fed a HCD and who received a placebo pellet (group II) revealed more atheromatous lesions, as indicated by the mean percentage of luminal encroachment and the mean lesion area in the hypercholesterolemic than in the animals fed a HCD but who received E2 pellets (group III). The area of atherosclerosis in the thoracic aorta was reduced by 70% in the E2-treated group (group III) compared with the HCD group receiving placebo pellets (group II) (Fig. 1 Left and Center). The intima:media (I:M) ratios also decreased after E2 treatment (group III vs. group II) (Fig. 1 Right).
Fig. 1.
Histological evaluation of the atherosclerotic area of thoracic aortas as indicated by the surface involvement, mean lesion area (percent of area occupied by lesion), and I:M ratio (Upper) and representative photographs (Lower). (Upper Left) Surface involvement of the atherosclerotic area of thoracic aortas from rabbits (group I, normal chow; group II, 0.5% HCD and placebo pellet; group III, 0.5% HCD and E2 pellet). ∗∗∗, P < 0.001. (Upper Center) Area occupied by the atherosclerotic area of thoracic aortas from the three groups of rabbits. ∗∗∗, P < 0.001. (Upper Right) I:M ratio of thoracic aortas from three groups of rabbits. ∗∗∗, P < 0.001. (Lower) Representative photographs of thoracic aortas from rabbits. (Lower Left) Group I. (Lower Center) Group II. (Lower Right) Group III. (Original magnification, ×40.)
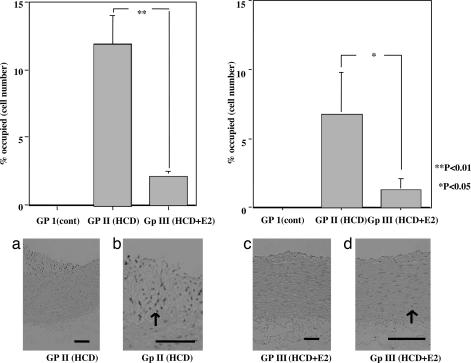
BrdU-Positive Neointima Lesion.
BrdU-positive cells, mainly composed of endothelial cells and intimal smooth muscle cells, were significantly decreased in group III compared with group II (Fig. 2).
Fig. 2.
Immunohistochemical analysis with the anti-BrdU-positive, neointimal area of the thoracic aortas of New Zealand White (NZW) rabbits from the atherosclerotic group. (Upper Left) Atherosclerotic area. (Upper Right) Nonatherosclerotic area. In group III, the number of positive cells was significantly decreased compared with group II. ∗, P < 0.05; ∗∗, P < 0.01. (Lower) Representative photographs of the thoracic aortas from rabbits. (Lower Left a and b) Group II. (Lower Right c and d) Group III. [Original magnification, ×100 (a and c); ×400 (b and d). Scale bars, 100 μm.] The arrows point to anti-BrdU-positive cells.
Immunocytochemical Analysis.
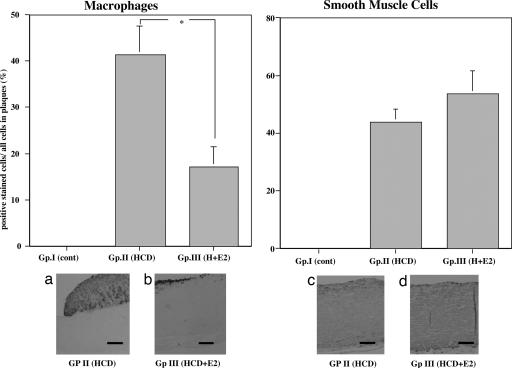
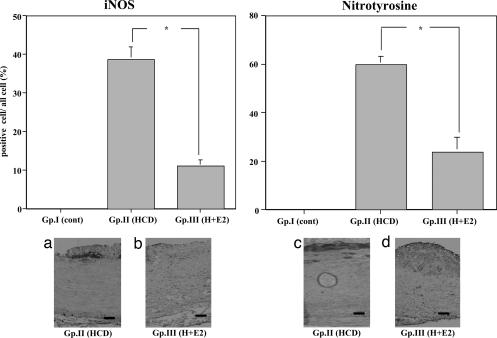
Smooth muscle cell α-actin, monocytes/macrophages, inducible NOS (iNOS), and nitrotyrosine (one of the reaction products of ONOO−).
The atheroma in the aorta was composed of many macrophages derived from foam cells and intimal smooth muscle cell proliferation (Fig. 3). A significant reduction in the atherosclerotic area, as well as a decrease in the relative number of macrophages, was observed in animals in the E2-treated group in this study (Fig. 3 Left). The number of smooth muscle cell α-actin-positive cells was not changed between groups II and III (Fig. 3 Right). The iNOS and nitrotyrosine-positive areas were decreased in the E2-treated group (group III) compared with the placebo group (group II) (Fig. 4). We observed iNOS expression in the T cells and macrophages in the advanced atherosclerotic plaque of the thoracic aortas of group II, consistent with previous data (17).
Fig. 3.
Immunohistochemical analysis with anti-macrophage and anti-smooth muscle cell α-actin monoclonal antibody of thoracic aortas of NZW rabbits from the atherosclerotic group. (Upper Left) Area occupied by macrophages in subintimal atherosclerotic plaque of thoracic aortas of groups I, II, and III rabbits. ∗, P < 0.05. (Upper Right) Area occupied by smooth muscle cell α-actin in subintimal atherosclerotic plaque of thoracic aortas of groups I, II, and III rabbits. (Lower) Representative photographs of thoracic aortas from rabbits. (Lower Left a) Group II. (Lower Left b) Group III. Macrophages were detected in both the core and fibrous cap in a section stained with a monoclonal antibody against rabbit macrophages. (Original magnification, ×100.) (Lower Right c) Group II. (Lower Right d) Group III. Smooth muscle cell α-actins were detected in the media and subintimal atherosclerotic plaque area of thoracic aortas of groups II and III rabbits. No significant difference between groups II and III was observed. (Original magnification, ×100. Scale bars, 25 μm.)
Fig. 4.
Immunohistochemical analysis with anti-iNOS and anti-nitrotyrosine monoclonal antibody of thoracic aortas of NZW rabbits from atherosclerotic group. (Upper) Area occupied by iNOS-positive cells (Left) and nitrotyrosine-positive cells (Right) in subintimal atherosclerotic plaque of thoracic aortas of rabbits. ∗, P < 0.05. (Lower) Representative photographs of thoracic aortas from rabbits. (Lower Left a) Group II. (Lower Left b) Group III. iNOS was detected adjacent to the necrotic core. (Original magnification, ×100. Scale bars, 25 μm.) (Lower Right c) Group II. (Lower Right d) Group III. Nitrotyrosine was detected in the subintimal atherosclerotic plaque area of the thoracic aortas. (Original magnification, ×100. Scale bars, 25 μm.)
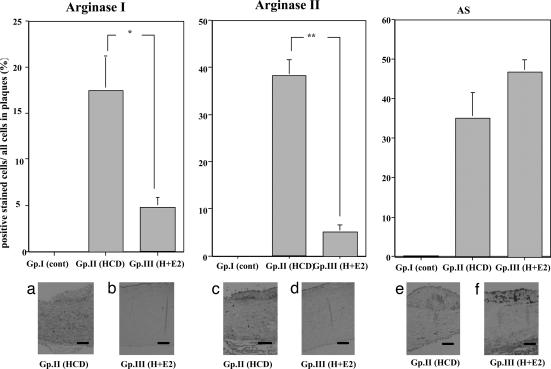
Arginase I, arginase II, and arginosuccinate synthetase.
The atheroma in the aorta expressed a large amount of arginase I and II, as well as arginosuccinate synthase in animals in group II; however, the expression of arginase I and II, but not arginosuccinate synthase, decreased in the aortas from group III (Fig. 5).
Fig. 5.
Distribution of arginase I and II and arginosuccinate synthase in atherosclerotic aortas. (Upper) Immunohistochemical analysis with anti-arginase I and II and arginosuccinate synthetase (AS) antibody of thoracic aortas of NZW rabbits from the different groups. ∗, P < 0.05 and ∗∗, P < 0.01. (Lower) Immunohistochemical analysis with anti-arginase I [(a) group II and (b) group III)] and anti-arginase II [(c) group II and (d) group III] monoclonal antibodies and the anti-arginosuccinate synthetase monoclonal antibody [(e) group II and (f) group III] of the thoracic aortas of NZW rabbits from the atherosclerotic group. (Original magnification, ×100. Scale bars, 25 μm.)
Discussion
Endothelial dysfunction leading to expression of adhesion molecules plays an integral part in the initiation of atherosclerosis. Expression of adhesion molecules on the endothelial surface leads to adhesion of monocytes to endothelial cells, which is one of the early steps in the development of atherosclerosis (18). Endothelial dysfunction is also characterized by impaired endothelium-derived nitric oxide (EDNO) production and impaired endothelium-dependent vasodilation (19). Only low concentrations of NO are normally produced by the endothelial cells physiologically, which protect the endothelial cell and, therefore, play an important role in attenuating the onset of atherosclerosis (20, 21). On the other hand, once atherosclerosis develops, activated macrophages are present in the lesion area, and these macrophages express iNOS (17). This process leads to relatively high concentrations of NO as well as superoxide (O2−), leading to formation of peroxynitrite (ONOO−) in the lesion area, which is toxic to the endothelial cells (22, 23). l-Arginine is the precursor to NO (13, 22), and therefore, l-arginine should either have a protective or deleterious role with regard to atherosclerosis depending on whether endothelial NOS (eNOS) or iNOS is expressed.
Chronic administration of l-arginine to HCD rabbits enhanced the synthesis of EDNO and reduced or reversed the progression of intimal lesions (24, 25). In these studies, the animals initially received 0.5% a HCD for only 10 weeks. Subsequently, l-arginine or vehicle was administered for an additional 13 weeks while the HCD was continued. We demonstrated that administration of l-arginine led to regression of the preexisting intimal lesions. More recently, it has been demonstrated in humans that oral supplementation with large amounts (6–21 g/day) of l-arginine, the precursor to EDNO (26), as well as intake of a nutrient bar enriched with l-arginine (26), designed to enhance EDNO, improved flow-mediated endothelium-dependent vasodilation in hypercholesterolemic individuals. Although l-arginine is the precursor to EDNO, there are other actions of l-arginine independent of NO production that may also have been the cause of improved flow-mediated endothelium-dependent vasodilation in hypercholesterolemic individuals.
In our studies, when the animals were fed a HCD for a much longer period, i.e., 15 weeks, there was an 8-fold increase in arginase I and II expression along with the expression of iNOS compared with animals fed normal chow. Arginase II expression is also up-regulated in rheumatoid arthritis (27, 28) in a way similar to that seen in our studies on atherosclerosis. It has been suggested that in cells where both arginase II and iNOS activity occurs, there is a reciprocal regulation, suggesting that agents that induce arginase II could down-regulate the levels of NO and divert l-arginine metabolism toward cell proliferation and/or tissue regeneration (28). Similarly, the concomitant expression of iNOS as well as arginase can markedly reduce basal NO synthesis in endothelial cells because of a decrease in the intracellular arginine content in these cells (28). In our study, increased arginase expression most likely led to increased cellular proliferation and reduced endothelial cell NO formation, and thus, the protective effects that the endothelial-derived NO has on the atherosclerotic process were absent.
Similar to our results in rabbits, an increase in arginase II enzyme activity in apolipoprotein E−/− mice has been found by another group of investigators (29). Furthermore, in these mice, l-arginine induced vasoconstriction in segments of mouse aorta. The contraction induced by l-arginine was much more pronounced in atherosclerotic apolipoprotein E−/− mice compared with control animals. These contractions were converted to relaxations in the presence of an arginase inhibitor (29). On the basis of our results and those of others (29), it is possible to speculate that after expression of arginase in atherosclerotic lesions, l-arginine administration may have a deleterious effect on the atherosclerotic process rather than the beneficial effect obtained in the absence of arginase expression.
We (1) and others (30, 31) have previously demonstrated that estrogens may attenuate atherogenesis by increasing NO production by endothelial cells (4–6). It has also been shown that estrogens can decrease the expression of TNFα-stimulated VCAM-1 expression by a NO-mediated mechanism (8, 32). This action of estrogens in increasing eNOS would allow l-arginine to be directed to the l-arginine–NO pathway, leading to attenuation of initiation of atherosclerosis. Results from this study further indicate that estrogens attenuate the expression of arginase II, which would blunt the l-arginine–polyamine pathway and prevent cell proliferation. This action would then allow l-arginine to be directed to the l-arginine–NO pathway, thereby offering another potential mechanism by which estrogen attenuates atherogenesis. E2 treatment did not affect the expression of arginosuccinate synthetase, indicating that estrogens do not affect the availability of l-arginine from citrulline.
The precise mechanism(s) by which E2 attenuates the expression of arginase is not known, and it was not assessed in our study. E2 can have varied effects on arginase activity. E2 benzoate evoked a 3-fold elevation in the arginase activity of the dorsal prostate, in contrast to the decreased arginase activity in the ventral prostate after E2 administration (33). However, the type of arginase was not indicated in the study. Further studies are needed to assess the mechanism(s) by which estrogen modulates arginase II activity.
In conclusion, the results from our studies indicate that after prolonged feeding of a HCD, arginase expression is increased in hyperlipidemic rabbits in the atherosclerotic lesion area, whereas the expression of arginase II is significantly reduced by simultaneous administration of E2. The increase in arginase II activity may account for the associated cellular proliferation by diverting l-arginine to form polyamines, whereas E2, by inhibiting arginase II expression, attenuates atherosclerosis by providing a substrate for eNOS to synthesize NO, which is atheroprotective. It is also possible that l-arginine may be beneficial in the early stages of atherosclerosis before the expression of arginase II, whereas it may have deleterious effects if administered later on when significant lesions have already developed, and arginase II, expressed as l-arginine is administered, would then lead to cell proliferation (34). Further studies are needed to assess whether the timing of l-arginine administration may be the key determining factor as to whether it would be atheroprotective or lead to deleterious effects.
Materials and Methods
Chemicals and Solutions.
Monoclonal antibodies against smooth muscle cell α-actin (HHF35) and monocytes/macrophages (RAM11) were purchased from Enzo Diagnostics and DAKO, respectively. Antibodies against iNOS, nitrotyrosine, arginase I, and arginosuccinate synthase were all purchased from Transduction Laboratories (Lexington, KY). Antibodies against arginase II were gifts from M. Gotoh and M. Mori (Kumamoto University School of Medicine) (35). BrdU, a thymidine analog that labels newly synthesized DNA, was purchased from Sigma. Peroxidase-conjugated anti-BrdU was purchased from DAKO.
Animals.
Female NZW rabbits weighing 3–3.5 kg were used. All animals were fed regular chow for 2 weeks. They were housed in individual cages and underwent oopherectomy with placement of either placebo or E2 pellets (10 mg, 60-day release). Eight weeks after the placement of pellets, new E2 or placebo pellets were placed, and the animals were killed at 15 weeks.
The method for oopherectomy was similar to that described in ref. 1. For diets, the animals were divided into three groups. Group I received normal chow. Group II received a 0.5% HCD for 15 weeks and a placebo pellet. Group III received a 0.5% HCD and an E2 pellet. The pellets were replaced by new pellets at the end of 8 weeks to ensure that the animals had E2 released from the pellets for the full duration of the study. At the end of the 15-week feeding period, animals were anesthetized. Anesthesia was initiated by with acepromazine (0.3 mg/kg i.v.) and ketamine (10 mg/kg i.v.) followed by isoflurane [2% (vol/vol) by inhalation]. For postoperative analgesia, the animals were also administered buprenorphine (0.03 mg/kg i.m.) twice each day for 2–3 days.
The animal protocol was formally approved by the Animal Research Committee of the University of California at Los Angeles and by the Nagoya University Graduate School of Medicine.
Determinations of Plasma Lipids and Estradiol Concentration.
To assess lipid and E2 levels, an aliquot of blood from each animal was collected into tubes containing EDTA. The total cholesterol and triglyceride levels were measured by enzymatic assays as described in refs. 36 and 37. The HDL-cholesterol was determined after precipitation with phosphotungstate/MgCl2 (37). The plasma concentration of E2 was examined as described in ref. 38.
Histological Evaluation of Aortic Atherosclerosis.
After 15 weeks of feeding, the animals were euthanized with pentobarbital (100 mg/kg i.v.). The descending thoracic aorta was quickly dissected out, and adjoining segments were either snap frozen in liquid nitrogen or preserved in formaldehyde. Cross sections of the descending thoracic aorta were stained with hematoxylin/eosin (SRL, Tokyo) to examine the endothelial lining and with van Gieson’s elastic stain (SRL) to determine the thickness of the intima. Morphometric analysis was performed as described by Weiner et al. (39). Briefly, the complete section of each block was projected onto a vertical surface with a projecting microscope. Six samples from each rabbit aorta were analyzed with the objective lens. The contours of the lumen and the internal elastic lamina were traced, and the tracings were digitized with a graphics tablet. The surface involvement by atherosclerotic lesion was calculated by dividing the lesion circumference by the circumference of the internal elastic lamina. The circumferences of the lesion area and normal area were defined as circumferences of each part of the internal elastic lamina. The area occupied by atherosclerotic lesions was defined as the percent area bounded by the lumen and the internal elastic lamina. The control luminal area was calculated from the perimeter of the internal elastic lamina as described in ref. 40. The I:M ratio was calculated (41). Data were transferred to a minicomputer (Macintosh iMac; Apple, San Jose, CA) for further analysis.
BrdU Incorporation and Immunohistochemistry.
BrdU was administered at 18 h (100 mg/kg s.c. and 30 mg/kg i.v.) and 12 h (30 mg/kg i.v.) before harvest. BrdU labeling was carried out on 5-μm frozen sections (42). Background staining was blocked by incubation with 5% normal goat serum for 30 min, and then the sections were incubated with a monoclonal antibody to BrdU (1:200; DAKO) at 4°C overnight followed by an alkaline phosphatase-conjugated goat anti-mouse IgG (1:200; Jackson ImmunoResearch) at room temperature for 1 h (42). The BrdU-labeled endothelial and smooth muscle cell nuclei, identified as elongated oval regions of immunoreactivity, were counted in five sequential sections from the thoracic artery of each rabbit. The percentage of BrdU-labeled endothelial cells was expressed as the ratio of vessels having BrdU-labeled endothelial and intimal smooth muscle cells to the total number of endothelial cells and intimal smooth muscle cell profiles per cross section (43).
Immunohistochemical Analysis.
Cross sections of the descending thoracic aorta were deparaffinized with xylene and dehydrated with graded alcohol (17). The specimens were preincubated for 30 min with methanol containing 0.3% hydrogen peroxide and washed for 10 min with PBS. The specimens were permeabilized with 0.1% Triton X-100 in PBS for 20 min and washed with PBS. They were then blocked with normal horse serum for 1 h and incubated with primary monoclonal antibody (for smooth muscle cell α-actin, monocytes/macrophages, iNOS, nitrotyrosine, arginase I, arginase II, and arginosuccinate synthetase) diluted in PBS for 60 min, and washed again with PBS. Negative controls included substitution of irrelevant antibodies for the primary antiserum/antibody. A biotinylated rabbit anti-mouse IgG (1:500 dilution) was incubated for 30 min and washed with PBS followed by avidin–biotin peroxidase complex reagent (ABC kit; Vector Laboratories) incubation for 30 min. The result was a brown peroxidase reaction product of diaminobenzidine. The cell nuclei were counterstained with methyl green (17). In the negative controls, either PBS or irrelevant antibodies replaced the primary antiserum. Each field was scored for the number of positive stained cells against each antibody in plaques on slides, and all cells in the plaques were calculated and analyzed statistically as described in ref. 17. From each section, five digital images were obtained with a 3CCD color camera (JVC; Victor Company of Japan, Tokyo) and Leitz microscope. The intensity and distribution patterns of the staining reaction were evaluated by two blinded, independent observers (T.E. and T.M.) using a semiquantitative staining score (graded as 0 = none, 1 = weak, 2 = moderate, and 3 = strong staining). The cells whose mean scores were higher than 2 were recognized as positive staining cells.
Data Analysis.
The results were expressed as the mean ± SEM. The spss/pc 6.01 software package (SPSS, Chicago) was used for collection, processing, and statistical analysis of all data. Statistical analysis was performed with the nonparametrical Wilcoxon signed-rank test for comparison of the means. The Spearman ρ coefficient was used to assess any significant correlations between the analyzed substances within the distinct groups. P < 0.05 was considered statistically significant.
Acknowledgments
This work was supported in part by Grant AG-15857 from the National Institute on Aging, National Institutes of Health (to G.C.) and by Grant-in-Aid 16406001 from the Ministry of Education, Science, and Culture of Japan (to T.H.).
Abbreviations
- E2
17β-estradiol
- EDNO
endothelium-derived nitric oxide
- eNOS
endothelial nitric-oxide synthase
- HCD
high-cholesterol diet
- I:M ratio
intima:media ratio
- iNOS
inducible nitric-oxide synthase
- MCP-1
monocyte chemoattractant protein 1
- NOS
nitric-oxide synthase
- NZW
New Zealand White
- VCAM-1
vascular cell adhesion molecule 1.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Nathan L., Pervin S., Singh R., Rosenfeld M., Chaudhuri G. Circ. Res. 1999;85:377–385. doi: 10.1161/01.res.85.4.377. [DOI] [PubMed] [Google Scholar]
- 2.Pervin S., Singh R., Rosenfeld M., Chaudhuri G., Nathan L. Arterioscler. Thromb. Vasc. Biol. 1998;18:1575–1582. doi: 10.1161/01.atv.18.10.1575. [DOI] [PubMed] [Google Scholar]
- 3.Simoncini T., Maffei S., Basta G., Barsacchi G., Genazzani A. R., Liao J. K., De Caterina R. Circ. Res. 2000;87:19–25. doi: 10.1161/01.res.87.1.19. [DOI] [PubMed] [Google Scholar]
- 4.Hayashi T., Fukuto J. M., Ignarro L. J., Chaudhuri G. Proc. Natl. Acad. Sci. USA. 1992;89:11259–11263. doi: 10.1073/pnas.89.23.11259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayashi T., Yamada K., Esaki T., Ishikawa T., Iguchi A. Biochem. Biophys. Res. Commun. 1995;214:847–855. doi: 10.1006/bbrc.1995.2364. [DOI] [PubMed] [Google Scholar]
- 6.Weiner C. P., Lizasoain I., Baylis S. A., Knowles R. G., Charles I. G., Moncada S. Proc. Natl. Acad. Sci. USA. 1994;91:5212–5216. doi: 10.1073/pnas.91.11.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Caterina R., Libby P., Peng H. B., Thannickal V. J., Rajavashisth T. B., Gimbrone M. A., Jr, Shin W. S., Liao J. K. J. Clin. Invest. 1995;96:60–68. doi: 10.1172/JCI118074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mukherjee T. K., Nathan L., Dinh H., Reddy S. T., Chaudhuri G. J. Biol. Chem. 2003;278:11746–11752. doi: 10.1074/jbc.M207800200. [DOI] [PubMed] [Google Scholar]
- 9.Zeiher A. M., Fisslthaler B., Schray-Utz B., Busse R. Circ. Res. 1995;76:980–986. doi: 10.1161/01.res.76.6.980. [DOI] [PubMed] [Google Scholar]
- 10.Fleming I., Busse R. Adv. Pharmacol. 1995;34:187–206. doi: 10.1016/s1054-3589(08)61086-8. [DOI] [PubMed] [Google Scholar]
- 11.Naruse K., Shimizu K., Muramatsu M., Toki Y., Miyazaki Y., Okumura K., Hashimoto H., Ito T. Arterioscler. Thromb. 1994;14:746–752. doi: 10.1161/01.atv.14.5.746. [DOI] [PubMed] [Google Scholar]
- 12.Quyyumi A. A., Dakak N., Andrews N. P., Husain S., Arora S., Gilligan D. M., Panza J. A., Cannon R. O., III J. Clin. Invest. 1995;95:1747–1755. doi: 10.1172/JCI117852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukuto J. M., Chaudhuri G. Annu. Rev. Pharmacol. Toxicol. 1995;35:165–194. doi: 10.1146/annurev.pa.35.040195.001121. [DOI] [PubMed] [Google Scholar]
- 14.Jenkinson C. P., Grody W. W., Cederbaum S. D. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1996;114:107–132. doi: 10.1016/0305-0491(95)02138-8. [DOI] [PubMed] [Google Scholar]
- 15.Pegg A. E., McCann P. P. Am. J. Physiol. 1982;243:C212–C221. doi: 10.1152/ajpcell.1982.243.5.C212. [DOI] [PubMed] [Google Scholar]
- 16.Hecker M., Sessa W. C., Harris H. J., Anggard E. E., Vane J. R. Proc. Natl. Acad. Sci. USA. 1990;87:8612–8616. doi: 10.1073/pnas.87.21.8612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esaki T., Hayashi T., Yamada K., Iguchi A. Atherosclerosis (Shannon, Irel.) 1997;128:39–46. doi: 10.1016/s0021-9150(96)05976-x. [DOI] [PubMed] [Google Scholar]
- 18.Ross R. N. Engl. J. Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 19.Chester A. H., O’Neil G. S., Moncada S., Tadjkarimi S., Yacoub M. H. Lancet. 1990;336:897–900. doi: 10.1016/0140-6736(90)92269-n. [DOI] [PubMed] [Google Scholar]
- 20.Khan B. V., Harrison D. G., Olbrych M. T., Alexander R. W., Medford R. M. Proc. Natl. Acad. Sci. USA. 1996;93:9114–9119. doi: 10.1073/pnas.93.17.9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koh K. K., Son J. W., Ahn J. Y., Lee S. K., Hwang H. Y., Kim D. S., Jin D. K., Ahn T. H., Shin E. K. Int. J. Cardiol. 2001;81:43–50. doi: 10.1016/s0167-5273(01)00527-7. [DOI] [PubMed] [Google Scholar]
- 22.Moncada S., Higgs A. N. Engl. J. Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 23.Beckman J. S., Koppenol W. H. Am. J. Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 24.Cooke J. P., Singer A. H., Tsao P., Zera P., Rowan R. A., Billingham M. E. J. Clin. Invest. 1992;90:1168–1172. doi: 10.1172/JCI115937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Candipan R. C., Wang B. Y., Buitrago R., Tsao P. S., Cooke J. P. Arterioscler. Thromb. Vasc. Biol. 1996;16:44–50. doi: 10.1161/01.atv.16.1.44. [DOI] [PubMed] [Google Scholar]
- 26.Maxwell A. J., Anderson B., Zapien M. P., Cooke J. P. Cardiovasc. Drugs Ther. 2000;14:309–316. doi: 10.1023/a:1007886725480. [DOI] [PubMed] [Google Scholar]
- 27.Huang L. W., Chang K. L., Chen C. J., Liu H. W. Kaohsiung J. Med. Sci. 2001;17:358–363. [PubMed] [Google Scholar]
- 28.Corraliza I., Moncada S. J. Rheumatol. 2002;29:2261–2265. [PubMed] [Google Scholar]
- 29.Ming X. F., Barandier C., Viswambharan H., Kwak B. R., Mach F., Mazzolai L., Hayoz D., Ruffieux J., Rusconi S., Montani J. P., Yang Z. Circulation. 2004;110:3708–3714. doi: 10.1161/01.CIR.0000142867.26182.32. [DOI] [PubMed] [Google Scholar]
- 30.Clarkson T. B., Hughes C. L., Klein K. P. Prog. Cardiovasc. Dis. 1995;38:189–198. doi: 10.1016/s0033-0620(95)80011-5. [DOI] [PubMed] [Google Scholar]
- 31.Alexandersen P., Haarbo J., Zandberg P., Jespersen J., Skouby S. O., Christiansen C. Hum. Reprod. 2003;18:1395–1403. doi: 10.1093/humrep/deg286. [DOI] [PubMed] [Google Scholar]
- 32.Hayashi T., Esaki T., Muto E., Sumi D., Thakur N. K., Jayachandran M., Iguchi A. Arterioscler. Thromb. Vasc. Biol. 2000;20:1613–1621. doi: 10.1161/01.atv.20.6.1613. [DOI] [PubMed] [Google Scholar]
- 33.Yamanaka H., Shimazaki J., Imai K., Sugiyama Y., Shida K. Endocrinol. Jpn. 1975;22:297–302. doi: 10.1507/endocrj1954.22.297. [DOI] [PubMed] [Google Scholar]
- 34.Li H., Meininger C. J., Kelly K. A., Hawker J. R., Jr, Morris S. M., Jr, Wu G. Am. J. Physiol. 2002;282:R64–R69. doi: 10.1152/ajpregu.2002.282.1.R64. [DOI] [PubMed] [Google Scholar]
- 35.Gotoh M., Mori M. J. Cell Biol. 1999;144:427–434. doi: 10.1083/jcb.144.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allain C. C., Poon I. S., Chen C. S. G. Clin. Chem. 1974;20:470–473. [PubMed] [Google Scholar]
- 37.Lipid Research Clinics. Manual of Laboratory Operations. 2nd Ed. Vol. 1. Bethesda: Natl. Inst. Health; 1982. DHEW Publ. No. (NIH) 76-628. [Google Scholar]
- 38.Gaskell S. J., Finlay E. M., Pike A. W. Biomed. Mass Spectrom. 1980;7:500–504. doi: 10.1002/bms.1200071109. [DOI] [PubMed] [Google Scholar]
- 39.Weiner B. H., Ockene I. S., Hoogasian J. J. N. Engl. J. Med. 1986;315:841–845. doi: 10.1056/NEJM198610023151401. [DOI] [PubMed] [Google Scholar]
- 40.Hayashi T., Fukuto J. M., Ignarro L. J., Chaudhuri G. J. Cardiovasc. Pharmacol. 1995;26:792–802. doi: 10.1097/00005344-199511000-00017. [DOI] [PubMed] [Google Scholar]
- 41.Kown M. H., Yamaguchi A., Jahncke C. L., Miniati D., Murata S., Grunenfelder J., Koransky M. L., Rothbard J. B., Robbins R. C. J. Thorac. Cardiovasc. Surg. 2001;121:971–980. doi: 10.1067/mtc.2001.112532. [DOI] [PubMed] [Google Scholar]
- 42.Ehsan A., Mann M. J., Acqua G. D., Tamura K., Braun-Dullaeus E., Dzau V. J. Circulation. 2002;105:1686–1692. doi: 10.1161/01.cir.0000013775.02396.93. [DOI] [PubMed] [Google Scholar]
- 43.Virag J. I., Murry C. E. Am. J. Pathol. 2003;163:2433–2440. doi: 10.1016/S0002-9440(10)63598-5. [DOI] [PMC free article] [PubMed] [Google Scholar]