Abstract
At the heart of photosynthetic reaction centers (RCs) are pairs of chlorophyll a (Chla), P700 in photosystem I (PSI) and P680 in photosystem II (PSII) of cyanobacteria, algae, or plants, and a pair of bacteriochlorophyll a (BChla), P870 in purple bacterial RCs (PbRCs). These pairs differ greatly in their redox potentials for one-electron oxidation, Em. For P680, Em is 1,100–1,200 mV, but for P700 and P870, Em is only 500 mV. Calculations with the linearized Poisson–Boltzmann equation reproduce these measured Em differences successfully. Analyzing the origin for these differences, we found as major factors in PSII the unique Mn4Ca cluster (relative to PSI and PbRC), the position of P680 close to the luminal edge of transmembrane α-helix d (relative to PSI), local variations in the cd loop (relative to PbRC), and the intrinsically higher Em of Chla compared with BChla (relative to PbRC).
Keywords: electron transfer, photosystem, redox potential, special pair, electrostatic energy
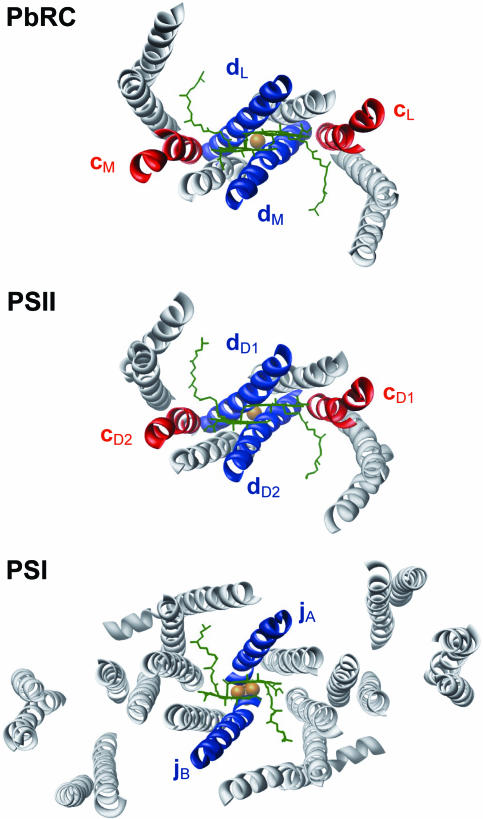
The essence of photosynthetic reaction centers (RCs) of photosystem I (PSI) and photosystem II (PSII) of cyanobacteria, green algae, and plants, as well as of purple bacterial RCs (PbRCs), are two homologous protein subunits (D1, D2) in PSII, (L, M) in PbRC, and the C-terminal RC domains of subunits (A, B) in PSI. The polypeptide chains of these subunits and the C-terminal domains of PSI are folded into five transmembrane α-helices (TMHs) in a semicircular arrangement, and the two subunits in each RC are interlocked in a handshake motif with comparable topography and related by a pseudo-twofold symmetry axis (Fig. 1).
Fig. 1.
Helices in RCs of PbRC, PSII, and PSI (view from the luminal side). Chlorophyll pairs are shown in green. RC subunits L, D1, A, M, D2, and B are shown in light gray. Helices d in PbRC/PSII and j in PSI (blue) provide axial ligands to PL/M/PD1/D2 and PA/B, respectively, which are indicated in black. Helices c (red) are shown in PbRC/PSII. The non-heme Fe in PbRC/PSII and the Fe4S4 cluster FX in PSI (orange) indicate the pseudo-C2 symmetry axis that is normal to the paper plane.
We consider here the pair of chlorophyll a (Chla) in PSI (Chla PA/B in P700) and in PSII (Chla PD1/D2 in P680) and the pair of bacteriochlorophyll a (BChla) in PbRC (BChla PL/M in P870), where light-driven charge separation results in positively charged radicals P700+·, P680+·, and P870+·, respectively. In PSI and PbRC, P700+· and P870+· are rereduced by small water-soluble proteins. By contrast, P680+· in PSII is rereduced by a redox-active tyrosine (D1-Tyr-161, YZ), which is subsequently reduced by electron transfer from the unique Mn4Ca cluster, where water is oxidized under release of atmospheric oxygen, protons, and electrons. Kinetic studies (1) and computations (2) yielded redox potentials for one-electron oxidation Em(P680) of 1,100–1,300 mV, high enough for P680+· to act as an electron acceptor for the different Mn4Ca redox states. According to recent studies, P680 probably consists of the Chla pair PD1/D2 or the two adjacent accessory Chla, ChlD1/D2 (3).
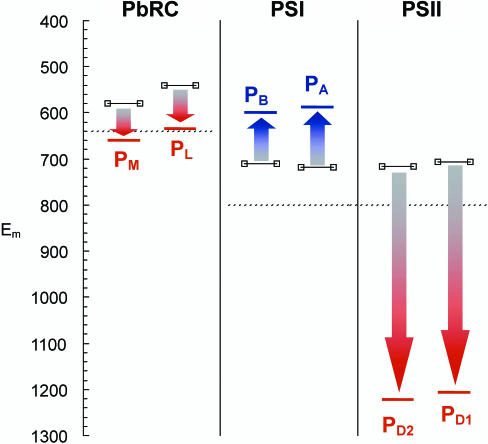
In contrast to PSII, with an unusually high Em(P680) of 1,100–1,300 mV (1, 2), the corresponding Em values in PbRC, Em(P870) = 500 mV (4), and in PSI, Em(P700) = 500 mV (5), are low. Part of these Em differences were associated with electronic coupling, which is weak between Chla in PD1/D2 but strong between Bchla in PL/M because of mutual overlap of BChla rings I. Indeed, in the PbRC mutant His(M202)Leu, where His-202 that coordinates BChla PM is lost, PM is replaced by bacteriopheophytin a, yielding a larger measured value of Em(PL) = 640 mV (6). A significant part of this Em difference (140 mV) may be due to absence of strong electronic coupling. In this regard, it is noteworthy that P700 in PSI features an Em of ≈500 mV (5), similar to Em(P870), whereas mutual overlap of Chla rings in P700 is absent in contrast to P870. Therefore, electronic coupling cannot explain the dramatic Em difference of 600 mV between P680 and P870/P700. Although BChla and Chla dissolved in CH2Cl2 exhibit Em(BChla) = 640 mV (7) and Em(Chla) = 800 mV (8, 9), respectively (see supporting information, which is published on the PNAS web site), there remains a gap of 440 mV between Em(P870) and Em(P680) that has to be explained.
Although nature uses the same type of cofactors (Chla) for PA/B in PSI and PD1/D2 in PSII, the protein environment modulates their Em such that Em(P700) ≈ 500 mV in PSI is ≈700 mV lower than Em(P680) = 1,200 mV in PSII. This redox potential difference is because in PSII, the oxidative power must be high enough to oxidize water with an Em of 820 mV, whereas in PSI and PbRC, high oxidative power is not needed but the reducing power of released electrons is maximized, and, simultaneously, oxidative damage of the protein environment due to positively charged dimer radicals is prevented, evident by the fact that Em is low for PA/B and PL/M. To elucidate this known but still unexplained difference in Em(P700), Em(P870), and Em(P680), we calculated Em in the RC of PSI, PbRC, and PSII by solving the linearized Poisson–Boltzmann equation for all atoms in the crystal structures (10–14) under identical computational conditions. Former theoretical work mainly contributed to unravel the energetics of the primary electron transfer events in PbRC [i.e., the relative energy of the P*B and P+B− states (15–18)]. The aim of the present study was to understand how nature invokes the dramatic differences in BChla and Chla redox potentials solely by the surrounding protein matrix in PbRC, PSI, and PSII.
Results and Discussion
Em(PL/M) in PbRC.
In WT PbRC, Em(P870) was measured to be 500 mV (4). We calculated averages of Em(PL) and Em(PM) for different crystal structures (10, 11) of WT PbRC from Rhodobacter sphaeroides and obtained Em(PL) = 635 ± 12 mV and Em(PM) = 660 ± 14 mV (Fig. 2). The experimentally observed larger spin density on PL [spin-density ratio ρ(PL)/ρ(PM) = 0.72/0.28 (19)] can be attributed to larger localization of the cationic state at PL, rendering Em(PL) < Em(PM). Although the calculated Em(PL) is slightly lower than Em(PM), the calculated Em difference is in the same range of error as derived from three different PbRC crystal structures. To explain the measured spin-density distribution on PL and PM, electronic and vibronic coupling must also be considered (20, 21). The present study provides Em only for monomer PL/M, without considering these influences.
Fig. 2.
Calculated Em(BChla) in PbRC and Em(Chla) in PSII (red horizontal bars) and PSI (blue horizontal bars). Dotted lines indicate the reference values measured for BChla and Chla dissolved in CH2Cl2: Em(BChla) = 640 mV (7) and Em(Chla) = 800 mV (8, 9). Horizontal bars with open squares at both ends refer to Em(BChla) and Em(Chla) calculated in protein dielectric volumes in the absence of atomic charges. The Em shift from uncharged protein dielectric volume to charged protein environment is indicated by the vertical arrows.
Because of large overlap of the BChla rings I in PL and PM, it was suggested that the π–π interactions of the BChla are strong in P870 of bacterial RC, whereas there is negligible overlap for the Chla in P700 of PSI (13). The corresponding pair in PbRC mutant His(M202)Leu (12) consists of BChla/bacteriopheophytin a (Bpheoa) at PL/M positions and is assumed to be a suitable model system that lacks electronic coupling between PL and PM. To estimate the electronic coupling effect on Em(P870), we calculated Em(PL) = 639 mV in the PbRC mutant His(M202)Leu (12), in excellent agreement with the measured value of 640 mV (6). In the present study, we calculated the Em for both monomer BChla in PL/M, without considering possible couplings between them (20, 21). Under these computational conditions, the electrostatic influence on BChla (PL) generated by BPheoa (PM) in mutant PbRC should be similar as that generated by BChla (PM) in WT PbRC, because both BChla and BPheoa have the same net charge of zero in their uncharged states. Replacement of PM ligand His at M202 by Leu deceases locally the polarity but not the charge. Hence, the effect on Em(PL) should be small. Thus, as suggested previously (6), Em(PL) in the PbRC mutant His(M202)Leu seems to refer to Em(PL) of the WT PbRC, ignoring possible coupling between PL and PM.
Em of RC Chlorophylls in PSI and PSII.
Our computations for PSI yielded Em(PA/B) = 587/599 mV (Fig. 2), ≈100 mV higher than the measured Em(P700) = 500 mV (reviewed in ref. 5) that we ascribe to neglect of electronic coupling. In PSI, the calculated Em(A−1 A/B) for the accessory Chla are 833/815 mV (supporting information), ≈220–250 mV higher than those calculated for PA/B.
In contrast, in PSII, the calculated Em(PD1) = 1,206 mV and Em(PD2) = 1,222 mV for the Chla pair are slightly lower than the respective values Em(ChlD1) = 1,262 mV and Em(ChlD2) = 1,320 mV for the accessory Chla (Fig. 4), indicating that the charge-separated state in PSII is stabilized with positive charge localized at PD1/D2 rather than at the accessory ChlD1/D2 showing >40 mV higher Em (22).
Main Contributions to the 600-mV Em Difference Between PD1/D2 in PSII and PA/B in PSI.
Peripheral protein subunits up-shifting Em(PD1/D2) by 200 mV.
Upon removal of all protein subunits except for the D1/D2 subunits of PSII harboring the RC, the calculated Em(PD1/D2) is down-shifted to 1,032/1,019 mV (Fig. 4), indicating that 170–200 mV of the 600-mV difference between Em(PD1/D2) and Em(PA/B) originates from the atomic charges and protein dielectric volume of all PSII subunits except for D1/D2. The protein volume is defined as the volume obtained by merging the volumes of the van der Waals spheres of all protein atoms by using charmm atomic radii. In protein dielectric volume, a homogeneous dielectric continuum of εp = 4 is considered. In the D1/D2/CP43/CP47 core of PSII, the calculated Em(PD1/D2) is 1,096/1,093 mV, resulting in an up-shift of 64/74 mV relative to the D1/D2 core. These Em(PD1/D2) are still significantly higher than Em(PA/B) = 587/599 mV calculated for the native PSI complex [Em(PA/B) = 593/610 mV for the PsaA/PsaB core]. In the following two paragraphs, we focus on the D1/D2 core, the simplified PSII system.
Negligible discrimination from protein dielectric volume.
The influence of the dielectric environment of the protein that might be possibly lower in PSII than PSI was speculated to be a major factor of the high Em of P680 in PSII by Hasegawa and Noguchi (23). However, Rutherford and Faller (24) suggested that there is no reason to assume that the dielectric environment in PSII is different compared with the other RC. One of the remarkable findings of the present study is that the Em(Chla) values calculated by considering merely the protein dielectric volume (i.e., the space covered by the merged van der Waals volumes of protein atoms) and neglecting atomic charges do not differ greatly between PSII and PSI, in agreement with the latter suggestion (24) (Figs. 2 and 4). Thus, in contrast to the apparent structural difference (Fig. 1), the substantial influence of protein dielectric volume (i.e., protein shape) on Em(Chla) is essentially the same in both proteins.
Em difference of 400–450 mV due to atomic charges.
The majority of the 600-mV Em difference between PD1/D2 and PA/B originates from the protein atomic charges. They are responsible for a dramatic up-shift of 325/303 mV for Em(PD1/D2) in PSII, as opposed to a down-shift of 125/101 mV for Em(PA/B) in PSI. Hence, the atomic charge distribution of the proteins yield a net Em difference of 400–450 mV between PD1/D2 and PA/B (Table 1). In the following, we describe the details of atomic charge influences for bacterial RC, PSI, and PSII.
Table 1.
Direct influence of cofactor/protein charges on Em(BChla) in PbRC (L/M) and Em(Chla) in PSI RC (PsaA/PsaB) and PSII RC (D1/D2)
| Components of protein | PbRC |
PsaA/PsaB |
D1/D2 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | L |
B |
A |
D2 |
D1 |
|||||||
| BM | PM | PL | BL | A−1B | PB | PA | A−1A | Ch1D2 | PD2 | PD1 | Ch1D1 | |
| Cofactors, a | −13 | 7 | 1 | −15 | 21 | −57 | −83 | 27 | 103 | 123 | 237 | 206 |
| Mn4Ca cluster | — | — | — | — | — | — | — | — | 47 | 100 | 214 | 160 |
| Side chains, b | −38 | −19 | 35 | 47 | −121 | −84 | −85 | −123 | 48 | −12 | −135 | −85 |
| Backbone, c | 22 | 93 | 59 | 23 | 71 | 40 | 43 | 62 | 150 | 192 | 223 | 80 |
| Total, a + b + c | −29 | 81 | 95 | 55 | −29 | −101 | −125 | −34 | 301 | 303 | 325 | 201 |
Mn4Ca Cluster and Side Chains in the RC of PSII.
In PSII, the direct influence of cofactors, especially of the Mn4Ca cluster coordinated to D1, up-shift Em(PD1) and Em(ChlD1) by 214 and 160 mV, respectively (Table 1), whereas the up-shift of Em(PD2) and Em(ChlD2) is much smaller (100 and 47 mV, respectively). Charged side chains in PSII RC down-shift Em(PD1) by 135 mV but leave Em(PD2) essentially invariant (Table 1), thereby partially compensating influences from the Mn4Ca cluster. Indeed, to energetically adjust the positively charged Mn4Ca cluster on the D1 side in PSII, there are more acidic and less basic residues on the D1 side than on the D2 side. For a detailed discussion of side-chain influence, see supporting information. These data suggest that the combined influences of the Mn4Ca cluster and side chains yield smaller Em differences of ≈100 mV between PD1 and PD2 (Table 1).
Influences of the TMHs Harboring the Chlorophyll Pair in PSI and PSII.
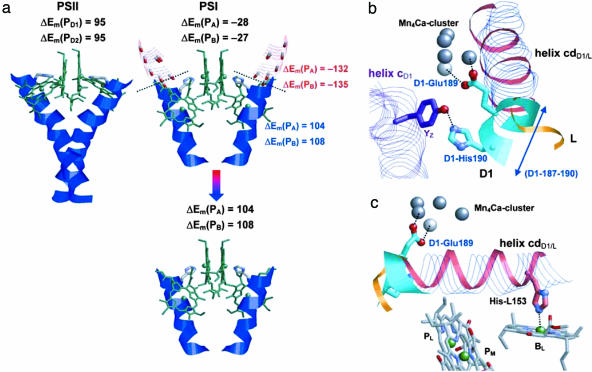
The up-shifts of Em(Chla) induced by protein backbone are significantly larger in the RC of PSII than of PSI (Table 1). The discussion below on PSII also holds true for PbRC. The strong influence of TMH dD1 in PSII, which up-shifts Em(PD1/D2) by 95 mV, is remarkable (supporting information). Notably, TMHs dD1/D2 provide the His-axial ligands to PD1/D2 (D1-His-198/D2-His-197). However, the corresponding TMHs j in PSI (PsaA 670–691/PsaB 650–671) engender down-shifts of Em(PA) and Em(PB) by 28 and 27 mV, respectively (Fig. 3a).
Fig. 3.
Specific protein components influencing the Chla pair redox potentials in PSI and PSII differently. (a) Different geometries of TMHs harboring His that axially coordinate PA/B in PSI (Right) or PD1/D2 in PSII (Left). Black type indicates Em shifts (ΔEm) due to the direct influence of backbone charges from the whole TMHs j or d on Em(PA/B) or Em(PD1/D2), respectively. ΔEm arising from the direct influence of backbone dipoles on removed and remaining parts of these TMHs are shown in red and blue type, respectively. (b) Arrangement of α-helix cdD1 on the D1 side in PSII relative to cdL in PbRC. The α-helices cd and c in PSII are shown by blue translucent ribbons, and in PbRC, they are shown by pink solid ribbons. The green turn cdD1-187–190 in PSII has no corresponding helix region in bacterial RC. The redox-active tyrosine YZ hydrogen-bonds to D1-His-190 and D1-Glu-189, which coordinate the Mn4Ca cluster. (c) Ligation of the accessory BCla (BL) in PbRC to His-L153 from α-helix cdL (solid green, pink, and orange ribbons). The corresponding His is absent in α-helix cdD1 of PSII (blue translucent ribbon and green turn with D1-Glu-189).
The TMHs d in PSII and TMHs j in PSI are of similar length, but the histidines that coordinate the Chla of PD1/D2 and PA/B are located at different positions. In PSII, these histidines are at the luminal ends of TMHs d, as opposed to their more central positions in TMHs j of PSI (red translucent parts of TMHs j in Fig. 3a). In TMH j of PSI upstream of these His ligands, there are still eight more residues (PsaA 670–677/PsaB 650–657) (red translucent ribbons in Fig. 3a) relative to the situation in PSII. The protein backbone dipoles of these eight residues in TMH j of PSI stabilize the PA/B+· charge state dramatically. After removing these eight residues, the remaining parts of TMHs j in PSI (blue solid ribbons in Fig. 3a) have a direct influence that up-shifts Em(PA) and Em(PB) by 104 and 108 mV, respectively. Similar up-shifts of 95 mV were computed as a direct influence originating from the entire TMH dD1/D2 for Em(PD1) and Em(PD2) in PSII (Fig. 3a). Hence, the charges of the structurally different parts of the TMH jA and jB backbone in PSI (red translucent ribbons in Fig. 3a) down-shift Em(PA/B) by ≈130–140 mV relative to Em(PD1/D2) in PSII (red numbers in Fig. 3a).
Influence of Luminal α-Helices cd on Em(PD1/D2) in PSII Relative to Em(PL/M) in PbRC.
The luminal α-helices cd and the segments connecting TMHs c and d in PSII (Fig. 3c) were proposed to play an important role in the energetics of P680+· (25–27). The α-helix cdD1 of PSII (D1-176–190) is four residues longer than the α-helix cdL of PbRC (L152–162) [i.e., D1-187–190 that up-shifts Em(PD1/D2) by 48/22 mV (Fig. 3b)]. There are other significant differences in this region between PbRC and PSII: (i) in PSII, D1-His-190/D2-His-189 (at or near the C termini of α-helices cdD1/D2) are H bond partners (D1-His-190 and D1-Glu-189) for the redox-active tyrosine YZ (D1-Tyr-161) located on TMH cD1 (Fig. 3b) and (ii) in PbRC, His-L153/His-M182 near the N termini of α-helices cdL/M are axial ligands for BChla of BChlL/M (Fig. 3c), whereas in PSII, the corresponding ChlD1/D2 possess no axial ligands. These structural differences in this region give rise to a difference of 90–110 mV between Em(PD1/D2) and Em(PL/M) (supporting information).
Conclusion
Em Difference of 600 mV Between PD1/D2 in PSII and PA/B in PSI.
The calculated Em(PD1/D2) for the complete PSII complex lies between 1,200 and 1,220 mV. Even for the D1/D2 RC alone, Em(PD1/D2) lies between 1,020 and 1,030 mV, which is still considerably high. Hence, the protein subunits peripheral to D1/D2 up-shift Em(PD1/D2) by 170–200 mV. This result contrasts with PSI, where the calculated Em(PA/B) in both the complete PSI complex and the RC formed by PsaA and PsaB lies between 590 and 600 mV. Elimination of the atomic charges in D1/D2 RC in PSII yields Em(PD1/D2) of 710–720 mV. Elimination of the atomic charges in the RC of PSI yields the same values for Em(PA/B) of 710 to 720 mV, indicating that the protein dielectric volumes of the RC in PSI and PSII do not give rise to a difference between Em(PA/B) and Em(PD1/D2).
The combination of charges of cofactors, side chains, and backbone in D1/D2 up-shifts Em(PD1/D2) by 300–330 mV, whereas the combination in the RC of PSI down-shifts Em(PA/B) by 100–130 mV (Table 1). As a consequence, the atomic charges in the protein environment give rise to a difference of 400–460 mV between Em(PA/B) and Em(PD1/D2). Specifically, the charges of the Mn4Ca cluster up-shift Em(PD1/D2) by 210/100 mV.
Relative to Em(PD1/D2), the protein backbone dipoles down-shift Em(PA/B) by 150–180 mV. Most remarkable are the different geometries of the TMHs that harbor the His-ligands for PD1/D2 (D1-His-198/D2-His-197) or PA/B (PsaA-His-680/PsaB-His-660). In TMH j of PSI, there are eight more residues (PsaA 670–677/PsaB 650–657) upstream of these His ligands relative to the situation in PSII. The protein backbone dipoles of these eight residues in TMH j of PSI stabilize the PA/B+· charge state dramatically, giving rise to a 130- to 140-mV down-shift in Em(PA/B) relative to Em(PD1/D2). In this regard, the TMH d in PSII and PbRC has the same influence on Em(PD1/D2) and Em(PL/M).
Em Difference of 600 mV Between PL/M in PbRC and PD1/D2 in PSII.
The calculated Em(PL/M) lies between 640 and 660 mV. This finding is consistent with the 640 mV measured for the Em(PL) of mutant His(M202)Leu of PbRC, which is generally assumed to yield the Em for the uncoupled monomers of the BChla pair (6). Thus, the measured Em(P870) in PbRC is lower by 140–160 mV than the computed value because of the neglect of electronic coupling between PL and PM in the latter case.
The peripheral subunits of D1/D2 in PSII up-shift Em(PD1/D2) by 170–200 mV, whereas no corresponding shift was found for PbRC that does not possess these subunits.
Relative to Em(PL/M), the protein backbone dipoles up-shift Em(PD1/D2) by 100–160 mV. The major part of this difference (90–110 mV) originates from the luminal cytoplasmic segments D1-176–195/D2-176–194 in PSII and L152–170/M179–199 in PbRC. The remaining 160 mV of the 600-mV difference between PSII and PbRC is due to the intrinsically different Em values of Chla and BChla.
Computational Procedures
Coordinates.
We used the crystal structures for PSI at 2.5-Å resolution (Protein Data Bank ID code 1JB0) (13) and PSII at 3.0-Å resolution (PDB ID code 2AXT) (14) from the thermophilic cyanobacterium Thermosynechococcus elongatus. For WT PbRC, we used the crystal structures of PbRC from R. sphaeroides at 2.65-Å resolution (PDB ID code 1PCR) (10), at 2.2-Å resolution (PDB ID code 1AIJ, the dark-adapted structure), and at 2.6-Å resolution (PDB ID code 1AIG, the light-exposed structure) (11). For the PbRC mutant His(M202)Leu, we used the crystal structure at 2.55-Å resolution (PDB ID code 1KBY) (12).
As described in previous applications (2, 28, 29), hydrogen atom positions were energetically optimized with charmm (30), keeping the positions of all nonhydrogen atoms fixed at crystallographically determined coordinates while all titratable groups were in their standard protonation states [i.e., acidic groups ionized and basic groups (including titratable histidines) protonated]. His residues that are ligands of Chla were treated as nontitratable with neutral charge. The cytochromes b559 and c550 in PSII were kept in the reduced state; the other redox-active cofactors were in the neutral charge states.
Atomic Partial Charges.
Atomic partial charges of the amino acids were adopted from the all-atom charmm22 (30) parameter set. For cofactors and residues whose charges are not available in charmm22, we used atomic partial charges from previous applications [PbRC (28), PSI (29), and PSII (2)].
For the Mn4Ca cluster, we used essentially the same charge model as previously (see ref. 2), where the previous crystal structures at 3.2-Å resolution (PDB ID code 1W5C) (31) and 3.5-Å resolution (PDB ID code 1S5L) (32) were used. Although the structure at 3.0-Å resolution (14) features an additional Ca2+ ion as a component of the Mn4Ca cluster as well as that at 3.5-Å resolution (32), the exact configuration of the Mn4Ca cluster remains unclear. In the previous computation for the structures at 3.2-Å and 3.5-Å resolution, despite their different atomic models, we used the same net charge of the Mn4Ca cluster for both structures. In the present study, although we assigned a charge of +2 to the newly determined Ca2+ ion, we used the same net charge of the Mn4Ca cluster as in ref. 2. All computations were performed in the S1 resting state of the Mn4Ca cluster with the corresponding charge distribution.
Computational Model for Chla/BChla.
The Chla of the dimer PA/B in PSI and of the pseudo-dimer PD1/D2 in PSII are ligated by histidines, whereas the accessory Chla (A−1A/B) in PSI and ChlD1/D2 in PSII are not. In case of the accessory Chla, we used the same atomic charges as in the previous studies for PSI (29) and PSII (2). The atomic charges for the His-ligated Chla for PD1/D2 and PA/B and the His-ligated BChla for PL/M and BChlL/M are listed in the supporting information.
Em(Chla) and Em(BChla) for one-electron oxidation have been experimentally measured in several solvents (reviewed in ref. 33). In the present study, we consider those measured in CH2Cl2 because only in CH2Cl2 are both Em(Chla) and Em(BChla) available. Em(Chla) was measured to be 800 mV (versus normal hydrogen electrode) in CH2Cl2 with tetrabutylammonium perchlorate as an electrolyte (8, 9). Taking into account the solvation energy difference between CH2Cl2 and water, we used the value of 698 mV as a reference Em for Chla in water (for details regarding the influence of electrolyte and water contamination, see supporting information). We evaluated the influence of a His ligand on Em(Chla) based on the calculated Em(Chla) of both model systems with and without His ligand and used the value of 585 mV as a reference Em for His-ligated Chla in water. For PA in PSI, Chla occurs as C132 epimer (Chla′) (34), for which experimental Em values are not available. Because of the increased steric energy between 132-methyl ester and 17-propionic ester, Chla’′ is thermodynamically slightly less stable than Chla (reviewed in ref. 35), which may result in a minor Em shift of, at most, a few tens of millivolts. Therefore, we used the same Em value as that used for conventional Chla. This approximation will not significantly affect the difference in Em(Chla) of ≈600 mV between PSI and PSII. Em(BChla) for one-electron oxidation was measured to be 640 mV (versus normal hydrogen electrode) in CH2Cl2 (7). As with Chla, we calculated the influence of the His ligand, yielding Em(BChla) = 427 mV as a reference Em for His-ligated BChla in water. For a discussion of Em((B)Chla) in different solvents, see ref. 33 and supporting information.
Computation of Em(Chla) and Em(BChla) in Proteins.
The computation of the energetics of the protonation pattern of titratable residues and cofactors in proteins is based on the electrostatic continuum model, in which the linearized Poisson–Boltzmann (LPB) equation is solved by the program mead from Bashford and Karplus (36). To sample the ensemble of protonation patterns by a Monte Carlo (MC) method, we used our own program, karlsberg (37, 38). The dielectric constant was set to εP = 4 inside the protein and εW = 80 for solvent and possible protein cavities. For evaluation of the dielectric constant in the protein, see supporting information. Crystal water could not be observed in the PSII structure at 3.0-Å resolution. All computations were performed at 300 K, pH 7.0, and an ionic strength of 100 mM. The LPB equation was solved by a three-step grid-focusing procedure with a starting, intermediate, and final grid of 2.5-, 1.0-, and 0.3-Å resolution. MC sampling yields the probabilities [Aox] and [Ared] of the oxidized and reduced states of the redox-active compound A, respectively. The Em(Chla) and Em(BChla) values in the protein environment were calculated from the Nernst equation. We varied the solvent potential such that we obtained an equal amount of both redox states ([Aox] = [Ared]) at the Em(A). For convenience, the computed Em values are given with millivolt accuracy, without implying that the last digit is significant. Systematic errors, which typically relate to specific conformations that may differ from the given crystal structures, can sometimes be considerably larger. Because the present study was performed under the same conditions as in previous computations, further details on error estimates and comparisons with the previous results can be obtained from refs. 2, 28, and 29.
Supplementary Material
Acknowledgments
We thank Dr. Dennis Diestler for improving the manuscript. This work was supported by Deutsche Forschungsgemeinschaft Sonderforschungsbereich 498 (Projects A4 and A5).
Abbreviations
- RC
reaction center
- PbRC
purple bacterial RC
- PSI
photosystem I
- PSII
photosystem II
- TMH
transmembrane α-helix
- Chla
chlorophyll a
- BChla
bacteriochlorophyll a.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Rappaport F., Guergova-Kuras M., Nixon P. J., Diner B. A., Lavergne J. Biochemistry. 2002;41:8518–8527. doi: 10.1021/bi025725p. [DOI] [PubMed] [Google Scholar]
- 2.Ishikita H., Loll B., Biesiadka J., Saenger W., Knapp E.-W. Biochemistry. 2005;44:4118–4124. doi: 10.1021/bi047922p. [DOI] [PubMed] [Google Scholar]
- 3.Groot M. L., Pawlowicz N. P., van Wilderen L. J. G. W., Breton J., van Stokkum I. H. M., van Grondelle R. Proc. Natl. Acad. Sci. USA. 2005;102:13087–13092. doi: 10.1073/pnas.0503483102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams J. C., Alden R. G., Murchison H. A., Peloquin J. M., Woodbury N. W., Allen J. P. Biochemistry. 1992;31:11029–11037. doi: 10.1021/bi00160a012. [DOI] [PubMed] [Google Scholar]
- 5.Brettel K. Biochim. Biophys. Acta. 1997;1318:322–373. [Google Scholar]
- 6.Allen J. P., Artz K., Lin X., Williams J. C., Ivancich A., Albouy D., Mattioli T. A., Fetsch A., Kuhn M., Lubitz W. Biochemistry. 1996;35:6612–6619. doi: 10.1021/bi9528311. [DOI] [PubMed] [Google Scholar]
- 7.Fajer J., Brune D. C., Davis M. S., Forman A., Spaulding L. D. Proc. Natl. Acad. Sci. USA. 1975;72:4956–4960. doi: 10.1073/pnas.72.12.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fajer J., Fujita I., Davis M. S., Forman A., Hanson L. K., Smith K. M. In: Electrochemical and Spectrochemical Studies of Biological Redox Components. Kadish K. M., editor. Vol. 201. Washington, DC: Am. Chem. Soc.; 1982. pp. 489–513. [Google Scholar]
- 9.Maggiora L. L., Petke J. D., Gopal D., Iwamoto R. T., Maggiora G. M. Photochem. Photobiol. 1985;42:69–75. [Google Scholar]
- 10.Ermler U., Fritzsch G., Buchanan S. K., Michel H. Structure (London) 1994;2:925–936. doi: 10.1016/s0969-2126(94)00094-8. [DOI] [PubMed] [Google Scholar]
- 11.Stowell M. H. B., McPhillips T. M., Rees D. C., Solitis S. M., Abresch E., Feher G. Science. 1997;276:812–816. doi: 10.1126/science.276.5313.812. [DOI] [PubMed] [Google Scholar]
- 12.Camara-Artigas A., Magee C., Goetsch A., Allen J. P. Photosynth. Res. 2002;74:87–93. doi: 10.1023/A:1020882402389. [DOI] [PubMed] [Google Scholar]
- 13.Jordan P., Fromme P., Witt H. T., Klukas O., Saenger W., Krauss N. Nature. 2001;411:909–917. doi: 10.1038/35082000. [DOI] [PubMed] [Google Scholar]
- 14.Loll B., Kern J., Saenger W., Zouni A., Biesiadka J. Nature. 2005;438:1040–1044. doi: 10.1038/nature04224. [DOI] [PubMed] [Google Scholar]
- 15.Creighton S., Hwang J. K., Warshel A., Parson W. W., Norris J. Biochemistry. 1988;27:774–781. [Google Scholar]
- 16.Parson W. W., Chu Z.-T., Warshel A. Biochim. Biophys. Acta. 1990;1017:251–272. doi: 10.1016/0005-2728(90)90192-7. [DOI] [PubMed] [Google Scholar]
- 17.Gunner M. R., Nicholls A., Honig B. J. Phys. Chem. 1996;100:4277–4291. [Google Scholar]
- 18.Zhang L. Y., Friesner R. A. Proc. Natl. Acad. Sci. USA. 1998;95:13603–13605. doi: 10.1073/pnas.95.23.13603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mattioli T. A., Williams J. C., Allen J. P., Robert B. Biochemistry. 1994;33:1636–1643. doi: 10.1021/bi00173a004. [DOI] [PubMed] [Google Scholar]
- 20.Johnson E. T., Müh F., Nabedryk E., Williams J. C., Allen J. P., Lubitz W., Breton J., Parson W. W. J. Phys. Chem. B. 2002;106:11859–11869. [Google Scholar]
- 21.Reimers J. R., Hush N. S. J. Am. Chem. Soc. 2004;126:4132–4144. doi: 10.1021/ja036883m. [DOI] [PubMed] [Google Scholar]
- 22.Dekker J. P., van Grondelle R. Photosynth. Res. 2000;63:195–208. doi: 10.1023/A:1006468024245. [DOI] [PubMed] [Google Scholar]
- 23.Hasegawa K., Noguchi T. Biochemistry. 2005;44:8865–8872. doi: 10.1021/bi050273c. [DOI] [PubMed] [Google Scholar]
- 24.Rutherford A. W., Faller P. Philos. Trans. R. Soc. London B. 2003;358:245–253. doi: 10.1098/rstb.2002.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manna P., LoBrutto R., Eijckelhoff C., Dekker J. P., Vermaas W. Eur. J. Biochem. 1998;251:142–154. doi: 10.1046/j.1432-1327.1998.2510142.x. [DOI] [PubMed] [Google Scholar]
- 26.Mulkidjanian A. Y. Biochim. Biophys. Acta. 1999;1410:1–6. doi: 10.1016/s0005-2728(98)00174-1. [DOI] [PubMed] [Google Scholar]
- 27.Keilty A. T., Vavilin D. V., Vermaas W. F. J. Biochemistry. 2001;40:4131–4139. doi: 10.1021/bi002772d. [DOI] [PubMed] [Google Scholar]
- 28.Ishikita H., Knapp E.-W. Proc. Natl. Acad. Sci. USA. 2005;102:16215–16220. doi: 10.1073/pnas.0503826102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishikita H., Knapp E.-W. J. Biol. Chem. 2003;278:52002–52011. doi: 10.1074/jbc.M306434200. [DOI] [PubMed] [Google Scholar]
- 30.Brooks B. R., Bruccoleri R. E., Olafson B. D., States D. J., Swaminathan S., Karplus M. J. Comput. Chem. 1983;4:187–217. [Google Scholar]
- 31.Biesiadka J., Loll B., Kern J., Irrgang K.-D., Zouni A. Phys. Chem. Chem. Phys. 2004;6:4733–4736. [Google Scholar]
- 32.Ferreira K. N., Iverson T. M., Maghlaoui K., Barber J., Iwata S. Science. 2004;303:1831–1838. doi: 10.1126/science.1093087. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe T., Kobayashi M. In: Chlorophylls. Scheer H., editor. Boca Raton, FL: CRC; 1991. pp. 287–303. [Google Scholar]
- 34.Watanabe T., Kobayashi M., Hongu A., Nakazato M., Hiyama T., Murata N. FEBS Lett. 1985;191:252–256. [Google Scholar]
- 35.Webber A. N., Lubitz W. Biochim. Biophys. Acta. 2001;1507:61–79. doi: 10.1016/s0005-2728(01)00198-0. [DOI] [PubMed] [Google Scholar]
- 36.Bashford D., Karplus M. Biochemistry. 1990;29:10219–10225. doi: 10.1021/bi00496a010. [DOI] [PubMed] [Google Scholar]
- 37.Rabenstein B. karlsberg, A Monte Carlo pH and Redox Titration of Proteins Program. Berlin: Free University Berlin; 1999. [Google Scholar]
- 38.Rabenstein B., Knapp E.-W. Biophys. J. 2001;80:1141–1150. doi: 10.1016/S0006-3495(01)76091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.