Abstract
Multidrug resistance (MDR) presents a major obstacle for the successful chemotherapy of cancer. Its emergence during chemotherapy is attributed to a selective process, which gives a growth advantage to MDR cells within the genetically unstable neoplastic cell population. The pleiotropic nature of clinical MDR poses a great difficulty for the development of treatment strategies that aim at blocking MDR at the tumor cell level. Targeting treatment to the nonmalignant vascular network—the lifeline of the tumor—is a promising alternative for the treatment of drug-resistant tumors. The present study demonstrates thatMDRin cancer can be successfully circumvented by photodynamic therapy (PDT) using an antivascular treatment protocol. We show that, although P-glycoprotein-expressing human HT29/MDR colon carcinoma cells in culture are resistant to PDT with Pd-bacteriopheophorbide (TOOKAD), the same treatment induces tumor necrosis with equal efficacy (88% vs 82%) in HT29/MDR-derived xenografts and their wild type counterparts, respectively. These results are ascribed to the rapid antivascular effects of the treatment, supporting the hypothesis that MDR tumors can be successfully eradicated by indirect approaches that bypass their inherent drug resistance. We suggest that with progress in ongoing clinical trials, TOOKAD-PDT may offer a novel option for local treatment of MDR tumors.
Keywords: multidrug resistance, P-glycoprotein, photodynamic therapy, TOOKAD, antivascular therapy
Introduction
Resistance to chemotherapy with structurally and functionally unrelated drugs is attributed to a selection process that originates from the genetic instability of cancer cell populations—the primary target of treatment—along with the selection pressure applied by chemotherapeutic agents [1]. Clinical studies have shown that multidrug resistance (MDR) is often associated with poor patient prognosis, underscoring the urgency for overcoming this problem [2]. The ultimate objective of MDR research is to improve treatment outcome by developing strategies that prevent the emergence or circumvent existing MDR [3]. However, the classic approaches that inhibit drug efflux mediated by various adenosine triphosphate (ATP)-dependent MDR transporters, such as P-glycoprotein (Pgp) and others [4], were so far unsuccessful. The main complications in these strategies are: 1) pharmacokinetic interactions between the MDR inhibitor and the anticancer drug; 2) inhibition of the same transport systems in healthy tissues, causing multiple adverse effects [3]; and 3) failure to overcome MDR by focusing on a specific pathway due to the multifactorial nature of clinical resistance [2]. This situation strengthens the necessity of developing new strategies that do not target the malignant cells directly, but rather aim at destroying nonmalignant tumor components that are crucial for tumor survival and development. A key system for such an indirect targeting is tumor vasculature, which is critical for maintaining tumor growth and development. In addition, endothelial cells are genetically stable and unlikely to develop MDR [5]. Antiangiogenic [1,6] and antivascular [5] cancer therapies are examples for strategies that target the blood vessels, and thus may provide a bypass for MDR. Several antiangiogenic agents, mostly inhibitors of vascular endothelial growth factor (VEGF), are now under extensive investigation as anticancer agents [7]. There is also a clinical report about the treatment of refractory multiple myeloma patients with thalidomide, in which response to treatment is attributed to an antiangiogenic mechanism [8]. The exact mechanism of anticancer activity of thalidomide is still unclear and may be contributed, for example, by immunomodulation. In contrast to antiangiogenic therapy that prevents neovascularization, antivascular chemotherapy [9] or photodynamic therapy (PDT) performed with high sensitizer concentration in the circulation [10,11] targets existing tumor blood vessels, leading to their occlusion with subsequent hypoxia, necrosis, and consequent tumor destruction [5].
PDT is a binary treatment modality consisting of systemic administration of a nontoxic substance—the photosensitizer (drug)—and local illumination of the target tumor at a wavelength that matches the sensitizer absorption maximum. Upon photosensitization, the molecule is excited and, by energy transfer to oxygen or by free radical-forming mechanisms, generates cytotoxic singlet oxygen and/or other highly reactive oxygen species (ROS) within the tumor. Such ROS react rapidly with vital cellular components (such as membranes, cytoskeleton, and DNA), causing cellular damage and death [12]. One of the advantages of PDT is the ability to selectively deliver local treatment, thus avoiding injury of healthy tissues, which is the greatest problem of conventional chemotherapies and radiotherapies.
We have synthesized a novel family of photosensitizers, derived from the photosynthetic pigment bacteriochlorophyll [13,14], notably Pd-bacteriopheophorbide (TOOKAD) [14], presently found in clinical trials for prostate cancer therapy in collaboration with Steba Biotech (Toussus Le-Noble, France). In comparison with most clinically used photosensitizers, the bacteriochlorophyll-based photosensitizers, including TOOKAD, exhibit advantageous photochemical and pharmacological characteristics, namely: 1) high extinction coefficient in the near-infrared (IR) (ε0 ∼ 105 at 763 nm), enabling treatment of bulky tumors to a depth of 2 cm [10,13,15]; and 2) rapid clearance from the circulation (T0.5=0.6 minute) and skin (no phototoxicity at times >1 hour after treatment; Koudinova et al., 2002, unpublished), which minimizes skin phototoxicity. In addition, the PDT treatment protocol where the sensitizer and the light are simultaneously administered was shown in our laboratory to be an antivascular modality that selectively induces tumor blood vessel occlusion and stasis within minutes of illumination [16,17]. This protocol leads to the development of local ischemia, culminating with necrosis (24–48 hours) and ultimate tumor eradication (weeks) [10,11,15,18].
In the present study, we demonstrate that although human HT29/MDR colon carcinoma cells are resistant to TOOKAD-PDT in culture, the same treatment has equal efficacy when applied to the respective MDR xenografts and their wild type (WT) counterparts. This proof of concept suggests that by targeting the tumor vasculature, cancer therapies such as TOOKAD-PDT can overcome MDR and provide effective treatment for these malignancies.
Materials and Methods
Cultured Cells
Human isogenic HT29/WT and MDR cells were obtained from Prof. I. Z. Cabantchik (Hebrew University of Jerusalem) and cultured at 37°C in a humidified atmosphere containing 8% CO2. The MDR cells were maintained in the presence of 300 ng/ml colchicine [19], which was washed from the cultured MDR cells 24 hours before the experiments.
Animals
Male CD1 nude mice (28–32 g) were housed in The Weizmann Institute animal facility and all experimental procedures were conducted according to institute guidelines (1996).
Tumor Model
HumanWT and MDR HT29 cell monolayers were scraped in saline, washed, resuspended in saline, and injected subcutaneously (4–6 x 106 cells/0.07 ml per mouse) into the back of the mice. Tumors reached a treatment size (6–8 mm) within 2 (WT) and 4–5 (MDR) weeks. Mice bearing tumors (≥15 mm) were euthanized by anesthetic overdose.
Light Sources
Light source 1 A-home built 250-W halogen lamp focused through a 4-cm water filter fitted with a cutoff filter (λ<650 nm) was used for in vitro studies.
Light source 2: A 763-nm diode laser (1 W; CERAMOPTEC, Bonn, Germany) was used for in vivo studies.
Photosensitizer
In vitro studies TOOKAD, synthesized in our laboratory [14], was dissolved in ethanol immediately before use and further diluted to the final concentration in culture medium containing 1% ethanol.
In vivo studies TOOKAD (2.5 mg/ml) was administered in 5% Cremophor El-based formulation (NEGMA-LERADS, Toussus-Le-Noble, France).
PDT Protocol
In vitro studies Cells (4–6 x 104 per well) were plated in 96-well plates and cultured for 24 hours. The cells were then preincubated in the dark (4 hours) with the indicated TOOKAD concentrations, washed with fresh medium, and illuminated from below at a dose of 12 J/cm2. After illumination, the cells were placed in the culture incubator and cell survival was determined after 24 hours using the neutral red viability assay.
In vivo studies Mice, anesthesized by intraperitoneal injection of 40 µl of a mixture of ketamine (Rhone Merieux, Lyon, France) and xylazine 2% (Vitamed, Hedera, Israel) (85:15 vol:vol), were injected intravenously with 10 mg/kg TOOKAD, and the tumor was immediately illuminated (field φ=14 mm) at a dose of 90 J/cm2. Tumor response (using local necrosis on day 8 post-PDT as an endpoint) was photographically recorded and tumor volume was assessed [20].
Controls Dark control—cells or mice treated with TOOKAD but not illuminated. Light control—cells illuminated without incubation with TOOKAD, or mice intravenously injected with vehicle and illuminated. Untreated control—no light or drug (served as 100% cell survival for in vitro studies).
Histology
Tumors were excised from sacrificed animals, fixed in 4% formaldehyde in phosphate-buffered saline (PBS) at room temperature (RT; 48 hours), and paraffin-embedded. Sections were prepared and stained with hematoxylin/eosin (HNE) using standard protocols.
Immunohistochemistry
Cultured cells Cells were grown on coverslips (48 hours), washed with PBS, and briefly fixed (5 minutes, 2% paraformaldehyde at 4°C). Samples were then blocked (1 hour, 2% BSA, and 20% horse serum in PBS at RT). Cells were stained for Pgp by overnight incubation with 20 mg/ml monoclonal anti-human Pgp antibodies (4E3; Dako, Carpinteria, CA) at 4°C, followed by treatment with alkaline phosphatase (AP)-conjugated goat anti-mouse antibodies (Promega, Madison, WI) using fast red (Sigma, St. Louis, MO) as a color substrate.
Tumor sections Paraffin-embedded sections were deparaffinized with xylene and rehydrated by serial 5-minute incubations in 100%, 95%, and 70% ethanol and water. Endogenous peroxidases were inactivated by incubation in 3% H2O2 (Merck, Darmstadt, Germany), followed by blocking with 1% bovine serum albumin (BSA; Sigma) and 2% goat serum in PBS. Sections were incubated overnight (4°C) with 20 µg/ml 4E3 Pgp, or with polyclonal anti-HNE antibodies (1:500; Calbiochem, San Diego, CA). Sections were then treated (1 hour at RT) with goat anti-mouse or goat anti-rabbit peroxidase-conjugated antibodies (Jackson, ME) using 3-amino-9-ethylcarbazole (AEC; Sigma) as color substrate.
Cells and sections were counterstained with 0.1% hematoxylin (Sigma). In the negative control, the primary antibodies were omitted. All intermediate washes were performed with PBS.
Light microscopy was performed using a microscope (Nikon Optiphot 2; Nikon, Tokyo, Japan) equipped with a digital camera (DVC Company, Austin, TX).
Preparation of Cell and Tissue Extracts
Cell lysates Cells were washed twice with cold PBS, scraped in RIPA [20 mM Tris-HCl, 137 mM NaCl, 10% glycerol, 0.1% sodium dodecyl sulfate (SDS), 0.5% deoxycholate, 1% Triton X-100, 2 mM EDTA, pH 8.0, 1 mM PMSF, 20 µM leupeptin], sonicated for 10 seconds, centrifuged at 2 x 104g (15 minutes), and stored at -20°C until use. All steps were carried out on ice.
Tumor extracts Tumors were homogenized in RIPA. Homogenates were kept at 4°C (15 minutes) and centrifuged at 2 x 104g (10 minutes). The supernatant was collected and stored at -20°C until use.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and Immunoblot Analysis
Proteins (30–100 µg protein per lane) were separated on 7.5% SDS-PAGE and blotted onto nitrocellulose membranes. Membranes were blocked with 10% milk and 1% goat serum in PBS. Pgp was identified using monoclonal anti-human Pgp antibodies (C219, 1:500; Alexis Biochem, Montreal, Canada) and peroxidase-conjugated or APconjugated goat anti-mouse IgG as secondary antibodies. Bands were visualized using ECL (Santa Cruz Biotechnology, Santa Cruz, CA) or incubation with fast red, respectively. α-Tubulin was detected with monoclonal anti-α-tubulin antibodies (Sigma).
Results
Cultured HT29/MDR Cells Are Resistant to TOOKAD-PDT
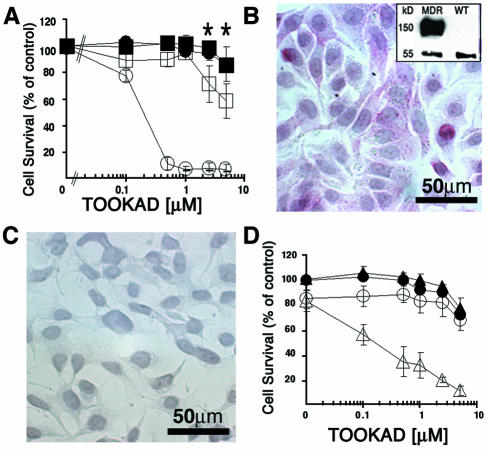
To demonstrate that the MDR phenotype renders HT29/MDR cells resistant to the direct effects of TOOKAD-PDT, we subjected cultured HT29/WT and MDR cells to TOOKAD-PDT in vitro. The WT cells responded to PDT in a TOOKAD concentrat ion-dependent manner (LD50=0.3 µM), whereas no response was seen in the absence of light (dark control) (Figure 1A). In contrast, the MDR cells did not respond to PDT under the same experimental conditions (LD50 >> 5 µM) (the apparent decrease in cell survival in the MDR/PDT group was statistically insignificant (P = .5) by two-way analysis of variance (ANOVA).
Figure 1.
Resistance of human HT29/MDR cells to TOOKAD-PDT. (A) Cells were preincubated in the dark with TOOKAD, washed, and illuminated. Closed circles, WT/dark; open circles, WT/PDT; closed squares, MDR/dark; open squares, MDR/PDT. The graphs represent the mean±SE of four independent experiments performed in triplicates. Cell survival is presented as a percentage of untreated control. *No significant difference between values (MDR/dark and MDR/PDT) by Fisher's LSD test. (B and C) Immunostaining for Pgp of cultured HT29 MDR and WT cells, respectively. (B, inset) Immunoblot of the respective cell lysates with anti-Pgp antibodies. Bands representing α-tubulin (55 kDa) and Pgp (170 kDa) are shown. (D) TOOKAD-PDT of HT29/MDR cells in the absence (closed squares, dark/VP-; open squares, PDT/VP-) or presence (closed triangles, dark/VP+; open triangles, PDT/VP+) of 50 µM VP. Cells were preincubated with VP for 30 minutes prior to the standard PDT protocol. The curves represent the mean±SE of three independent experiments performed in triplicates. Cell survival is presented as percent of untreated control.
The Pgp drug transporter is overexpressed in MDR cells [21]. To verify the presence of this marker in the HT29/MDR variants, we subjected cells to immunostaining, and cell lysates to SDS-PAGE and immunoblotting using monoclonal anti-Pgp antibodies. As expected, Pgp staining was observed in the MDR (Figure 1B, inset) but not in WT cells (Figure 1C). It was thus presumed that if the resistance to TOOKAD-PDT is indeed Pgp-dependent, then preincubation of the MDR cells with verapamil (VP), a known inhibitor of Pgp [22], should sensitize them to the cytotoxic treatment. Indeed, we found that VP-pretreated HT29/MDR cells responded to TOOKAD-PDT (Figure 1D), specifically confirming the role of the Pgp efflux pump in the resistance of these cultured MDR cells to TOOKAD-PDT.
MDR HT29 Xenografts Are Sensitive to PDT with TOOKAD
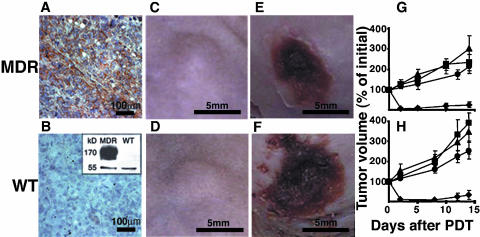
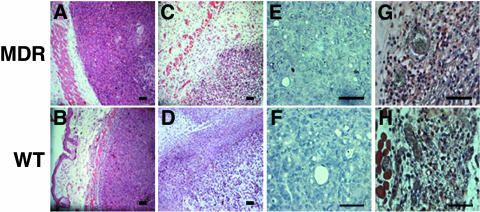
It was hypothesized that although MDR cells are resistant to PDT in vitro, they should be susceptible to TOOKAD-PDT-mediated ablation of blood vessels in vivo. To test this idea, we subcutaneously implanted MDR or WT HT29 cells in mice and examined the effect of TOOKAD-PDT on the tumors in vivo. To verify the maintenance of the MDR phenotype in the xenografts, we examined Pgp expression by immunohistochemical analysis of tumor sections (Figure 2, A and B) and by immunoblotting of tumor homogenates using anti-Pgp antibodies (Figure 2B, inset). Only tumors derived from MDR cells showed positive staining for Pgp, whereas the WT tumors were negative, illustrating that the MDR phenotype was indeed maintained in vivo. Tumors that reached treatment size (Figure 2, C and D) were subjected to TOOKAD-PDT and the response to treatment was monitored by clinical and histopathological means. Both variants responded positively, as judged by necrosis on day 8 post-PDT (Figure 2, E and F). Further follow-up of tumor size showed growth inhibition of both WT and MDR tumors by PDT (Figure 2, G and H). In contrast, tumors in the control groups continued to grow (Figure 2, G and H) Furthermore, the observed efficacy of treatment on the MDR (88.2%) and WT (82.4%) tumors was almost identical (Table 1). When also considering animals with incomplete response (necrosis covering only part of the tumor), these results amount to (16/17, 94.1%) and (17/17, 100%) respectively. Histopathological examination of both xenograft variants in comparison with untreated controls showed degenerative changes in the entire neoplastic population with clear-cut necrosis, including pyknosis and karyolysis in about 30–40% of the cells, 24 hours post-PDT [Figure 3, A and B (before PDT); Figure 3, C and D (24 hours after PDT)]. There were edema and mixed inflammatory infiltration in the surrounding tissues. Blood vessels were dilated and scattered necrotic debris was visible in the vicinity and within the walls of some dermal and subcutaneous vessels, away from the tumor together with extensive hemorrhage observed in both tumor types.
Figure 2.
Response of HT29 MDR and WT xenografts to TOOKAD-PDT. (A and B) Pgp immunostaining of MDR and WT xenografts. (B, inset) Immunoblot of respective tumor homogenates with anti -Pgp antibodies. Tubulin (55 kDa) and Pgp (170 kDa). (C and D) Tumors before and (E and F) 8 days after PDT. (G and H) Tumor growth curves starting from day of treatment (day 0). Diamonds, PDT; squares, light control; triangles, dark control; circles, untreated control. The number of mice per group was as follows: PDT (WT and MDR, n= 17 each), dark control (WT and MDR, n =6 each), untreated (WT and MDR, n= 4 each), and light control (WT, n= 8 and MDR, n =5). Bars represent mean±SE.
Table 1.
The Response of Human HT29 WT and MDR Xenografts to PDT with TOOKAD.
| Tumor Type | Number of Animals | Response (Necrosis on Day 8) | |
| Complete | Partial | ||
| MDR | 17 | 15 (88.2%) | 1 (5.9%) |
| WT | 17 | 14 (82.3%) | 3 (17.6%) |
Figure 3.
Histological sections of MDR and WT xenografts. (A and B) HNE staining before and (C and D) 24 hours after PDT. (E and F) HNE immunostaining before and (G and H) 24 hours after PDT. Bars= 50 µm.
One of the common features of PDT-induced damage is local lipid peroxidation (LPO), which can be assessed immunohistochemically using 4-hydroxy-2-nonenal (HNE) as marker [10]. Analysis of PDT-induced LPO indicates that the degree of photodamage was similar in both tumor variants (Figure 3, E–H). Thus, both clinically and histopathologically, MDR and WT tumors responded similarly to PDT. The response of the MDR tumor variant to TOOKAD-PDT is therefore consistent with the antivascular activity of this treatment, which targets the nonmalignant host blood vessels—the only common denominator of the two tumor variants.
Discussion
In this study, we hypothesized that the inherent resistance of HT29/MDR cells to PDT with TOOKAD can be effectively circumvented when the respective xenografts are treated with antivascular treatment protocol. To prove this point in a controlled manner, we first demonstrated that in culture, HT29/MDR cells exhibit resistance to TOOKAD-PDT, whereas the respective WT cells are fully responsive (Figure 1A). We secondly grafted the same cells to CD1 nude mice and demonstrated that TOOKAD-PDT induces necrosis in xenografts derived from resistant HT29/MDR and isogenic WT counterparts with equal efficacy (Figure 2 and Table 1). Pgp expression was positively correlated with the MDR phenotype of the cultured MDR cells (Figure 1B) and with resistance to TOOKAD-PDT (Figure 1A), which was abolished by the inhibition of TOOKAD efflux by VP (Figure 1D). However, Pgp expression in vivo (Figure 2, E and B, inset) did not correlate with the tumor phenotype, where the MDR tumors responded well to TOOKAD-PDT (Figure 2, E and G; Figure 3, C and G). This finding is consistent with the notion that the PDT protocol used here does not target the tumor cells, but rather the host-derived vasculature, which is identical in both tumors. PDT with TOOKAD, like its predecessor bacteriochlorophyll serine [11], was shown to eradicate solid tumors by vascular destruction and blood stasis [16,17].
Furthermore, we previously showed that the antitumor activity of TOOKAD-PDT is secondary to the photodynamic induction of local hypoxia and necrosis [10] and is likely to be independent of the tumor type. In contrast to TOOKAD, most other photosensitizers used in experimental and clinical cancer therapies were designed to selectively accumulate in and destroy the malignant cells and tumors upon illumination [23]. It is, therefore, not surprising that under culture conditions, PDT with sensitizers, such as Photofrin or copper benzochlorin iminium salt (CuBI), is generally [24,25], although not always [26], ineffective against MDR cells but effective against their parental WT variants, as is the case with TOOKAD. Although other sensitizers (Foscan, hypericine) were shown to exert vascular effects in animal models, the ability of this effect by itself to induce complete tumor eradication was not conclusive [27,28]. Verteporfin, however, is an agent designed as a specific antivascular modality and was introduced for treatment of age-related macular degeneration [29]. However, PDT with hydrophilic sensitizers such as monocationic porphyrin (MCP) and 5-aminolaevulinic acid (5-ALA) was found to be cytotoxic for both Pgp-expressing MDR and their parental WT variants in culture [30,31]. The sensitivity of MDR cells to these agents is thought to be because they are not recognized by Pgp. In agreement with these findings, the charged aluminum disulfonated phthalocyanine (AlS2Pc) was found to be effective against MDR and WT murine tumors [32], yet no specific mechanism was indicated. It appears that the response to PDT with classic sensitizers may vary with tumor type, the nature of the sensitizer, and the specific MDR mechanism involved, limiting their use in the treatment of MDR tumors. In contrast, antivascular PDT with TOOKAD is predicted to be independent of tumor type and MDR mechanism. To the best of our knowledge, however, no controlled in vivo studies with clinically relevant photosensitizers reported successful treatment of MDR tumors. One can anticipate that the abovementioned PDT agents bearing antivascular activity will behave similarly to TOOKAD in the treatment of MDR tumors depending on: 1) whether their sole antivascular action is sufficient to cause tumor eradication, as is the case for TOOKAD; and 2) if the respective MDR cells in culture are resistant to their photodynamic activity. The emergence of drug resistance in tumor therapy is a likely consequence of selection pressure promoted by chemotherapeutic drugs on the neoplastic component of the tumor, typically characterized by high mutation rates and genetic instability. In an attempt to develop strategies that circumvent this therapeutic risk, it was logical to approach the tumor indirectly by targeting its blood supply. Tumor-associated endothelial cells are nonmalignant and, as such, are less likely to develop resistance [1]. Their selective targeting by TOOKAD-PDT was, therefore, expected to succeed in cases where conventional cancer therapies failed.
Thus, results presented here support the hypothesis that by targeting the tumor vasculature, TOOKAD-PDT circumvents drug resistance of malignant tumor cells, permitting effective treatment of MDR tumors. Moreover, we presume that the brief, single, 10-minute PDT protocol itself is not likely to induce drug resistance.
In summary, this study suggests a new, promising strategy for effective treatment of drug-resistant tumors, based on targeting of the tumor blood vessels by TOOKAD-PDT. We hope that with progress in ongoing clinical trials, TOOKAD-PDT will also improve the treatment outcome for patients with MDR tumors and enable cancer treatment where conventional chemotherapy fails.
Acknowledgements
Y.S. is the incumbent of the Tillie and Charles Lubin Professorial Chair in Biochemical Endocrinology. M.L. is the incumbent of the Harold L. Korda Professorial Chair in Biology. A.S. is the incumbent of the Robert and Yadele Sklare Professional Chair in Biochemistry. All the studies described here were performed by D.P. in partial fulfillment of her MSc thesis requirements at the Feinberg Graduate School of The Weizmann Institute of Science. We thank Prof. Edna Schechtman for help in the statistical analysis and Dr. Ori Brenner for assisting with the histopathologic analysis. We also thank Dana Ravid for helpful discussions.
Footnotes
This work was supported by STEBA-BIOTECH (France).
Equal contribution.
References
- 1.Kerbel RS. Inhibition of tumor angiogenesis as a strategy to circumvent acquired resistance to anti-cancer therapeutic agents. Bio-Essays. 1991;13:31–36. doi: 10.1002/bies.950130106. [DOI] [PubMed] [Google Scholar]
- 2.Lehnert M. Clinical multidrug resistance in cancer: a multifactorial problem. Eur J Cancer. 1996;32A:912–920. doi: 10.1016/0959-8049(96)00069-x. [DOI] [PubMed] [Google Scholar]
- 3.Liscovitch M, Lavie Y. Cancer multidrug resistance: a review of recent drug discovery research. IDrugs. 2002;5:349–355. [PubMed] [Google Scholar]
- 4.Krishna R, Lawrence DM. Multidrug resistance MDR in cancer. Mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur J Pharm Sci. 2000;11:265–283. doi: 10.1016/s0928-0987(00)00114-7. [DOI] [PubMed] [Google Scholar]
- 5.Chaplin DJ, Dougherty GJ. Tumor vasculature as a target for cancer therapy. Br J Cancer. 1999;80:57–64. [PubMed] [Google Scholar]
- 6.Folkman J. Clinical application of research on angiogenesis. N Engl J Med. 1995;333:1757–1763. doi: 10.1056/NEJM199512283332608. [DOI] [PubMed] [Google Scholar]
- 7.Ferrara N, Alitalo K. Clinical applications of angiogenic growth factors and their inhibitors. Nat Med. 1999;5:1359–1364. doi: 10.1038/70928. [DOI] [PubMed] [Google Scholar]
- 8.Kneller A, Raanani P, Hardan I, Avigdor A, Levi I, Berkowicz M, Ben-Bassat I. Therapy with thalidomide in refractory multiple myeloma patients—the revival of an old drug. Br J Haematol. 2000;108:391–393. doi: 10.1046/j.1365-2141.2000.01835.x. [DOI] [PubMed] [Google Scholar]
- 9.Tozer GM, Kanthou C, Parkins CS, Hill SA. The biology of the combretastatins as tumour vascular targeting agents. Int J Exp Pathol. 2002;83:21–38. doi: 10.1046/j.1365-2613.2002.00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koudinova NV, Pinthus JH, Brandis A, Brenner O, Bendel P, Ramon J, Eschhar Z, Scherz A, Salomon Y. Photodynamic therapy with Pd-bacteriopheophorbide TOOKAD: successful in vivo treatment of human prostate small cell carcinoma xenografts. Int J Cancer. 2003;104:782–789. doi: 10.1002/ijc.11002. [DOI] [PubMed] [Google Scholar]
- 11.Zilberstein J, Schreiber S, Bloemers MCWM, Bendel P, Neeman M, Schechtman E, Kohen F, Scherz A, Salomon Y. Antivascular treatment of solid melanoma tumors with bacteriochlorophyll-serine-based photodynamic therapy. J Photochem Photobiol. 2001;73:257–266. doi: 10.1562/0031-8655(2001)073<0257:atosmt>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 12.Ackroyd R, Clive K, Brown N, Reed M. The history of photodetection and photodynamic therapy. Photochem Photobiol. 2001;74:656–669. doi: 10.1562/0031-8655(2001)074<0656:thopap>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 13.Scherz A, Feodor L, Salomon Y. Chlorophyll and bacteriochlorophyll derivatives, their preparation and pharmacological compositions comprising them. US Patent 5,650,292. 1997 [Google Scholar]
- 14.Scherz A, Salomon Y, Scheer H, Brandis A. Palladiumsubstituted bacteriochlorophyll derivatives and use thereof. US Patent Application No. 09/857,772, US allowed 2003. 1999 [Google Scholar]
- 15.Chen Q, Huang Z, Luck D, Beckers J, Brun PH, Wilson BS, Scherz A, Salomon Y, Hetzel FW. Preclinical studies in normal canine prostate of a novel palladium-bacteriopheophorbide WST09 photosensitizer for photodynamic therapy of prostate cancer. Photochem Photobiol. 2002;76:438–445. doi: 10.1562/0031-8655(2002)076<0438:PSINCP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 16.Gross S, Schertz A, Neeman M, Salomon Y. Photodynamic therapy PDT of solid tumors with Pd-bacteriopheophorbide WST09: functional imaging by blood oxygen level-dependant BOLD MRI; Proceedings of the 10th Scientific Meeting of the International Society for Magnetic Resonance in Medicine ISMRM, Honolulu, HI CD-ROM 2154; 2002. [Google Scholar]
- 17.Mazor O, Brandis A, Gross S, Hami R, Vakrat Y, Koudinova N, Gladysh E, Kostenitz G, Orenstien A, Salomon Y, Scherz A. Pd-bacteriopheophorbide TOOKAD, a novel anti-vascular agent for photodynamic therapy of tumors: in vitro and in vivo studies. The 3rd Congress of the Federation of Israel Societies for Experimental Biology, Book of Abstracts, Eilat, Israel. 2002;231 [Google Scholar]
- 18.Schreiber S, Gross S, Brandis A, Harmelin A, Rosenbach-Belkin V, Scherz A, Salomon Y. Local photodynamic therapy PDT of rat C6 glioma xenografts with Pd-bacteriopheophorbide leads to decreased metastases and increased animal cure compared with surgery. Int J Cancer. 2002;99:279–285. doi: 10.1002/ijc.10299. [DOI] [PubMed] [Google Scholar]
- 19.Breuer W, Slotki IN, Ausiello DA, Cabantchik IZ. Induction of multidrug resistance downregulates the expression of CFTR in colon epithelial cells. Am J Physiol. 1993;265:C1711–C1715. doi: 10.1152/ajpcell.1993.265.6.C1711. [DOI] [PubMed] [Google Scholar]
- 20.Gleave ME. Serum prostate specific antigen levels in mice bearing human prostate LNCaP tumors are determined by tumor volume and endocrine and growth factors. Cancer Res. 1992;52:1598–1605. [PubMed] [Google Scholar]
- 21.Gottesman MM. How cancer cells evade chemotherapy: Sixteenth Richard and Hinda Rosenthal Foundation Award Lecture. Cancer Res. 1993;53:747–754. [PubMed] [Google Scholar]
- 22.Tsuruo T, Iida H, Tsukagoshi S, Sakurai Y. Overcoming of vincristine resistance in P388 leukemia in vivo and in vitro through enhanced cytotoxicity of vincristine and vinblastine by verapamil. Cancer Res. 1981;41:1967–1972. [PubMed] [Google Scholar]
- 23.Macdonald IJ, Dougherty TJ. Basic principles of photodynamic therapy. J Porphyr Phthalocyanines. 2001;5:105–129. [Google Scholar]
- 24.Singh G, Wilson BC, Sharkey SM, Browman GP, Deschamps P. Resistance to photodynamic therapy in radiation induced fibrosarcoma-1 and Chinese hamster ovary multidrug resistant cells in vitro. Photochem Photobiol. 1991;54:307–312. doi: 10.1111/j.1751-1097.1991.tb02021.x. [DOI] [PubMed] [Google Scholar]
- 25.Kessel D, Hampton J, Fingar V, Morgan A. Tumor versus vascular photodamage in rat tumor model. J Photochem Photobiol B Biol. 1998;45:25–27. doi: 10.1016/S1011-1344(98)00155-9. [DOI] [PubMed] [Google Scholar]
- 26.Dellinger M, Moreno G, Salet C, Tapiero H, Lampidis TJ. Cytotoxic and photodynamic effects of Photofrin on sensitive and multidrug resistant Freund leukaemia cells. Int J Radiat Biol. 1992;62:735–741. doi: 10.1080/09553009214552691. [DOI] [PubMed] [Google Scholar]
- 27.Chen B, Roskams T, Witte PAM. Antivascular tumor eradication by hypericine-mediated photodynamic therapy. Photochem Photobiol. 2002;76:509–513. doi: 10.1562/0031-8655(2002)076<0509:atebhm>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 28.Cramers P, Ruevekamp M, Oppelaar H, Dalesio O, Baas P, Stewart FA. Foscan uptake and tissue distribution in relation to photodynamic efficacy. Br J Cancer. 2003;88:283–290. doi: 10.1038/sj.bjc.6600682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fingar VH, Kik PK, Haydon PS, Cerrito PB, Tseng M, Abang E, Wieman TJ. Analysis of acute vascular damage after photodynamic therapy using benzoporphyrin derivative BPD. Br J Cancer. 1999;79:1702–1708. doi: 10.1038/sj.bjc.6690271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kessel D, Woodburn K, Henderson BW, Chang CK. Sites of photodamage in vivo and in vitro by a cationic porphyrin. Photochem Photobiol. 1995;62:875–881. doi: 10.1111/j.1751-1097.1995.tb09150.x. [DOI] [PubMed] [Google Scholar]
- 31.Li W, Zhang WJ, Ohnishi K, Yamada I, Ohno R, Hashimoto K. 5-Aminolaevulinic acid-mediated photodynamic therapy in multidrug resistant leukemia cells. J Photochem Photobiol B Biol. 2001;60:79–86. doi: 10.1016/s1011-1344(01)00124-5. [DOI] [PubMed] [Google Scholar]
- 32.Canti G, Lattuada D, Morelli S, Nicolin A, Cubeddu R, Taroni P, Valentini G. Efficacy of photodynamic therapy against doxorubicin-resistant murine tumors. Cancer Lett. 1995;93:255–259. doi: 10.1016/0304-3835(95)03818-h. [DOI] [PubMed] [Google Scholar]