Abstract
Rad54 protein is a Snf2-related dsDNA-specific ATPase essential for homologous recombination mediated by Rad51 protein, the eukaryotic RecA ortholog. Snf2-related enzymes couple ATP hydrolysis with translocation on dsDNA to remodel or dissociate a wide variety of protein–dsDNA complexes. Rad54 and Rad51 interact through species-specific contacts and mutually stimulate their biochemical activities. Specifically, Rad51 bound to dsDNA, the product of homologous recombination after DNA-strand exchange, stimulates the Rad54 ATPase up to 6-fold, leading to the turnover of Rad51 in the product complex. Electron microscopy visualized the Rad51–Rad54 interaction on dsDNA, showing that an oligomeric form of Rad54 associates preferentially with termini of the Rad51–dsDNA filament. Our data support a mechanism of processive dsDNA–Rad51 filament dissociation by the translocating Rad54 protein.
Keywords: recombination, ATPase, Snf2, like proteins
Homologous recombination (HR) is a high-fidelity, template-dependent pathway involved in the nonmutagenic tolerance of DNA damage, the repair of complex DNA damage, and the recovery of stalled and collapsed replication forks (1, 2). The Rad52 group proteins define the HR pathway. Initially, RPA, the eukaryotic ssDNA-binding protein, binds to ssDNA exposed by double-strand break processing or discoordination of leading and lagging strands at stalled replication forks. Mediator proteins (Rad52, Brca2, and Rad51 paralogs) orchestrate the assembly of Rad51 protein on ssDNA to form the nucleoprotein filament, which performs homology search and DNA-strand invasion to prime repair synthesis by using the undamaged sister chromatid or homolog as a template. After resolution of the pairing intermediates, HR has restored the contiguity of the chromosomes with a crossover or noncrossover outcome.
Eukaryotes contain a large number of Snf2-related proteins (3, 4). This group of proteins shares structural and sequence similarity with SF2 DNA helicases, but they are unable to catalyze strand separation typical for DNA helicases (5). Instead, these proteins likely translocate on dsDNA inducing topological changes. Using the energy of ATP hydrolysis, their action leads to the remodeling or dissociation of protein–dsDNA complexes (4, 6). Prominent members of this group are the chromatin-remodeling factors Snf2, Isw1, Isw2, or Chd1. Each eukaryotic genome codes for a variety of Snf2-related proteins. Saccharomyces cerevisiae alone has 17 paralogs, and a number of these proteins appear to function outside a nucleosome remodeling context; for example, the Mot1 protein was found to dissociate the TATA box-binding protein from dsDNA (7). DNA repair pathways are richly endowed with specific, largely nonredundant Snf2-related proteins that function in transcription-coupled repair (Cockayne’s Syndrome B/Rad26 in S. cerevisiae; ref. 8), postreplication repair (Rad5; ref. 9), genomewide nucleotide excision repair (Rad16; ref. 10), recombinational repair (Rad54; ref. 2), and meiotic recombination (Rdh54/Tid1; ref. 2).
Rad54 is a critical member of the Rad52 group, and its absence leads to catastrophic sensitivity to double-strand breaks in yeast (2, 11, 12). This protein displays robust dsDNA-specific ATPase activity, which is essential for its in vivo function (13–15). Rad54 engages in a specific, albeit transient, interaction with Rad51 protein that leads to stimulation of the Rad51 in vitro recombination activity by Rad54 and the stimulation of the Rad54 ATPase, as well as topological activity by the Rad51 nucleoprotein filament (16–23). After DNA-strand exchange, Rad51 is found in a product complex on the heteroduplex DNA. Rad51 filaments on dsDNA are the preferred cofactor for the Rad54 ATPase, leading to a 6-fold stimulation compared with protein-free DNA. The resulting dissociation of Rad51 from dsDNA (22, 23) might be required to allow access for DNA polymerases to prime DNA synthesis at the invading 3′ OH end. Here, we visualize by transmission electron microscopy (TEM) the interaction of Rad54 protein with the Rad51–dsDNA filament showing that Rad54 interacts preferentially with a terminus of the Rad51–nucleoprotein complex. The biochemical analyses and the structures of Snf2-related proteins (24, 25) are consistent with a model that an oligomeric form of Rad54 translocates on protein-free DNA toward the terminus of the Rad51–dsDNA filament, where its ATPase activity is enhanced to processively dissociate Rad51 from dsDNA.
Results
Rad54 Oligomerizes on dsDNA into Complexes Comprising a Variable Number of Monomers.
The complex of human Rad54 protein with dsDNA was visualized by atomic force microscopy as particles of heterogeneous size with an average corresponding to at least a trimer of Rad54 (26). Dimerization and oligomerization of S. cerevisiae Rad54 protein in the presence of dsDNA (13) suggests that it may form nucleoprotein complexes with a similar architecture. To visualize the interaction between active Rad54 and the Rad51–dsDNA filament, we established reaction conditions where Rad54 formed distinct, nonaggregating particles that displayed robust ATPase activity (Figs. 4 and 5, which are published as supporting information on the PNAS web site). The results indicate that at a DNA to Rad54 ratio of 60 bp per Rad54 protein (90 monomers per 5.3-kbp DNA molecule), the Rad54 ATPase displayed half-maximal activity approaching maximal activity at ratio of >300 bp per Rad54 monomer (Fig. 4c).
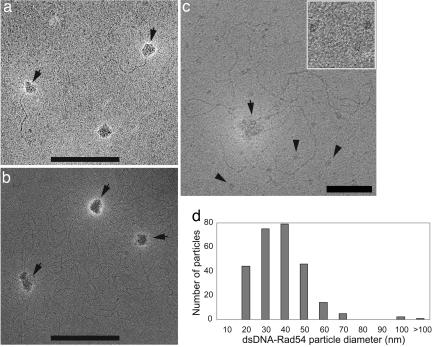
To visualize Rad54 protein on dsDNA, we used TEM of unfixed negatively stained nucleoprotein complexes. Glutaraldehyde fixation neither affected the appearance of the Rad54–dsDNA complexes nor resulted in their better preservation. Complexes were assembled at a ratio optimal for ATPase activity (600 bp per Rad54 monomer), where ≈60% of the DNA molecules were proteinbound (Fig. 4 b and c). Assuming uniform distribution of Rad54, this ratio corresponds to at least nine Rad54 monomers per 5.3-kbp φX174 DNA molecule. Rad54 formed particles on φX174 (5.3 kbp) and linear 600-bp dsDNA fragments but not on 90-bp-long oligonucleotides (Fig. 1a and data not shown). The latter substrate still supported robust Rad54 ATPase activity (50% of φX174 DNA), and the addition of Rad51 enhanced the Rad54 ATPase activity severalfold (data not shown). This finding suggests either a short lifetime of the Rad54 oligomer on 90 bp DNA, consistent with the previously reported DNA length dependence of ATP hydrolysis by Rad54, ISWI, and RSC/Sth1 (27–29) or selective preservation of Rad54 bound to larger DNA molecules during EM specimen casting.
Fig. 1.
TEM visualization of DNA-bound Rad54. (a) Unfixed complexes of untagged Rad54 on 600-bp DNA molecules formed at a ratio of 600 bp per monomer. (b) Unfixed complexes of GST-tagged Rad54 on duplex φX174 DNA formed at a ratio of 600 bp per monomer. In a and b, arrows point to DNA-bound Rad54 particles. Because it is often impossible to follow the entire path of negatively stained dsDNA, a proportion of species with more than one Rad54 particle per DNA cannot be determined in our analysis. (Scale bars: 200 nm.) (c) DNA-unbound Rad54 particles are uniform in size. Unfixed complexes of nicked circular duplex φX174 DNA and wild-type GST-Rad54 were prepared at a ratio of 600 bp per monomer. Shown is a specimen negatively stained with ammonium molybdate. A DNA-bound oligomeric Rad54 particle is seen at the center and is marked by an arrow. Multiple DNA-unbound smaller Rad54 species are distributed across the image (some are marked by arrowheads). Three representative smaller Rad54 species are enlarged in Inset (50 × 50 nm). (Scale bar: 50 nm.) All EM micrographs are shown in reverse contrast to enhance visibility. (d) Histogram of size distribution of DNA-bound untagged Rad54 particles (n = 266) from a. The diameter was calculated from contour-length values measured for individual particles making an approximation of their spherical shape.
The majority of the DNA molecules observed by TEM were in association with particles of Rad54 protein. Typically, Rad54-bound DNA contained one to two and sometimes up to four protein particles per DNA molecule. The overall architecture of the complexes was similar to those of human Rad54 on circular dsDNA observed by atomic force microscopy (26). Like human Rad54 protein, the DNA-bound yeast Rad54 particles were heterogeneous in size with a somewhat irregular globular shape ranging from 15 to >100 nm in diameter (Fig. 1). This size heterogeneity suggests that individual DNA-bound Rad54 particles can contain a variable number of Rad54 monomers. In the absence of DNA and occasionally in the presence of DNA, we observed a Rad54 species with a smaller diameter that was uniform in shape and size and not associated with DNA (Fig. 1c and data not shown).
Similar to nucleoprotein complexes of human Rad54 (26), a fraction of the DNA-bound yeast Rad54 particles were in contact with multiple regions on the same DNA molecule forming topologically separated domains (Fig. 1a). We also observed species where Rad54 was associated with more than one DNA molecule. The formation of these species was Rad54 concentration-dependent (data not shown). In contrast to human Rad54 (26), nucleoprotein complexes of yeast Rad54 assembled in the presence or absence of ATP were morphologically indistinguishable. Also, nucleoprotein complexes of ATPase-deficient Rad54-K341R protein did not visibly differ from those formed by the wild-type protein (data not shown). This finding is consistent with the observation that Rad54 binding to DNA is independent of ATP binding/hydrolysis.
To address the possibility that the GST tag may have induced multimerization of Rad54 and be the reason for the considerable size heterogeneity of DNA-bound Rad54 particles, we purified untagged Rad54 protein. Real-time monitoring of ATP hydrolysis during protease digestion in liquid phase showed that removal of the GST tag did not cause a drop in the Rad54 ATPase ratio. Also, purified untagged Rad54 retained its ability to stimulate Rad51-mediated DNA-strand exchange in vitro (data not shown). TEM examination showed that the overall architecture of nucleoprotein complexes of untagged Rad54 was very similar to that of the GST-tagged protein (Fig. 1 a and b).
To minimize the formation of complexes involving multiple DNA molecules and to increase the number of molecules for the analysis of particle size distribution, we assembled Rad54 nucleoprotein complexes on short, 600-bp-long dsDNA. TEM examination found that complexes comprised one and, less frequently, two Rad54 particles per DNA molecule (Fig. 1a). Surprisingly, Rad54 was able to form topologically separated domains (DNA loops) on such short linear DNA (Fig. 1a). Particle analysis showed that removal of the GST tag reduced, but not completely eliminated, the heterogeneity of the DNA-bound Rad54 particles in shape and size. The diameter of Rad54 particles varied mostly between 20 and 50 nm (Fig. 1d).
Rad54 Protein Preferentially Interacts with a Partially Saturated Rad51-DNA Filament.
The Rad54 ATPase activity displays an enhanced mode on partial Rad51–dsDNA filaments with 6-fold stimulated ATPase activity over modes on protein-free DNA or saturated Rad51–dsDNA filaments (23). This finding suggests that tertiary complexes of different architecture were formed. On saturated Rad51–dsDNA filaments, Rad54 is likely to contact the filament via lateral protein–protein contacts or gain access to DNA hidden in the major groove of the filament by squeezing between Rad51 protomers. On partially Rad51-covered DNA, Rad54 also may bind to the protein-free DNA regions and filament termini. Here, we studied the physical interactions of Rad54 protein with fully and partially saturated Rad51–dsDNA filaments and characterized Rad51–Rad54–dsDNA complexes by nucleoprotein gel analysis.
First, Rad54 protein was reacted with Rad51–dsDNA complexes formed at a ratio of 4 bp per Rad51 monomer corresponding to 75% of filament saturation, which results in partially and some completely Rad51-covered DNA molecules. Two supershifted species were detected on nucleoprotein gels (Fig. 6a, which is published as supporting information on the PNAS web site). We investigated the supershifted complexes by using 600-bp linear DNA fully (0.4 bp per Rad51 monomer; Fig. 6b) and partially (4 bp per Rad51 monomer; Fig. 6c) covered with Rad51 protein. Upon addition of Rad54, the major band representing saturated Rad51 complexes was supershifted partially to a lower mobility smear but only at the highest Rad54 concentrations (60 and 120 Rad54 molecules per DNA fragment). Under these conditions, the Rad54 ATPase also was increased (data not shown), indicating that at these concentrations, Rad54 protein may displace Rad51 and be activated to the enhanced ATPase mode. The rather weak supershift of the major band of saturated Rad51–dsDNA filaments by Rad54, even at close to equimolar Rad51/Rad54 ratios, suggests either poor preservation of ternary complexes by glutaraldehyde fixation or their transient character, or both.
At the subsaturating ratio of 4 bp per Rad51 monomer, a broad, smear-like distribution of partially Rad51-covered species is seen. At the same time, a substantial portion (up to 40%) of the 600-bp DNA molecules remains protein-free, most likely because of limits imposed by Rad51 filament nucleation on short DNA (Fig. 6c, left lane). At low Rad54 concentrations, we detected both smeared supershifted species and those trapped at the gel origin. At higher Rad54 concentrations, almost all protein-bound DNA was either trapped at the gel origin or could not enter the gel. We quantified the formation of ternary complexes by the disappearance of Rad51-bound DNA (saturated filament in Fig. 6b; partial filament in Fig. 6c). Rad54 binding to protein-free DNA in the presence of partial Rad51–dsDNA filaments (Fig. 6c) and in the absence of Rad51 (gel not shown) was measured by the disappearance of the protein-free DNA band. Control experiments excluded the presence of nuclease/phosphatase activities that could interfere with the assay, and dissociation of the protein–DNA complexes by SDS/proteinase K quantitatively recovered the labeled input DNA (data not shown). We found that Rad54 protein exhibited a preference for binding to partial Rad51–dsDNA filaments compared with saturated filaments. At higher Rad54 to DNA ratios, Rad54 bound partial Rad51–dsDNA filaments as well or better than protein-free DNA. The Rad51–Rad54–dsDNA complexes assembled at subsaturating (30 bp per Rad51) and saturating (3 bp per Rad51) ratios did not aggregate (Fig. 5), making them suitable for analysis by TEM.
Rad54 Protein Localizes to Termini of Rad51–dsDNA Filaments.
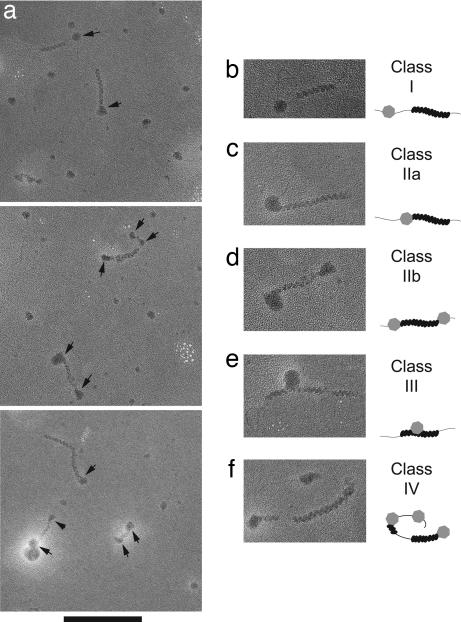
TEM showed that Rad54 protein forms complexes on dsDNA, which can be distinguished easily from the Rad51 filament (Fig. 1). Under our reaction conditions, the prevailing majority of Rad51-covered DNA molecules contained a single-filament patch of varying length, whereas <4% of the DNA molecules contained two or more filament patches (see Fig. 2a). Of 227 Rad51-covered molecules examined, 66% (151) were in association with one or more Rad54 particles (Table 1). We distinguished several types of contacts of Rad54 with partial Rad51–dsDNA complexes (Fig. 2 b–f). Seventy-five percent of the filaments made direct contacts with Rad54, either laterally (class III) or with filament ends (classes IIa and IIb), and 25% of filaments made indirect contacts (class I) with Rad54 spaced by protein-free DNA regions. The bias of direct versus indirect association rules out the possibility of circumstantial binding of Rad54 to DNA partially covered with Rad51. Of those Rad51–dsDNA filaments contacting Rad54 directly, the great majority of contacts (>86%) were via filament termini, and only <14% were lateral contacts. At this resolution, it is also possible that particles classified as lateral association actually are bound to protein-free DNA between short filament segments. Given the variation in size of the Rad54 particles, we counted only those terminal contacts, where the diameter of Rad54 particles was persuasively larger than the width of the Rad51 filament (≈10 nm; ref. 30). Of all Rad51 filaments making terminal contacts with Rad54, 78% had contacts at one end and 22% at both ends of the filament (Table 1). Overall, the prevalence of terminal over lateral association of Rad54 with Rad51–dsDNA filament suggests that Rad54 protein approaches the filament from the terminus.
Fig. 2.
Rad54 protein is targeted to the termini of partial dsDNA–Rad51 filament. (a) Partial Rad51 filaments on 600-bp DNA assembled at a ratio of 7.5 bp per Rad51 monomer were reacted with untagged Rad54 protein at a ratio of 600 bp per Rad54 monomer. Specimens were prepared without fixation. Arrows mark Rad54 protein particles in direct and indirect contact with Rad51 nucleoprotein filaments (see Table 1 for complete quantitation). (Scale bar: 200 nm.) (b–f) Classification of contacts of Rad54 protein with partial dsDNA–Rad51 filaments. Shown are typical images of species along with their schematic drawings. (b) Indirect contact of Rad54 with Rad51 filament where the Rad54 particle is separated from the filament by a protein-free DNA region. Direct Rad54 contact with one (c) and both (d) Rad51 filament termini. (e) Direct lateral contact of Rad54 with the Rad51 filament. (f) A combination of two direct terminal (both at single end) and one indirect contact on a DNA molecule containing two patches of Rad51 filament and three Rad54 particles. All EM micrographs are shown in reverse contrast to enhance visibility.
Table 1.
Summary of EM analysis of Rad54 interaction with Rad51–dsDNA filaments
| Species description | Number | Percent |
|---|---|---|
| Rad51-covered DNA molecules | 227 | 100 |
| In association with Rad54 particles (Rad51–Rad54 species) | 151 of 227 | 66.5 |
| Rad51–Rad54 species: | ||
| With single Rad51 filament | 146 of 151 | 96.7 |
| With two or more Rad51 filaments | 5 of 151 | 3.3 |
| With single Rad54 particle | 113 of 151 | 74.8 |
| With two or more Rad54 particles | 38 of 151 | 25.2 |
| Rad54 particles associated with Rad51-containing molecules | 193 | 100 |
| Direct contacts with filament (terminal or lateral) (classes IIa, IIb, III) | 145 of 193 | 75.1 |
| Indirect contacts with filament (spaced by protein-free DNA) (class I) | 48 of 193 | 24.9 |
| Rad51 filaments associated with Rad54 particles | 156 | 100 |
| Direct contacts with Rad54 (terminal or lateral) | 118 of 156 | 75.6 |
| Terminal contacts (classes IIa and IIb) | 101 of 118 | 85.6 |
| Terminal at one end (class IIa) | 79 of 101 | 78.2 |
| Terminal at both ends (class IIb) | 22 of 101 | 21.8 |
| Lateral contacts (class III) | 17 of 118 | 14.4 |
| 51-54 species with two or more Rad54 particles | 38 | 100 |
| All indirect contacts with filament | 6 of 38 | 15.8 |
| All terminal contacts with filament (class IV) | 23 of 38 | 60.5 |
| Combination of terminal, lateral, or indirect contacts | 9 of 38 | 23.7 |
Particle analysis was performed by using 32 micrographs acquired from the specimen shown in Fig. 2. Only Rad51-covered DNA molecules were analyzed. Molecules with hard-to-discern short Rad51 filaments (with less than three zigzags of helical repeat) were omitted. Examples and a schematic drawing for each species (classes I–IV) are shown in Fig. 2 b–f. To minimize counting errors, Rad51-covered DNA molecules, Rad51 filaments, and Rad54 particles were scored independently. When analyzing Rad51–Rad54 species with two or more Rad54 particles, those containing three Rad54 particles per DNA (<3% of Rad51–Rad54 species) were assigned to the corresponding class with two Rad54 particles on the basis of best match.
Rad51–dsDNA Filament Disassembly Occurs via Rad54 Translocation and Is Controlled by the Extent of Filament Saturation.
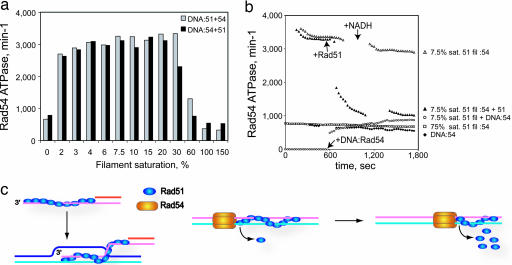
We recently showed that the rate of Rad54 ATPase on Rad51–dsDNA complexes and the efficiency of Rad51 displacement from dsDNA by Rad54 differed on partially and fully saturated Rad51–dsDNA filaments (22, 23). At very low Rad51 concentrations, changes in the order of protein addition can cause a delay in the Rad54 ATPase activation. Across a range of subsaturating Rad51 concentrations, the Rad54 ATPase attains a maximal level of activity. Passing a certain threshold in Rad51 to DNA ratio toward filament saturation, both the Rad54 ATPase and the efficiency of filament displacement decreases. To address the nature of Rad51 to DNA ratio-dependent activity of Rad54 protein, we performed a set of titration experiments with different orders of Rad54 addition. The data showed that in a window of Rad51 to DNA ratios, where all DNA molecules ought to contain Rad51–dsDNA filaments, the Rad54 ATPase activity attained a maximal level irrespective of the order of addition (Fig. 3a). A plausible explanation is that mobile Rad54 particles, which bound the template earlier, quickly reach the ends of the immobile Rad51 filaments, as long as the DNA/Rad51 ratio procures at least one filament per DNA molecule. To distinguish whether Rad54 targets the filament termini via 3D diffusion from solution or via translocation along the DNA lattice, we assayed the stimulation of Rad54 ATPase by partially saturated dsDNA–Rad51 filaments, where nucleoprotein complexes of Rad51 and Rad54 were assembled on separate DNA molecules (Fig. 3b). In this case, the Rad54 ATPase activity did not significantly differ from that of the Rad54–dsDNA complex in the absence of Rad51. Hence, Rad54 remains committed over the time measured to its initial Rad51-free DNA template with little transfer to partial Rad51 filaments via diffusion, consistent with previous observations (22). Together, these results provide strong support for Rad54 translocation on DNA.
Fig. 3.
Rad54 activity is controlled by the extent of saturation of the dsDNA–Rad51 filaments. ATPase kinetics were performed in standard ATPase reaction buffer supplemented with the ATP regeneration system. (a) Effect of order of component addition on Rad54 ATPase interacting with dsDNA–Rad51 filaments of different saturation. Gray bars, direct order of addition where dsDNA–Rad51 complexes were assembled on duplex φX174 DNA (2.3 μM) with varying amounts of Rad51 protein. Measurement of ATPase kinetics was started upon addition of GST-Rad54 protein (5 nM). Black bars, inverted order of addition where DNA first was incubated with Rad54 protein. ATPase kinetics was started upon addition of Rad51 protein. The rates of ATP hydrolysis were calculated from time intervals within the first 6 min of kinetics. A 100% filament saturation corresponds to a ratio of 3 bp per Rad51 monomer. (b) Real-time ATPase kinetics with different substrate combinations. Complexes were formed between duplex φX174 DNA (4.6 μM), GST-Rad54 protein (5 nM), and Rad51 protein at different DNA/protein ratios. ♦ DNA was reacted with Rad54 in the absence of Rad51. □ and ▵, Rad51 was assembled on DNA at 4 and 40 bp per Rad51 monomer (75 and 7.5% filament saturation). ATPase kinetics was initiated upon Rad54 addition. ▴, after the first 600 sec of kinetics, an aliquot of complexes represented by ▵ was challenged by the addition of extra Rad51 to achieve 75% filament saturation. ○, Rad51 filament at 7.5% saturation was assembled on 2.3 μM DNA. An equimolar amount of DNA incubated separately with 5 nM Rad54 then was added. Arrows show the time points of the addition of extra Rad51 protein, DNA–Rad54 complex, and NADH to replenish the ATP regeneration system. (c) Model for the interaction of Rad54 with Rad51–dsDNA filaments to turnover the Rad51–dsDNA product complex of HR. (Left) Rad51-mediated DNA-strand exchange between a processed DSB and a dsDNA target, resulting in a D loop with Rad51 bound to the product dsDNA. The disposition of Rad54 on the Rad51–ssDNA filament is not known, and Rad54 is not drawn (see Discussion). (Right) Anchoring of Rad54 particles to one end of the Rad51–dsDNA filament may result in Rad54 pumping DNA away from the filament stripping Rad51 off the duplex DNA.
At higher Rad51 to DNA ratios, the decreased Rad54 ATPase may result either from failure to initiate the enhanced ATPase mode and/or the inability to maintain it. To test the latter possibility, we first initiated enhanced Rad54 ATPase on partial Rad51–dsDNA filaments assembled at a ratio of 40 bp per Rad51 monomer (Fig. 3b). The loss of Rad54 ATPase was observed soon after the addition of further Rad51 to a ratio of 4 bp per Rad51 monomer (75% saturation). Hence, Rad54 fails to initiate and maintain the enhanced ATPase mode on saturated Rad51–dsDNA filaments. At the tested saturation level at least 25% of DNA remains protein-free, and it appears unlikely that the observed inhibition can be explained by the inability of Rad54 to access free DNA or by a lack of filament ends. Note that Rad51 displays considerably lower cooperativity in DNA binding than RecA protein, leading to filaments that only partially occupy a DNA molecule at subsaturating concentrations (23, 31). The rate-limiting initial assembly of oligomeric Rad54 on dsDNA is followed by fast translocation until it reaches the terminus of the Rad51 filament on DNA and starts displacing it. The length of the filament per se should not affect the velocity of the reaction provided that an adequate ATP pool is supplied, as was the case here (22). Indeed, the robust Rad54 ATPase over a wide range of Rad51 to DNA ratios (Fig. 3a) is indicative of processivity in displacing Rad51 from dsDNA. The observed down-regulation of the Rad54 ATPase after addition of additional Rad51 can be explained by a topological constraint. Filaments are bulk structures anchoring DNA at their entry/exit points and not allowing superhelical tension to propagate. Short free DNA regions between filaments will behave topologically similar to minicircles, where the transition of DNA twist into supercoiling becomes energetically unfavorable and chromatin remodeling by the yeast Snf2 complex was found very inefficient (32).
Discussion
Here we visualize by TEM the architectural disposition of the interaction between a presumably active Rad54 particle and its bona fide in vivo target, the Rad51–dsDNA filament, which is the first view of the interaction of a Snf2-related enzyme with its remodeling substrate. Together with the previous Rad54 studies (11–14, 18–26, 33, 34), the present work suggests that Rad54 assembles into homomultimeric particles that processively dissociate Rad51–dsDNA filaments by translocating on DNA toward a terminus of the filament, providing a first glimpse at the interaction of the Rad51 filament with a relevant interaction partner. This function of Rad54 may serve to turn over Rad51 protein after DNA-strand exchange.
Mechanism of Rad54 Function.
Yeast Rad54 formed characteristic particles on dsDNA. The ATPase data suggest that the Rad54 particles observed by TEM likely represent a functional form of Rad54. At present, it is difficult to estimate the number of Rad54 monomers per particle, because the active fraction of Rad54 molecules is unknown. Calculations of the particle volume and deriving its possible molecular weight from the apparent diameter would yield an overestimate because of the contribution of staining and flattening caused by surface adsorption to the diameter. Also it is unclear whether Rad54 assembles into active particles with varying subunit composition or with a defined stoichiometry. In the latter case, the heterogeneity in particle size (and subunit number) would represent the formation of subassemblies/aggregates, which has been observed with SV40 large T antigen, a hexameric DNA helicase (35).
The oligomeric nature of a functional Rad54 is likely to endow the enzyme with higher processivity during translocation and Rad51 filament dissociation than would be expected for other complexes that exhibit a heteromultimeric architecture with a single motor subunit and modest ATP hydrolysis rates (36). The processivity also would explain the template commitment identified for Rad54 and its inability to quickly shuttle between DNA molecules (Figs. 3 and 4d; ref. 22). FtsK, a bacterial motor protein that pumps DNA during bacterial cell division, displays very fast translocation on dsDNA (up to 5 kbp/sec) with considerable processivity and forms particles that are presumably even bigger than the Rad54 particles described here, because they are visible in light microcopy (37).
A number of biochemical observations provide indirect evidence that Rad54 translocates on dsDNA (reviewed in refs. 11 and 12), and recent single-molecule experiments provide direct evidence for Rad54 translocation on duplex DNA (38). We cannot entirely exclude that some Rad54 particles translocated from a lateral association (class III) to the terminus of the filaments (classes IIa and IIb) and became trapped there or bound directly from solution to the filament terminus. There were relatively few lateral associations (17 class III particles of 193) but a much more significant number of class I particles (48 of 193), where the Rad54 particle was separated from the filament terminus by a short stretch of protein-free DNA (Table 1 and Fig. 2). In the possible model of lateral association, translocation along the filament, trapping at the end, this class I would not be expected to occur. Hence, we favor the interpretation that the class I particles represent the intermediate of Rad54 translocating along dsDNA to the filament terminus, where they accumulated because of the specific interaction between Rad51 and Rad54. Therefore, we propose that Rad54 translocates along dsDNA to the terminus of the Rad51 filament, where it processively dissociates the Rad51 filament (Fig. 3c). The structures of the zebrafish Rad54 protein (24) and a Snf2-related protein from Sulfolobus (25) provide support for such a mechanism that involves DNA sequence-independent tracking along the minor groove of the DNA.
Rad54 Function in the Context of Rad51-Mediated DNA-Strand Exchange.
The central reaction in HR is the formation of a nucleoprotein filament on ssDNA by a homologous pairing and DNA-strand exchange protein, RecA in bacteria and Rad51 in eukaryotes. Invasion of the homologous duplex DNA to allow priming of DNA synthesis restores lost sequence information and the continuity of the broken DNA (Fig. 3c; refs. 2 and 31). Rad54 and its dsDNA-dependent ATPase function are essential for Rad51-mediated recombination in S. cerevisiae in vivo, and several models have been proposed for the mechanism of Rad54 including nucleosome removal, opening of the target DNA, sliding of the target DNA during homology search, and Rad51 turnover after DNA-strand exchange (reviewed in refs. 11 and 12). The interaction with the presynaptic Rad51–ssDNA filament targets Rad54 to the pairing site (20, 39, 40), where Rad51 will be dsDNA-bound after DNA-strand exchange (Fig. 3c). It is unclear how exactly Rad54 is delivered to the pairing site by the Rad51–ssDNA filament (lateral or terminal association or both). We were unable to gain structural information on the Rad54–Rad51–ssDNA ternary complex because of the extreme instability of the ssDNA–Rad51 filament and aggregation of a ternary complex during TEM specimen casting (data not shown).
Unlike the dynamic dsDNA–RecA complex, which quickly turns over by ATP hydrolysis (31, 41), S. cerevisiae Rad51 protein remains stably bound to dsDNA even under ATP hydrolysis conditions (22). Rad54 was shown to dissociate Rad51 from dsDNA employing an enhanced mode of ATPase activity that was induced by interaction with duplex-bound Rad51 (22, 23). Here, we show that under these conditions Rad54 forms oligomeric particles that associate preferentially with the termini of the Rad51–dsDNA filament. The Rad51–ssDNA filament forms with a defined polarity, resulting in two structurally nonequivalent ends (ref. 31; Fig. 3c). During recombination, this structural disposition is maintained in the product complex. One terminus is likely the specific protein interaction site of both proteins. The interaction with the Rad51 filament terminus tethers the Rad54 particle, where it might work similar to FtsK in pumping dsDNA (Fig. 3c). Rad54 possibly pumps DNA either away from the filament or into the filament. Pumping DNA into the filament might dissociate Rad51 by torsional stress, similar to the bulge diffusion model discussed for chromatin remodeling factors (4). Alternatively, when Rad54 pumps DNA away from the filament, it may act as a “protein wire-stripper” on dsDNA. Its affinity for the Rad51 filament terminus would provide Rad54 with substrate specificity by using this generic mechanism. This latter model (Fig. 3c) is supported by the structural and functional similarity between Rad54 (and other Snf2-related proteins) and DNA helicases that pump a single strand of DNA acting as a “DNA wire-stripper” (5). A postsynaptic role of Rad54 is supported by genetic data (reviewed in ref. 11) and a role in the turnover of the Rad51–dsDNA product complex is consistent with the specific association of Rad54 with Rad51 and their mutual stimulation of biochemical activities. This model does not exclude other possible functions of Rad54 during presynapsis and synapsis (reviewed in refs. 11 and 12) or in the dissociation of other protein–dsDNA complexes, including nucleosomes (27, 42, 43) that might interfere with recombination.
Materials and Methods
Recombinant Proteins.
S. cerevisiae GST-Rad54 and Rad51 were purified as described in ref. 21. The GST tag was removed by human rhinovirus PreScission, resulting in a predicted Rad54 protein that contained five additional amino acids at its N terminus. Untagged Rad54 was purified further by chromatography on a hydroxyapatite column. Remnants of GST and uncleaved protein were removed glutathione Sepharose affinity chromatography. The flowthrough was concentrated and stored at –80°C.
DNA Substrates.
Ninety-base-pair DNA fragments were obtained by annealing of two complementary 90-nt oligonucleotides. Six hundred-base-pair DNA fragments were PCR-amplified from an RFI φX174 DNA template by using the following primers: forward, 5′-GTC TTC ATT TCC ATG CGG TG-3′; reverse, 5′-TTA TCG AAG CGC GCA TAA AT-3′. Larger DNA fragments were obtained by linearization of φX174 DNA with restriction enzymes. Fragments were radiolabeled to high specific activity by using T4 DNA polynucleotide kinase, and a spike of 10,000–20,000 cpm was added per reaction with a known molar quantity of unlabeled substrate.
ATP Hydrolysis Assays.
ATPase activity of Rad54 protein was assayed in the NADH-coupled ATP hydrolysis assay as described in ref. 44.
Nucleoprotein Gel Assay.
The DNA–Rad54 complexes were formed at the indicated amounts of GST-tagged or -untagged Rad54 protein and various radiolabeled dsDNA substrates (usually at 2 μM concentration) in 10–12 μl of Rad54 reaction buffer containing 25 mM triethanolamine acetate (TEA), pH 7.5, 13 mM magnesium acetate, 1.8 mM DTT, 5 mM ATP, and 100 μg/ml BSA for 15 min at room temperature. Complexes of dsDNA and Rad51 protein were formed at 30°C in Rad54 reaction buffer (15-min incubation time). When indicated, samples were fixed by the addition of freshly prepared 5% glutaraldehyde buffered in 0.1 M TEA (pH 7.5; 0.25% final concentration) followed by a 10-min incubation, or left on ice without fixation before loading on agarose gels. Gels were run for 90 min at 4 V/cm, dried, and analyzed on the PhosphorImager (Molecular Devices).
Nucleoprotein Network Assay.
The formation of nucleoprotein networks of Rad54 protein and DNA or Rad54 protein and DNA–Rad51 complexes was assayed as described in ref. 45.
TEM of Nucleoprotein Complexes.
For TEM, complexes of dsDNA with Rad51 or Rad54 proteins were prepared as described above, except BSA was omitted from the reaction buffers. Spreading and negative staining of glutaraldehyde-fixed complexes were performed as described in refs. 46 and 47. Briefly, to prepare specimens of native (unfixed) complexes, spreading was performed directly in the reaction buffer. A 10- to 12-μl drop of the reaction mixture was placed onto a strip of Parafilm. Freshly glow-discharged carbon support then was floated onto the drop for 5–10 sec, briefly washed by floating on a surface of 5 mM magnesium acetate, and placed on the top of a 40-μl drop of 2% uranyl acetate solution for 1 min. The excess stain was wiped off the side by using filter paper, and the grids were dried by air. Glow discharge was done in air atmosphere by using an EMS100X discharger (Electron Microscopy Sciences) according to manufacturer’s instructions. Specimens were imaged by using a Philips CM-120 TEM operated at 80 kV acceleration voltage. Images are shown as negatives and were recorded by using low-dose procedures on photographic film (Kodak SO-163) at a magnification of ×35,000, or on a 2,048 × 2,048 pixel Gatan charge-coupled device camera at ×52,000 nominal magnification. The diameter of Rad54 protein particles was measured by using the digital micrograph (Gatan, Pleasanton, CA) software system.
Supplementary Material
Acknowledgments
We thank Steve Kowalczykowski for generously making available his equipment and communicating results before his study’s publication and Henning Stahlberg, Kirk Ehmsen, Xiao-Ping Zhang, Mike Rolfsmeier, Kristina Herzberg, and Tammy Doty for helpful discussions and comments on the manuscript. This work was supported by National Institutes of Health Grant GM58015 (to W.-D.H.).
Abbreviations
- HR
homologous recombination
- TEM
transmission electron microscopy.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Michel B., Grompone G., Flores M. J., Bidnenko V. Proc. Natl. Acad. Sci. USA. 2004;101:12783–12788. doi: 10.1073/pnas.0401586101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Symington L. S. Microbiol. Mol. Biol. Rev. 2002;66:630–670. doi: 10.1128/MMBR.66.4.630-670.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker P. B., Hörz W. Annu. Rev. Biochem. 2002;71:247–273. doi: 10.1146/annurev.biochem.71.110601.135400. [DOI] [PubMed] [Google Scholar]
- 4.Flaus A., Owen-Hughes T. Curr. Opin. Gen. Dev. 2004;14:165–173. doi: 10.1016/j.gde.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Singleton M. R., Wigley D. B. J. Bacteriol. 2002;184:1819–1826. doi: 10.1128/JB.184.7.1819-1826.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pazin M. J., Kadonaga J. T. Cell. 1997;88:737–740. doi: 10.1016/s0092-8674(00)81918-2. [DOI] [PubMed] [Google Scholar]
- 7.Auble D. T., Hansen K. E., Mueller C. G. F., Lane W. S., Thorner J., Hahn S. Genes Dev. 1994;8:1920–1934. doi: 10.1101/gad.8.16.1920. [DOI] [PubMed] [Google Scholar]
- 8.Van Gool A. J., Verhage R., Swagemakers S. M. A., Vandeputte P., Brouwer J., Troelstra C., Bootsma D., Hoeijmakers J. H. J. EMBO J. 1994;13:5361–5369. doi: 10.1002/j.1460-2075.1994.tb06871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson R. E., Henderson S. T., Petes T. D., Prakash S., Bankmann M., Prakash L. Mol. Cell. Biol. 1992;12:3807–3818. doi: 10.1128/mcb.12.9.3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schild D., Glassner B. J., Mortimer R. K., Carlson M., Laurent B. C. Yeast. 1992;8:385–395. doi: 10.1002/yea.320080506. [DOI] [PubMed] [Google Scholar]
- 11.Heyer W. D., Li X. R., Rolfsmeier M., Zhang X.-P. Nucleic Acids Res. 2006 doi: 10.1093/nar/gkl481. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan T. L. R., Kanaar R., Wyman C. DNA Repair. 2003;2:787–794. doi: 10.1016/s1568-7864(03)00070-3. [DOI] [PubMed] [Google Scholar]
- 13.Petukhova G., Van Komen S., Vergano S., Klein H., Sung P. J. Biol. Chem. 1999;274:29453–29462. doi: 10.1074/jbc.274.41.29453. [DOI] [PubMed] [Google Scholar]
- 14.Swagemakers S. M. A., Essers J., de Wit J., Hoeijmakers J. H. J., Kanaar R. J. Biol. Chem. 1998;273:28292–28297. doi: 10.1074/jbc.273.43.28292. [DOI] [PubMed] [Google Scholar]
- 15.Clever B., Schmuckli-Maurer J., Sigrist M., Glassner B., Heyer W.-D. Yeast. 1999;15:721–740. doi: 10.1002/(SICI)1097-0061(19990630)15:9<721::AID-YEA414>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 16.Clever B., Interthal H., Schmuckli-Maurer J., King J., Sigrist M., Heyer W. D. EMBO J. 1997;16:2535–2544. doi: 10.1093/emboj/16.9.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang H., Xie Y. Q., Houston P., Stemke-Hale K., Mortensen U. H., Rothstein R., Kodadek T. J. Biol. Chem. 1996;271:33181–33186. doi: 10.1074/jbc.271.52.33181. [DOI] [PubMed] [Google Scholar]
- 18.Van Komen S., Petukhova G., Sigurdsson S., Stratton S., Sung P. Mol. Cell. 2000;6:563–572. doi: 10.1016/s1097-2765(00)00055-1. [DOI] [PubMed] [Google Scholar]
- 19.Van Komen S., Petukhova G., Sigurdsson S., Sung P. J. Biol. Chem. 2002;277:43578–43587. doi: 10.1074/jbc.M205864200. [DOI] [PubMed] [Google Scholar]
- 20.Mazin A. V., Bornarth C. J., Solinger J. A., Heyer W.-D., Kowalczykowski S. C. Mol. Cell. 2000;6:583–592. doi: 10.1016/s1097-2765(00)00057-5. [DOI] [PubMed] [Google Scholar]
- 21.Solinger J. A., Lutz G., Sugiyama T., Kowalczykowski S. C., Heyer W.-D. J. Mol. Biol. 2001;307:1207–1221. doi: 10.1006/jmbi.2001.4555. [DOI] [PubMed] [Google Scholar]
- 22.Solinger J. A., Kiianitsa K., Heyer W.-D. Mol. Cell. 2002;10:1175–1188. doi: 10.1016/s1097-2765(02)00743-8. [DOI] [PubMed] [Google Scholar]
- 23.Kiianitsa K., Solinger J. A., Heyer W. D. J. Biol. Chem. 2002;277:46205–46215. doi: 10.1074/jbc.M207967200. [DOI] [PubMed] [Google Scholar]
- 24.Thoma N. H., Czyzewski B. K., Alexeev A. A., Mazin A. V., Kowalczykowski S. C., Pavletich N. P. Nat. Struct. Mol. Biol. 2005;12:350–356. doi: 10.1038/nsmb919. [DOI] [PubMed] [Google Scholar]
- 25.Dürr H., Körner C., Müller M., Hickmann V., Hopfner K. P. Cell. 2005;121:363–373. doi: 10.1016/j.cell.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 26.Ristic D., Wyman C., Paulusma C., Kanaar R. Proc. Natl. Acad. Sci. USA. 2001;98:8454–8460. doi: 10.1073/pnas.151056798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaskelioff M., Van Komen S., Krebs J. E., Sung P., Peterson C. L. J. Biol. Chem. 2003;278:9212–9218. doi: 10.1074/jbc.M211545200. [DOI] [PubMed] [Google Scholar]
- 28.Whitehouse I., Stockdale C., Flaus A., Szczelkun M. D., Owen-Hughes T. Mol. Cell. Biol. 2003;23:1935–1945. doi: 10.1128/MCB.23.6.1935-1945.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saha A., Wittmeyer J., Cairns B. R. Genes Dev. 2002;16:2120–2134. doi: 10.1101/gad.995002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogawa T., Yu X., Shinohara A., Egelman E. H. Science. 1993;259:1896–1899. doi: 10.1126/science.8456314. [DOI] [PubMed] [Google Scholar]
- 31.Bianco P. R., Tracy R. B., Kowalczykowski S. C. Front. Biosci. 1998;3:570–603. doi: 10.2741/a304. [DOI] [PubMed] [Google Scholar]
- 32.Gavin I., Horn P. J., Peterson C. L. Mol. Cell. 2001;7:97–104. doi: 10.1016/s1097-2765(01)00158-7. [DOI] [PubMed] [Google Scholar]
- 33.Petukhova G., Stratton S., Sung P. Nature. 1998;393:91–94. doi: 10.1038/30037. [DOI] [PubMed] [Google Scholar]
- 34.Tan T. L. R., Essers J., Citterio E., Swagemakers S. M. A., de Wit J., Benson F. E., Hoeijmakers J. H. J., Kanaar R. Curr. Biol. 1999;9:325–328. doi: 10.1016/s0960-9822(99)80142-0. [DOI] [PubMed] [Google Scholar]
- 35.Dean F. B., Dodson M., Echols H., Hurwitz J. Proc. Natl. Acad. Sci. USA. 1987;84:8981–8985. doi: 10.1073/pnas.84.24.8981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith C. L., Horowitz-Scherer R., Flanagan J. F., Woodcock C. L., Peterson C. L. Nat. Struct. Biol. 2003;10:141–145. doi: 10.1038/nsb888. [DOI] [PubMed] [Google Scholar]
- 37.Pease P. J., Levy O., Cost G. J., Gore J., Ptacin J. L., Sherratt D., Bustamante C., Cozzarelli N. R. Science. 2005;307:586–590. doi: 10.1126/science.1104885. [DOI] [PubMed] [Google Scholar]
- 38.Amitani I., Baskin R. J., Kowalczykowski S. Mol. Cell. 2006 doi: 10.1016/j.molcel.2006.05.009. in press. [DOI] [PubMed] [Google Scholar]
- 39.Solinger J. A., Heyer W.-D. Proc. Natl. Acad. Sci. USA. 2001;98:8447–8453. doi: 10.1073/pnas.121009898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mazin A. V., Alexeev A. A., Kowalczykowski S. C. J. Biol. Chem. 2003;278:14029–14036. doi: 10.1074/jbc.M212779200. [DOI] [PubMed] [Google Scholar]
- 41.Cox J. M., Tsodikov O. V., Cox M. M. PLoS Biol. 2005;3:e52. doi: 10.1371/journal.pbio.0030052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alexeev A., Mazin A., Kowalczykowski S. C. Nat. Struct. Biol. 2003;10:182–186. doi: 10.1038/nsb901. [DOI] [PubMed] [Google Scholar]
- 43.Alexiadis V., Kadonaga J. T. Genes Dev. 2003;16:2767–2771. doi: 10.1101/gad.1032102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kiianitsa K., Solinger J. A., Heyer W.-D. Anal. Biochem. 2003;321:266–271. doi: 10.1016/s0003-2697(03)00461-5. [DOI] [PubMed] [Google Scholar]
- 45.Tsang S. S., Chow S. A., Radding C. M. Biochemistry. 1985;24:3226–3232. doi: 10.1021/bi00334a023. [DOI] [PubMed] [Google Scholar]
- 46.Di Capua E., Engel A., Stasiak A., Koller T. J. Mol. Biol. 1982;157:87–103. doi: 10.1016/0022-2836(82)90514-9. [DOI] [PubMed] [Google Scholar]
- 47.Stasiak A., Di Capua E., Koller T. J. Mol. Biol. 1981;151:557–564. doi: 10.1016/0022-2836(81)90010-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.