Abstract
In maturing T lineage cells, the helix–loop–helix protein E47 has been shown to enforce a critical proliferation and developmental checkpoint commonly referred to as β selection. To examine how E47 regulates cellular expansion and developmental progression, we have used an E2A-deficient lymphoma cell line and DNA microarray analysis to identify immediate E47 target genes. Hierarchical cluster analysis of gene expression patterns revealed that E47 coordinately regulates the expression of genes involved in cell survival, cell cycle progression, lipid metabolism, stress response, and lymphoid maturation. These include Plcγ2, Cdk6, CD25, Tox, Gadd45a, Gadd45b, Gfi1, Gfi1b, Socs1, Socs3, Id2, Eto2, and Xbp1. We propose a regulatory network linking Janus kinase (JAK)/signal transducer and activator of transcription (STAT)-mediated signaling, E47, and suppressor of cytokine signaling (SOCS) proteins in a common pathway. Finally, we suggest that the aberrant activation of Cdk6 in E47-deficient T lineage cells contributes to the development of lymphoid malignancy.
Keywords: E2A, Gfi, SOCS, Xbp1
Lymphocyte development is regulated, in part, by a distinct class of helix–loop–helix (HLH) proteins, named E proteins (1). Four E proteins, E12, E47, E2-2, and HEB, are expressed in developing lymphocytes. E12 and E47 are encoded by one gene, designated as E2A, and arise through differential splicing in an exon encoding the HLH domain. E2A-deficient mice exhibit a complete block in B cell development at the onset of lineage commitment (2–4). T cell development is partially blocked in E2A-deficient mice before the onset of T cell receptor (TCR) β V(D)J gene rearrangement (5–7).
E proteins, and E2A proteins in particular, play critical roles in regulating developmental progression at the pre-TCR and TCR checkpoints (8, 9). Signals emanating from the pre-TCR and TCR complex act to suppress E protein DNA-binding activity, induce the expression of the E protein inhibitor Id3, and lower the abundance of E47 protein levels (10). Lowering the dose of E47 has been demonstrated to release the block in differentiation and proliferation observed in thymocytes with defects in pre-TCR expression and pre-TCR-mediated signaling (8, 9, 11). The E proteins also function at the TCR checkpoint, because an E2A deficiency has been shown to accentuate positive selection (12). In contrast, positive selection in Id3-ablated mice is blocked, albeit partially (13). Taken together, these data suggest that the E2A proteins act as gatekeepers at the pre-TCR and TCR checkpoints, and passage through these checkpoints requires pre-TCR or TCR-mediated modulation of E2A activity.
E proteins also function as tumor suppressors. E2A-deficient mice rapidly develop thymic lymphoma (5, 14). Reintroduction of E2A activity into cell lines adapted from lymphomas that developed in E2A-ablated mice caused rapid apoptosis (15). In contrast, enforced expression of E47 in conjunction with Bcl-2 induced cell cycle arrest, suggesting a direct role for E2A in the suppression of cell growth (11). As a first approach to determine the mechanism by which E47 inhibits cellular proliferation and promotes developmental progression, we have used oligonucleotide microarrays to analyze the global patterns of gene expression in E2A-deficient lymphomas in the absence and presence of E47. Cluster analysis identified groups of genes that were regulated by E47 and shared biochemical or physiological properties. These included genes involved in cell survival, cell cycle progression, stress response, lipid metabolism, αβ, and natural killer T (NKT) cell function. Based on these observations, we suggest that E47 functions in molecular pathways to coordinate cell survival, cell growth, and developmental maturation. We identified cyclin-dependent kinase 6 (Cdk6) as an E47 target gene, providing a potential mechanism underlying a role for E47 in cell cycle progression. Socs1 and Socs3 were also regulated by E47, and we propose that E47 functions in a cytokine receptor-mediated regulatory network to regulate Socs gene expression. Interestingly, the regulation of two E47 targets, Hes1 and Gfi1, reflects a conserved regulatory network used in Drosophila melanogaster sensory organ development.
Results
Global Gene Expression Patterns in E2A-Deficient Lymphomas in Response to E47 Activity.
Our previous observations have indicated a critical role for the E2A proteins in cell survival, growth, and early thymocyte development (1). However, the mechanisms underlying E47-mediated control of proliferation and differentiation remain to be elucidated. As a first approach to this question, we have used oligonucleotide microarrays to identify genes regulated by E47 in E2A-deficient lymphoma cell lines. For this study, we used the 1F9 cell line, originally isolated from E2A-deficient thymomas (15). To identify E47 target genes, 1F9 cells were transduced with a retrovirus carrying an E47/estrogen receptor (E47ER) hybrid protein. Control cells were transduced with virus carrying the basic HLH region but lacking the N-terminal transactivation domain. Both retroviral constructs also directed the expression of human CD25 (hCD25) to allow rapid isolation of transduced cells (16). One day after infection, cells were incubated with 4-hydroxytamoxifen (4-OHT) for a period of 6 hours, to activate the E47ER fusion protein. hCD25-positive cells were purified by using magnetic beads, and RNA was isolated and used to generate probes for hybridization to murine oligonucleotide microarrays. Approximately 225 genes were identified whose transcript levels were modulated by the enforced expression of E47 (data not shown). The expression pattern associated with E47 expression appeared to reflect the role of E47 in cell cycle regulation and T lineage developmental maturation, as demonstrated by the induction of pTα, Hes1, Xbp1, Eto2, Gadd45a, Gadd45b, Ets2, Mef2b, Gfi1, Gfi1b, Id2, Socs1, Socs3, and Rorγ, as well as repression of Cdk6, Pou2af1, Icos, Gata3, Rorα, Foxo1, Lmo4, CD25, and Tox (Fig. 1). To verify the observations obtained from the microarray analysis, we have used quantitative PCR analysis to quantitatively determine transcript levels of CD25, Socs3, Gadd45a, GAdd45b, and Rorγ (Fig. 4, which is published as supporting information on the PNAS web site, and results not shown). The data point to a role for E47 in contributing to the transcriptional program that controls lymphoid development by acting upstream of transcriptional regulators known to play key roles in various proliferation and developmental pathways, including Hes1, Ets2, Gata3, Pou2af1, Foxo1, Xbp1, Rorγ, Gfi1, Gfi1b, and Tox.
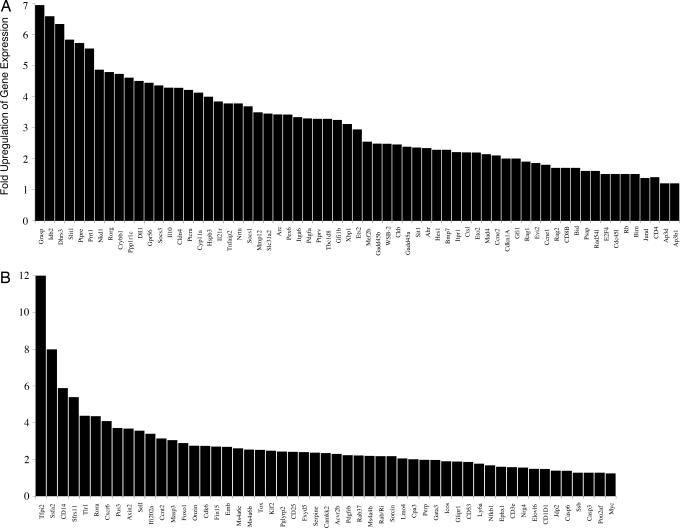
Fig. 1.
E47 target genes expressed in an E2A-deficient lymphoma cell line. The 1.F9 cell line was transduced with retrovirus encoding E47 or a truncated form of E47 encoding the basic HLH domain. RNA was isolated from transduced cells and analyzed for target gene expression by using microarray analysis. Selected activated and repressed target genes by the overexpression of E47 are depicted on the top (A) and bottom (B), respectively. Values indicated on the vertical axis show the fold induction or repression of E47 target transcript levels.
E47 Regulates the Expression of the Cell Cycle Regulator Cdk6.
To link genes that act together in a physiological pathway, hierarchical clustering analysis of gene expression patterns was performed on group samples according to their shared biochemical and functional pathways. Interestingly, this analysis revealed a large cluster of genes previously shown to play critical roles in cell cycle progression and cell survival. Among these were Cdk6, p21, retinoblastoma (Rb), cyclin E1, cyclin E2, and E2F4 (Table 1). The repression of Cdk6 transcription upon enforced expression of E47 was particularly striking. Specifically, Cdk6 transcript levels were reduced by a factor of 2-fold, 6 hours after tamoxifen treatment (Table 1). This result was further corroborated by quantitative PCR analysis (Fig. 4). Because Cdk6 activity acts to phosphorylate Rb to regulate cell cycle progression, we examined the phosphorylation status of Rb using Western blotting and Rb-specific antibodies. One day after induction, Rb phosphorylation significantly declined, consistent with cell cycle arrest of E2A-deficient lymphomas (R.S. and I.E., unpublished observations). E47 also induced the expression of p21, Rb, and E2F4, albeit modestly (Table 1). The growth arrest and DNA damage inducible genes, Gadd45a and Gadd45b, were also coordinately induced by E47 expression (Table 1 and data not shown). Taken together, these data indicate that a subset of genes involved in cell cycle progression are regulated by E47 in E2A-deficient lymphoma cells, most notably Gadd45 members and Cdk6.
Table 1.
Cluster of E47 targets potentially regulating cell growth in E2A-deficient lymphomas
| Identified target | Function | Fold modulation |
|---|---|---|
| Gadd45a | Growth arrest and DNA-damage-inducible 45 α | 2.4 |
| Gadd45b | Growth arrest and DNA-damage-inducible 45 β | 2.2 |
| Ccne2 | Cyclin E2 | 2.1 |
| Cdkn1A | Cyclin-dependent kinase inhibitor 1A (p21) | 2.0 |
| Ccne1 | Cyclin E1 | 1.8 |
| Cdc45L | Cell division cycle homolog (S. cerevisiae like) | 1.5 |
| Rb | Retinoblastoma gene | 1.5 |
| E2F4 | E2F transcription factor | 1.5 |
| Jund1 | Jun protooncogene related gene D1 | 1.3 |
| c-myc | Myelocytomatosis oncogene | −1.4 |
| Axin2 | Axin2 | −3.7 |
| Casp3 | Caspase 3 | −1.3 |
| Cdk6 | Cyclin-dependent kinase 6 | −2.6 |
| Bid | BH3-interacting domain death agonist | 1.7 |
| Bim | Bcl2-interacting mediator of cell death | 1.5 |
| Casp 6 | Caspase 6 | −1.4 |
| Perp-pending | p53 apoptosis effector related to PMP22 | −2.0 |
E47 Modulates the Expression of Genes Involved in Cell Survival, Lipid Metabolism, and T Lineage Maturation.
E47 targeted the expression of genes involved in cell survival, including Bid, Bim, caspase-3, and caspase-6 (Table 1). Hierarchical clustering of E47 target signatures 6also revealed groups of genes that act during various stages in T lineage development. These include CD25, Rag1, Rag2, Socs1, Socs3, CD3ε, and CD8b (Table 2). Several transcriptional regulators that function to promote lymphoid development at various stages, including Gata3, Foxo1, Rorγ, Gfi1, Ets2, and Xbp1, were also among the immediate targets of E47 (Table 2). Interestingly, we found that clustering analysis showed gene expression signatures that grouped samples according to their shared roles in NKT cell development, including CD1d, AP3D, AP3B1, cathepsin, and prosaposin (Table 2). Furthermore, this analysis revealed that E47 induced the expression of a subset of genes involved in lipid biosynthesis, notably Plcγ2 (Table 3). Collectively, this analysis elucidated biochemical and functional pathways in which E47 acts to control cell cycle progression, cell survival, lipid biosynthesis, and T lineage maturation.
Table 2.
Cluster of E47 targets potentially regulating hematopoiesis and immune response
| Identified target | Function | Fold modulation |
|---|---|---|
| Rag1 | Recombination-activating gene 1 | 1.9 |
| Rag2 | Recombination-activating gene 2 | 1.7 |
| CD8b | CD8 antigen | 1.7 |
| CD3ε | CD3ϵ polypeptide | −1.6 |
| CD25 | Interleukin 2 receptor α | −2.7 |
| Rorγ | RAR-related orphan receptor γ | 4.3 |
| Socs3 | Suppressor of cytokine signaling 3 | 3.9 |
| Socs1 | Suppressor of cytokine signaling 1 | 3.5 |
| Xbp1 | X-box-binding protein 1 | 3.1 |
| Ets2 | E26 avian leukemia oncogene 2 | 3.0 |
| Mef2b | Myocyte enhancer factor 2B | 2.7 |
| Gfi1 | Growth factor independent 1 | 2.0 |
| Gfi1b | Growth factor independent 1b | 2.7 |
| Gata3 | GATA-binding protein 3 | −2.0 |
| Foxo1 | Forkhead box O1 | −2.9 |
| Ctsl | Cathepsin L | 2.2 |
| Psap | Prosaposin | 1.6 |
| AP3D | Adaptor-related protein complex 3, δ subunit | 1.2 |
| AP3B1 | Adaptor-related protein complex 3, β1 subunit | 1.2 |
| CD1D | CD1D1 antigen | −1.5 |
Table 3.
Cluster of E47 targets regulating genes involved in lipid metabolism
| Identified target | Function | Fold modulation |
|---|---|---|
| Cyp11a | Cytochrome P450 | 14.7 |
| Plcγ2 | Phospholipase Cγ2 | 2.8 |
| Dgke | Diacylglycerol kinase ε | 2.0 |
| Cerk | Ceramide kinase | 1.9 |
| Psap | Prosaposin | 1.6 |
| Pafah1B3 | Platelet-activating factor acetylhydrolase 1B | 1.5 |
| Gpam | Glycerol-3-phosphate acyltransferase | 1.5 |
| SC4mol | Sterol-C4-methyl oxidase-like | −1.4 |
| Cpt1β | Carnitine palmitoyltransferase 1 | −1.4 |
| Gm2a | Gm2 ganglioside activator protein | −1.8 |
| Gpld1 | Glycosylphosphatidylinositol phospholipase D1 | −11.8 |
| Sult2B1 | Sulfotransferase 2B | −41.9 |
E2A Proteins Are Required for the Complete Inhibition of CD25 Expression in Immature Single-Positive (ISP) and Double-Positive (DP) Thymocytes.
The data described above indicate that CD25 is repressed by E47. CD25 is expressed at relatively high levels in the double-negative thymocyte DN2 and DN3 compartments but is rapidly down-regulated during the maturation of murine thymocytes from the DN3 to DP stages, such that the vast majority of DP cells do not express detectable levels of CD25. The CD25 levels in E47-deficient DN2 and DN3 cells are similar to that of wild-type thymocytes, indicating that E47 activity is not essential to induce CD25 expression in the DN compartment. However, the data described above raise the possibility that E47 activity is required to repress CD25 transcription upon β selection. To investigate this possibility, we monitored the expression of CD25 in E2A-deficient thymocytes using flow cytometry. Interestingly, we observed that a large fraction of E2A-deficient ISP and DP thymocytes expressed low but significant levels of CD25 (Fig. 2A). These data suggest that E47 functions in both the ISP and DP cell stages to suppress CD25 expression.
Fig. 2.
Aberrant expression of CD25 levels in E47−/− thymocytes. (A) Thymocytes from E47−/− and +/− littermates were stained with antibodies against CD8a, CD4, TCRβ, and CD25 and analyzed by flow cytometry. Shown are the CD25 staining histograms for CD8highCD4low TCRβ-immature single-positive thymocytes (Upper Left), CD8highCD4high DP (Upper Right), CD4highCD8low CD4SP (Lower Left), and CD8highCD4lowTCRhigh mature CD8SP (Lower Right). Black tracings: E47−/−. Gray tracings: E47+/−. Solid tracings: anti-CD25 staining. Dotted tracings: isotype control staining. (B) Thymocytes from BrdU pulse-labeled E47−/− and E47+/− littermates were stained for CD8a, CD4, CD25, and either BrdU or CD69. Histograms depict CD25 fluorescence in CD8high CD4high BrdU+ (BrdU+ DPs, Left), total DPs (Center), and CD8highCD4highCD69high (CD69highDPs, Right). Black solid tracings: E47−/−. Gray dotted tracings: E47+/−.
To examine whether E47 regulated CD25 gene expression in DP thymocytes, we prepared RNA from E47-null, heterozygous, and wild-type DP cells that were purified by electronic sorting. We then used these RNA samples to generate cDNA and measured CD25 transcript levels using quantitative PCR. Strikingly, CD25 transcripts were found to be present at ≈8-fold higher levels in E47−/− DP thymocytes than in either the wild-type or heterozygous littermate samples (data not shown). These data suggest that E2A proteins repress CD25 in DP thymocytes largely through pretranslational mechanisms. Thus passage through the β selection checkpoint modifies E2A activity, leading to changes in the subset of genes regulated by E47.
These observations suggested that complete repression of CD25 in E2A-deficient thymocytes might be initiated during maturation within the DP stage. To test this hypothesis, we used flow cytometry-based strategies to analyze CD25 expression within both relatively immature and mature subsets of DP thymocytes from E47-null and heterozygous littermates. To define immature DP thymocytes, we injected mice with BrdU and performed thymus extraction. We then stained these BrdU-pulsed thymocytes with antibodies against CD4, CD8, CD25, and BrdU. Thymocyte proliferation largely ceases shortly after progression to the DP stage, thus a short pulse of BrdU will be incorporated only by the most immature DP thymocytes (17). We observed that nearly all BrdU+ DP thymocytes from E47−/− mice expressed relatively high levels of CD25 (Fig. 2B). To identify relatively mature populations of DP cells, we stained thymocytes using antibodies against CD4, CD8, and CD25 together with either anti-TCRβ or CD69. Thymocytes up-regulate both TCR and CD69 expression just before exit from the DP stage, thus the most mature fractions of the DP population can be identified by increased levels of either TCR or CD69. We observed that either TCR moderate/high or CD69+ DP thymocytes from E47-null mice expressed relatively low levels of CD25 (Fig. 2B and data not shown). These data demonstrate that maturation events occurring within the DP stage are necessary for the down-regulation of CD25 in E2A-deficient thymocytes.
The Expression of a Substantial Proportion of E47 Target Genes Is Modulated During the DN3 to DN4 Transition.
The data described above indicate that E47 is required to repress CD25 expression beyond the pre-TCR checkpoint. As a first approach to identify additional E47 target genes that are regulated during β selection, we have compared the programs of gene expression regulated by E47 in the 1F9 cell line with that of genes whose expression patterns are modulated during β selection (18). We observed 13 genes whose mRNA levels were significantly elevated in DN4 cells, whereas the transcript levels encoded by 74 genes were lower in DN4 cells (H.T.P., unpublished observations). Interestingly, ≈10% of the genes modulated in expression levels during the DN3 to DN4 cell stages were also regulated by E47 in E2A-deficient lymphomas (Fig. 5, which is published as supporting information on the PNAS web site). In addition to CD25, this common set of target genes included Gpr56, Dhrs3, Ptpre, Socs3, Hes1, Xbp1, L-selectin, and Rorγ. Taken together, these data indicate that the expression of a significant fraction of E47 target genes in E2A-deficient lymphomas is modulated during β selection.
Discussion
Previous observations have indicated a critical role for E47 in lymphocyte survival, growth, and developmental progression. Additionally, E47 has been demonstrated to function as a tumor suppressor, because E47-deficient mice rapidly develop T cell lymphoma. These observations brought into question how E2A proteins regulate such a diverse set of cellular activities. The data presented here identify previously unrecognized E47 target genes that may provide insight into how E47 acts to regulate cell cycle progression, cytokine-mediated signaling, lipid metabolism, stress response, survival, and T lineage maturation.
We have shown that E47 is necessary to prevent the developmental progression of DN3 thymocytes in the absence of pre-TCR signaling, and that signaling from the TCR and pre-TCR complexes acts to down-regulate E47-binding activity (8, 10, 11). These data led us to conclude that TCR signals function largely to inhibit E47 activity, which in turn allows for thymocyte developmental progression. However, we now show that E47 activity is required to repress CD25 expression upon transition from the DN to DP cell stages. In addition, we also observed that L-selectin is down-regulated by E47 in 1F9 cells as well as during β selection. Furthermore, Rorγ transcripts, which are induced by E47 in 1F9 cells, are also up-regulated after passage of the β selection checkpoint. These observations necessitate that we revise our model of the effects of pre-TCR signaling on E2A protein activity (Fig. 3A). Although it is clear that pre-TCR signaling acts to relieve the inhibitory effects of E2A proteins on much of the differentiation program induced by β selection, it is now also apparent that signals arising as a consequence of pre-TCR expression elicit novel E protein activities.
Fig. 3.
Model diagrams indicating regulation and function of E47 activity. (A) Model diagram depicting the effects of pre-TCR signaling on E2A function. Signals from the pre-TCR complex abrogate the inhibitory effect of E2A proteins on the proliferation and differentiation of DN3 thymocytes to the DP stage. However, pre-TCR signaling also induces a function of E2A proteins in the down-regulation of CD25 expression. The asterisk indicates novel E-protein activities in response to pre-TCR signaling. Genes regulated by E47 in E2A-deficient lymphomas and during β selection are indicated. (B) Model diagram showing a regulatory network involving cytokine receptor-mediated signaling, E47, and SOCS activities in a common pathway. (C) Similarities of regulatory networks in D. melanogaster sensory organ development and T lineage cells.
We observed that E47 regulates a significant number of transcripts encoding factors involved in cell cycle progression. These include p21 and Cdk6. A role for E47 in modulating p21 expression has been described (19). However, lymphomagenesis in p21-deficient mice is rare and in fact appears to be promoted rather than being suppressed by p21 in ataxia telangiectasia mutated-deficient mice (20). Thus, it appears unlikely that E47 acts to prevent the development of lymphoma through the induction of p21 expression. A more attractive candidate is Cdk6. Cdk6 mRNA levels decreased 2-fold within a 6-hour period. Furthermore, we have observed that cells in which E47 activity has been enforced for 24 hours expressed increased levels of hypophosphorylated Rb, an effect consistent with a reduction in Cdk6 activity (data not shown). These data provide a possible mechanism for how E47 expression promotes cell cycle arrest (11).
These results bring into question whether the regulation of Cdk6 transcription by E47 plays a role in the development of human T-acute lymphoblastoid leukemia (T-ALL). The most common defect with human T-ALL is the activation of the TAL/SCL1 and TAL/SCL2 (TAL1 and TAL2, respectively) genes (21, 22). The TAL gene products are members of the HLH family and readily form heterodimers with E2A. They become aberrantly activated in T lineage cells upon somatic genomic rearrangement. We have previously suggested that the TAL protooncogenes act to promote human T-ALL by suppressing the ability of E2A to induce downstream target gene expression (5). Mouse models using E protein-deficient mice and transgenic mice expressing TAL1 in the thymocyte compartment have provided further support for this hypothesis and show interestingly common patterns of gene expression with E47 target genes, including RORγ, RAG2, and CD3ε (23–25). Our data also imply that TAL proteins may act to prevent the repression of Cdk6 by E47 as well, consistent with the presence of high-affinity E2A/Tal1-binding sites that are present in the Cdk6 promoter region. Thus TAL proteins may promote T-ALL by interfering with otherwise repressive effects of E2A proteins on Cdk6 expression, and it will be important to examine the potential role of Cdk6 in the development of human T-ALL.
Two modulators of E protein activity, Id2 and Eto2, were activated by E47 expression. Id2 functions by interfering with the DNA-binding activity of E47, whereas Eto2 acts to repress target gene expression upon interacting with the E47 N-terminal transactivation domain (26, 27). The activation of Id2 by E47 is reminiscent of the feedback mechanism regulating IκB expression by NF-κB, and it will be interesting to examine how E47, Id2, and Eto2 act in a regulatory network to control cell growth and cell fate (28).
The high mobility group box-containing transcription factor Tox, is also negatively regulated by E47 activity. Tox expression is transiently activated during both β and positive selection (29). Thymocyte development in transgenic mice overexpressing Tox shows significant similarities with that observed for E2A-deficient mice, raising the possibility that TCR-mediated signaling, E2A, and Tox are linked in a linear pathway (30).
Our microarray analysis showed that the transcriptional regulator Xbp1 is regulated by E47. Xbp1 functions to promote the development of activated B lineage cells into antibody-secreting cells, regulates the unfolded protein response, and promotes phospholipid biosynthesis (31, 32). Additionally, clustered analysis showed that E47 also modulates the expression of a significant subset of genes involved in lipid metabolism and cell growth. It is conceivable that during maturation of mature B lineage cells into plasma cells, E47 activity is modulated to induce the expression of Xbp1 and genes involved in lipid biogenesis to promote development of the plasma cell secretory apparatus. Likewise, it would be of interest to determine the role of Xbp1 during β selection.
Several genes that are coordinately, albeit modestly, regulated by E47 are involved in NKT cell function. Among these are CD1d, AP3D, AP3B1, prosaposin, and cathepsin L. AP3D and AP3B interact with the cytoplasmic tail of the CD1 proteins and function to deliver CD1B to late endosomes and lysosomes (33). Prosaposins act as endosomal lipid transfer proteins to edit Cd1b-bound lipid antigens (34). The lysosomal cysteine protease cathepsin L is essential to promote NKT cell development (35). The coordinate regulation of genes involved in NKT cell development and lipid metabolism is intriguing, and a significant role for E proteins in NKT cell development cannot be excluded.
Interestingly, the expression of Socs1 and Soc3 was substantially induced by E47 expression. SOCS proteins are feedback suppressors of the Janus kinase (JAK) and signal transducer and activator of transcription (STAT) signaling module. Cytokine signaling induces the expression of SOCS proteins that act in turn to antagonize further signaling. In vivo experiments have indicated that SOCS1 and SOCS3 act in response to IL-7, IL-6, IL-4, IL-12, IL-15, and granulocyte colony-stimulating factor (36). Our data indicate that E47 regulates the expression of SOCS proteins, and it will be particularly interesting to examine whether JAK/STAT signaling directly or indirectly activates E47 expression, which in turn may act to induce SOCS gene expression (Fig. 3B). E2A proteins have been demonstrated to play a critical role in the IL-7-dependent proliferation of pro-B lineage cells (37). Furthermore, modulation of IL-7 signaling has been shown to be a required step in B lineage commitment (38). It is conceivable that E47 acts in prepro-B and pro-B cells to regulate SOCS expression to modulate IL-7Rα-mediated signaling. Likewise, E proteins may promote the survival of IL-7-dependent developing double-negative thymocytes by modulating Socs gene expression (Fig. 3B).
The induction of Gfi1, Gfi1b, and Hes1 expression by E47 is intriguing, because a similar regulatory network regulates Drosophila sensory organ development (Fig. 3C). In D. melanogaster, sensory organ development is regulated in part by the proneural proteins, achaete and scute, that form heterodimers with daughterless, a protein closely related to E47, to regulate Enhancer of Split transcription (39). Similarly, our data demonstrate that E47 acts to induce Hes1 expression in T lineage cells. Sensory organ development also requires the activity of a zinc-finger-containing protein named Senseless. The expression of Senseless is regulated by the proneural genes that act as heterodimers with daughterless (40). Notably, here we demonstrate that two mammalian homologues of Senseless, Gfi1 and Gfi1b, are regulated by E47 in T lineage cells. A role for E47 in modulating the expression of Gfi1 was not unexpected, because Gfi1-null mutant mice show similar defects in thymocyte development as described for E47-deficient mice (12, 41). Taken together, these data indicate that an ancient regulatory network underlying Drosophila sensory organ development has been conserved in mammalian cells. It will be interesting to determine how this network acts in developing T cells to modulate developmental progression and cellular expansion.
Collectively, these data provide a conceptual framework in which E47 acts to regulate cell cycle regulation, cell survival, and developmental progression. They also suggest novel and unexpected functions for E47, including the control of lipid biosynthesis, cytokine-mediated signaling, and stress response.
Methods
Retroviral Infection and Purification of Transduced Cells.
Retroviral supernatant preparation and spin infection into the E2A−/− lymphoma line 1.F9 were performed as described (15). For preparation of 4-hydroxy tamoxifen-treated RNA samples, cells were spin-infected with E47-ER-Tac or basic HLH-ER-Tac virus and cultured for 20 hours in Iscove’s modified Dulbecco’s media without phenol red plus 5% FBS. The retrovirally transduced cells were then cultured for an additional 6 hours with or without 1 μM 4-hydroxytamoxifen before harvest and isolation of transduced cells by magnetic selection of human CD25 (Tac antigen), as described (16).
RNA Purification and Microarray Analysis.
Isolated RNA samples were converted into double-stranded cDNA. The resulting cDNA samples were then used to synthesize biotinylated cRNA, which in turn was hybridized to codelink mouse oligonucleotide arrays (Amersham Pharmacia). After hybridization and washing, the arrays were incubated with streptavidin-coupled fluorophore, washed, scanned, and analyzed using codelink analysis software. Expression levels from scanned images were determined by using corgon software (42, 43). Genes with P values for presence of ≤0.1 were considered for further analysis. Expression levels from all experimental conditions were normalized simultaneously by using the multi-loess technique, as described (43). We calculated the absolute and relative changes of the expression levels for every gene and sorted the genes based on their interest statistics. The interest statistic design was based on the software package focus (44).
Supplementary Material
Acknowledgments
We thank Jessica Novak for technical assistance and Carol Katayama for help with oligonucleotide design for quantitative PCR. This work was supported by National Institutes of Health grants to C.M. and H.T.P. and a Lymphoma Research Foundation fellowship to I.E.
Abbreviations
- DP
double positive
- DN
double negative
- HLH
helix–loop–helix
- Cdk6
cyclin-dependent kinase 6
- TCR
T cell receptor
- NKT
natural killer T
- T-ALL
T-acute lymphoblastoid leukemia.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Murre C. Nat. Immunol. 2005;6:1079–1086. doi: 10.1038/ni1260. [DOI] [PubMed] [Google Scholar]
- 2.Bain G., Robanus Maandag E., Izon D., Armsen D., Kruisbeek A., Weintraub B. C., Krop I., Schlissel M. S., Feeney A. J., van Roon M., et al. Cell. 1994;79:885–892. doi: 10.1016/0092-8674(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 3.Zhuang Y., Soriano P., Weintraub H. Cell. 1994;79:875–884. doi: 10.1016/0092-8674(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 4.Kee B. L., Quong M. W., Murre C. Immunol. Rev. 2000;175:138–149. [PubMed] [Google Scholar]
- 5.Bain G., Engel I., Robanus Maandag E. C., te Riele H. P. J., Voland J. R., Sharp L. L., Chun J., Murre C. Mol. Cell Biol. 1997;17:4782–4791. doi: 10.1128/mcb.17.8.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heemskerk M. H. M., Blom B., Nolan G, Stegmann A. P. A., Bakker A. Q., Weijer K., Res P. C. M., Spits H. J. Exp. Med. 1997;186:1597–1602. doi: 10.1084/jem.186.9.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barndt R. J., Dai M., Zhuang Y. Mol. Cell Biol. 2000;20:6677–6685. doi: 10.1128/mcb.20.18.6677-6685.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engel I., Johns C., Bain G., Rivera R. R., Murre C. J. Exp. Med. 2001;194:733–745. doi: 10.1084/jem.194.6.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim D., Xu M., L. Nie L., Peng X. C., Jimi E., Voll R. E., Nguyen T., Ghosh S., Sun X. H. Immunity. 2002;16:9–21. doi: 10.1016/s1074-7613(02)00264-9. [DOI] [PubMed] [Google Scholar]
- 10.Bain G., Cravatt C., Loomans C., Alberola-Ila J., Hedrick S. M., Murre C. Nat. Immunol. 2001;2:165–171. doi: 10.1038/84273. [DOI] [PubMed] [Google Scholar]
- 11.Engel I., Murre C. EMBO J. 2004;23:202–211. doi: 10.1038/sj.emboj.7600017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bain G., Quong M. W., Solow R., Hedrick S. M., Murre C. J. Exp. Med. 1999;190:1605–1616. doi: 10.1084/jem.190.11.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rivera R. R., Johns C. J., Quan J., Johnson R. S., Murre C. Immunity. 2000;12:17–26. doi: 10.1016/s1074-7613(00)80155-7. [DOI] [PubMed] [Google Scholar]
- 14.Yan W., Young A. Z., Soares V. C., Kelley R., Benezra R., Zhaung Y. Mol. Cell Biol. 1997;17:7317–7327. doi: 10.1128/mcb.17.12.7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engel I., Murre C. Proc. Natl. Acad. Sci. USA. 1999;96:996–1001. doi: 10.1073/pnas.96.3.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sayegh C. E., Quong M. W., Agata Y., Murre C. Nat. Immunol. 2003;4:586–594. doi: 10.1038/ni923. [DOI] [PubMed] [Google Scholar]
- 17.Penit C., Lucas B., F. Vasseur F. J. Immunol. 1995;154:5103–5113. [PubMed] [Google Scholar]
- 18.Tabrizifard S., Olaru A., Plotkin J., Fallahi-Sichani M., Livak F., Petrie H. T. J. Immunol. 2004;173:1094–1102. doi: 10.4049/jimmunol.173.2.1094. [DOI] [PubMed] [Google Scholar]
- 19.Prabhu S., Ignatova A., Park S. T, Sun X. H. Mol. Cell Biol. 1997;17:5888–5896. doi: 10.1128/mcb.17.10.5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cueva E. D., Garcia-Cao I., Herranz M., Lopez P., Garcia-Palencia P., Flores J. M., Serranto M., Fernandez-Piqueras J., Martin-Caballero J. Oncogene. 2006;6:1–5. doi: 10.1038/sj.onc.1209432. [DOI] [PubMed] [Google Scholar]
- 21.Begley C. C., Aplan P. D., Denning S. M., Haynes F., Wadman T. A., Kirsch I. L. Proc. Natl. Acad. Sci. USA. 1989;86:10128–10321. doi: 10.1073/pnas.86.24.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Q., Cheng J., Tsai N., Buchanan N., Schneider G., Carroll A., Crist W., Ozanne B., Siciliano M., Baer R. EMBO J. 1990;9:415–424. doi: 10.1002/j.1460-2075.1990.tb08126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Neil J., Shank J., Cusson N., Murre C., Kelliher M. Cancer Cell. 2004;5:587–597. doi: 10.1016/j.ccr.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 24.Ferrando A. A., Look A. T. Semin. Hematol. 2003;40:274–280. doi: 10.1016/s0037-1963(03)00195-1. [DOI] [PubMed] [Google Scholar]
- 25.Ferrando A. A, Neuberg D. S, Staunton J, Loh M. L, Raimondi S. C, Behm F. G, Pui C. H, Downing J. R, Gilliland D. G, Lander E. S, et al. Cancer Cell. 2002;1:75–87. doi: 10.1016/s1535-6108(02)00018-1. [DOI] [PubMed] [Google Scholar]
- 26.Massari M. E., Jennings P. A., Murre C. Mol. Cell Biol. 1996;16:121–129. doi: 10.1128/mcb.16.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J., Kalkum M., Yamamura S., Chait B. T., Roeder R. G. Science. 2004;305:1286–1289. doi: 10.1126/science.1097937. [DOI] [PubMed] [Google Scholar]
- 28.Hoffmann A., Levchenki A., Scott M. L., Baltimore D. Science. 2002;8:1241–1250. doi: 10.1126/science.1071914. [DOI] [PubMed] [Google Scholar]
- 29.Aliahmad P., Kaye J. Immunol. Rev. 2006;209:253–273. doi: 10.1111/j.0105-2896.2006.00345.x. [DOI] [PubMed] [Google Scholar]
- 30.Wilkinson B., Chen J., Han P., Rufner K. M., Goularte O. D., Kaye J. Nat. Immunol. 2002;3:272–280. doi: 10.1038/ni767. [DOI] [PubMed] [Google Scholar]
- 31.Relmold A. M., Iwakoshi N. M., Manis J., Vallabhajosyula P., Szommolany-Tsuda E., Friend D., Grusby M. J., Alt F., Glimcher L. Nature. 2001;412:300–307. doi: 10.1038/35085509. [DOI] [PubMed] [Google Scholar]
- 32.Sriburi R., Jackowski S., Mori K., Brewer J. W. J. Ce ll Biol. 2004;167:23–25. doi: 10.1083/jcb.200406136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moody D. B., Porcelli S. A. Nat. Rev. Immunol. 2003;3:11–22. doi: 10.1038/nri979. [DOI] [PubMed] [Google Scholar]
- 34.Zhou D., Cantu C., Sagiv Y., Schrantz N., Kulkarni A. B., Qi X., Mahuran D. J., Morales C. R., Grabowski G. A., Benlagha K., et al. Science. 2004;303:523–527. doi: 10.1126/science.1092009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Honey K., Benlagha K., Beers C., Forbush K., Teyton L., Kleijmeer M. J., Bendelac A. Nat. Immunol. 2002;3:1069–1074. doi: 10.1038/ni844. [DOI] [PubMed] [Google Scholar]
- 36.Chen P., Losman J. A., Rothman P. Immunity. 2000;13:287–290. doi: 10.1016/s1074-7613(00)00028-5. [DOI] [PubMed] [Google Scholar]
- 37.Seet C. S., Brumbaugh R. L., Kee L. J. Exp. Med. 2004;199:1689–1700. doi: 10.1084/jem.20032202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Purohit S. J., Stephan R. P., Kim H., Herrin B. R., Gartland L., Klug C. A. EMBO J. 2003;22:5511–5521. doi: 10.1093/emboj/cdg522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bailey A. M., Posakony J. W. Genes Dev. 1995;9:2609–2622. doi: 10.1101/gad.9.21.2609. [DOI] [PubMed] [Google Scholar]
- 40.Nolo R., Abbott L. A., Bellen H. J. Cell. 2000;102:349–362. doi: 10.1016/s0092-8674(00)00040-4. [DOI] [PubMed] [Google Scholar]
- 41.Yucel R., Karsunky H., Klein-Hitpass L., Moroy T. J. Exp. Med. 2003;197:831–844. doi: 10.1084/jem.20021417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sasik R., Calvo E., Corbeil J. J. Bioinformatics. 2002;18:1633–1640. doi: 10.1093/bioinformatics/18.12.1633. [DOI] [PubMed] [Google Scholar]
- 43.Sasik R., Woelk C. H., Corbeil J. J. Mol. Endocrinol. 2004;33:1–9. doi: 10.1677/jme.0.0330001. [DOI] [PubMed] [Google Scholar]
- 44.Cole S. W., Galic Z., Zack J. A. Bioinformatics. 2003;14:1808–1816. doi: 10.1093/bioinformatics/btg242. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.