Abstract
We investigated whether the recombinant Borrelia Western blot test previously described (B. Wilske, C. Habermann, V. Fingerle, B. Hillenbrand, S. Jauris-Heipke, G. Lehnert, I. Pradel, D. Rössler, and U. Schulte-Spechtel, Med. Microbiol. Immunol. 188:139-144, 1999) can be improved by the addition of VlsE and additional DbpA and OspC homologues. By using a panel of sera from 36 neuroborreliosis patients and 67 control patients, the diagnostic sensitivity of the recombinant immunoblot test was significantly increased (86.1% versus 52.7%) without loss of specificity and was higher (86.1% versus 63.8%) than that of the conventional whole-cell lysate immunoblot test (U. Hauser, G. Lehnert, R. Lobentanzer, and B. Wilske, J. Clin. Microbiol. 35:1433-1444, 1997). Improvement was mainly due to the presence of VlsE and DbpA.
Diagnosis of Lyme borreliosis primarily depends on clinical symptoms and on serological findings. Though serological tests are widely used, they are still poorly defined regarding sensitivity, specificity, and standardization. In both the United States and Europe, a two-step approach is recommended by the Centers for Disease Control and Prevention and the German Society for Hygiene and Microbiology, respectively. The first step is a sensitive enzyme-linked immunosorbent assay (ELISA). In cases resulting in a reactive first test, a Western immunoblot test is performed (2, 11, 20). This implies that the immunoblot test must be highly reliable, with high specificity. In immunoblot tests using whole-cell lysate (conventional blot tests), reliable identification of diagnostic bands is very difficult due to difficulties in distinguishing specific and nonspecific reactivities of antigens with similar molecular weights. In contrast, evaluation of blot tests using recombinant selected proteins is reliable and easy. However, up to now the conventional blot test has been superior to the recombinant test in sensitivity (18).
In a previous study, Wilske et al. described the use of the following recombinant antigens for serodiagnostic immunoblot tests: p83/100 derived from strain PKo (Borrelia afzelii); p39 (BmpA) and OspC from strains PKa2 (B. burgdorferi sensu stricto), PBi (B. garinii, OspA-type 4), and PKo; p41i (internal flagellin fragment) from PKo and PBi; p58 derived from PBi; and Osp17 from PKo (18). In the present study, we investigated whether the additional use of three further recombinantly expressed highly immunogenic proteins, decorin binding protein A (DbpA) derived from B. garinii strain PBr (OspA-type 3), VlsE from B. burgdorferi sensu stricto strain PKa2, and OspC from B. garinii strain 20047, can improve the previously described recombinant immunoglobulin G (IgG) immunoblot test. VlsE, a recently detected lipoprotein of B. burgdorferi sensu lato, was shown to undergo antigenic variation (21). However, ELISA studies with American Lyme disease patients and a limited panel of European patients indicated that VlsE is a highly sensitive diagnostic antigen with conserved immunogenic epitopes (12, 14). DbpA is a major in vivo-expressed lipoprotein of B. burgdorferi sensu lato with high sequence heterogeneity (15). Therefore, and since neuroborreliosis in Europe is associated with B. garinii in 60 to 70% of cases (17), we wanted to investigate whether the use of DbpA from a B. garinii strain in addition to DbpA from a B. afzelii strain (formerly Osp17); (18) can improve the sensitivity of the recombinant immunoblot test in patients with neuroborreliosis. We also asked whether the sensitivity of the blot test can be improved by the use of an additional B. garinii OspC besides the OspC from strain PBi, since B. garinii OspCs are rather heterogeneous (17). Furthermore, results from the new recombinant blot test were compared with results from the conventional whole-cell lysate immunoblot test (5). In this study, sera from patients with early neuroborreliosis (neuroborreliosis stage II) were investigated, since a considerable fraction of these samples have been negative in the previous tests.
Cultivation and sources of strains PKa2, PBr, and 20047 as used in this study have been described previously (19). Cloning of the vlsE gene from strain PKa2 was performed using primer F4120 (5′-CGGGATCCAAGTTGCTGATAAGGACGACCC-3′) containing a BamHI restriction site and primer R4121(5′-CGGAAGCTTCAATCATGAGGGCATAGTCGTGTCCATACA-3′) with a HindIII restriction site (the BamHI and HindIII restriction sequences, respectively, are underlined). The amplified fragment (1,227 bp without a leader sequence) was ligated into BamHI- and HindIII-treated pQE30 vector, which contains a sequence encoding an N-terminal His6 tag. The recombinant plasmid was transformed into Escherichia coli SURE (Stratagene, Amsterdam, The Netherlands). Using the sequence of the dbpA gene of B. garinii PBr (GenBank accession no. AF069281) (15), we constructed a plus-strand primer, FdbpA-A1 (5′-GAGGGATCCATCATGGGCTTAACAGGAGAAACTAA-3′) (the recognition sequence for BamHI is underlined), and a minus-strand primer, RdbpA-B1 (5′-AAACTGCAGTTAATGGTGATGGTGATGGTGTGTAGTAGTAGCAGTTTTGGC-3′) (the recognition sequence for PstI is underlined; amplification using the sequence indicated with double underlining resulted in a C-terminal His6 tag). The resulting amplification product was 495 bp in length. The fragment was ligated into BamHI-PstI-treated pUHE21 vector, and the recombinant plasmid was transformed into E. coli XL1-Blue. Using standard ospC primers from our laboratory as described previously (9), the ospC gene from strain 20047 was amplified without a leader sequence. The expression of dbpA, vlsE, and ospC in recombinant E. coli XL1-Blue and SURE was induced by the addition of isopropyl-β-d-thiogalactopyranoside. VlsE and DbpA were purified using an FPLC system (Pharmacia Biotech, Freiburg, Germany). Recombinant proteins containing a His6 tag (DbpA and VlsE) were subjected to affinity chromatography on a NiSO4-loaded IMAC column (Fractogel EMD Chelat; Merck, Darmstadt, Germany) as described previously (10, 16). Recombinant OspC of strain 20047 was purified first by anion exchange chromatography (DEAE-Sepharose) and then by cation exchange (Fractogel SO3).
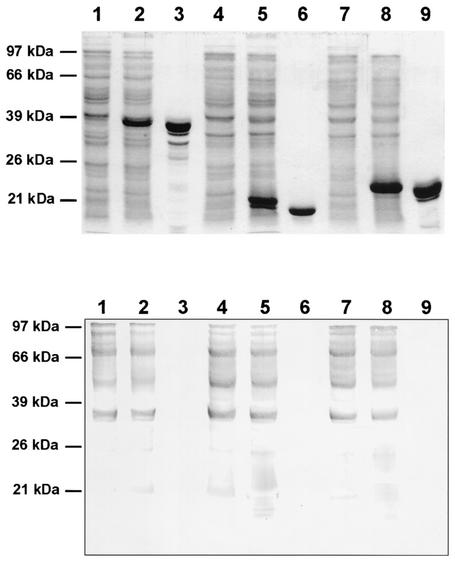
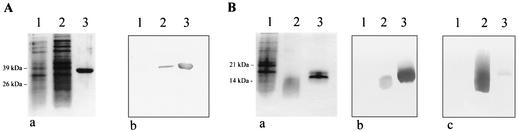
E. coli clones were obtained which effectively expressed DbpA, VlsE, and OspC from strains PBr, PKa2, and 20047, respectively (Fig. 1). At this stage of the study, the expression of the VlsE clone was controlled using an anti-VlsE-positive serum from an American patient (laboratory of B.J.). A clone expressing VlsE from B. burgdorferi sensu stricto strain B31 generated in the same laboratory (1) served as a positive control. The serum recognized VlsEs from the recombinant clones; E. coli without a vlsE insert gave a negative result (data not shown). Purified proteins of DbpA (PBr), VlsE (PKa2), and OspC (20047) clones were obtained by chromatography without contamination of other proteins (Fig. 1). Even in the immunoblot test with a high-titered immune serum against E. coli, no contaminating proteins were detected (Fig. 1). Purified PKa2 VlsE was reactive with a rabbit immune serum raised against the recombinant PKa2 VlsE (Fig. 2), and purified OspC was reactive with the OspC-specific monoclonal antibody L22 C11 (17) (data not shown). Purified DbpAs from strains PBr and PKo were reactive with rabbit immune sera against DbpAs from strains PBr and PKo, respectively. Reactivities of the homologous sera were very strong, whereas reactivities were very weak with the heterologous sera (Fig. 2). PBr DbpA was not reactive with monoclonal antibody L17 G2, whereas PKo DbpA was strongly reactive (data not shown), as previously described (10).
FIG. 1.
Purification of recombinant antigens. (Top panel) SDS-PAGE results for VlsE (lane 1, uninduced recombinant E. coli whole-cell lysate; lane 2, induced recombinant E. coli; lane 3, purified protein), DbpA (lane 4, uninduced recombinant E. coli whole-cell lysate; lane 5, induced recombinant E. coli; lane 6, purified protein), and OspC (lane 7, uninduced recombinant E. coli whole-cell lysate; lane 8, induced recombinant E. coli; lane 9, purified protein). (Bottom panel) Immunoblot test results with immune serum against E. coli. Antigens used were as described for the top panel.
FIG. 2.
Reactivity of recombinant proteins with rabbit immune sera. (A) Reactivity of recombinant VlsE with homologous rabbit immune serum. (a) SDS-PAGE results for E. coli whole-cell lysate without vlsE insert (lane 1), induced recombinant E. coli (lane 2), and purified protein (lane 3). (b) Immunoblot test results with immune serum against recombinant VlsE. Antigens used were as described for panel a. (B) Reactivity of recombinant DbpA from strains PBr and PKo with homologous and heterologous immune sera, respectively. (a) SDS-PAGE results for E. coli whole-cell lysate (lane 1), purified DbpA strain PBr (lane 2), and purified DbpA strain PKo (lane 3). (b) Immunoblot test results with immune serum against DbpA strain PKo. Antigens used were as described for panel a. (c) Immunoblot test results with immune serum against DbpA strain PBr. Antigens used were as described for panel a.
In this study we used sera from a clinically well-defined serum panel previously investigated in the studies of Hauser et al. (5) and Wilske et al. (18). The sera used were from the following groups of patients: (i) 36 neuroborreliosis patients (stage II) with positive intrathecal Borrelia-specific antibody production and/or positive IgM ELISA results (confirmed by immunoblot testing) and (ii) negative-control patients, whose sera comprised 49 serum samples from blood donors, 8 serum samples positive for rheumatoid factor, and 10 serum samples from syphilis patients. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and conventional and recombinant immunoblot tests were performed as previously described (5, 18). A concentration of 12.5% was used for all gels. Human and anti-E. coli rabbit serum samples were diluted 1:200, and bound IgG antibodies were detected with horseradish peroxidase-labeled anti-human IgG purchased from Dakopatts (Copenhagen, Denmark). Goat immune serum against rabbit Igs (Dakopatts) was used for detection of rabbit antibodies against VlsE and DbpA (10). For the whole-cell lysate immunoblot test, the antigen was B. afzelii isolate PKo. This strain expresses the immunodominant antigens OspC and DbpA in culture (5). Immunoblot strips for use with the older recombinant blot test contained the following recombinant proteins: p83/100 derived from strain PKo (B. afzelii); p39 (BmpA) and OspC from strains PKa2 (B. burgdorferi sensu stricto), PBi (B. garinii, OspA type 4), and PKo; p41i (internal flagellin fragment) from PKo and PBi; p58 derived from PBi; and DbpA from PKo (18). For the newer immunoblot test, an additional strip was produced containing the following antigens: DbpA (strain PBr), OspC (strain 20047) and VlsE (strain PKa2). The newer immunoblot test also included use of the antigens from the older immunoblot test. Thus, all of the relevant proteins—those from the older test and the additional new proteins—were evaluated for the newer blot test. For statistical analysis, Fisher's exact test for dichotomous variables was performed.
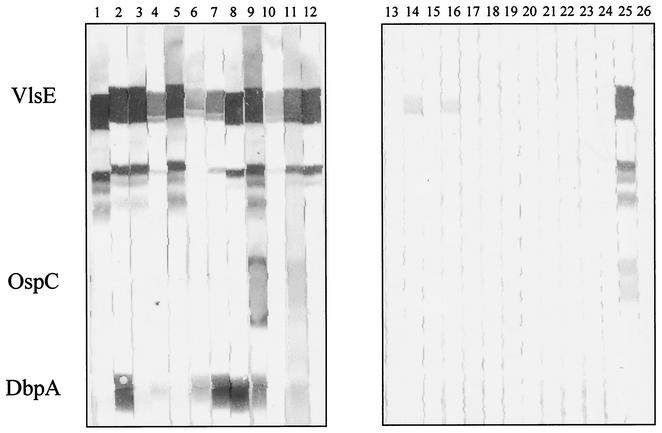
Results of the tests are shown in Table 1. Figure 3 shows examples of immunoreactivity for 12 sera from patients with neuroborreliosis and 12 control sera with the new antigens. Neuroborreliosis serum no. 9 was reactive with all three new recombinant proteins. Note the minor bands below the VlsE band; these bands apparently represent degradation products of VlsE (also seen in the Coomassie staining results shown in Fig. 1) and not E. coli contaminants, because they were detected only with strongly reacting VlsE antibody-positive sera and not with the immune serum against E. coli or VlsE antibody-negative sera. For all immunoblot tests, a two-band criterion (at least two reactive bands) was used to document a positive result. Use of a one-band criterion would result in very low specificity. In the control group, 10 of 67 samples had at least one positive band (resulting in a specificity of only 85%) but none had two positive bands. Overall, we found an increase in sensitivity (from 52.7 to 86.1%) when the three new proteins were added. The difference is significant (P = 0.004). The new recombinant blot test was also more sensitive than blot tests which used whole-cell lysate antigens (86.1% versus 63.8%). However, this difference was not significant (P = 0.055). The recombinant VlsE was the most sensitive antigen (83.3%), followed by DbpA from PBr (44.4%) (Table 2). Interestingly, the combination of different DbpAs was highly effective, since the two proteins showed low levels of cross-reactivity. At least one of the DbpAs was reactive in 69.4% of the neuroborreliosis sera. OspC from strain 20047 was reactive with only 3 (8.3%) of the 36 sera (Table 2).
TABLE 1.
Comparison of the newer recombinant immunoblot test with the older recombinant test and the whole-cell lysate immunoblot test
| Group | Total no. of serum samples | No. (%) of positive serum samples by the:
|
||
|---|---|---|---|---|
| Newer recombinant immunoblot test | Older recombinant immunoblot test | Whole-cell lysate immunoblot test | ||
| Neuroborreliosis stage II | 36 | 31 (86.1) | 19 (52.7) | 23 (63.8) |
| Controla | 67 | 0 | 0 | 2 |
The control group consisted of 49 healthy persons, 8 patients with syphilis, and 10 patients positive for rheumatoid factor.
FIG. 3.
Newer recombinant immunoblot test. Test results for reactivity of VlsE, OspC (strain 20047), and DbpA (strain PBr) with sera from patients with neuroborreliosis stage II (lanes 1 to 12) are shown. Lanes 13 to 24, negative-control sera; lane 25, positive control; lane 26, negative control. Note that the minor bands between VlsE and OspC represent degradation products of VlsE.
TABLE 2.
Immunoreactivity of VIsE and Osp17 (DbpA) in the recombinant immunoblot testa
| Group | Total no. of serum samples | No. (%) of serum samples with positive results for immunoreactivity of:
|
|||
|---|---|---|---|---|---|
| VIsE | Osp17 (DbpA) with:
|
||||
| PKo | PBr | PKo and/or PBr | |||
| Neuroborreliosis | 36 | 30 (83.3) | 14 (38.8) | 16 (44.4) | 25 (69.4) |
| Control | 67 | 4 (6.0) | 2 (3.0) | 2 (3.0) | 4 (6.0) |
In tests of reactivity of OspC (20047), only three neuroborreliosis sera gave positive results; all controls gave negative results.
In contrast to the United States, where causative strains are very homogeneous, the heterogeneity of the borreliae is a specific problem in Europe. This is especially true for the diagnosis of early neuroborreliosis (stage II), in which causative strains are very heterogeneous (4, 17) and, in contrast to late disease (5), the immune response recognizes only few antigens. We were able to show that both VlsE and the new DbpA homologue from strain PBr are important antigens for improvement of the diagnostic sensitivity of the previous recombinant blot test. The two different DbpAs complement each other, since only a few serum samples were reactive with both proteins. This shows that DbpA has type-specific epitopes which are diagnostically relevant. This has also been shown recently in two other studies (3, 6). Notably, VlsE is expressed by the borreliae in the mammalian host or upon contact with mammal cells but not in the unfed tick (7, 8, 21). VlsE was detected from cultured borreliae only with highly sensitive detection methods (e.g., chemiluminescence) but not in blot tests which used horseradish peroxidase conjugates (unpublished data). As shown previously (10), many strains do not express DbpA (Osp17) in culture. The low or variable level of expression of preferentially in vivo-expressed proteins in culture is a big problem for conventional immunoblot tests using lysates from in vitro-cultured borreliae. With the recombinant immunoblot test presented here, we now have a sensitive and easy-to-standardize confirmation test as recommended for the two-step approach for serodiagnosis of Lyme disease in Europe. It is an open question whether the sensitivity of the IgG immunoblot test can be improved further. It might be interesting to test the antigenicity of additional VlsE proteins derived from B. garinii or B. afzelii to investigate whether epitopes restricted to single species or types are relevant for serodiagnosis. This might be relevant, since the immunodominant C-terminal region is conserved among B. burgdorferi sensu lato species to only a limited extent (13).
Acknowledgments
We thank Ruth Hillermann for excellent technical work, Rendi Murphree Bacon for the VlsE positive control, and Jürgen Heesemann for generous support.
REFERENCES
- 1.Bacon, R. M., B. J. Biggerstaff, M. Schriefer, R. D. Gilmore, M. T. Philipp, A. Steere, G. P. Wormser, A. R. Marques, and B. J. B. Johnson. Improved serodiagnosis of Lyme disease by kinetic ELISAs using recombinant VlsE1 or peptide antigens of Borrelia burgdorferi compared with two-tiered testing. J. Infect. Dis., in press. [DOI] [PMC free article] [PubMed]
- 2.Centers for Disease Control and Prevention. 1995. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. Morb. Mortal. Wkly. Rep. 44:590.. [PubMed] [Google Scholar]
- 3.Cinco, M., M. Ruscio, and F. Rapagna. 2000. Evidence of Dbps (decorin binding proteins) among European strains of Borrelia burgdorferi sensu lato and in the immune response of LB patient sera. FEMS Microbiol. Lett. 183:111-114. [DOI] [PubMed] [Google Scholar]
- 4.Eiffert, H., A. Ohlenbusch, H.-J. Christen, R. Thomssen, A. Spielman, and F.-R. Matuschka. 1995. Nondifferentiation between Lyme disease spirochetes from vector ticks and human cerebrospinal fluids. J. Infect. Dis. 171:476-479. [DOI] [PubMed] [Google Scholar]
- 5.Hauser, U., G. Lehnert, R. Lobentanzer, and B. Wilske. 1997. Interpretation criteria for standardized Western blots for three European species of Borrelia burgdorferi sensu lato. J. Clin. Microbiol. 35:1433-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heikkilä, T., I. Seppälä, H. Saxen, J. Panelius, H. Yrjänäinen, and P. Lahdenne. 2002. Species-specific serodiagnosis of Lyme arthritis and neuroborreliosis due to Borrelia burgdorferi sensu stricto, B. afzelii, and B. garinii by using decorin binding protein A. J. Clin. Microbiol. 40:453-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hudson, C. R., J. G. Frye, F. D. Quinn, and F. C. Gherardini. 2001. Increased expression of Borrelia burgdorferi vlsE in response to human endothelial cell membranes. Mol. Microbiol. 41:229-239. [DOI] [PubMed] [Google Scholar]
- 8.Indest, K. J., J. K. Howell, M. B. Jacobs, D. Scholl-Meeker, S. J. Norris, and M. T. Philipp. 2001. Analysis of Borrelia burgdorferi vlsE gene expression and recombination in the tick vector. Infect. Immun. 69:7083-7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jauris-Heipke, S., R. Fuchs, M. Motz, V. Preac-Mursic, E. Schwab, E. Soutschek, G. Will, and B. Wilske. 1993. Genetic heterogenity of the genes coding for the outer surface protein C (OspC) and the flagellin of Borrelia burgdorferi. Med. Microbiol. Immunol. 182:37-50. [DOI] [PubMed] [Google Scholar]
- 10.Jauris-Heipke, S., B. Roessle, G. Wanner, C. Habermann, D. Roessler, V. Fingerle, G. Lehnert, R. Lobentanzer, I. Pradel, B. Hillenbrand, U. Schulte-Spechtel, and B. Wilske. 1999. Osp17, a novel immunodominant outer surface protein of Borrelia afzelii: recombinant expression in Escherichia coli and its use as a diagnostic antigen for serodiagnosis of Lyme borreliosis. Med. Microbiol. Immunol. 187:213-219. [DOI] [PubMed] [Google Scholar]
- 11.Johnson, B. J. B., K. E. Robbins, R. E. Balley, B.-L. Cao, S. L. Sviat, R. B. Craven, L. W. Mayer, and D. T. Dennis. 1996. Serodiagnosis of Lyme disease: accuracy of a two-step approach using a flagella-based ELISA and immunoblotting. J. Infect. Dis. 174:346-353. [DOI] [PubMed] [Google Scholar]
- 12.Liang, F. T., E. Aberer, M. Cinco, L. Gern, C. M. Hu, Y. N. Lobet, M. Ruscio, P. E. Voet, V. E. Weynants, and M. T. Philipp. 2000. Antigenic conservation of an immunodominant invariable region of the VlsE lipoprotein among European pathogenic genospecies of Borrelia burgdorferi SL. J. Infect. Dis. 182:1455-1462. [DOI] [PubMed] [Google Scholar]
- 13.Liang, F. T., L. C. Bowers, and M. T. Philipp. 2001. C-terminal invariable domain of VlsE is immunodominant but its antigenicity is scarcely conserved among strains of Lyme disease spirochetes. Infect. Immun. 69:3224-3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang, F. T., A. C. Steere, A. R. Marques, B. J. B. Johnson, J. N. Miller, and M. T. Philipp. 1999. Sensitive and specific serodiagnosis of Lyme disease by enzyme-linked immunosorbent assay with a peptide based on an immunodominant conserved region of Borrelia burgdorferi VlsE. J. Clin. Microbiol. 37:3990-3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberts, W. C., B. A. Mullikin, R. Lathigra, and M. S. Hanson. 1998. Molecular analysis of sequence heterogeneity among genes encoding decorin binding proteins A and B of Borrelia burgdorferi sensu lato. Infect. Immun. 66:5275-5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roessler, D., U. Hauser, and B. Wilske. 1997. Heterogeneity of BmpA (P39) among European isolates of Borrelia burgdorferi sensu lato and influence of interspecies variability on serodiagnosis. J. Clin. Microbiol. 35:2752-2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilske, B., U. Busch, H. Eiffert, V. Fingerle, H.-W. Pfister, D. Rössler, and V. Preac-Mursic. 1996. Diversity of OspA and OspC among cerebrospinal fluid isolates of Borrelia burgdorferi sensu lato from patients with neuroborreliosis in Germany. Med. Microbiol. Immunol. 184:195-201. [DOI] [PubMed] [Google Scholar]
- 18.Wilske, B., C. Habermann, V. Fingerle, B. Hillenbrand, S. Jauris-Heipke, G. Lehnert, I. Pradel, D. Rössler, and U. Schulte-Spechtel. 1999. An improved recombinant IgG immunoblot for serodiagnosis of Lyme borreliosis. Med. Microbiol. Immunol. 188:139-144. [DOI] [PubMed] [Google Scholar]
- 19.Wilske, B., V. Preac-Mursic, U. B. Göbel, B. Graf, S. Jauris-Heipke, E. Soutschek, E. Schwab, and G. Zumstein. 1993. An OspA serotyping system for Borrelia burgdorferi based on reactivity with monoclonal antibodies and OspA sequence analysis. J. Clin. Microbiol. 31:340-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilske, B., L. Zöller, V. Brade, M. Eiffert, U. B. Göbel, G. Stanek, and H. W. Pfister. 2000. MIQ 12 Lyme-Borreliose, p. 1-59. In H. Mauch and R. Lütticken (ed.), Qualitätsstandards in der mikrobiologisch-infektiologischen Diagnostik. Urban & Fischer Verlag, Munich, Germany.
- 21.Zhang, J.-R., J. M. Hardham, A. G. Barbour, and S. J. Norris. 1997. Antigenic variation in Lyme disease borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell 89:275-285. [DOI] [PubMed] [Google Scholar]