Abstract
We have conducted a prospective controlled multicenter study to evaluate differences in the levels of clinical utility of the tuberculous glycolipid (TBGL) serodiagnostic test and the nucleic acid amplification test in patients with smear-negative active pulmonary tuberculosis (TB). The TBGL test and the PCR test were individually not so useful for the rapid diagnosis of smear-negative active pulmonary TB. However, clinical utility was considerably improved by using the TBGL test and the PCR test in combination, especially in patients with smear-negative and culture-negative active pulmonary TB and in patients with minimally advanced lesions.
For early detection and treatment of tuberculosis (TB) patients before transmission of TB bacilli, it is important to improve diagnostic techniques. Recently, nucleic acid amplification (NAA) tests have been successfully established as methods for rapid and accurate diagnosis of active pulmonary TB (6, 13, 14). For patients with smear-negative pulmonary TB, however, NAA diagnostic sensitivity was reported to be approximately 50%. Sensitivity was found to be extremely low (5 to 20%) in smear- and culture-negative cases (2, 3, 4, 9, 10). A new serological tuberculous glycolipid (TBGL) test has been developed, and this test has been reported to be useful for the rapid diagnosis of active pulmonary TB (8, 11). We considered rapid diagnosis using the TBGL test to be effective for patients with smear-negative active pulmonary TB, especially when combined with the NAA test. For this report, we have conducted a prospective controlled multicenter study to evaluate the clinical usefulness of the NAA and TBGL tests.
Patients were referred as potential participants for the study between April 1999 and March 2000. They were diagnosed (according to clinical symptoms and signs and chest X-ray findings during the first visit in each respiratory institute) as having smear-negative active TB. We obtained informed consent before participation and prospectively studied all patients. Patients with a medical history of smear tests positive for acid-fast bacilli were excluded from this study. Patients who were defined to be smear positive at the admission examination and patients with nontuberculous mycobacteriosis, cured pulmonary TB, or pleurisy were excluded. All patients requiring anti-TB chemotherapy were admitted to the hospitals. Patients with other respiratory diseases (such as lung cancer, pulmonary bacterial infections other than mycobacterial, or interstitial pneumonia) who were undergoing differential diagnosis for active pulmonary TB were enrolled as controls during same period. Additional evaluation procedures, such as bronchoscopy, were performed at the discretion of the treating physician. Hospital records were reviewed to abstract demographic data and clinical information. Eligibility and clinical records of each enrolled patient were reviewed at an extramural meeting. No patients identified as having human immunodeficiency virus infection were included in this study.
Sputum specimens for both smear staining and culturing were obtained on three consecutive days after admission. The sputum specimens were digested and decontaminated with a solution of 2% sodium hydroxide (NaOH) and N-acetyl-l-cysteine (NALC) and then centrifuged for 15 min at 3,000 × g. The supernatant was removed, and the remaining sediment was mixed in a 1:10 dilution with sterile water. The processed sample was stained with Auramine O fluorochrome and examined by fluorescent microscopy. Ziehl-Neelsen acid-fast staining was used to confirm the presence of acid-fast bacilli. Mycobacterial cultures were grown by inoculating 0.1 ml of the processed sample into tubes of Ogawa medium (Nissui Pharmaceutical Co., Ltd., Tokyo, Japan) (12) and/or into the liquid medium of a Mycobacteria Growth Indicator Tube system (Beckton Dickinson) according to the manufacturers' instructions. Identification to species level of Mycobacterium tuberculosis and other nontuberculous mycobacteria (NTM) was determined by a DNA-DNA hybridization method with a DDH Mycobacteria apparatus (Kyokuto Pharmaceuticals, Tokyo, Japan).
The Roche Amplicor Mycobacterium test (Amplicor MTB) was performed according to the manufacturer's instructions (Roche Diagnostics, Laval, Quebec, Canada). Sputum specimens for NAA were collected on admission. Aliquots of 0.1 ml were prepared from all sputum specimens for NAA. The TBGL serodiagnostic test (Kyowa Medex Co., Ltd., Tokyo, Japan) was performed as described previously (11). Serum specimens were obtained 1 day before chemotherapy and stored at −20°C until assay of TBGL antibody titers. All technicians carried out the NAA and the enzyme-linked immunosorbent assay such that they were blind to the final diagnosis.
Active TB was confirmed when cultures of respiratory secretions (sputum or bronchial lavage) were positive for M. tuberculosis. Active clinical TB (smear negative and culture negative) was confirmed when there was a compatible abnormal chest X ray which showed improvement, as judged by independent review of the chest X ray, after treatment for 2 to 3 months with three or four antituberculosis drugs. Agreement of three specialists in their separate respiratory institutions was required to confirm the clinical diagnosis. The extent of TB lesions was classified according to the guideline of the Committee on Revision of Diagnostic Standards (5). A meeting of an investigative commission was held during which the investigators confirmed the criteria for diagnosis, especially by X ray. According to the American Thoracic Society TB classification system (1, 7), the active clinical TB group was class 3. NTM was diagnosed, in accord with American Thoracic Society criteria (15), by isolation of potentially pathogenic NTM from the respiratory secretions (sputum or bronchial lavage). Diagnoses of other respiratory diseases (cancer, lung infections, other bronchial diseases, etc.) were based on chest X rays compatible with the original diagnosis, diagnoses by the treating physicians, and all other clinical information.
Statistical analyses were performed with a computer-based statistical package (Statcel; OMS, Ltd., Saitama, Japan). The values are expressed as means ± standard deviations (SD) and were determined by a Student t test or a multiple comparison test (Scheffe's F test) for a single factor. Differences in sensitivity between diagnostic methods were evaluated by a Fisher's exact probability test.
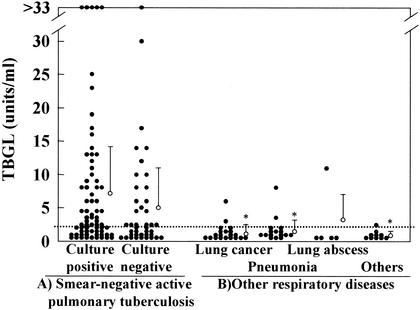
As shown in Table 1, 111 patients were diagnosed with smear-negative active pulmonary TB. There were 70 patients who were culture positive for M. tuberculosis and 41 who were culture negative. The samples from the smear-negative active pulmonary TB group gave significantly (P < 0.05) higher TBGL antibody titers than those in the other respiratory disease group (Table 1 and Fig. 1), leading to a high level of specificity (88.5%) as reported previously (11).
TABLE 1.
Subject profiles and TBGL antibody titers
| Patient groupa | No. of patients (male/female) | Age (mean ± SD) | Anti-TBGL titer (U/ml) (mean ± SD) |
|---|---|---|---|
| Smear-negative active pulmonary TB | 111 (78/33) | 61.0 ± 20.7 | 6.2 ± 8.4 |
| Culture positive | 70 (51/19) | 63.2 ± 20.3 | 7.0 ± 9.0 |
| Culture negative | 41 (27/14) | 57.3 ± 20.8 | 5.0 ± 7.3 |
| Other respiratory disease | 52 (52/16) | 66.9 ± 18.4 | 1.2 ± 1.9b |
| Lung cancer | 21 (17/4) | 70.3 ± 13.6 | 1.0 ± 1.2b |
| Pneumonia | 16 (8/8) | 62.9 ± 26.3 | 1.5 ± 1.8b |
| Lung abscess | 5 (3/2) | 58.0 ± 10.7 | 2.4 ± 4.7 |
| Others | 10 (8/2) | 70.7 ± 13.9 | 0.7 ± 0.7b |
Among the patients who were referred as potential participants for the study, those who were defined to be smear positive for active pulmonary TB (94 patients) and those with nontuberculous mycobacteriosis (61 patients), pleuritis (8 patients), or old TB (10 patients) were excluded.
P < 0.05 versus smear-negative pulmonary TB.
FIG. 1.
Distribution of TBGL antibody titers in patient groups. The dotted line indicates a cutoff point (2 U/ml). Closed circles indicate individual values. Open circles indicate means plus SD. Asterisks indicate values significantly different from those for patients with smear-negative active TB (P < 0.05).
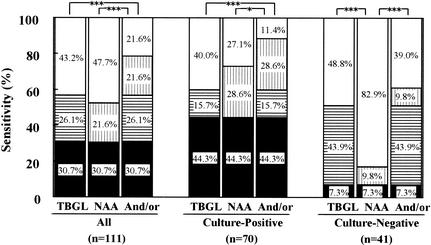
The sensitivity of the TBGL test was compared with that of the NAA test for the smear-negative active pulmonary TB group (Fig. 2). For this group, the sensitivity of the TBGL test (56.8%) was comparable to that of the NAA test (52.3%). In the subgroup of culture-positive cases, the sensitivity of the NAA test was higher (72.9%) than that of the TBGL test (60.0%). In the subgroup of culture-negative cases, however, the sensitivity of the NAA test was significantly lower (17.1%) than that of the TBGL test (51.2%). Using the TBGL test and the NAA test combined, the sensitivity of diagnosis for smear-negative active pulmonary TB was significantly increased to 78.4%. In patients with smear-negative and culture-positive active pulmonary TB, the sensitivity of the NAA test was 72.9%, but when used in combination with the TBGL test, the sensitivity was significantly increased and diagnosed 88.6% of patients as having active pulmonary TB. For patients with smear- and culture-negative pulmonary TB, using the combined TBGL and NAA tests significantly improved sensitivity (61%). In fact, 30 of 53 (56.6%) smear-negative and NAA-negative cases and 18 of 34 (53.0%) smear-negative, culture-negative, and NAA-negative cases were TBGL positive.
FIG. 2.
Sensitivity of TBGL test and NAA test, either alone or in combination, for patients with smear-negative active pulmonary TB. Symbols: open, not detected; vertical stripe, detected by NAA alone; horizontal stripe, detected by the TBGL test alone; closed, detected by both the TBGL test and NAA. Significant differences are indicated (*, P < 0.05; ***, P < 0.001).
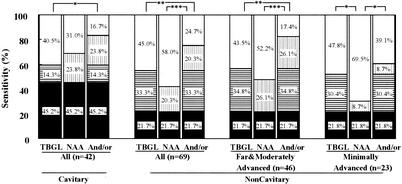
The sensitivities of the TBGL test and the NAA test were compared on the basis of the presence or absence of cavitary lesions in chest radiographs of patients, as shown in Fig. 3. In 42 cases of patients with cavitary lesions, the sensitivity of the NAA test was slightly higher (69.0%) than that of the TBGL test (59.5%). In 69 cases of noncavitary lesions, an assessment was performed according to the spread of lesions, demonstrating that the sensitivity of the TBGL test was higher than that of the NAA test, especially in cases with minimally advanced lesions.
FIG. 3.
Sensitivity of TBGL test and NAA test, either alone or in combination, according to chest X-ray findings for patients with smear-negative active pulmonary TB. The extent of TB lesions was classified according to the guideline of the Committee on Revision of Diagnostic Standards (5). Symbols: open, not detected; vertical stripe, detected by NAA alone; horizontal stripe, detected by the TBGL test alone; closed, detected by both the TBGL test and NAA. Significant differences are indicated (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
The use of the TBGL test in combination with the NAA test significantly improved the sensitivity of diagnosis of pulmonary TB from 42.0 to 75.3%. In the subgroup of cases with minimally advanced active pulmonary TB without cavitary lesions, the use of both tests in combination significantly improved sensitivity from 30.5 to 60.9%. Also, in terms of chest radiographic findings, the TBGL test was more sensitive than the NAA test for the cases with noncavitary lesions, irrespective of the spread of lesions. These results indicated that the serodiagnostic test can improve the differential diagnosis of smear-negative TB, especially at early stages before tuberculous bacilli can be detected in the sputum.
The TBGL test and the PCR test were not so useful individually for the rapid diagnosis of smear-negative active pulmonary TB. However, clinical utility was considerably improved by using the TBGL test and the PCR test in combination, especially in patients with smear-negative and culture-negative active pulmonary TB and in patients with minimally advanced lesions.
Acknowledgments
R.M. is one of the Clinical Trial of Anti-TBGL Antibody (CTAT) investigators. Other CTAT investigators include Souichirou Yokota (Toneyama National Hospital, Osaka, Japan), Masako Wada (Research Institution of Tuberculosis, Japan Anti-Tuberculosis Association, Tokyo), Ryou Yoneyama (Higashi-Saitama National Hospital, Saitama, Japan), Takefumi Saitoh (Seiransou National Hospital, Saitama, Japan), Kazuko Machida (Tokyo National Hospital, Tokyo, Japan), Eiro Tsubura (Osaka Hospital, Japan Anti-Tuberculosis Association, Tokyo), Mitsunori Sakatani, (Kinkichuo National Hospital, Osaka, Japan), Shin Kawahara (Minami-Okayama National Hospital, Okayama, Japan), Noriharu Motoki (Higashi-Kouchi National Hospital, Kouchi, Japan), Koutaro Ouizumi (First Department of Internal Medicine, Kurume University School of Medicine, Fukuoka, Japan), et al.
REFERENCES
- 1.Bass, J. B., L. S. Farer, P. C. Hopewell, R. F. Jacobs, and D. E. Snider. 1990. Diagnostic standards and classification of tuberculosis. Am. Rev. Respir. Dis. 142:725-735. [DOI] [PubMed] [Google Scholar]
- 2.Bradley, S. P., S. L. Reed, and A. Catanzaro. 1996. Clinical efficacy of the amplified Mycobacterium tuberculosis direct test for the diagnosis of pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 153:1606-1610. [DOI] [PubMed] [Google Scholar]
- 3.Catanzaro, A., and Conference Faculty. 1997. Rapid diagnostic tests for tuberculosis. What is the appropriate use? Am. J. Respir. Crit. Care Med. 155:1804-1814. [DOI] [PubMed] [Google Scholar]
- 4.Chin, D. P., D. M. Yajko, W. K. Hadley, C. A. Sanders, P. S. Nassos, J. J. Madej, and P. C. Hopewell. 1995. Clinical utility of a commercial test based on the polymerase chain reaction for detecting Mycobacterium tuberculosis in respiratory specimens. Am. J. Respir. Crit. Care Med. 151:1872-1877. [DOI] [PubMed] [Google Scholar]
- 5.Committee on Revision of Diagnostic Standards. 1969. Diagnostic standards and classification of tuberculosis. National Tuberculosis and Respiratory Disease Association of the U.S.A., New York, N.Y.
- 6.D'Amato, R. F., A. A. Wallman, L. H. Hochstein, P. M. Colaninno, M. Scardamaglia, E. Ardila, M. Ghouri, K. Kim, R. C. Patel, and A. Miller. 1995. Rapid diagnosis of pulmonary tuberculosis by using Roche AMPLICOR Mycobacterium tuberculosis PCR test. J. Clin. Microbiol. 33:1832-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunlap, N. E., J. Bass, P. Fujiwara, P. Hopewell, C. R. Horsburgh, M. Salfinger, and P. M. Simone. 2000. Diagnostic standards and classification of tuberculosis in adults and children. Am. J. Respir. Crit. Care Med. 161:1376-1395. [DOI] [PubMed] [Google Scholar]
- 8.Kawamura, M., N. Sueshige, K. Imayoshi, I. Yano, R. Maekura, and H. Kohno. 1997. Enzyme immunoassay to detect antituberculous glycolipid antigen (anti-TBGL antigen) antibody in serum for diagnosis of tuberculosis. J. Clin. Lab. Anal. 11:140-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koga, H. 1996. Clinical usefulness of molecular techniques for diagnosis of mycobacteriosis. Kekkaku 71:687-692. (In Japanese.) [PubMed] [Google Scholar]
- 10.Koga, H. 1997. Clinical evaluation of a reagent for detection of DNA of mycobacterium tuberculosis complex using the ligase chain reaction (LCR) method. J. Jpn. Assoc. Infect. Dis. 71:1246-1251. [DOI] [PubMed] [Google Scholar]
- 11.Maekura, R., Y. Okuda, M. Nakagawa, T. Hiraga, S. Yokota, M. Ito, I. Yano, H. Kohno, M. Wada, C. Abe, T. Toyoda, T. Kishimoto, and T. Ogura. 2001. Clinical evaluation of anti-tuberculous glycolipid immunoglobulin G antibody assay for rapid serodiagnosis of pulmonary tuberculosis. J. Clin. Microbiol. 39:3603-3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sato, K. 1993. Comparison of BACTEC and Ogawa media for culture of mycobacteria from sputum specimens. Kekkaku 68:345-349. (In Japanese.) [PubMed] [Google Scholar]
- 13.Stauffer, F., R. Mutschlechner, P. Hasenberger, S. Stadlbauer, and H. Schinko. 1995. Detection of Mycobacterium tuberculosis complex in clinical specimens by a commercial polymerase chain reaction kit. Eur. J. Clin. Microbiol. Infect. Dis. 14:1046-1051. [DOI] [PubMed] [Google Scholar]
- 14.Vuorinen, P., A. Miettinen, R. Vuento, and O. Hällström. 1995. Direct detection of Mycobacterium tuberculosis complex in respiratory specimens by Gen-Probe Amplified Mycobacterium Tuberculosis Direct Test and Roche Amplicor Mycobacterium Tuberculosis Test. J. Clin. Microbiol. 33:1856-1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallace, R. J., J. Glassroth, D. E. Griffith, K. N. Olivier, J. L. Cook, and F. Gordin. 1997. Diagnosis and treatment of disease caused by nontuberculous mycobacteria. Am. J. Respir. Crit. Care Med. 156:S1-S25. [DOI] [PubMed] [Google Scholar]