Abstract
Focal adhesion kinase (FAK) prevents apoptosis in many cell types. We have reported that tyrosine residues in FAK are dephosphorylated and FAK is degraded during mannitol-induced apoptosis in human neuroblastoma cells. Several studies suggest that FAK dephosphorylation and degradation are separate events. The current study defines the relationship between FAK dephosphorylation and degradation in neuroblastoma cells using okadaic acid (OA). OA, a serine phosphatase inhibitor, promotes serine/threonine phosphorylation, which in turn blocks tyrosine phosphorylation. OA induced focal adhesion loss, actin cytoskeleton disorganization, and cellular detachment, which corresponded to a loss of FAK Tyr397 phosphorylation. These changes preceded caspase-3 activation, Akt and MAP kinase activity loss, protein ubiquitination, and cellular apoptosis. Insulin-like growth factor-I prevented mannitol-induced, but not OA-induced, substrate detachment and FAK Tyr397 dephosphorylation, and the effects of OA on FAK Tyr397 phosphorylation were irreversible. The proteolytic degradation of FAK is temporally distinct from its tyrosine dephosphorylation, occurring when apoptotic pathways are already initiated and during a generalized destruction of signaling proteins. Therefore, agents resulting in the dephosphorylation of FAK may be beneficial for therapeutic treatment, irrespective of FAK protein levels, as this may result in apoptosis, which cannot be prevented by growth factor signaling.
Keywords: Neuroblastoma, focal adhesion kinase, insulin-like growth factor-I, okadaic acid, apoptosis
Introduction
Neuroblastoma, a pediatric tumor arising from improper differentiation of the neural crest, is largely chemoresistant in children over 12 months of age [1]. Long-term survival rates are inadequate, and over 40% of children die of metastatic disease [2], with primary metastatic sites including the bone, liver, and lungs [3–5]. Many chemotherapeutic agents induce a caspase-8-dependent apoptotic death in tumor cells [6]. However, neuroblastoma tumors often have defects in caspase-8-mediated apoptotic pathways, or overexpress antiapoptotic proteins of the Bcl-2 family, which may explain their chemoresistant behavior [7]. Therefore, in our laboratory, we have focused on alternative ways to induce apoptosis in human neuroblastoma cells, primarily to understand which biochemical pathways to target during neuroblastoma treatment.
We have shown that mannitol, which causes hyperosmotic stress in cells, induces apoptosis in both neuroblastic (N-type) and substrate-adherent (S-type) cells derived from heterogeneous neuroblastoma tumors [8–10]. Mannitol induces caspase-3 activation within 3 to 9 hours, DNA fragmentation within 12 hours, and membrane alterations by 24 hours after treatment [8–10]. We have also shown that mannitol treatment leads to morphological changes by 4 hours, with disruptions in actin cytoskeleton and degradation of both the survival protein Akt and focal adhesion kinase (FAK) [11]. This coincides with a loss of focal adhesion sites, cell detachment, and eventual cell death [11]. FAK activation appears to be essential in preventing anoikis [12], a form of apoptosis that occurs when anchoragedependent cells detach from their substrate [13]. Therefore, these changes have led us to postulate that mannitol induces an anoikis-like death through its effects on FAK.
FAK is a cytoplasmic tyrosine kinase central in many critical cellular pathways [12,14,15]. FAK is tyrosine-phosphorylated upon integrin ligand binding [16] or downstream of ligand binding by growth factor receptors [17,18]. FAK is located at focal adhesion sites where it forms complexes with downstream signaling molecules, such as phosphatidylinositol-3-kinase, Grb2, Shc, Src, and paxillin [16]. FAK activation at focal adhesion sites leads to cytoskeletal reorganization, cellular adhesion, and survival.
Microinjection of an anti-FAK antibody or FAK antisense oligonucleotides, preventing FAK activation, induces apoptosis [15,19]. Conversely, overexpression of FAK prevents apoptosis caused by cellular detachment [14], ultraviolet irradiation [20], hydrogen peroxide, or etoposide [21]. Results from previous studies suggest that FAK dephosphorylation and degradation are both coupled to anoikis [22]. However, other studies suggest that FAK dephosphorylation and degradation are not directly connected [23]. Therefore, to help clarify the role of FAK in neuroblastoma cell death, we examined its modification during anoikis induced by okadaic acid (OA), an inhibitor of the serine/threonine protein phosphatases 1 (PP1) and 2A (PP2A). OA induces apoptosis in a wide variety of cell types, including mouse keratinocytes [24], renal epithelial tumor cells [25], oral squamous carcinoma cells [26,27], lung fibroblasts [28], and cultured neuronal cells [29,30]. OA-induced apoptosis occurs through several mechanisms, including phosphorylation of p53 [31] and upregulation of the Fas receptor [26]. OA also results in caspase-2, caspase-3, caspase-7, and caspase-9 in multiple cell types, with no activation of caspase-8 [32]. However, the major observation in OA-treated cells is the disruption of the actin cytoskeleton [33,34]. Therefore, in this study, we examined the effect of OA on FAK phosphorylation, FAK degradation, and apoptosis to determine the role of FAK in neuroblastoma cell apoptosis.
In the current study, we show that OA induces a loss of focal adhesions, disorganization of the actin cytoskeleton, cellular detachment, and cellular rounding. These events correspond with a loss of FAK phosphorylation on Tyr397, serine phosphorylation and degradation of paxillin, activation of caspase-3, loss of Akt and MAP kinase, protein ubiquitination, and detection of apoptosis by flow cytometry. Insulinlike growth factor-I (IGF-I) prevents mannitol-induced, but not OA-induced, substrate detachment and FAK Tyr397 dephosphorylation, and the effects of OA on FAK Tyr397 phosphorylation were irreversible. These results suggest that OA causes an irreversible change in paxillin or another upstream signaling molecule that disrupts focal adhesion complexes and prevents FAK activation and autophosphorylation. Finally, the proteolytic degradation of FAK is temporally distinct from its tyrosine dephosphorylation, occurring when apoptotic pathways are already initiated and during a generalized destruction of signaling proteins. These results suggest that FAK dephosphorylation and degradation are separable processes. Therefore, agents resulting in the dephosphorylation of FAK may be beneficial for therapeutic treatment, irrespective of FAK protein levels, as this may result in apoptosis, which cannot be prevented by growth factor signaling.
Materials and Methods
Materials
Antibodies against phosphorylated FAK (pFAK, pY397, and pS722) were purchased from Biosource International (Camarillo, CA). Anti-FAK monoclonal and PY20 antiphosphotyrosine antibodies were purchased from Transduction Laboratories (Lexington, KY). Antibodies against phosphorylated and unphosphorylated Akt and mitogen-activated protein kinase (MAPK) were from Cell Signaling Technology (Beverly, MA). Anticaspase-3 antibody was from BD Phar-Mingen (San Diego, CA). Antibodies against actin, ubiquitin, and FAK (polyclonal); secondary antibodies for Western immunoblotting; and protein A/G agarose beads were from Santa Cruz Biotechnology (Santa Cruz, CA). Texas Red-conjugated phalloidin, biotin-labeled secondary antibodies, and fluorescein isothiocyanate (FITC)-labeled avidin were from Vector Laboratories (Burlingame, CA). OA and other inhibitors were purchased from Calbiochem (La Jolla, CA). Recombinant human IGF-I was kindly provided by Cephalon, Inc. (Westchester, PA).
Cell Culture
SH-EP human neuroblastoma cells were maintained in DMEM containing 10% calf serum. SH-EP cells transfected with vector (pSFFV) or IGF-IR cDNA [35] were maintained in DMEM containing 10% calf serum and 0.25 mg/ml G418. The cells were serum-starved for 4 hours before each experiment.
Immunoblotting, Immunoprecipitation, and Immunocytochemisty
Immunoblotting and immunoprecipitation were performed as previously described [36]. All experiments were repeated at least three times and typical representative results are presented in the figures. Immunocytochemistry was performed as described previously [37] using an anti-FAK monoclonal antibody and Texas Red phalloidin.
Flow Cytometry
The percentage of apoptotic cells was measured as the percentage of sub-G0 DNA using flow cytometry as described previously [10]. All results are expressed as the mean percentage of apoptotic cells of at least three experiments ± the standard error of the mean (SEM).
Results
OA Induces Cell Detachment, Actin Stress Fiber Disruption, and Loss of Focal Adhesion Sites in SH-EP Human Neuroblastoma Cells
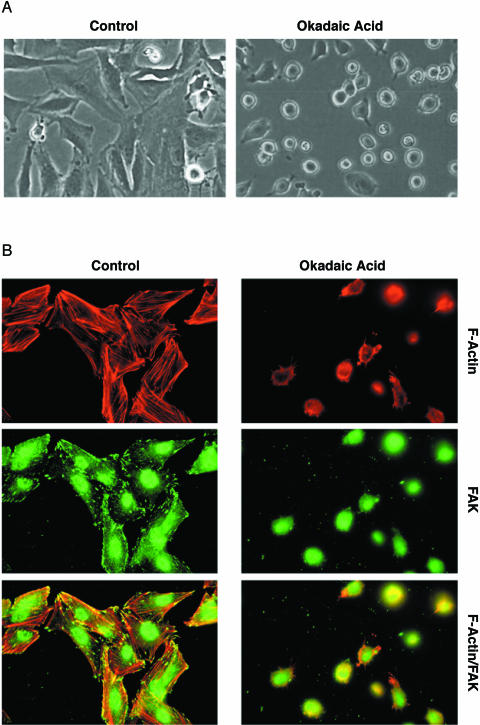
We recently reported that hyperosmotic media induce apoptosis in neuroblastoma cells [9,38–40]. One of the earliest events observable with mannitol treatment is disruption of the actin cytoskeleton [11]. The phosphatase inhibitors OA and cytostatin, which inhibit PP1 and PP2A, cause a similar disruption of the actin cytoskeleton [33,34] and induce apoptosis [41–43]. Therefore, we were interested in whether comparable biochemical pathways were involved in mannitol-and OA-induced morphological changes and apoptosis. For these studies, we utilized SH-EP human neuroblastoma cells, which we have previously used to characterize mannitol-induced apoptosis [10,11]. In initial studies, SH-EP cells were serum-starved for 4 hours and then treated for 4 hours with or without 250 nM OA. The control untreated cells were elongated and well spread, and most of the cells were attached to the substrate. However, upon OA treatment, SH-EP cells rounded up and detached from the substrate (Figure 1A). Control cells contained well-organized actin stress fibers, as visualized through staining with Texas Red phalloidin (Figure 1B, left panel), whereas the actin cytoskeleton was disrupted in OA-treated cells. Immunostaining for the focal adhesion protein FAK revealed a typical punctate pattern at the sites of focal adhesion in control cells. However, this punctate pattern was lost in OA-treated cells, suggesting a loss of focal adhesion formation. These results indicate that initial cellular events occurring after OA treatment are a loss of actin stress fibers and focal adhesions concomitant with rounding up and detachment of cells, similar to our observations in mannitol-treated cells [11].
Figure 1.
OA causes detachment of SH-EP cells. (A) Phase contrast microscopy of SH-EP neuroblastoma cells serum-starved for 4 hours and treated for 4 hours with or without 250 nM OA. (B) The cells were treated as in (A), fixed, and stained with Texas red phalloidin and anti-FAK antibody.
OA Induces Concentration- and Time-Dependent Dephosphorylation of FAK
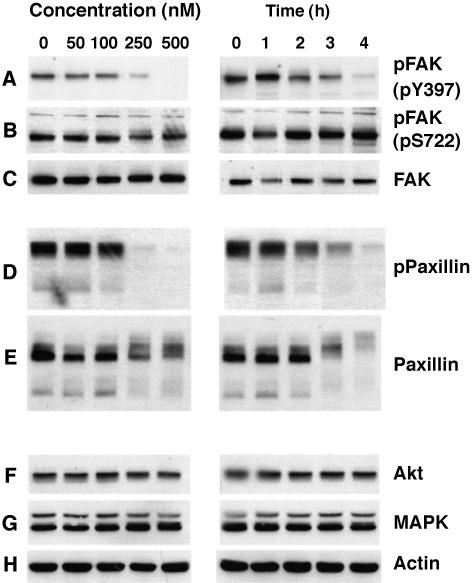
Our initial results suggested that FAK is modified by OA treatment. Tyr397 phosphorylation indicates FAK activation, as FAK phosphorylates itself on Tyr397 [44]. Therefore, we utilized an anti-phospho-Tyr397-specific antibody to determine the effect of OA on FAK tyrosine phosphorylation and activity. OA treatment caused a concentration- and timedependent decrease in the phosphorylation of FAK at Tyr397 (Figure 2A). FAK tyrosine phosphorylation decreased at OA concentrations between 100 and 250 nM, and Tyr397 phosphorylation was completely lost at 500 nM. FAK Tyr397 phosphorylation began to decrease after 2 to 3 hours of OA treatment, and this site was completely dephosphorylated after 4 hours (Figure 2A). In contrast, little change occurred in FAK Ser722 phosphorylation (Figure 2B), and the levels of FAK protein remained constant during OA treatment (Figure 2C).
Figure 2.
OA induces time- and concentration-dependent dephosphorylation of FAK. SH-EP cells were serum-starved for 4 hours and treated with increasing concentrations of OA for 4 hours (left panel), or with 250 nM OA for the indicated times (right panel). Equal amounts of protein from whole cell lysates were separated by SDS-PAGE, transferred to nitrocellulose, and analyzed by immunoblotting with the indicated antibodies (A–H).
We next examined the effect of OA on paxillin, a focal adhesion protein that associates with and targets FAK to focal adhesions [45]. OA caused a time- and concentration-dependent loss of paxillin tyrosine phosphorylation (Figure 2D). In contrast to FAK, OA also appeared to induce the degradation of paxillin, or at least a reduction in its levels (Figure 2E). The decrease in paxillin expression occurred in parallel with reduced electrophoretic mobility, suggesting that OA enhanced the phosphorylation of paxillin on serine or threonine. Finally, OA treatment did not cause a change in the levels of Akt, Erk1/2, or actin (Figure 2F–H), demonstrating that OA does not cause a generalized degradation of cellular proteins.
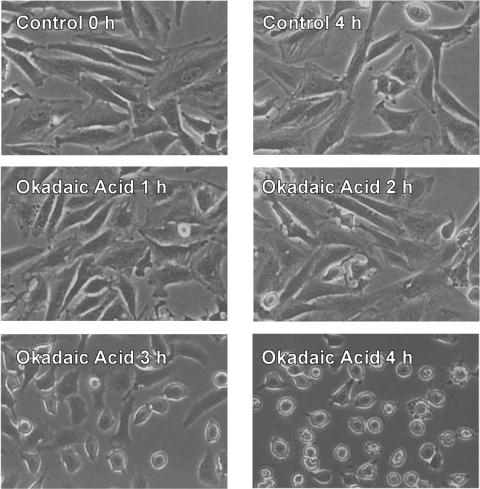
FAK Tyr397 dephosphorylation coincided with morphological changes induced by OA. Specifically, the cells began to round up and lose their attachment within 3 hours of OA treatment (Figure 3), the same time point at which FAK phosphorylation begins to decrease. Furthermore, most of the cells were detached after 4 hours, when the Tyr397 of FAK is completely dephosphorylated. These results strongly suggest that FAK tyrosine phosphorylation is connected to morphological and cytoskeletal changes caused by OA.
Figure 3.
OA induces time-dependent detachment of SH-EP cells. Serum-starved SH-EP cells were incubated with or without 250 nM OA. Phase contrast images were taken at the indicated times.
Influences of IGF-IR Signaling on OA-Induced FAK Dephosphorylation and Morphological Changes
The activation of IGF-IR is important for transformation and tumor progression in several tumor types [46,47]. IGF-IR activation and downstream signaling promote both cell proliferation [48–53] and cell survival [54,55] and rescue cells from apoptosis caused by a wide variety of agents [56]. In fact, we have shown that IGF-I treatment or IGF-IR overexpression prevents mannitol-induced apoptosis in SH-EP cells [8–11,39]. Furthermore, IGF-IR activation prevents mannitol-induced FAK dephosphorylation and degradation [11]. IGF-I treatment [42] or IGF-IR overexpression [43] also prevents OA-induced apoptosis in other cell lines. Therefore, we examined the effect of IGF-IR overexpression and activation on OA-induced changes in SH-EP cells.
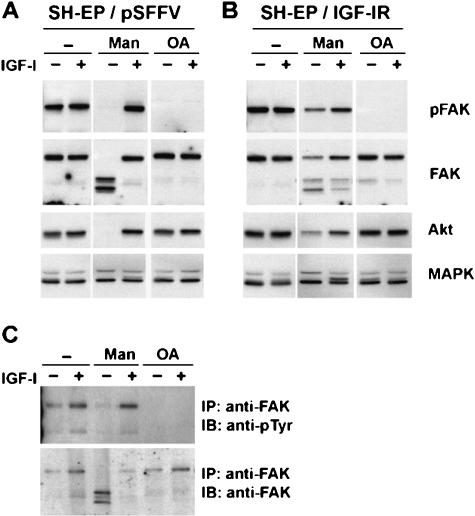
When serum-starved control SH-EP cells transfected with empty pSFFV vector (SHEP/pSFFV cells) were treated with IGF-I, there was a slight increase in both Tyr397 (Figure 4A) and total FAK tyrosine phosphorylation (Figure 4C). In agreement with our previous report [11], mannitol induced a complete degradation of FAK, as well as a loss of both Tyr397 and total tyrosine phosphorylation. Akt is also degraded during mannitol treatment. These effects of mannitol on FAK and Akt were prevented by IGF-I treatment. Like mannitol, OA induced both Tyr397 and total tyrosine dephosphorylation. However, in contrast to mannitol, OA did not cause the degradation of FAK or Akt. IGF-I treatment, although effective against mannitolinduced FAK dephosphorylation, did not prevent OAinduced FAK dephosphorylation. Neither mannitol nor OA caused a change in the level of Erk 1/2. As previously reported [11], IGF-IR overexpression reduced the mannitol-stimulated dephosphorylation of FAK on Tyr397 (Figure 4B) but was unable to prevent OA-induced FAK Tyr397 dephosphorylation, whether or not IGF-I was present. In addition, IGF-IR overexpression reduced mannitol-induced FAK and Akt degradation. Collectively, these results show that OA-induced FAK tyrosine dephosphorylation cannot be rescued by IGF-IR activation. This contrasts directly with the effects of mannitol, which can be reversed by IGF-IR activation.
Figure 4.
Effect of IGF-I on OA-induced FAK dephosphorylation. Vectortransfected (A and C) or IGF-IR-transfected (B) SH-EP cells were treated for 4 hours with or without 300 mM mannitol (Man) or 250 nM OA in the presence or absence of 10 nM IGF-I. Whole cell lysates were analyzed by immunoblotting with indicated antibodies (A and B). (C) Whole cell lysates were immunoprecipitated with an anti-FAK polyclonal antibody and then analyzed by immunoblotting with an antiphosphotyrosine (pTyr) antibody (upper panel). The immunoblot was stripped and reblotted with an anti-FAK monoclonal antibody (lower panel). IB = immunoblot; IP = immunoprecipitation.
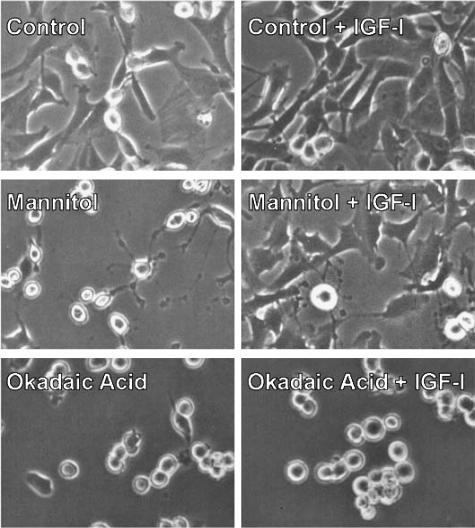
Given that IGF-I was able to rescue mannitol-induced, but not OA-induced, FAK tyrosine dephosphorylation, we suspected that IGF-I might also prevent the morphological changes caused by mannitol but not OA. Mannitol induced cell detachment and morphological changes associated with apoptosis, including membrane blebbing, loss of contacts with neighboring cells, rounding up, and detachment from the substrate (Figure 5), similar to our previous report [11]. As expected, these changes were prevented by IGF-I treatment. Like mannitol, OA induced the rounding up and detachment of cells from the substrate. However, unlike cells exposed to mannitol, cells treated with OA did not display the extensive membrane blebbing typical of apoptosis [11]. Moreover, IGF-I was unable to prevent OA-induced rounding and loss of substrate attachment.
Figure 5.
Effect of IGF-I on OA-induced morphological changes and cell detachment. Serum-starved vector-transfected SH-EP cells were treated for 4 hours with 300 mM mannitol or 250 nM OA in the presence or absence of 10 nM IGF-I. Representative phase contrast images are shown.
Influences of Integrin Signaling on OA-Induced FAK Dephosphorylation
FAK is activated downstream of both growth factor receptors [17,18] and integrins [16]. Therefore, because growth factor receptor activation failed to prevent OA-induced FAK dephosphorylation, we next examined the effect of integrin ligand binding on FAK phosphorylation. SH-EP cells grown on fibronectin display slightly elevated FAK Tyr397 compared with cells grown on plastic (Figure 6).
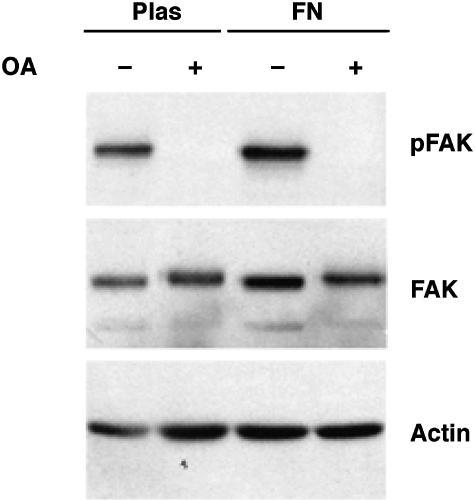
Figure 6.
Effect of integrin activation on OA-induced FAK dephosphorylation. SH-EP cells were grown on plastic (Plas) or fibronectin (FN) for 3 days. After serum starvation, the cells were treated for 4 hours with 250 nM OA, and whole cell lysates were analyzed by immunoblotting with anti-phospho-FAK (pY397), anti-FAK, or antiactin antibodies.
Treatment of SH-EP cells grown on fibronectin with 250 nM OA for 4 hours caused a complete loss of Tyr397 phosphorylation without affecting the protein levels of FAK or actin. These results demonstrate that fibronectin activation of integrins cannot prevent OA-induced FAK tyrosine dephosphorylation, and that the effects of OA are not limited to cells growing on a plastic surface. Therefore, unlike mannitol treatment, neither growth receptor activation nor integrin ligand binding prevents OA-induced FAK dephosphorylation.
Reversibility of OA-Induced FAK Dephosphorylation
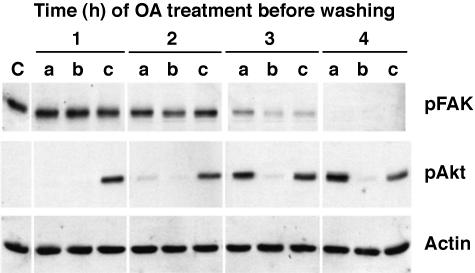
To determine whether the effects of OA on FAK phosphorylation are reversible, we treated cells with 250 nM OA for 1 to 4 hours, then changed the medium to fresh DMEM. FAK dephosphorylation on Tyr397 cannot be reversed after 2 hours of OA treatment (Figure 7). We also examined the phosphorylation of Akt, which is thought to be dephosphorylated by PP2B [57]. As expected, Akt phosphorylation was enhanced by OA, and switching the medium to fresh DMEM returned Akt phosphorylation to basal levels. IGF-I stimulated Akt phosphorylation in the fresh medium, even after 4 hours of OA treatment, despite the fact that the cells are completely rounded up at this time point. These results show that the effects of OA on FAK are irreversible, suggesting that OA causes a permanent covalent modification of FAK or another upstream signaling molecule. Irreversibility is not a general feature of OA treatment, as its effects on Akt are readily reversible.
Figure 7.
Reversibility of OA-induced FAK dephosphorylation. Serum-starved SH-EP cells were treated with OA for 1 to 4 hours. Cell lysates were collected (a) immediately after OA treatment, or cells were washed and incubated for 3 hours in (b) DMEM or (c) DMEM+10 nM IGF-I. Equal amounts of whole cell lysates were separated by SDS-PAGE, transferred to nitrocellulose, and analyzed by immunoblotting with anti-phospho-FAK (pY397), anti-phospho-Akt, or antiactin antibodies.
Short-Term Effects of OA on Apoptotic Pathways in SH-EP Cells
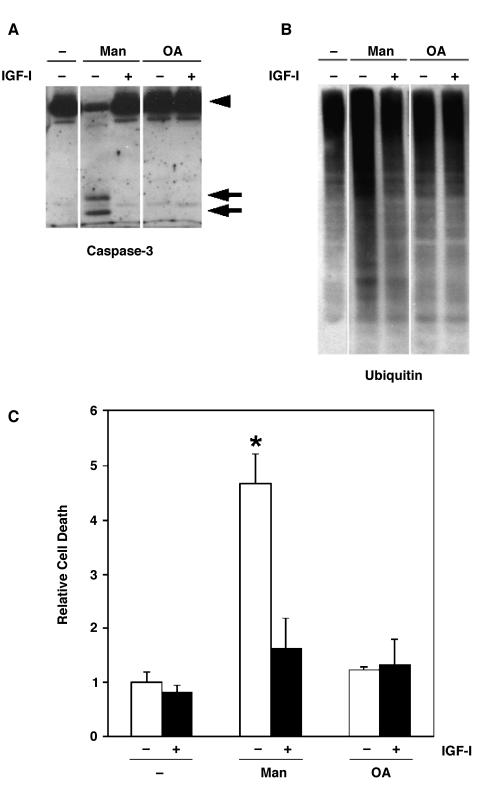
Our previous studies showed that caspases are actively involved in mannitol-induced or glucose-induced apoptosis [10,58,59]. Furthermore, caspase-3 cleavage has been linked to OA-induced apoptosis in other cell types [25,28,60]. Therefore, we examined the effect of OA on caspase-3 cleavage in SH-EP cells (Figure 8A). We also analyzed the effects of OA on the proteasome/ubiquitin pathway (Figure 8B), which participates in apoptosis and the breakdown of cellular proteins [61,62]. After 4 hours of OA treatment, when FAK is fully dephosphorylated and the cells have completed rounding up and detachment, we did not observe caspase-3 cleavage, accumulation of ubiquitinated proteins, or apoptosis (Figure 8). In contrast, a 4-hour treatment with mannitol caused caspase-3 cleavage, protein ubiquitination, and subsequent apoptosis—effects that were blocked by IGF-I (Figure 8) [11].
Figure 8.
Effect of OA on apoptotic pathways. SH-EP cells were treated for 4 hours with or without 300 mM mannitol or 250 nM OA in the presence or absence of 10 nM IGF-I. Whole cell lysates were analyzed by immunoblotting with (A) anti-caspase-3 or (B) antiubiquitin antibodies. Intact (arrowhead) and cleaved (arrow) forms of caspase-3 are indicated. (C) SH-EP cells were treated as above, and the number of apoptotic cells was measured by flow cytometry.
Long-Term Effects of OA on Apoptotic Pathways in SH-EP Cells
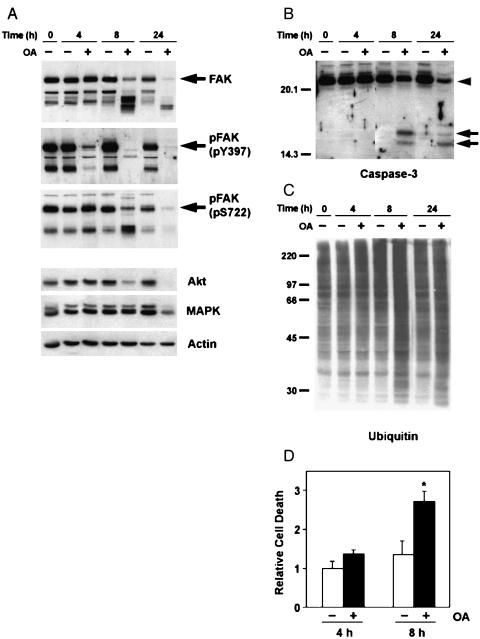
Cells denied anchorage will undergo a form of apoptosis called anoikis [13]. Surprisingly, although OA acid treatment induces cellular detachment, it does not stimulate caspase-3 cleavage, ubiquitination, or accumulation of apoptotic cells. We therefore suspected that the induction of apoptosis was delayed beyond the 4-hour time point. Indeed, when SH-EP cells were treated with 250 nM OA for 8 hours (4 hours after they lost substrate adhesion), they began to show biochemical changes characteristic of apoptosis. First, treatment with OA for 8 hours induced the cleavage of FAK (Figure 9A). This cleavage of FAK was complete after 24 hours of OA treatment. We previously reported a similar breakdown of FAK and Akt during mannitol-induced apoptosis in SH-EP cells [11]. In contrast to the degradation of FAK, dephosphorylation of FAK Tyr397 is easily observed after 4 hours of OA treatment, although, like FAK degradation, it continues with extended OA treatment. Little change in FAK Ser722 phosphorylation is observed at the 4-hour time point; however, it also decreases at later time points. These results suggest that FAK dephosphorylation progresses at later (8–24 hours) time points, although it is difficult to determine whether the loss of anti-pTyr397 and anti-pSer722 immunoblotting is truly due to continued dephosphorylation, or simply a loss of the antigenic epitope due to proteolytic cleavage. Finally, long-term OA treatment also caused substantial degradation of Akt and Erk 1/2, but not actin, suggesting that extended treatment with OA induces a general degradation of signaling proteins in SH-EP cells.
Figure 9.
Time course of OA-induced apoptosis. SH-EP cells were treated for 4 to 24 hours with or without 250 nM OA. (A–C) Whole cell lysates were analyzed by immunoblotting with indicated antibodies. Intact (arrowhead) and cleaved (arrow) forms of caspase-3 are indicated. (D) SH-EP cells were treated as above and the number of apoptotic cells was measured by flow cytometry.
Similar to FAK cleavage, caspase-3 cleavage, although not observable after 4 hours of OA treatment, was readily observed within 8 hours (Figure 9B). Likewise, protein ubiquitination is not apparent after 4 hours of OA treatment, but is easily detected after 8 hours (Figure 9C). These results confirm that apoptotic pathways are triggered only after extended treatment with OA. In agreement with these findings, flow cytometry revealed that OA causes substantial apoptosis only after 8 hours (Figure 9D).
Discussion
Anoikis is an apoptotic process that occurs in cells denied cell-matrix or cell-cell attachment [63]. Although many characteristics of apoptosis are recapitulated in anoikis, the caspases, which are critical for executing the apoptotic program, are activated much later in anoikis [64]. Integrin activation of FAK appears particularly crucial for preventing anoikis [65]. However, once anoikis has been initiated, other signaling pathways may protect cells from death, including Akt activation [66], IGF-I treatment [67], and insulin receptor overexpression [68]. We have previously shown that exposure of neuroblastoma cells to mannitol causes cell detachment, actin cytoskeleton alterations, FAK dephosphorylation and degradation, and apoptosis, suggesting an anoikis-like death [11]. In the current study, we investigated the events following OA treatment, a known apoptosis inducer, to determine the role of cellular detachment and FAK dephosphorylation in neuroblastoma cell death.
To begin, we examined the effects of OA on the morphology of SH-EP neuroblastoma cells. The initial effect of OA is rounding up of cells and loss of substrate adhesion. These effects are associated with disorganization of the actin cytoskeleton, loss of focal adhesions, tyrosine dephosphorylation of FAK, and degradation of paxillin. At later times, OA induces apoptosis, which is associated with cleavage of FAK, caspase-3, and other signaling proteins, as well as the accumulation of ubiquitinated protein. Thus, tyrosine dephosphorylation and proteolysis of FAK are temporally distinct events associated with different effects of OA.
The morphological effects of OA treatment in SH-EP cells are similar to the effects of OA in other cell types [25,33,34,69]. However, unlike other reports [25,33,69], we did not observe extensive OA-induced membrane blebbing, a characteristic of cells undergoing apoptosis, which is readily detected in mannitol-treated SH-EP cells [11]. Failure to observe OA-induced membrane blebbing is likely due to a delay in the induction of apoptosis until after the 4-hour time point at which we analyzed cell morphology. In fact, caspase-3 cleavage, protein ubiquitination, and the accumulation of apoptotic cells were apparent only after 8 hours of treatment with OA. Our results are supported by studies of OA-induced apoptosis in other cell types. In ERC-18 renal epithelial cells, OA induces cell detachment within 2 hours, caspase cleavage within 6 hours, and membrane blebbing within 6 to 10 hours [25]. This time course is very similar to our results. In this report, they divided the apoptotic process into early, intermediate, and late events, and suggested that different apoptotic and necrotic pathways are involved in each process. Similarly, human lung fibroblasts do not show caspase-3 activation, DNA fragmentation, or the translocation of phosphatidylserine to the outer plasma membrane leaflet until 12 hours after OA treatment [28]. Finally, OA leads to apoptosis in human oral squamous carcinoma cells after 24 hours [26]. These results, along with our findings, suggest that the induction of apoptosis by OA is indirect, occurring subsequent to a loss of focal adhesions and substrate attachment. This is consistent with an anoikis-like death, in which contact-dependent cells undergo apoptosis after loss of substrate attachment [12]. However, it is still possible that other apoptotic signals that we did not test, including p53 activation [31] or expression of Fas ligand and its receptor [26], are also involved in OA-induced apoptosis in our cells.
The biochemical changes in FAK support our hypothesis that OA-induced apoptosis occurs subsequent to substrate detachment similar to anoikis. OA treatment caused a rapid dephosphorylation of FAK on tyrosine at times (3–4 hours after OA addition) when focal adhesions and the actin cytoskeleton are disrupted. We also observed the degradation, tyrosine dephosphorylation, and apparent serine/threonine phosphorylation of paxillin at these early time points. Such a connection between cell detachment, cytoskeletal disorganization, and tyrosine dephosphorylation of FAK and paxillin is in agreement with our previous findings and many other reports [22,70]. Integrin stimulation prevents apoptosis in numerous cell types [63], potentially through increased attachment of cells to their substrate. In fact, we have previously shown that β1 integrin prevents mannitol-induced apoptosis in SH-EP neuroblastoma cells [71]. However, adhesion of SH-EP cells to fibronectin, the primary extracellular matrix substrate for β1 integrin, did not prevent OAinduced dephosphorylation of FAK Tyr397. Therefore, neither IGF-IR expression and activation, nor integrin stimulation is effective at preventing OA-induced apoptosis.
FAK, Akt, and MAP kinase are degraded at later time points (8–24 h after OA addition) when caspase-3 is cleaved and activated, protein ubiquitination is increased, and apoptosis is readily observed. FAK and Akt cleavage may be carried out directly by activated caspase-3 [22,72,73]. Levkau et al. [73] noted that cleavage of FAK appears to precede and contribute to cellular detachment during apoptosis. Similarly, caspase-dependent cleavage of focal adhesion proteins leads to cellular detachment during neuronal apoptosis [74]. However, in other cells, cellular detachment and loss of focal adhesions are not due to caspase cleavage of FAK, but rather, caspase-mediated cleavage of FAK occurs after the loss of focal adhesions [22]. Similarly, we observe FAK degradation only when apoptosis is already occurring (8–24 h after OA addition), not during the loss of focal adhesion and cell-substrate attachment (3–4 h after OA addition).
OA-induced dephosphorylation FAK Tyr397 was irreversible, and occurred prior to the observed proteolytic cleavage of FAK. This suggests that OA causes some other permanent change in FAK or another upstream signaling molecule. One likely candidate is the degradation of paxillin observed in parallel with FAK Tyr397 dephosphorylation. Indeed, paxillin is thought to target FAK to focal adhesions [45], and its destruction could account for the loss of FAK activation and focal adhesion function. A similar degradation of paxillin preceding apoptosis has been observed in neuronoma 476-16 cells [75].
Phosphorylation of FAK at Tyr397 is thought to suppress apoptosis by binding and activating phosphatidylinositol-3-kinase, which, in turn, stimulates the antiapoptotic activity of Akt [14,21,76,77]. Therefore, the OA-induced tyrosine dephosphorylation of FAK Tyr397 may cause apoptosis by reducing the activity of phosphatidylinositol-3-kinase. This is exacerbated by the proteolytic degradation of FAK and Akt that occurs after apoptotic pathways are initiated.
Like integrin activation, IGF-IR activation leads to downstream activation of the phosphatidylinositol-3-kinase/Akt pathway and provides a signal for cellular survival [40]. Indeed, in our previous [11] and current studies, we showed that IGF-I treatment prevents mannitol-induced apoptosis, FAK cleavage and dephosphorylation, caspase-3 activation, and protein ubiquitination. However, IGF-I was unable to prevent the events induced by OA. This contrasts with the ability of IGF-I to prevent OA-induced apoptosis in mouse embryo fibroblasts [43], or in rat cerebellar neurons [42]. One possible explanation for this difference may be the concentrations of OA used. Specifically, the studies in mouse embryo fibroblasts and rat cerebellar neurons utilized 5 to 25 nM OA, whereas we used 50 to 500 nM OA. For this reason, we examined the effect of 25 nM OA. Although it takes more than 48 hours for 25 nM OA to cause morphological changes in SH-EP cells, IGF-I is ineffective at preventing the effects of this concentration of OA (data not shown). Therefore, it is more likely that the differences are due to cell-specific sensitivity to OA responses to IGF-I or focal adhesion components. Another possibility is that OA targets the IGF-IR, interfering with its tyrosine kinase activity, as occurs with ethanol treatment in SH-SY5Y neuroblastoma cells [78,79].
OA may cause changes in FAK phosphorylation and function by blocking PP1, PP2A, or both phosphatases. FAK associates with PP1 [80], and the phosphorylation of FAK on serine disrupts its association with other focal adhesion proteins [81,82]. However, we did not observe an increase in the phosphorylation of FAK on Ser722, nor did we observe a reduction in its mobility on sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). This suggests that OA does not enhance the phosphorylation of FAK on serine or threonine. A second possibility is that OA stimulates FAK tyrosine dephosphorylation via increased serine phosphorylation of the tyrosine phosphatase SHP2 (also known as PTP1D or Syp) [83,84]. As mentioned previously, tyrosine dephosphorylation of FAK can disrupt signaling via the phosphatidylinositol-3-kinase/Akt survival pathway. Although this may contribute to FAK dephosphorylation, this does not explain the irreversibility of OA-induced FAK tyrosine dephosphorylation.
A more plausible mechanism explanation for the effects of OA involves changes in paxillin. OA inhibits PP2A, which is known to cause the hyperphosphorylation of paxillin on serine [81]. In fact, we found that OA reduces the mobility of paxillin onSDS-PAGE,consistent with its hyperphosphorylation on serine. Serine phosphorylation of paxillin, in turn, can disrupt the formation of FAK/Src/paxillin complexes, thereby preventing FAK activation and signaling [81]. Interestingly, we found that the apparent serine phosphorylation of paxillin also coincides with its degradation. We therefore speculate that OA-induced serine phosphorylation targets paxillin for destruction, in a manner similar to NFκB [85]. The degradation of paxillin, in turn, would prevent FAK from associating with focal adhesions, blocking FAK activation and autophosphorylation. This simple model could explain why the effects of OA on FAK tyrosine phosphorylation are irreversible and insensitive to IGF-IR activation.
In summary, we have shown that OA causes both degradation and dephosphorylation of FAK on Tyr397. Dephosphorylation of FAK Tyr397 coincides with a loss of focal adhesions, disorganization of the actin cytoskeleton, degradation of paxillin, cell rounding, and detachment from the substrate. The degradation of FAK, however, follows the loss of cell-substrate attachment and occurs during active apoptosis and a general breakdown of signaling molecules including Akt and MAP kinase. The effects of OA on FAK Tyr397 phosphorylation cannot be prevented by IGF-I and are irreversible. Therefore, it appears that OA causes a permanent change in an upstream signaling molecule, perhaps degradation of paxillin, which is responsible for the loss in FAK activation and tyrosine phosphorylation. Finally, these studies suggest that OA causes apoptosis primarily by disrupting cell adhesion.
Acknowledgements
The authors thank Tracy Schwab for helpful discussion and Judith Boldt for editorial and secretarial assistance.
Footnotes
This work was supported by the National Institutes of Health (NS38849 and NS36778), the Juvenile Diabetes Research Foundation Center for the Study of Complications in Diabetes, and the Program for Understanding Neurological Diseases (PFUND).
References
- 1.Bown N. Neuroblastoma tumour genetics: clinical and biological aspects. J Clin Pathol. 2001;54:897–910. doi: 10.1136/jcp.54.12.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Westermann F, Schwab M. Genetic parameters of neuroblastomas. Cancer Lett. 2002;184:127–147. doi: 10.1016/s0304-3835(02)00199-4. [DOI] [PubMed] [Google Scholar]
- 3.Philip T. Overview of current treatment of neuroblastoma. Am J Pediatr Hematol Oncol. 1992;14:97–102. doi: 10.1097/00043426-199205000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Tanabe M, Ohnuma N, Iwai J, Yoshida H, Takahashi H, Maie M, Etoh K, Kawamura K. Bone marrow metastasis of neuroblastoma analyzed by MRI and its influence on prognosis. Med Pediatr Oncol. 1995;24:292–299. doi: 10.1002/mpo.2950240505. [DOI] [PubMed] [Google Scholar]
- 5.DuBois SG, Kalika Y, Lukens JN, Brodeur GM, Seeger RC, Atkinson GM, Haase GM, Black CT, Perez C, Shimada H, Gerbing R, Stram DO, Matthay KK. Metastatic sites in stage IV and IVS neuroblastoma correlate with age, tumor biology, and survival. J Pediatr Hematol Oncol. 1999;21:181–189. doi: 10.1097/00043426-199905000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Kim PK, Mahidhara R, Seol DW. The role of caspase-8 in resistance to cancer chemotherapy. Drug Resist Updat. 2001;4:293–296. doi: 10.1054/drup.2001.0223. [DOI] [PubMed] [Google Scholar]
- 7.Teitz T, Lahti JM, Kidd VJ. Aggressive childhood neuroblastomas do not express caspase-8: an important component of programmed cell death. J Mol Med. 2001;79:428–436. doi: 10.1007/s001090100233. [DOI] [PubMed] [Google Scholar]
- 8.Matthews CC, Feldman EL. Insulin-like growth factor I rescues SH-SY5Y human neuroblastoma cells from hyperosmotic induced programmed cell death. J Cell Physiol. 1996;166:323–331. doi: 10.1002/(SICI)1097-4652(199602)166:2<323::AID-JCP10>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 9.van Golen CM, Feldman L. Insulin-like growth factor I is the key growth factor in serum that protects neuroblastoma cells from hyperosmotic-induced apoptosis. J Cell Physiol. 2000;182:24–32. doi: 10.1002/(SICI)1097-4652(200001)182:1<24::AID-JCP3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 10.van Golen CM, Castle VP, Feldman EL. IGF-I receptor activation and Bcl-2 overexpression prevent early apoptotic events in human neuroblastoma. Cell Death Differ. 2000;7:654–665. doi: 10.1038/sj.cdd.4400693. [DOI] [PubMed] [Google Scholar]
- 11.Kim B, Feldman EL. Insulin-like growth factor I prevents mannitol-induced degradation of focal adhesion kinase and Akt. J Biol Chem. 2002;277:27393–27400. doi: 10.1074/jbc.M201963200. [DOI] [PubMed] [Google Scholar]
- 12.Kornberg L, Earp HS, Parsons JT, Schaller M, Juliano RL. Cell adhesion or integrin clustering increases phosphorylation of a focal adhesion-associated tyrosine kinase. J Biol Chem. 1992;267:23439–23442. [PubMed] [Google Scholar]
- 13.Frisch SM, Ruoslahti E. Integrins and anoikis. Curr Opin Cell Biol. 1997;9:701–706. doi: 10.1016/s0955-0674(97)80124-x. [DOI] [PubMed] [Google Scholar]
- 14.Frisch SM, Vuori K, Ruoslahti E, Chan-Hui PY. Control of adhesion-dependent cell survival by focal adhesion kinase. J Cell Biol. 1996;134:793–799. doi: 10.1083/jcb.134.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hungerford JE, Compton MT, Matter ML, Hoffstrom BG, Otey CA. Inhibition of pp125FAK in cultured fibroblasts results in apoptosis. J Cell Biol. 1996;135:1383–1390. doi: 10.1083/jcb.135.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schlaepfer DD, Hauck CR, Sieg DJ. Signaling through focal adhesion kinase. Prog Biophys Mol Biol. 1999;71:435–478. doi: 10.1016/s0079-6107(98)00052-2. [DOI] [PubMed] [Google Scholar]
- 17.Rankin S, Rozengurt E. Platelet-derived growth factor modulation of focal adhesion kinase (p125FAK) and paxillin tyrosine phosphorylation in Swiss 3T3 cells. Bell-shaped dose response and cross-talk with bombesin. J Biol Chem. 1994;269:704–710. [PubMed] [Google Scholar]
- 18.Casamassima A, Rozengurt E. Insulin-like growth factor I stimulates tyrosine phosphorylation of p130(Cas), focal adhesion kinase, and paxillin. Role of phosphatidylinositol 3′-kinase and formation of a p130(Cas) Crk complex. J Biol Chem. 1998;273:26149–26156. doi: 10.1074/jbc.273.40.26149. [DOI] [PubMed] [Google Scholar]
- 19.Xu LH, Owens LV, Sturge GC, Yang X, Liu ET, Craven RJ, Cance WG. Attenuation of the expression of the focal adhesion kinase induces apoptosis in tumor cells. Cell Growth Differ. 1996;7:413–418. [PubMed] [Google Scholar]
- 20.Chan PC, Lai JF, Cheng CH, Tang MJ, Chiu CC, Chen HC. Suppression of ultraviolet irradiation-induced apoptosis by overexpression of focal adhesion kinase in Madin-Darby canine kidney cells. J Biol Chem. 1999;274:26901–26906. doi: 10.1074/jbc.274.38.26901. [DOI] [PubMed] [Google Scholar]
- 21.Sonoda Y, Matsumoto Y, Funakoshi M, Yamamoto D, Hanks SK, Kasahara T. Anti-apoptotic role of focal adhesion kinase (FAK). Induction of inhibitor-of-apoptosis proteins and apoptosis suppression by the overexpression of FAK in a human leukemic cell line, HL-60. J Biol Chem. 2000;275:16309–16315. doi: 10.1074/jbc.275.21.16309. [DOI] [PubMed] [Google Scholar]
- 22.van de Water B, Nagelkerke JF, Stevens JL. Dephosphorylation of focal adhesion kinase (FAK) and loss of focal contacts precede caspase-mediated cleavage of FAK during apoptosis in renal epithelial cells. J Biol Chem. 1999;274:13328–13337. doi: 10.1074/jbc.274.19.13328. [DOI] [PubMed] [Google Scholar]
- 23.Kabir J, Lobo M, Zachary I. Staurosporine induces endothelial cell apoptosis via focal adhesion kinase dephosphorylation and focal adhesion disassembly independent of focal adhesion kinase proteolysis. Biochem J. 2002;367:145–155. doi: 10.1042/BJ20020665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elegbede JA, Hayes K, Schell K, Oberley TD, Verma AK. Induction of apoptosis and inhibition of papilloma formation may signal a new role for okadaic acid. Life Sci. 2002;71:421–436. doi: 10.1016/s0024-3205(02)01701-0. [DOI] [PubMed] [Google Scholar]
- 25.Kolb TM, Chang SH, Davis MA. Biochemical and morphological events during okadaic acid-induced apoptosis of Tsc2-null ERC-18 cell line. Toxicol Pathol. 2002;30:235–246. doi: 10.1080/019262302753559579. [DOI] [PubMed] [Google Scholar]
- 26.Goto K, Fukuda J, Haneji T. Okadaic acid stimulates apoptosis through expression of Fas receptor and Fas ligand in human oral squamous carcinoma cells. Oral Oncol. 2002;38:16–22. doi: 10.1016/s1368-8375(00)00134-2. [DOI] [PubMed] [Google Scholar]
- 27.Fujita M, Seta C, Fukuda J, Kobayashi S, Haneji T. Induction of apoptosis in human oral squamous carcinoma cell lines by protein phosphatase inhibitors. Oral Oncol. 1999;35:401–408. doi: 10.1016/s1368-8375(99)00013-5. [DOI] [PubMed] [Google Scholar]
- 28.Leira F, Vieites JM, Vieytes MR, Botana LM. Apoptotic events induced by the phosphatase inhibitor okadaic acid in normal human lung fibroblasts. Toxicol In Vitro. 2001;15:199–208. doi: 10.1016/s0887-2333(01)00013-3. [DOI] [PubMed] [Google Scholar]
- 29.Kim DH, Hong HN, Lee JH, Park HS. Okadaic acid induces cycloheximide and caspase sensitive apoptosis in immature neurons. Mol Cell. 2000;10:83–89. doi: 10.1007/s10059-000-0083-8. [DOI] [PubMed] [Google Scholar]
- 30.Nuydens R, De Jong M, Van Den Kieboom G, Heers C, Dispersyn G, Cornelissen F, Nuyens R, Borgers M, Geerts H. Okadaic acid-induced apoptosis in neuronal cells: evidence for an abortive mitotic attempt. J Neurochem. 1998;70:1124–1133. doi: 10.1046/j.1471-4159.1998.70031124.x. [DOI] [PubMed] [Google Scholar]
- 31.Long X, Wu G, Gaa ST, Rogers TB. Inhibition of protein phosphatase-1 is linked to phosphorylation of p53 and apoptosis. Apoptosis. 2002;7:31–39. doi: 10.1023/a:1013508811252. [DOI] [PubMed] [Google Scholar]
- 32.Rossini GP, Sgarbi N, Malaguti C. The toxic responses induced by okadaic acid involve processing of multiple caspase isoforms. Toxicon. 2001;39:763–770. doi: 10.1016/s0041-0101(00)00202-6. [DOI] [PubMed] [Google Scholar]
- 33.Fiorentini C, Matarrese P, Fattorossi A, Donelli G. Okadaic acid induces changes in the organization of F-actin in intestinal cells. Toxicon. 1996;34:937–945. doi: 10.1016/0041-0101(96)00025-6. [DOI] [PubMed] [Google Scholar]
- 34.Leira F, Alvarez C, Vieites JM, Vieytes MR, Botana LM. Study of cytoskeletal changes induced by okadaic acid in BE(2)-M17 cells by means of a quantitative fluorimetric microplate assay. Toxicol In Vitro. 2001;15:277–282. doi: 10.1016/s0887-2333(01)00021-2. [DOI] [PubMed] [Google Scholar]
- 35.Singleton JR, Randolph AE, Feldman EL. Insulin-like growth factor I receptor prevents apoptosis and enhances neuroblastoma tumorigenesis. Cancer Res. 1996;56:4522–4529. [PubMed] [Google Scholar]
- 36.Kim B, Leventhal PS, Saltiel AR, Feldman EL. Insulin-like growth factor-I-mediated neurite outgrowth in vitro requires MAP kinase activation. J Biol Chem. 1997;272:21268–21273. doi: 10.1074/jbc.272.34.21268. [DOI] [PubMed] [Google Scholar]
- 37.Leventhal PS, Shelden EA, Kim B, Feldman EL. Tyrosine phosphorylation of paxillin and focal adhesion kinase during insulin-like growth factor-I-stimulated lamellipodial advance. J Biol Chem. 1997;272:5214–5218. doi: 10.1074/jbc.272.8.5214. [DOI] [PubMed] [Google Scholar]
- 38.Russell JW, Feldman EL. Insulin-like growth factor-I prevents apoptosis in sympathetic neurons exposed to high glucose. Horm Metab Res. 1998;31:90–96. doi: 10.1055/s-2007-978704. [DOI] [PubMed] [Google Scholar]
- 39.Matews CC, Odeh H, Feldman EL. Insulin-like growth factor-I is an osmoprotectant in human neuroblastoma cells. Neuroscience. 1997;79:525–534. doi: 10.1016/s0306-4522(96)00611-2. [DOI] [PubMed] [Google Scholar]
- 40.Delaney CL, Russel JW, Cheng H-L, Feldman EL. Insulinlike growth factor-I and over expression of Bcl-xL prevent glucosemediated apoptosis in Schwann cells. J Neuropathol Exp Neurol. 2001;60:147–160. doi: 10.1093/jnen/60.2.147. [DOI] [PubMed] [Google Scholar]
- 41.Leira F, Alvarez C, Vieites JM, Vieytes MR, Botana LM. Characterization of distinct apoptotic changes induced by okadaic acid and yessotoxin in the BE(2)-M17 neuroblastoma cell line. Toxicol In Vitro. 2002;16:23–31. doi: 10.1016/s0887-2333(01)00095-9. [DOI] [PubMed] [Google Scholar]
- 42.Fernandez-Sanchez MT, Garcia-Rodriguez A, Diaz-Trelles R, Novelli A. Inhibition of protein phosphatases induces IGF-1-blocked neurotrophin-insensitive neuronal apoptosis. FEBS Lett. 1996;398:106–112. doi: 10.1016/s0014-5793(96)01192-1. [DOI] [PubMed] [Google Scholar]
- 43.D'Ambrosio C, Valentinis B, Prisco M, Reiss K, Rubini M, Baserga R. Protective effect of the insulin-like growth factor I receptor on apoptosis induced by okadaic acid. Cancer Res. 1997;57:3264–3271. [PubMed] [Google Scholar]
- 44.Parsons JT, Schaller MD, Hildebrand J, Leu T-H, Richardson A, Otey C. Focal adhesion kinase: structure and signalling. J Cell Sci. 1994;107(Suppl 18):109–113. doi: 10.1242/jcs.1994.supplement_18.16. [DOI] [PubMed] [Google Scholar]
- 45.Turner CE. Paxillin and focal adhesion signalling. Nat Cell Biol. 2000;2:E231–E236. doi: 10.1038/35046659. [DOI] [PubMed] [Google Scholar]
- 46.Zumkeller W, Schwab M. Insulin-like growth factor system in neuroblastoma tumorigenesis and apoptosis: potential diagnostic and therapeutic perspectives. Horm Metab Res. 1999;31:138–141. doi: 10.1055/s-2007-978711. [DOI] [PubMed] [Google Scholar]
- 47.Baserga R. The insulin-like growth factor I receptor: a key to tumor growth? Cancer Res. 1995;55:249–252. [PubMed] [Google Scholar]
- 48.Kim B, Cheng H-L, Margolis B, Feldman EL. Insulin receptor substrate 2 and Shc play different roles in insulin-like growth factor I signaling. J Biol Chem. 1998;273:34543–34550. doi: 10.1074/jbc.273.51.34543. [DOI] [PubMed] [Google Scholar]
- 49.Kim B, Feldman EL. Differential regulation of focal adhesion kinase and mitogen-activated protein kinase tyrosine phosphorylation during insulin-like growth factor-I-mediated cytoskeletal reorganization. J Neurochem. 1998;71:1333–1336. doi: 10.1046/j.1471-4159.1998.71031333.x. [DOI] [PubMed] [Google Scholar]
- 50.Leventhal PS, Feldman EL. Insulin-like growth factors as regulators of cell motility: signaling mechanisms. Trends Endocrinol Metab. 1997;8:1–6. doi: 10.1016/s1043-2760(96)00202-0. [DOI] [PubMed] [Google Scholar]
- 51.De Meyts P, Wallach B, Christoffersen CT, Urso B, Gronskov K, Latus L-J, Yakushiji F, Ilondo MM, Shymko RM. The insulin-like growth factor-I receptor. Structure, ligand-binding mechanism and signal transduction. Horm Res. 1994;42:152–169. doi: 10.1159/000184188. [DOI] [PubMed] [Google Scholar]
- 52.Cheatham B, Kahn CR. Insulin action and insulin signaling network. Endocr Rev. 1995;16:117–142. doi: 10.1210/edrv-16-2-117. [DOI] [PubMed] [Google Scholar]
- 53.Miller TM, Tansey MG, Johnson EM, Jr, Creedon DJ. Inhibition of phosphatidylinositol 3-kinase activity blocks depolarizationand insulin-like growth factor I-mediated survival of cerebellar granule cells. J Biol Chem. 1997;272:9847–9853. doi: 10.1074/jbc.272.15.9847. [DOI] [PubMed] [Google Scholar]
- 54.Liu X, Turbyville T, Fritz A, Whitesell L. Inhibition of insulin-like growth factor I receptor expression in neuroblastoma cells induces the regression of established tumors in mice. Cancer Res. 1998;58:5432–5438. [PubMed] [Google Scholar]
- 55.Reiss K, D'Ambrosio C, Tu X, Tu C, Baserga R. Inhibition of tumor growth by a dominant negative mutant of the insulin-like growth factor I receptor with a bystander effect. Clin Cancer Res. 1998;4:2647–2655. [PubMed] [Google Scholar]
- 56.Humbel RE. Insulin-like growth factors I and II. Eur J Biochem. 1990;190:445–462. doi: 10.1111/j.1432-1033.1990.tb15595.x. [DOI] [PubMed] [Google Scholar]
- 57.Ivaska J, Nissinen L, Immonen N, Eriksson JE, Kahari VM, Heino J. Integrin alpha 2 beta 1 promotes activation of protein phosphatase 2A and dephosphorylation of Akt and glycogen synthase kinase 3 beta. Mol Cell Biol. 2002;22:1352–1359. doi: 10.1128/mcb.22.5.1352-1359.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Delaney CL, Cheng H-L, Feldman EL. Insulin-like growth factor-I prevents caspase mediated apoptosis in Schwann cells. J Neurobiol. 1999;41:540–548. doi: 10.1002/(sici)1097-4695(199912)41:4<540::aid-neu9>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 59.Widmann C, Gibson S, Johnson GL. Caspase-dependent cleavage of signaling proteins during apoptosis. J Biol Chem. 1998;273:7141–7147. doi: 10.1074/jbc.273.12.7141. [DOI] [PubMed] [Google Scholar]
- 60.Li DW, Xiang H, Mao YW, Wang J, Fass U, Zhang XY, Xu C. Caspase-3 is actively involved in okadaic acid-induced lens epithelial cell apoptosis. Exp Cell Res. 2001;266:279–291. doi: 10.1006/excr.2001.5223. [DOI] [PubMed] [Google Scholar]
- 61.Wojcik C. Proteasomes in apoptosis: villains or guardians? Cell Mol Life Sci. 1999;56:908–917. doi: 10.1007/s000180050483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Orlowski RZ. The role of the ubiquitin-proteasome pathway in apoptosis. Cell Death Differ. 1999;6:303–313. doi: 10.1038/sj.cdd.4400505. [DOI] [PubMed] [Google Scholar]
- 63.Frisch SM, Screaton RA. Anoikis mechanisms. Curr Opin Cell Biol. 2001;13:555–562. doi: 10.1016/s0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
- 64.Wang P, Valentijn AJ, Gilmore AP, Streuli CH. Early events in the anoikis programme occur in the absence of caspase activation. J Biol Chem. 2003;278:19917–19925. doi: 10.1074/jbc.M210337200. [DOI] [PubMed] [Google Scholar]
- 65.Ruoslahti E. Fibronectin and its integrin receptors in cancer. Adv Cancer Res. 1999;76:1–20. doi: 10.1016/s0065-230x(08)60772-1. [DOI] [PubMed] [Google Scholar]
- 66.Bachelder RE, Wendt MA, Fujita N, Tsuruo T, Mercurio AM. The cleavage of Akt/protein kinase B by death receptor signaling is an important event in detachment-induced apoptosis. J Biol Chem. 2001;276:34702–34707. doi: 10.1074/jbc.M102806200. [DOI] [PubMed] [Google Scholar]
- 67.Valentinis B, Reiss K, Baserga R. Insulin-like growth factor-I-mediated survival from anoikis: role of cell aggregation and focal adhesion kinase. J Cell Physiol. 1998;176:648–657. doi: 10.1002/(SICI)1097-4652(199809)176:3<648::AID-JCP22>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 68.Prisco M, Romano G, Peruzzi F, Valentinis B, Baserga R. Insulin and IGF-I receptors signaling in protection from apoptosis. Horm Metab Res. 1999;31:80–89. doi: 10.1055/s-2007-978703. [DOI] [PubMed] [Google Scholar]
- 69.Boe R, Gjertsen BT, Vintermyr OK, Houge G, Lanotte M, Doskeland SO. The protein phosphatase inhibitor okadaic acid induces morphological changes typical of apoptosis in mammalian cells. Exp Cell Res. 1991;195:237–246. doi: 10.1016/0014-4827(91)90523-w. [DOI] [PubMed] [Google Scholar]
- 70.Parsons JT, Martin KH, Slack JK, Taylor JM, Weed SA. Focal adhesion kinase: a regulator of focal adhesion dynamics and cell movement. Oncogene. 2000;19:5606–5613. doi: 10.1038/sj.onc.1203877. [DOI] [PubMed] [Google Scholar]
- 71.van Golen CM, Soules ME, Grauman AR, Feldman EL. N-Myc overexpression leads to decreased b1 integrin expression and increased apoptosis in human neuroblastoma cells. Oncogene. 2003;22:2664–2673. doi: 10.1038/sj.onc.1206362. [DOI] [PubMed] [Google Scholar]
- 72.Gervais FG, Thornberry NA, Ruffolo SC, Nicholson DW, Roy S. Caspases cleave focal adhesion kinase during apoptosis to generate a FRNK-like polypeptide. J Biol Chem. 1998;273:17102–17108. doi: 10.1074/jbc.273.27.17102. [DOI] [PubMed] [Google Scholar]
- 73.Levkau B, Herren B, Koyama H, Ross R, Raines EW. Caspase-mediated cleavage of focal adhesion kinase pp125FAK and disassembly of focal adhesions in human endothelial cell apoptosis. J Exp Med. 1998;187:579–586. doi: 10.1084/jem.187.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lesay A, Hickman JA, Gibson RM. Disruption of focal adhesions mediates detachment during neuronal apoptosis. Neuroreport. 2001;12:2111–2115. doi: 10.1097/00001756-200107200-00014. [DOI] [PubMed] [Google Scholar]
- 75.Marushige Y, Marushige K. Alterations in focal adhesion and cytoskeletal proteins during apoptosis. Anticancer Res. 1998;18:301–307. [PubMed] [Google Scholar]
- 76.Sonoda Y, Kasahara T, Yokota-Aizu E, Ueno M, Watanabe S. A suppressive role of p125FAK protein tyrosine kinase in hydrogen peroxide-induced apoptosis of T98G cells. Biochem Biophys Res Commun. 1997;241:769–774. doi: 10.1006/bbrc.1997.7895. [DOI] [PubMed] [Google Scholar]
- 77.Tamura M, Gu T, Danen EH, Takino T, Miyamoto S, Yamada KM. PTEN interactions with focal adhesion kinase and suppression of the extracellular matrix-dependent phosphatidylinositol 3-kinase/Akt cell survival pathway. J Biol Chem. 1999;274:20693–20703. doi: 10.1074/jbc.274.29.20693. [DOI] [PubMed] [Google Scholar]
- 78.Seiler AE, Ross BN, Rubin R. Inhibition of insulin-like growth factor-1 receptor and IRS-2 signaling by ethanol in SH-SY5Y neuroblastoma cells. J Neurochem. 2001;76:573–581. doi: 10.1046/j.1471-4159.2001.00025.x. [DOI] [PubMed] [Google Scholar]
- 79.Hallak H, Seiler AE, Green JS, Henderson A, Ross BN, Rubin R. Inhibition of insulin-like growth factor-I signaling by ethanol in neuronal cells. Alcohol Clin Exp Res. 2001;25:1058–1064. [PubMed] [Google Scholar]
- 80.Fresu M, Bianchi M, Parsons JT, Villa-Moruzzi E. Cellcycle-dependent association of protein phosphatase 1 and focal adhesion kinase. Biochem J. 2001;358:407–414. doi: 10.1042/0264-6021:3580407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Young MR, Kolesiak K, Meisinger J. Protein phosphatase-2A regulates endothelial cell motility and both the phosphorylation and the stability of focal adhesion complexes. Int J Cancer. 2002;100:276–282. doi: 10.1002/ijc.10491. [DOI] [PubMed] [Google Scholar]
- 82.Yamakita Y, Totsukawa G, Yamashiro S, Fry D, Zhang X, Hanks SK, Matsumura F. Dissociation of FAK/p130(CAS)/c-Src complex during mitosis: role of mitosis-specific serine phosphorylation of FAK. J Cell Biol. 1999;144:315–324. doi: 10.1083/jcb.144.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vadlamudi RK, Adam L, Nguyen D, Santos M, Kumar R. Differential regulation of components of the focal adhesion complex by heregulin: role of phosphatase SHP-2. J Cell Physiol. 2002;190:189–199. doi: 10.1002/jcp.10054. [DOI] [PubMed] [Google Scholar]
- 84.Rocchi S, Gaillard I, Van Obberghen E, Chambaz EM, Vilgrain I. Adrenocorticotrophic hormone stimulates phosphotyrosine phosphatase SHP2 in bovine adrenocortical cells: phosphorylation and activation by cAMP-dependent protein kinase. Biochem J. 2000;352(Part 2):483–490. [PMC free article] [PubMed] [Google Scholar]
- 85.Karin M, Ben Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]