Abstract
A competitive enzyme-linked immunosorbent assay (C-ELISA) which detects antibodies unique to rinderpest virus (RPV) has been developed. This test can differentiate antibodies against RPV and those against peste des petits ruminants virus. The recombinant RPV hemagglutinin (H)-protein C-ELISA (recH C-ELISA) is based on the ability of a well-characterized monoclonal antibody (MAb) produced with the soluble, secreted form of the H protein (Sec H protein) of RPV made in a baculovirus expression system to compete with the binding of RPV antibodies in the serum of vaccinated or infected, recovered animals to the Sec H protein. The B-cell epitope recognized by the MAb corresponds to amino acids 575 to 583 on the H protein, which is not present on the antigenically closely related peste des petits ruminants virus hemagglutinin-neuraminidase protein. Initially, a positive-negative threshold cutoff value for percent inhibition of 34 was established with 500 known RPV-negative serum samples. The recH C-ELISA was developed with the enzyme immunoassay software of a commercial RPV C-ELISA kit. Comparative analysis of the test results for 700 serum samples obtained with the commercial kit gave a sensitivity of 112.4% and a specificity of 72.4%. Variations in percent inhibition values were observed for the two assay systems. These variations may have been due to the undefined amount of antigen present in the commercial kit as well as the use of a different MAb. The recH C-ELISA detected more positive serum samples compared to the number detected by the commercial kit, with the results confirmed by a virus neutralization test. Thus, recH C-ELISA is a sensitive tool for RPV serosurveillance in disease eradication programs.
Rinderpest (RP), popularly known as cattle plague, is one of the oldest known acute and fatal viral diseases of domestic livestock like cattle, buffaloes, and wild ungulates of the order Artiodactyla. RP has been eradicated from most parts of the world by a vigorous policy of vaccination, seromonitoring, and serosurveillance and by slaughter and segregation programs. The disease is endemic in some parts of North Africa (southern Somalia and northeastern Kenya), South Asia (Pakistan, Afghanistan, and Yemen), and the Russian Federation (Amur region) (from the website of the Emergency Prevention System for Transboundary Animal and Plant Pests and Diseases, Food and Agriculture Organization [http://www.fao.org/waicent/faoinfo/agricult/agr/agah/empres/info/rinderp/pak99.htm]).
RP virus (RPV), the causative agent of RP, is a member of the genus Morbillivirus in the family Paramyxoviridae. The morbilliviruses, which comprise important vertebrate pathogens, form a closely related and serologically cross-reactive genus. RPV and peste des petits ruminants virus (PPRV) are two distinct but antigenically closely related morbilliviruses. PPR disease is widely distributed in sub-Saharan Africa, the Arabian Peninsula, and the Indian subcontinent (17, 18). Apart from protecting the bovine population against RPV infection, PPRV also causes subclinical infection in large ruminants, which act as carriers of infection to sheep and goats (2). RPV also infects small ruminants in India, often producing subclinical infection (5). This poses problems in the differentiation of the two diseases serologically, which is necessary in countries where both diseases coexist.
Differentiation of RPV and PPRV can be done by using cDNA probes derived from the nucleocapsid protein gene of each virus (6), as well as by competitive enzyme-linked immunosorbent assay (C-ELISA) with monoclonal antibodies (MAbs) directed against proteins of each virus (8). A C-ELISA which uses a MAb specific for the nucleocapsid protein of RPV was developed for detection of RPV antibodies in the sera of cattle and small ruminants (9). Unlike the virus neutralization test (VNT), the C-ELISA detects RPV-specific antibodies without showing a cross-reaction. A blocking ELISA method with two neutralizing MAbs has also been developed for the detection of PPRV-specific antibodies in caprine and ovine sera (15). This technique was developed because VNT, the only available serological test specific for PPRV and the cross-reactive RPV (14), is time-consuming and unaffordable for most laboratories in regions where both peste des petits ruminants and RP are endemic.
The surface glycoproteins of RPV induce virus-neutralizing antibodies in cattle (11, 13, 19), and hence, detection of these antibodies in sera is a direct measure of the immune status of the animals that have been vaccinated against or that have recovered from RPV infection. Since PPRV cross-reacts with RPV, the use of MAbs against the hemagglutinin (H) protein of RPV or the hemagglutinin-neuraminidase (HN) protein of PPRV which recognize a unique epitope on the H or HN protein would form the basis of a sensitive, differential serological test for the seromonitoring of animals for both viral infections. Anderson and McKay (3) have obtained unique MAbs and have developed a C-ELISA method for the differential diagnosis of these two viral infections. However, since this assay uses crude antigens derived from infected cell lysates, the method suffers from variations in the amount of H protein made in infected cells in different batches, often leading to inconsistent results.
In the present work, we have developed a recombinant RPV H-protein (recH) C-ELISA for RP serosurveillance which uses an extensively characterized RPV H-protein MAb (12) whose epitope on the H protein of RPV has been mapped and has not been found to be present on the PPRV HN protein. A baculovirus recombinant form of the H protein that does not anchor to the membrane was used as the antigen to take advantage of its relative purity, since the protein is secreted in serum-free medium when it is expressed in insect cells, and its expression could be maintained at a constant level.
MATERIALS AND METHODS
Cells and viruses.
Spodoptera frugiperda 21 (Sf-21) cells were grown in TC-100 insect cell culture medium (Gibco BRL, Gaithersburg, Md.) supplemented with 10% fetal bovine serum (Gibco BRL). Hybridoma cells were cultured in Iscove's modified Dulbecco's medium (Gibco BRL) supplemented with 10% fetal bovine serum. The method for the generation of a recombinant baculovirus that expressed the soluble, secreted form of the H protein (Sec H protein) has been described earlier (11), and the recombinant baculovirus was propagated in Sf-21 cells. Vero cells were cultured in Dulbecco's modified Eagle's medium (Himedia, Mumbai, India) supplemented with 5% fetal calf serum (Gibco-BRL). RPV (RBOK vaccine strain) was obtained from the Institute of Animal Health and Veterinary Biologicals, Bangalore, India, and PPRV Nig 75/1 was obtained from A. Diallo, Centre de coopération internationale en recherche agronomique pour le développement—Département d’élevage et de médecine vétérinaire, Montipellier, France; and both of them were propagated on Vero cells.
Serum samples.
Sera collected from 1,200 cattle were used in the present study. Of these, 700 serum samples were from animals that had been more than 4 years old when the sera were collected 2 months after vaccination with an RPV vaccine and 500 were from heifer calves ages 1 to 2 years that had been born after RPV vaccination had ceased. All these serum samples and 100 serum samples collected from sheep 2 months after vaccination were obtained from the national serum bank facility of the Project Directorate on Animal Disease Monitoring and Surveillance and were frozen at −20°C.
These serum samples are referred as field serum samples, since the samples were collected from animals vaccinated in the field during the seromonitoring phase of the National Project on Rinderpest Eradication and represent samples from animals from across India.
Preparation of Sec H protein.
Sf-21 cells were gradually adapted to serum-free medium (Sf-900 II SFM; Gibco-BRL). The cells were then infected with the recombinant baculovirus expressing the Sec H protein (11) at a multiplicity of infection of 10. The culture supernatant was collected after 72 h when a more than 90% cytopathic effect was observed. The supernatant was clarified by centrifugation at 4,000 × g for 30 min. The Sec H protein was titrated, aliquoted, freeze-dried, and stored at −20°C until further use in the C-ELISA.
RPV H -protein-specific MAb.
An RPV H-protein-specific MAb (MAb D2F4) was generated by using immunoaffinity-purified Sec H protein. MAb D2F4 maps to a region from amino acids 575 to 583 on the H protein which is unique to the RPV H protein and is an immunodominant epitope (12).
Other reagents.
An anti-mouse immunoglobulin horseradish peroxidase (HRP) conjugate was procured from Boehringer Mannheim (Mannheim, Germany); o-phenylenediamine dihydrochloride (OPD) was procured from Sigma Chemical Co. (St. Louis, Mo.), hydrogen peroxide was procured from Merck, (Mumbai, India), and 96-well Maxisorp ELISA plates were procured from Nunc (Roskilde, Denmark).
Reference assay kit.
An RPV C-ELISA kit developed at the Institute of Animal Health (Pirbright, United Kingdom) (3) and marketed by Biologicals Diagnostic Supplies Ltd. (Ayrshire, United Kingdom) was used as the reference kit in standardizing the recH C-ELISA. The software provided with the commercial kit was used for analysis of the test results.
Sensitivity and specificity of recH C-ELISA.
To calculate the sensitivity and specificity of the recH C-ELISA, we made use of the results for the 700 serum samples from vaccinated cattle obtained by both assays. The results obtained with the commercial kit were considered the “gold standard” (i.e., true positives and true negatives); and the following formulas were used to determine the sensitivity and specificity of the recH C-ELISA: sensitivity = (number of samples with positive results by recH C-ELISA/number of true-positive samples) × 100, and specificity = (number of samples with negative results by recH C-ELISA/number of true-negative samples) × 100.
Indirect ELISA.
ELISA plates were coated with lysates of Vero cells infected with RPV RBOK or PPRV Nig 75/1 at a concentration of 1 μg/well or with culture supernatants containing the Sec H protein (5 μg/ml; estimated by a standard method [10]) overnight at 4°C. The plate was washed in phosphate-buffered saline (PBS) thrice and blocked with blocking buffer (3% bovine gelatin plus 0.1% Tween 20 in PBS) for 1 h at 37°C. Serial twofold dilutions of the RPV H protein-specific MAb from ascitic fluid and 1:100 dilutions of PPRV-vaccinated sheep sera in PBS were used as the primary antibody, and the plates were incubated at 37°C for an hour. The plates were then washed, appropriately diluted anti-mouse whole immunoglobulin HRP and/or anti-goat immunoglobulin HRP were added, and the plates were incubated at 37°C for an hour. The reaction was developed by using OPD and H2O2, and the reaction was stopped with 2 N H2SO4. The plates were read at 490 nm in an ELISA reader.
C-ELISA.
The recH C-ELISA was performed essentially as described previously (3). Briefly, the baculovius recombinant Sec H protein of RPV prepared in serum-free medium was used as the antigen for the C-ELISA. Each well of a 96-well microtiter plate was coated with the antigen (5 μg/ml) at 4°C overnight and treated with blocking buffer (PBS supplemented with 0.1% [vol/vol] Tween 20 and 0.3% [vol/vol] normal bovine serum seronegative for RPV) for 1 h at 37°C. After the plate was washed five times in PBS (0.2× PBS), 50 μl of test serum was added in duplicate at a dilution of 1:100 in blocking buffer to the respective wells of the plate. Strongly positive, weakly positive, and negative bovine sera and a MAb with 0% competition were used as controls. MAb control wells (0% competition) contained antigen, MAb, and enzyme conjugate (no test serum). Following incubation at 37°C for 1 h on an orbital shaker, the plates were washed and anti-mouse immunoglobulin HRP conjugate (at a predetermined dilution) was added. After a final incubation, substrate and chromogen (OPD, H2O2) were added and the color was allowed to develop for 10 to 15 min. The absorbance values were read in an ELISA reader at 490 nm and analyzed with the software for an enzyme immunoassay (Biologicals Diagnostic Supplies Ltd.), and the optical densities (ODs) were converted to percent inhibition (PI) values by using the following formula: (100 − [OD in test well/OD in 0% competition well]) × 100.
Standardization of recH protein C-ELISA reagents and determination of cutoff PI value.
A checkerboard titration was carried out to establish the optimal working dilutions of antigen, serum, MAb, and immunoconjugate for use in the recH C-ELISA.
To establish a positive-negative threshold PI value, 500 serum samples from unvaccinated heifer calves (age, 1 to 2 years old) known to be negative for RPV were tested by the recH C-ELISA. The mean + 2 standard deviation × 2 PI value for these samples was considered the positive-negative cutoff value (16).
Determination of optimal MAb and serum dilutions.
Two serum samples (one strongly positive and one strongly negative) were chosen on the basis of their reactivities in tests with the reference kit. These sera were obtained as follows: anti-RPV H-protein-hyperimmune serum was made by injecting a single dose of recombinant H protein from extracellular RPV (500 μg) (11) without adjuvant into two RPV-seronegative crossbred Holstein × Friesian male calves (age, 1 year). The calves were tested serologically; and after 3 months, when the titer was high, a large amount of blood was collected and the serum was separated, aliquoted, and stored at −20°C. This undiluted, hyperimmune serum was considered the strongly positive serum control. Preimmune sera from the same calves were used as the negative control. The moderately positive control was prepared by appropriately diluting the hyperimmune serum in RPV-negative serum.
It has previously been determined for another morbillivirus C-ELISA (16) and for the reference kit used in the present study that serum dilutions of 1:10 to 1:20 compete well with the MAb. However, we observed that at a test serum dilution of 1:100 the recombinant antigen reacted well without background inhibitory activity. Therefore, for establishment of the optimal MAb dilution, the sera were tested at a fixed dilution of 1:100 against serial twofold dilutions of MAbs. Similarly, the optimal serum dilution was determined by testing serial twofold dilutions of the sera against the optimal MAb dilution. The PI values thus generated by the C-ELISA were plotted against the MAb dilution and the serum dilution. The optimal dilution of each reagent was determined to be the highest dilution that yielded the maximum differential in PI values between positive and negative sera.
Comparison of recH C-ELISA and reference kit.
The 700 serum samples from vaccinated animals were tested for the RPV antibody by using both the reference kit and the newly developed recH C-ELISA. The results of both assays were read by using the software for an enzyme immunoassay to determine whether each sample was positive or negative (cutoff PI values, 50 for the reference kit and 34 for the recH C-ELISA).
Field validation of recH C-ELISA.
A field validation of the prototype assay was undertaken by five laboratories, which are located in different states in India. These laboratories were supplied with the prototype kit containing coded field bovine serum samples (10 positive and 30 negative) for validation.
Serum neutralization assay.
A few of the field test serum samples which were found to be positive only by the recH C-ELISA and negative with the commercial RPV C-ELISA kit were tested for their abilities to neutralize RPV (RBOK strain) infectivity in vitro on Vero cells by a standard VNT method (4). Development of a cytopathic effect was monitored by light microscopy, and the titers were expressed as the reciprocal of the highest dilution of serum which neutralized virus infectivity by 50%.
RESULTS
Optimal dilutions of reagents for recH C-ELISA.
The ability of MAbs to compete with serum antibody depends not only on the specificities of their binding to antigens but also on the relative amounts of these reagents in the reaction mixture. It was thus important to titrate both of these critical reagents to determine the combination that yielded the best discrimination between positive and negative control sera. A MAb dilution of 1:5,000 and a test serum dilution of 1:100 were determined to be optimal. The 1:100 dilution identified RPV-positive and -negative samples on par with the commercial kit with the least background activity compared to that obtained with a dilution of 1:10, which was previously used in a C-ELISA with an infected cell extract as the antigen (3, 16).
A Sec H protein dilution of 1:150 and an anti-mouse conjugate dilution of 1:2,000 were determined to be optimal, while the control sera was used at a dilution of 1:10, similar to the dilution used in the reference kit. The control sera used in the recH C-ELISA were reanalyzed at a dilution of 1:10 by using the reference kit. The strongly positive serum had a PI value of 84, and the negative serum had a PI value of 10. The moderately positive control serum was prepared by mixing 1 part anti-RPV H protein immune serum in 3 parts negative serum and had a PI value of 60.
Determination of cutoff PI value for recH C-ELISA.
The positive-negative cutoff PI value of 34 was arbitrarily set by adding 2 standard deviations to the mean PI for 500 serum samples from heifer calves ages 1 to 2 years (Fig. 1).
FIG. 1.
Establishment of a positive-negative cutoff point for the C-ELISA. Each bar represents the PI values for negative serum samples grouped in classes of 10 samples each. The mean PI value for all negative serum samples (n = 500) was 16 + (2 standard deviations) 0.9 × 2 = 34 and was considered the cutoff PI value (vertical dotted line).
As our goal was also to differentiate RPV antibodies from PPRV antibodies, we also screened 100 serum samples from sheep vaccinated against PPRV (after confirmation of the presence of PPRV antibodies by indirect ELISA) for the absence of RPV antibodies by the recH C-ELISA. Use of a cutoff PI value of 34 also found that all PPRV-positive sera were negative for RPV antibodies, as desired, due to the unique MAb to the RPV H protein used as the competing antibody in the recH C-ELISA. Furthermore, this confirmed that the established cutoff PI value of 34 is appropriate for the recH C-ELISA.
Comparison of commercial C-ELISA kit and recH C-ELISA.
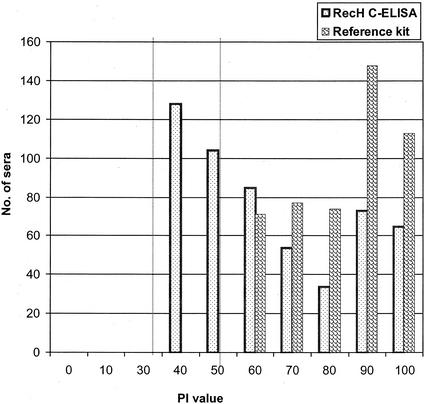
A comparative analysis of the commercial C-ELISA kit and the recH C-ELISA was carried out to establish diagnostic parameters like sensitivity and specificity and to study the distribution of PI values obtained by both assays, as the recH C-ELISA uses recombinant antigen and the H-protein MAb used in the recH C-ELISA is different from that used in the commercial kit. The results of both assays for 700 serum samples collected after vaccination indicated that 77.6% of the animals were serologically positive for RPV antibodies by the recH C-ELISA, while 69% of the animals were positive by use of the commercial kit; all 500 serum samples from unvaccinated animals were found to be negative by both the assays. The bar diagram depicting the PI values for the positive serum samples among the 700 serum samples screened by both assays indicated that there is a large degree of variation between these two assays, even though there was little qualitative variation in the test results (Fig. 2).
FIG. 2.
PI values for serum samples from vaccinated calves (n = 700) screened for the presence or absence of RPV antibodies both by the recH C-ELISA and with a commercial ELISA kit. Each pair of bars represents the comparative distributions of PI values obtained by both assays for only positive serum samples grouped in classes of 10 samples each. The vertical dotted lines represent the cutoff PI values for the recH C-ELISA and reference C-ELISA (left and right lines, respectively).
The serum neutralization assay was performed with RPV (RBOK strain)-infected Vero cells and 60 serum samples which were found to be positive exclusively by the recH C-ELISA. The results indicated that 45 of the 60 (75%) serum samples with various VN titers were capable of neutralizing virus infectivity (Fig. 3), while 8 were unfit for the assay and the remaining 7 did not neutralize virus infectivity.
FIG. 3.
Bar diagram showing the serum neutralization titers of RPV-positive serum samples positive by the recH C-ELISA and negative with the commercial C-ELISA kit.
DISCUSSION
RPV and PPRV infections occur in ruminant populations in East Africa, South Asia, sub-Saharan Africa, the Arabian Peninsula, and the Middle East (1, 7, 18). Serology is a major epidemiological tool used to detect RPV and PPRV infections in ruminant populations, in which the clinical symptoms of RPV and PPRV infections overlap those caused by the viruses that cause bovine viral diarrhea in bovines and foot and mouth disease and blue tongue in sheep and goats. Even virus isolation or confirmation of the presence of antigen is not always possible because of a lack of sophisticated laboratories to carry out such tasks.
The C-ELISA is a form of indirect ELISA being considered as the only efficient serological technique that can differentiate antibodies to antigenically closely related viruses by competitive inhibition of binding of unique epitope-specific MAbs to the antigen by sera from infected or vaccinated animals. The versatility of C-ELISA is that it can be applied to antibodies from any species, and even sera unfit for VNT can be tested. The availability of a simple, fast, reliable, and inexpensive test would provide a tool that various laboratories can readily use for diagnostic and epidemiological purposes. The recH C-ELISA described in the present work fits that role, and it also differentiates antibodies induced by RPV from those induced by PPRV.
The antigen (Sec H protein)-coated plates used for the recH C-ELISA retained their activities even after 1 year, without any degradation, which solved the inherent problem with the use of recombinant antigens in diagnostics, which are normally prone to rapid degradation upon repeated freezing-thawing and after longer periods of storage. This is the first report of the use of a baculovirus recombinant secretory antigen produced in insect cells for diagnostic purposes. As the antigen used in the assay described here can be quantified, batch-to-batch variations in the test results do not occur, as has been the case with the commercial kit, in which an infected cell lysate is used as the antigen, although its concentrations are not known. The results obtained by the recH C-ELISA, which uses a cutoff PI value of 34, and the reference kit, which uses a PI cutoff value of 50, are comparable. The epitope on the H protein for the MAb used in this work is from amino acids 575 to 583, is not present on PPRV HN, and is immunodominant (12). The uniqueness of the RPV H-protein-specific MAb was proven serologically by its ability to differentiate RPV antibodies from PPRV antibodies in the recH C-ELISA.
Even though the results of screening of 1,200 samples by both assays appear to be almost identical, the PI values for sera tested by both assays varied from 50 to 98%, which is due to the nature of the different antigens and the different MAbs used, which may result in variations in the amount of inhibition caused by field sera specific for the individual epitopes. Among the serum samples from vaccinated animals (n = 700), the recH C-ELISA detected larger numbers of animals carrying RPV antibodies, even when the test serum was diluted 1:100, compared to the number detected by the commercial kit, which uses a 1:10 dilution. Field validation of the performance of the recH C-ELISA in all six laboratories that participated in the validation of this assay gave consistent results (data not shown).
The recH C-ELISA detected 8.6% more positive serum samples than the commercial kit, and this enhanced sensitivity was further confirmed by selective screening of those 60 additional positive samples by VNT, which confirmed that 75% of them were true positives, with the virus neutralization titers ranging from 10 to 160. However, the actual lineage of the field virus strain is not known. The recH C-ELISA had a sensitivity of 112.4% and a specificity of 72.4%.
In order to further prove the efficiency of the recH C-ELISA, we used 18 serum samples from animals convalescing from RP from the Department of Biotechnology, Veterinary College, Chennai, India, and found that all of them reacted by both assays (the recH C-ELISA and the assay with the commercial kit). However, the actual lineage or serotype of the RPV in these serum samples is unknown. Similarly, satisfactory results were obtained with 38 convalescent-phase serum samples from animals infected with PPRV.
In conclusion, the recH C-ELISA developed in the present work could be used globally for RPV serosurveillance purposes in regions where the National Project on Rinderpest Eradication is still in existence. The task of serosurveillance could be better accomplished with a well-defined kit, such as the one described in the present work, along with other assays.
Acknowledgments
This work was supported by a grant from the National Project on Rinderpest Eradication, Government of India.
We thank deputy director general (animal science) and the assistant director general (animal health), ICAR, New Delhi, and the national project coordinator, National Project on Rinderpest Eradication, for help and cooperation.
REFERENCES
- 1.Abu-Elzein, E. M. E., M. M. Hassanein, A. I. Al-Afaleq, M. A. Abdelhadi, and F. M. J. Honsawai. 1990. Isolation of peste des petits ruminants from goats in Saudi Arabia. Vet. Rec. 127:309.. [PubMed] [Google Scholar]
- 2.Anderson, E. C., A. Hassan, T. Barrett, and J. Anderson. 1990. Observation on the pathogenicity for sheep and goats and the transmissibility of the strain of virus isolated during the rinderpest outbreaks in Sri Lanka in 1987. Vet. Microbiol. 21:309-318. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, J., and J. A. McKay. 1994. The detection of antibodies against peste des petits ruminants virus in cattle, sheep and goats and the possible implications to rinderpest control programmes. Epidemiol. Infect. 112:225-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrett, T., G. J. Belsham, S. M. Subbarao, and S. A. Evans. 1989. Immunization with a vaccinia recombinant expressing the F protein protects rabbits from challenge with a lethal dose of rinderpest virus. Virology 170:11-18. [DOI] [PubMed] [Google Scholar]
- 5.Barrett, T., and P. B. Rossiter. 1989. Rinderpest: the disease and its impact on humans and animals. Adv. Virus Res. 53:89-110. [DOI] [PubMed] [Google Scholar]
- 6.Diallo, A., T. Barrett, M. Barbron, S. M. Subbarao, and W. P. Taylor. 1989. Differentiation of rinderpest and peste des petits ruminants viruses using specific cDNA clones. J. Virol. Methods 23:127-136. [DOI] [PubMed] [Google Scholar]
- 7.Lefevre, P. C., A. Diallo, F. Schenkel, S. Hussein, and G. Staak. 1991. Serological evidence of peste des petits ruminants in Jordan. Vet. Rec. 1:28-110. [DOI] [PubMed] [Google Scholar]
- 8.Libeau, G., and P. C. Lefevre. 1990. Comparison of rinderpest and peste des petits ruminants viruses using anti-nucleoprotein monoclonal antibodies. Vet. Microbiol. 25:1-16. [DOI] [PubMed] [Google Scholar]
- 9.Libeau, G., A. Diallo, D. Calvez, and P. C. Lefevre. 1992. A competitive ELISA using anti-N monoclonal antibodies for specific detection of rinderpest antibodies in cattle and small ruminants. Vet. Microbiol. 31:147-160. [DOI] [PubMed] [Google Scholar]
- 10.Lowry, O. H., N. J. Rosebrough, A. J. Farr, and R. J. Randall,. 1951. Protein measurement with Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 11.Naik, S., and M. S. Shaila. 1997. Characterization of membrane-bound and membrane anchor-less forms of hemagglutinin glycoprotein of rinderpest virus expressed by baculovirus recombinants. Virus Genes 14:95-104. [DOI] [PubMed] [Google Scholar]
- 12.Renukaradhya, G. J., S. Mitra-Kaushik, G. Sinnathamby, M. Rajasekhar, and M. S. Shaila. 2001. Mapping of B-cell epitopes of hemagglutinin protein of rinderpest virus. Virology 298:214-223. [DOI] [PubMed] [Google Scholar]
- 13.Romero, C. H., T. Barrett, R. P. Kitching, V. M. Carn, and D. N. Black. 1994. Protection of cattle against rinderpest and lumpy skin disease with a recombinant capripoxvirus expressing the fusion protein gene of rinderpest virus. Vet. Rec. 135:152-154. [DOI] [PubMed] [Google Scholar]
- 14.Rossiter, P. B., D. M. Jesssett, and W. P. Taylor. 1985. Microneutralization systems for use with different strains of peste des petits ruminants virus and rinderpest virus. Trop. Anim. Health Prod. 17:75-81. [DOI] [PubMed] [Google Scholar]
- 15.Saliki, J. T., L. Libeau, J. A. House, C. A. Mebus, and E. J. Dubovi. 1993. Monoclonal antibody-based blocking enzyme-linked immunosorbent assay for specific detection and titration of peste des petits ruminants antibody in caprine and ovine sera. J. Clin. Microbiol. 31:1075-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saliki, J. T., and T. W. Lehenbauer. 2001. Monoclonal antibody-based competitive enzyme-linked immunosorbent assay for detection of morbillivirus antibody in marine mammal sera. J. Clin. Microbiol. 39:1877-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaila, M. S., D. Shamaki, M. A. Forsyth, A. Diallo, L. Goatley, P. Kitching, and T. Barrett. 1996. Geographic distribution and epidemiology of peste des petits ruminants viruses. Virus Res. 43:149-153. [DOI] [PubMed] [Google Scholar]
- 18.Taylor, W. P. 1984. The distribution and epidemiology of peste des petits ruminants. Prev. Vet. Med. 2:157-166. [Google Scholar]
- 19.Yilma, T., D. Hsu, L. Jones, S. Owens, M. Grubman, C. Mebus, M. Yamanaka, and B. Dale. 1988. Protection of cattle against rinderpest with infectious vaccinia virus recombinants expressing the HA or F gene. Science 242:1058-1061. [DOI] [PubMed] [Google Scholar]