Abstract
2-Methoxyestradiol (2-ME2) was reported to elicit both stimulation and inhibition of tumor angiogenesis and growth depending on the dosage used. However, the mechanism(s) of the biphasic action of 2-ME2 has been elusive. Here we describe a regulatory role of vascular endothelial growth factor-A (VEGF-A) in the biphasic effects on estrogen receptor (ER)+ GH3 rat pituitary tumor cells and MCF-7 human breast tumor cells depending on the dosage of 2-ME2 used. We observed that acute exposure to 2-ME2, irrespective of dosage, did not alter cellular proliferation, but enhanced the VEGF-A mRNA level. As the treatment duration increased, biphasic effect was elicited. A concentration of 1 µM 2-ME2 increased both cell proliferation and VEGF-A levels in these cells, whereas higher doses exhibited reversed impact. A low dose of 2-ME2 also increased the VEGF-A mRNA expression in ER-α-transfected human mammary epithelial cells (HMECs). The effect was reversed in ER- cells. The enhanced expression of VEGF-A mRNA could be blocked by the pure estrogen antagonist, ICI 182,780, and reveal that the upregulation of VEGF-A expression by 2-ME2 is mediated through ER-α. Furthermore, the biphasic effect of 2-ME2 on cell proliferation can be modulated by administrating VEGF-A antibodies or VEGF-A proteins. Studies also demonstrate that the VEGF-A protein, induced by 2-ME2, is functionally active and upregulates the proliferation of adjacent endothelial cells.
Keywords: 2-methoxyestadiol, VEGF, ER-alpha, human mammary epithelial cells, transfection
Introduction
2-Methoxyestradiol (2-ME2) is an endogenous estrogen (i.e., 17β-estradiol) metabolite produced by sequential hydroxylation of parent compounds followed by methylation by catechol-O-methyltransferase in the liver [1–3]. 2-ME2 is synthesized in mammalian tissues and represents a crucial step in the elimination of potentially damaging catechol estrogen from proliferating cells [4]. It has strong binding affinity for sex hormone-binding globulin (SHBG) in the blood [2,5]. However, unlike the parent compounds and their other metabolites, this molecule has a low affinity for estrogen receptors (ERs), thus having minimal estrogenic activity [1,2]. 2-ME2 has been considered as a promising anticancer drug candidate [6] as it reveals antiproliferative and apoptotic activities against rapidly growing tumors and endothelial cells by triggering the induction or suppression of genes that elicit apoptosis or cell proliferation [3,7–12]. 2-ME2 reduced the cell motility, migration, and adhesion of various drug-sensitive and drug-resistant tumor cell lines [13]. It suppressed the growth of certain murine tumors by inhibiting tumor cell proliferation and angiogenesis [10,14,15]. Furthermore, a Phase I clinical trial of this compound showed no serious drug-related unpleasant effects, while exhibiting a significant reduction in bone pain and analgesic intake in some breast cancer patients [6]. A Phase II randomized trial of 2-ME2 in hormone-refractory prostate cancer patients demonstrated the stabilization and/or reduction of prostate-specific antigen after treatment [6].
Despite the potential anticancer features of 2-ME2, recent studies have revealed dose-dependent dual effects of this compound. For example, 1-µM doses (or less) of 2-ME2 exhibit proliferative effects in MCF-7 breast tumor cells, whereas higher doses of 2-ME2 inhibit cell proliferation [12]. Similarly, in vivo studies have shown both stimulation and inhibition of tumor growth by this compound depending on the dosage used [14,15]. Together, these studies suggest a complex nature of action of this molecule, and indicate that in addition to the known signaling pathways [6,16–20], there may be additional pathways, which have not yet been elucidated, that exert biphasic effects of 2-ME2.
2-ME2 is a potent inhibitor of estrogen-induced tumor angiogenesis and tumorigenesis in F344 rat pituitaries, and this inhibitory activity is strongly associated with the downregulation of vascular endothelial growth factor-A (VEGF-A) expression [14]. Subsequently, similar results were observed by other studies in the SCID mouse model [21], and suggested that the inhibition of the local release of VEGF-A was an important event required to block tumor growth. However, the direct involvement of VEGF-A during the dose-dependent modulation of tumor cell growth and proliferation by 2-ME2 is unknown. The aim of the present study was to investigate the dose-dependent effect of 2-ME2 on VEGF-A expression in different cell lines, either ER+ or ER-, and to correlate these results with cell proliferation to determine if ER was involved in this process. Moreover, an additional aim was to evaluate the protective role of VEGFA, if any, against cell death exerted by 2-ME2.
Materials and Methods
Chemicals
2-ME2 was a gift from EntreMed, Inc. (Rockville, MD). A stock solution was prepared in absolute ethanol and stored at -20°C. Dulbecco's modified Eagle's medium (DMEM), human recombinant VEGF-A protein, VEGF monoclonal antibody, and cell dissociation solution were purchased from Sigma (St. Louis, MO). Fetal bovine serum (FBS) was purchased from HyClone Laboratories (Logan, UT). BrdU enzyme-linked immunosorbent assay (ELISA) kits and digoxigenin (DIG) high-prime DNA labeling kits were purchased from Roche Applied Science (Indianapolis, IN). [3H]Thymidine (specific activity: 84,000 mCi/mmol) was purchased from Amersham Life Science (Arlington, IL).
Cell Lines and Culture Conditions
GH3 rat pituitary tumor cells, human breast tumor-derived MCF-7 epithelial tumor cells, and MIA-PaCa-2 pancreatic adenocarcinoma cells were obtained from the American Type Cell Culture Collection (ATCC; Rockville, MD). ER-α-transfected or vehicle-transfected or nontransformed mammary epithelial cells were gifts from Dr. Deborah Zajchowski (Berlex Biosciences, Richmond, CA), Bovine capillary endothelial cells (BCECs) were kindly provided by Dr. Dipak K. Banerjee (University of Puerto Rico, San Juan, PR). GH3 cells were routinely maintained in DMEM supplemented with 15% horse serum and 2.5% FBS. MCF-7 cells and BCECs were grown in DMEM, supplemented with 10% FBS and 1% penicillin-streptomycin (Sigma). Transfected cells were grown in chemically defined medium. Cells were passed weekly, at a split ratio of 1:4, and subcultured into 25-cm2 T-flasks. The cultures were maintained in a humidified incubator with 5% CO2 at 37°C.
[3H]Thymidine Incorporation Assay
[3H]Thymidine was added to each control and treated well at a rate of 0.5 µCi/ml (1 µl/well) 24 hours prior to harvesting the cells. Cells were harvested in glass fiber filter papers using a Millipore vacuum system (Millipore, Bedford, MA) and radioactive thymidine incorporation was measured using a liquid scintillation counter.
BrdU Cell Proliferation Assay
The BrdU cell proliferation assay was performed according to the instructions provided by the manufacturer. Briefly, experimental cells were exposed to BrdU for 24 hours, and then fixed in FixDenat solution for 20 minutes at room temperature. Cells were incubated with anti-BrdU-POD solution for 90 minutes at room temperature followed by washing three times with washing solution. Cells were incubated with substrate solution until color development was sufficient for photometric detection.
mRNA Extraction and Northern Blot Analysis
Total RNA from different cells was extracted using the Trizol reagent (Life Technologies, Grand Island, NY) extraction procedure, as described previously [22]. Ten micrograms of total RNA was fractionated by electrophoresis in 1% agarose gels containing formaldehyde and transferred to super charge nylon membrane (Schleicher and Schuell, Keene, NH). Membranes were hybridized with nonradioactive polymerase chain reaction-generated DIG-labeled VEGF-A and glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) gene-specific probes and washed according to the protocol provided by the manufacturer of the DIG high-prime DNA labeling and detection kit. VEGF-A and GAPDH mRNA expression were quantitated by densitometric analyses using the Gel Expert software program (NucleoTech, San Francisco, CA).
Western Blot Analysis
Immunoreactive proteins corresponding to VEGF or actin were identified from total protein by Western immunobloting using specific monoclonal antibodies. The protein extraction and Western blot technique were essentially carried out as described previously [23]. Signals were quantitated by scanning the film using Scan Jet scanner, and the intensity of each band was measured using the Gel Expert software (NeucleoTech).
Indirect Coculture
Indirect coculture was performed using the Transwell culture system (0.4 µm pore size; Corning Coster, Cambridge, MA). BCECs (1 x 105) were seeded in the lower chamber, and allowed to grow in DMEM with 10% FBS until the culture became 60% confluent before being maintained in phenol red-free DMEM devoid of FBS for 24 hours. In a separate culture, GH3 cells were grown in DMEM with 10% FBS in the presence or absence of 1 or 5 µM 2-ME2 for 3 days. Following 2-ME2 treatment, cells were grown in fresh medium containing no serum for 4 hours; the media were collected, centrifuged at 250g for 2 minutes, and added into the upper chambers of the Transwell systems along with detached GH3 cells. The cells were detached by a nonenzymatic cell dissociation solution. The coculture was maintained for 24 hours. Radioactive thymidine was added into the culture prior to 24 hours of the cell proliferation assay. BCECs cultured in the same series without tumor cells in the upper chamber served as negative controls.
Results
Differential Response of 2-ME2 on the Proliferation of ER+ and ER- Tumor Cells
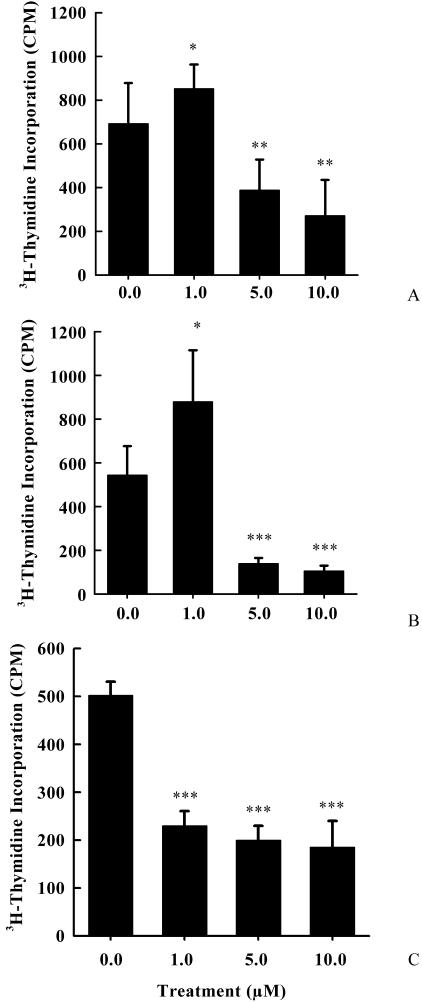
Existing evidence indicates that 2-ME2 perturbs the proliferation of different cells, including a variety of tumor cells, endothelial cells, and vascular smooth muscle cells [17,24]. However, dose-dependent responses are diverged from cell to cells. Comparative studies have demonstrated that the IC50 value for 2-ME2 is different for different cell lines [17,25]. Paradoxically, discrepancies in IC50 values for the same cell type have also been reported [17,25]. Furthermore, recent studies have reported a biphasic effect of 2-ME2 on MCF-7 cell proliferation [12]. These variable findings suggested that the culture conditions might be one of the crucial factors for this discrepancy. Therefore, in the present studies, we first evaluated the impact of 2-ME2 on radioactive thymidine incorporation into the replicating DNA in ER+ GH3 rat pituitary tumor cells and MCF-7 breast tumor cells, and ER- MIA-PaCa-2 pancreatic adenocarcinoma cells. After 3 days, 5 to 10 µM concentrations of 2-ME2 significantly inhibited radioactive thymidine incorporation in both ER+ and ER- tumor cells (Figure 1). The radioactive thymidine incorporation was reduced compared to untreated controls, by 1.8-, 3.9-, and 2.5-fold in 2-ME2 (5 µM)-treated GH3, MCF-7, and MIA-PaCa-2 cells, respectively (Figure 1). In contrast, after 3 days of exposure to 1 µM 2-ME2, radioactive thymidine incorporation was significantly (paired two-tailed Student's t-test) elevated by 1.3-fold in GH3 and 1.6-fold in MCF-7 cells, compared to vehicle-treated cells (Figure 1). The effect was reversed in MIA-PaCa-2 cells.
Figure 1.
Dose-dependent effects of 2-ME2 on cell proliferation in rat pituitary tumor-derived GH3 tumor cells, human breast tumor-derived MCF-7 tumor cells, and human pancreatic adenocarcinoma MIA-PaCa-2 cells. Exponentially growing cells in DMEM containing 10% serum were exposed to various concentrations of 2-ME2 for 3 days, and cell proliferation was determined by measuring the [3H]thymidine incorporation into DNA. (A) GH3 rat pituitary tumor cells. (B) MCF-7 human breast tumor cells. (C) MIA-PaCa-2 pancreatic adenocarcinoma cells. Data displayed as mean ± SD from three separate experiments. P value was determined by Student's t-test. *P < .05 vs control; **P < .01 vs control; ***P < .001 vs control.
The modulation of radioactive thymidine incorporation into the DNA of either cell by 2-ME2 is time-dependent, being undetected within 2 to 24 hours of exposure (data not shown). Moreover, the impact of 2-ME2 on proliferation is also dependent upon the cell density being minimal at higher densities (i.e., 70–80% confluent culture).
Proliferation-Dependent Changes in VEGF-A mRNA and Protein Expression
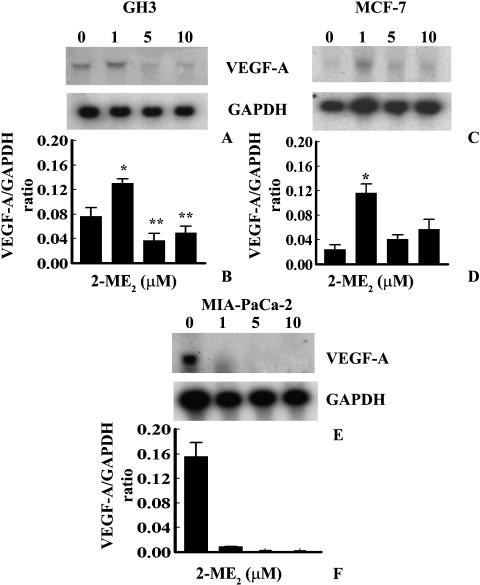
Vascular endothelial growth factors are able to modulate the proliferation of both normal and tumor cells including endothelial cells [26], ductal epithelial cells of the pancreas [27,28], and gastric adenocarcinoma cells [29]. Moreover, our previous studies as well as recent in vitro studies have demonstrated that VEGF-A expression was downregulated by 2-ME2 during the inhibition of estrogen-induced rat pituitary tumor growth [14,30]. We investigated the status of VEGF-A mRNA and proteins during the biphasic effects of 2-ME2 on DNA synthesis in ER+ GH3 rat pituitary tumor cells and MCF-7 human breast tumor cells, and ER- MIA-PaCa-2 pancreatic adenocarcinoma cells. 2-ME2, at 1 µM concentration, significantly enhanced VEGF-A mRNA expression in ER+ GH3 and MCF-7 cells (Figure 2, A–D). In contrast, expression in MIA-PaCa-2 cells was reversed (Figure 2, E and F). Higher concentrations (i.e., 5 or 10 µM concentrations) of 2-ME2 significantly (paired two-tailed Student's t-test) decreased the VEGF-A mRNA expression in GH3 cells and MIA-PaCa-2 cells compared to vehicle-treated controls (Figure 2). Ironically, the effects on VEGF-A mRNA expressions were apparently undetected by Northern blot analyses in MCF-7 cells (Figure 2, C and D). This may be due to the low-level constitutive expression of VEGF-A mRNA.
Figure 2.
Dose-dependent effects of 2-ME2 on VEGF-A mRNA expressions in GH3 rat pituitary tumor cells, MCF-7 human breast tumor cells, and MIA-PaCa-2 human pancreatic adenocarcinoma cells. Exponentially growing cells in DMEM containing 10% serum were exposed to various concentrations of 2-ME2 for 3 days, and VEGF-A mRNA expression was determined by Northern blot analyses using nonradioactive DIG-labeled probe. (A), (C), and (E) represent VEGF-A and GAPDH mRNA levels in untreated and treated GH3, MCF-7, and MIA-PaCa-2 cells respectively. (B), (D), and (F) represent arbitrary values indicating VEGF-A mRNA levels. Data displayed as mean±SD from three separate experiments. P value was determined by Student's t-test. *P < .05 vs control; **P < .01 vs control.
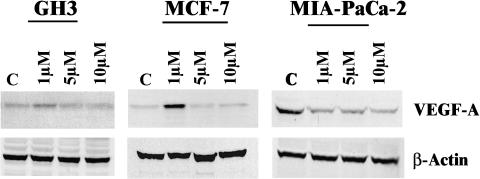
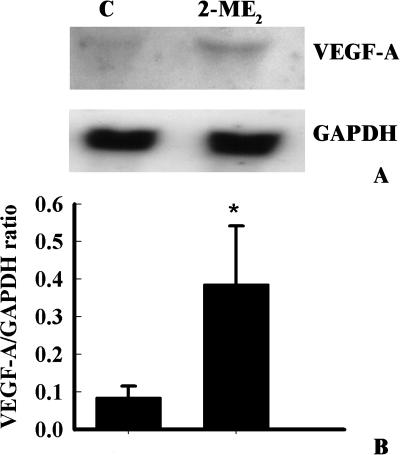
The effect on the expression of VEGF-A at the protein level indicated an induction in GH3 and MCF-7 cells at 1 µM 2-ME2 concentration. In contrast, the impact of 2-ME2 at higher doses was not markedly severe in GH3 and MCF-7 cells as observed in MIA-PaCa-2 cells (Figure 3).
Figure 3.
Chronic effects of various doses of 2-ME2 on the levels of VEGF-A protein in different tumor cell lines. Exponentially growing cells (i.e., GH3 or MCF-7 or MIA-PaCa-2 cells) in DMEM containing 10% serum were exposed to one of the three concentrations (i.e., 1.0, 5.0, and 10.0 µM) of 2-ME2 for 3 days. After exposure, proteins were extracted from different cells and 50 µg of total cell lysates was prepared for Western immunoblot analysis. VEGF-A protein levels were determined by chemiluminescent immunoblotting with monoclonal anti-human VEGF-A. The photograph exhibits a single representative blot showing VEGF-A and β-actin levels in untreated and 2-ME2-treated cells.
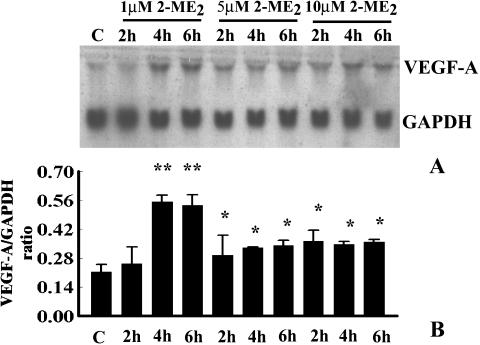
Acute treatment of GH3 cells with various doses (i.e., 1, 5, or 10 µM) of 2-ME2 augments the VEGF-A mRNA levels significantly after 4 hours of exposure, compared to control (Figure 4). The expression was steadily and significantly increased until 24 hours (Figure 4). The impact was maximal at the 1-µM dose (Figure 4B). Similar effects were also observed in MCF-7 cells. However, the effect was undetected in MIA-PaCa-2 cells (data not shown).
Figure 4.
Time- and dose-dependent effects of 2-ME2 on VEGF-A mRNA expression in GH3 cells. Semiconfluent GH3 cells were exposed to various doses of 2-ME2 (i.e., 1, 5, and 10 µM) for different times as indicated; 0.1% ethanol-treated culture was considered as the untreated control (C). After treatment, total RNA was extracted and VEGF-A mRNA expression was determined by Northern blot analyses using nonradioactive DIG-labeled probe. Data displayed as mean ± SD from three separate experiments. (A) Single representative blot showing VEGF-A and GAPDH expressions in untreated and 2-ME2-treated cells. (B) Arbitrary values indicate VEGF-A mRNA level. Data displayed as mean±SD in each case. P value was determined by Student's t-test. P < .01 compared to untreated control.
The Upregulation of VEGF-A mRNA Expression by 2-ME2 Is Meditated Through ERs
To determine whether 2-ME2 (1 µM)-induced upregulation of VEGF-A mRNA expressions in ER+ cells was mediated through an ER, GH3 cells were exposed to a pure antiestrogen, ICI 182,780, concomitantly with 2-ME2 (1 µM) for 3 days (Figure 5). The ICI 182,780 completely precluded the induction of VEGF-A mRNA expression to the basal levels by 2-ME2 in GH3 cells (Figure 5), indicating that the 2-ME2-induced VEGF-A mRNA upregulation is ER-dependent. To further confirm the involvement of ER, the impact of 2-ME2 on ER-α-transfected human mammary epithelial cells (ER-α+ HMECs) was evaluated. ER-α+ HMECs and vector only-transfected cells were exposed to 1 µM 2-ME2 for 3 days, and VEGF-A mRNA levels were determined using Northern blot analyses. As expected, VEGF-A mRNA levels were elevated significantly in ER-α+ HMECs (Figure 6), whereas the effect was reversed in ER-α- HMECs (data not shown). These studies demonstrate that the upregulation of VEGF-A mRNA by 2-ME2 is mediated through ER-α.
Figure 5.
Effect of pure antiestrogen ICI 182,780 on 2-ME2-induced upregulation of VEGF-A mRNA expression in GH3 cells. (A) GH3 cells were exposed to 1 µM 2-ME2 alone or in combination with 1 µM ICI 182,780 for 3 days. Total RNA was isolated and analyzed by Northern blotting using nonradioactive DIG-labeled PCRgenerated probes for VEGF-A and GAPDH. (B) The arbitrary values indicate VEGF-A mRNA concentrations. Data displayed as mean ± SD from three separate experiments. P value was determined by Student's t-test. *P < .01 vs control.
Figure 6.
Effect of 2-ME2 on ER-α-transfected HMECs. (A) ER-α-transfected cells were exposed to 1 µM 2-ME2 or ethanol vehicle for 3 days. Total RNA was isolated and analyzed by Northern blotting using nonradioactive DIG-labeled PCR-generated probes for VEGF-A and GAPDH. (B) The arbitrary values indicate VEGF-A mRNA concentrations. Data displayed as mean ± SD from three separate experiments. P value was determined by Student's t-test. *P < .001 vs control.
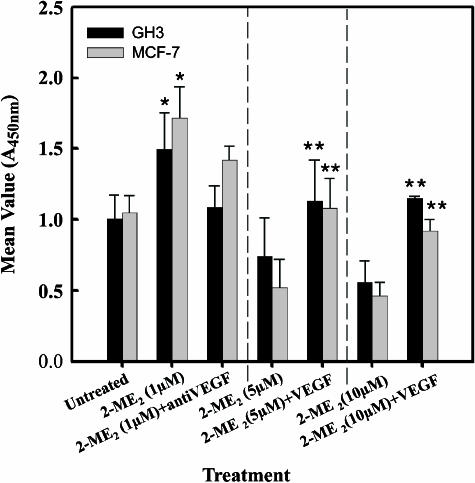
Impact of Anti-VEGF-A Antibodies and Recombinant Human VEGF-A Proteins on the Effects of 2-ME2
A previous study [31] has shown that VEGF-A is able to protect endothelial cells from the antiangiogenic therapy of decetaxel, a related compound of taxane extracted from Taxus baccata. However, 2-ME2 could diminish the protective effect of VEGF [31], and encourages us to determine whether the upregulation of VEGF induced by 2-ME2 was functionally relevant. To test this, exponentially growing cells in DMEM containing 10% FBS were exposed to either 1 µM 2-ME2 in the presence or absence of huMAb-VEGF-A (humanized monoclonal antibody directed against VEGF-A; 100 ng/ml), or 5 or 10 µM concentrations of 2-ME2 with or without recombinant VEGF-A protein. After 3 days of exposure, cell proliferation was measured using BrdU ELISA. Similar to the radioactive thymidine incorporation studies (Figure 1), BrdU incorporations were elevated in both GH3 and MCF-7 cells after exposure to 1 µM 2-ME2 for 3 days, whereas higher doses (i.e., 5 or 10 µM) significantly reduced BrdU incorporations in both cell types (Figure 7). Simultaneous addition of monoclonal anti-VEGF antibody with 1 µM 2-ME2 partially, but significantly, reduced (Student's two-tail test) BrdU incorporation in GH3 and MCF-7 cells when compared to the incorporation seen with 2-ME2 alone. In contrast, when cells were exposed to higher doses of 2-ME2, along with recombinant VEGF-A protein (10 ng/ml), the inhibitory action of 2-ME2 was reduced significantly (Student's two-tail test) by 1.9-fold in GH3 and 2.1-fold in MCF-7 cells (Figure 7). Together, these results indicate the involvement of VEGF-A in the modulation of tumor cell proliferation by 2-ME2.
Figure 7.
Effects of anti-VEGF antibody and recombinant VEGF protein on the action of 2-ME2 on the proliferation of GH3 and MCF-7 cells. Exponentially growing cells in DMEM containing 10% serum were exposed to various concentrations of 2-ME2 in the presence or absence of VEGF antibodies or recombinant VEGF protein for 3 days, and then cell proliferation was determined using BrdU ELISA assay. Data displayed as mean ± SD from three separate experiments. P value was determined by Student's t-test. *P < .05 vs control; **P < .01 vs 2-ME2-treated cells.
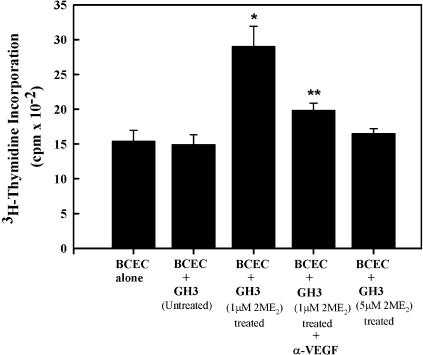
2-ME2-Induced VEGF-A Protein Secretion by Tumor Cells Increased Endothelial Cell Proliferation Through a Paracrine Pathway
To determine if the 2-ME2-induced release of VEGF-A from tumor cells can modulate neighboring endothelial cells, we carried out an indirect coculture experiment. BCECs were grown in the lower chamber for 48 hours, or until the culture became 60% confluent, and then grown for an additional 24 hours in serum-free conditions that kept the environment growth hormone-free. In a separate culture, GH3 cells were grown in DMEM containing 10% serum and 1 or 5 µM 2-ME2 for 3 days. After treatment, the GH3 cells with medium were seeded in the upper chamber for 24 hours, as described in the Materials and Methods section, and endothelial cell proliferation was determined by measuring the radioactive thymidine incorporation into the cells. The results indicate that when BCECs were indirectly cocultured with GH3 cells exposed to 1 µM 2-ME2, the incorporation of radioactive thymidine increased by 2.06-fold. In contrast, radioactive thymidine incorporation levels were unaltered in BCECs grown in the presence of GH3 cells exposed to a high dose of 2-ME2 (Figure 8). Moreover, the addition of huMAb-VEGF-A into the culture reversed the proliferative effect of GH3 cells that had been exposed to 1 µM 2-ME2.
Figure 8.
Effect of 2-ME2-induced VEGF-A in GH3 cells on neighboring endothelial cell proliferation. The effect was determined by indirect coculture using Transwell culture system. The detailed experimental procedure has been described in the Materials and Methods section. Endothelial cell proliferation was determined by measuring the radioactive thymidine incorporation using a liquid scintillation counter. Data displayed as mean±SD from three separate experiments. P value was determined by Student's t-test. *P < .05 vs untreated control; **P < .01 vs 2-ME2-treated cells.
Discussion
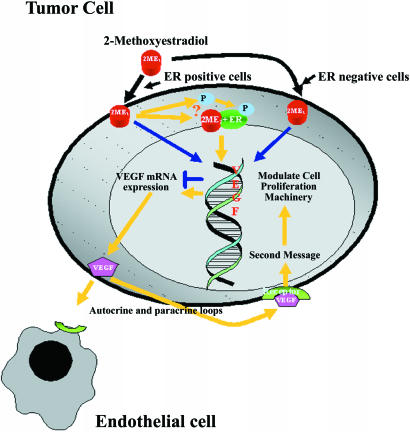
2-ME2 is traditionally an antiproliferative agent and exclusively interacts with proliferative cells modulating target genes associated with proliferation and apoptosis through complex basal transcriptional machineries [7,10,16–19,24,32]. The antiproliferative actions, as well as less toxic effects, increased the potential therapeutic value of 2-ME2. Interestingly, results of two recent in vivo and in vitro studies depart from the existing impression of 2-ME2 action and indicated that it can, depending on the dosage applied, exert either proliferative or antiproliferative effects on mammary tumor growth in rats, and proliferation of human mammary tumor-derived MCF-7 tumor epithelial cells [12,15]. However, the precise mechanisms of these actions have not yet been fully elucidated. Here, we report the novel findings that 2-ME2 is able to activate a signal pathway in steroid-responsive tumor cells, which leads to their growth and survival and also augments the proliferation of endothelial cells by a paracrine pathway (Figure 9).
Figure 9.
A diagram to illustrate the concept of biphasic effects of 2-ME2 and possible mechanism of action of 2-ME2 in ER+ and hormone-responsive tumor cells. ER = estrogen receptor-α; P = parent compound.
The cell proliferation studies demonstrated that 2-ME2 exhibits biphasic effects on cell proliferation in hormoneresponsive tumor cell lines. Higher dosage (i.e., 5 or 10 µM) of 2-ME2 inhibits tumor cell proliferation. However, at the dose of 1 µM, it significantly increases the radioactive thymidine in ER+ GH3 rat pituitary tumor cells and MCF-7 human mammary tumor cells after 3 days of exposure, whereas the effect was reversed in ER- MIA-PaCa-2 pancreatic adenocarcinoma cells. This result is consistent with previous studies [12]. 2-ME2 does not alter the incorporation of radioactive thymidine in these cells after acute exposure to a different dosage of 2-ME2. We therefore sought to determine the signal that was induced by 2-ME2 and act as a tumor cell survivor factor. Our recent studies have suggested that 2-ME2-induced inhibition of estrogen-induced rat pituitary tumor growth is mediated through the downregulation of VEGF-A expression [14]. This is an essential growth factor for estrogen-induced tumor angiogenesis and carcinogenesis [33], and also modulates the growth of various cancer cells by the autocrine and paracrine pathways [29,34,35]. VEGF-A mRNA and protein levels can be modulated by either potent long-acting, or weak or impeded short-acting estrogens by ER-dependent or ER-independent pathways [22,36,37]. This suggests that VEGF-A was the signaling agent responsible for the effects seen with 2-ME2. Because 2-ME2 has low affinity to bind with classical ER [12,24] and also because extensive demethylation of 2-ME2 in the body was reported [15], we have explored the effects of 2-ME2 on VEGF-A mRNA expression and protein synthesis. The results obtained indicate that, irrespective of the dosage, acute exposure (4, 6, or 24 hours) to 2-ME2 increases VEGFA transcription in ER+ GH3 cells. However, chronic treatment invoked a biphasic effect. At the dose of 1 µM 2-ME2, VEGF-A mRNA and protein levels were increased markedly in GH3 cells as well as in MCF-7 cells. Because the pure estrogen antagonist, ICI 182,780, was able to counteract the positive response of VEGF-A to 2-ME2, we suggest that an action of ER was involved. When cells were exposed to higher dosage of 2-ME2, the levels of VEGF-A mRNA were markedly reduced when compared to the basal level of untreated GH3 and MIA-PaCa-2 cells. Nevertheless, the inhibitory effect of higher dosage of 2-ME2 was virtually unaltered in MCF-7 cells. This could be explained by the remarkably low basal levels of VEGF-A mRNA in MCF-7 cells. The similar explanation could be applicable to the VEGF-A protein levels. Together, this study demonstrated a biphasic impact of 2-ME2 on VEGF-A genes, and enhanced expression of VEGF-A mRNA and proteins in steroid-responsive cells is mediated through classical ER. This observation was confirmed by additional studies indicating that 2-ME2 was able to upregulate the VEGF-A expression in ER-α-transfected HMECs, but not in vehicle-transfected cells or ER- MIAPaCa-2 pancreatic adenocarcinoma cells where the impact was basically reversed. There has been a difficulty in establishing how 2-ME2 modulates the biphasic expression of the VEGF-A gene in steroid-responsive cells, but it appears that the concentration and time of exposure are crucial. One could argue that when tumor cells are exposed to a low concentration of 2-ME2, it binds directly to the ER, which ultimately augments the expression of VEGF-A. Alternatively, tumor cells could produce a catechol estrogen by demethylating 2-ME2, which could bind the ER and increase VEGF-A transcription (Figure 9). Similar explanations could be invoked for results with cells exposed to higher dosage of 2-ME2 for short periods of time. As the exposure time increased, cytotoxic effects might alter functions of multiple genes and their signaling pathways that ultimately change the cascade of events associated with the demethylation process as well as with apoptosis.
If VEGF-A was the agent that was modulated by 2-ME2, then it would be logical to examine the effect of increasing its concentration using recombinant protein, or inhibiting its action by use of a specific anti-VEGF-A antibody. We found that anti-VEGF-A antibodies partially reversed the proliferative effect of 2-ME2 in both GH3 and MCF-7 cells. However, the addition of VEGF-A protein into the culture was able to block the inhibitory action of 2-ME2. Consequently, these studies indicate that a threshold level of VEGF-A and its signal is specific and crucial for protecting tumor cells from apoptotic death. Because the effect is not absolute, it is likely that other, as yet undefined, factors are also important in these events.
VEGF-A, a selective endothelial cell growth factor, is synthesized and secreted by various tumor cells and by several transplantable animal tumors [38–40]. Upon secretion from tumor cells, this growth factor may contribute to angiogenic stimulation by interacting with tumor vessel endothelial cell surface receptors [40,41]. Therefore, we examined whether 2-ME2-induced VEGF-A expression in tumor cells is functionally active and enhances the proliferation of neighboring endothelial cells. The indirect coculture studies revealed that enhanced expression of VEGF-A released from tumor cells in response to 2-ME2 interacted with adjacent capillary endothelial cells and eventually increased the endothelial cell proliferation.
In conclusion, this is the first report to indicate that the bifunctional action of 2-ME2 is directly linked to the VEGF pathway, as depicted in Figure 9. Although we do not know yet the immediate, proximate molecular changes that may require exerting the effect, it is clear that the pathway we have proposed here will be of importance in explaining the overall mechanism of action of this molecule. Expectantly, these studies can further its optimal use for the treatment of endocrine-related cancers such as breast cancer. Moreover, therapeutic use of 2-ME2 may be most effective when administered in combination with an anti-VEGF-A agent, at least against a subset of breast cancers.
Acknowledgements
We thank Dr. Donald Johnson for fruitful collaboration in the review of the manuscript. This work is dedicated in recognition of our beloved teacher and philosopher, Professor Samar Chakraborty, PhD.
Abbreviations
- 2-ME2
2-methoxyestradiol
- BCEC
bovine capillary endothelial cell
- CSPD
disodium 3-(4-methoxyspiro{1,2-dioxetane-3,2-(5-chloro)tricyclo[3.3.1.1,7]decan}-4-yl)phenyl phosphate
- DIG
digoxigenin
- ER
estrogen receptor
- GAPDH
glyceraldehyde-3-phosphatedehydrogenase
- HMEC
human mammary epithelial cell
- MOPS
3-(N-morpholino)propanesulfonic acid
- 1 x PBS
1 x phosphate-buffered saline
- PMSF
phenylmethylsulfonyl fluoride
- VEGF-A
vascular endothelial growth factor-A
- huMAb-VEGF-A
humanized monoclonal antibody directed against VEGF-A
Footnotes
This work is supported by NIH/NCI CA87680, V.A. merit review grant, the Midwest Biomedical Research Foundation grant, the EntreMed, Inc. research grant, and the University of Kansas Medical Center departmental research grant.
Both authors have equal contributions to this work.
Present address: Zoology Department, Rammohan College, Kolkata, India.
References
- 1.Brueggemeier RW, Singh U. Inhibition of rat liver microsomal estrogen 2-hydroxylase by 2-methoxyestrogens. J Steroid Biochem. 1989;33:589–593. doi: 10.1016/0022-4731(89)90045-9. [DOI] [PubMed] [Google Scholar]
- 2.Zhu BT, Conney AH. Is 2-methoxyestradiol an endogenous estrogen metabolite that inhibits mammary carcinogenesis? Cancer Res. 1998;58:2269–2277. [PubMed] [Google Scholar]
- 3.Gelbke HP, Knuppen R. The excretion of five different 2-hydroxyoestrogen monomethyl ethers in human pregnancy urine. J Steroid Biochem. 1976;7:457–463. doi: 10.1016/0022-4731(76)90112-6. [DOI] [PubMed] [Google Scholar]
- 4.Dawling S, Roodi N, Mernaugh RL, Wang X, Parl FF. Catechol-O-methyltransferase (COMT)-mediated metabolism of catechol estrogens: comparison of wild-type and variant COMT isoforms. Cancer Res. 2001;61:6716–6722. [PubMed] [Google Scholar]
- 5.Avvakumov GV, Grishkovskaya I, Muller YA, Hammond GL. Crystal structure of human sex hormone-binding globulin in complex with 2-methoxyestradiol reveals the molecular basis for high affinity interactions with C-2 derivatives of estradiol. J Biol Chem. 2002;277:45219–45225. doi: 10.1074/jbc.M207762200. [DOI] [PubMed] [Google Scholar]
- 6.Lakhani NJ, Sarkar MA, Venitz J, Figg WD. 2-Methoxyestradiol, a promising anticancer agent. Pharmacotherapy. 2003;23:165–172. doi: 10.1592/phco.23.2.165.32088. [DOI] [PubMed] [Google Scholar]
- 7.Zoubine MN, Weston AP, Johnson DC, Campbell DR, Banerjee SK. 2-Methoxyestradiol-induced growth suppression and lethality in estrogen-responsive MCF-7 cells may be mediated by down regulation of p34cdc2 and cyclin B1 expression. Int J Oncol. 1999;15:639–646. doi: 10.3892/ijo.15.4.639. [DOI] [PubMed] [Google Scholar]
- 8.Lottering ML, Haag M, Seegers JC. Effects of 17 betaestradiol metabolites on cell cycle events in MCF-7 cells. Cancer Res. 1992;52:5926–5932. [PubMed] [Google Scholar]
- 9.Lottering ML, de Kock M, Viljoen TC, Grobler CJ, Seegers JC. 17Beta-estradiol metabolites affect some regulators of the MCF-7 cell cycle. Cancer Lett. 1996;110:181–186. doi: 10.1016/s0304-3835(96)04489-8. [DOI] [PubMed] [Google Scholar]
- 10.Fotsis T, Zhang Y, Pepper MS, Adlercreutz H, Montesano R, Nawroth PP, Schweigerer L. The endogenous oestrogen metabolite 2-methoxyoestradiol inhibits angiogenesis and suppresses tumour growth. Nature. 1994;368:237–239. doi: 10.1038/368237a0. [DOI] [PubMed] [Google Scholar]
- 11.Golebiewska J, Rozwadowski P, Spodnik JH, Knap N, Wakabayashi T, Wozniak M. Dual effect of 2-methoxyestradiol on cell cycle events in human osteosarcoma 143B cells. Acta Biochim Pol. 2002;49:59–65. [PubMed] [Google Scholar]
- 12.Lavallee TM, Zhan XH, Herbstritt CJ, Kough EC, Green SJ, Pribluda VS. 2-Methoxyestradiol inhibits proliferation and induces apoptosis independently of estrogen receptors alpha and beta. Cancer Res. 2002;62:3691–3697. [PubMed] [Google Scholar]
- 13.Sattler M, Quinnan LR, Pride YB, Gramlich JB, Chu SC, Even GC, Kraeft SK, Chen LB, Salgia R. 2-Methoxyestradiol alters cell motility, migration, and adhesion. Blood. 2003;102:289–296. doi: 10.1182/blood-2002-03-0729. [DOI] [PubMed] [Google Scholar]
- 14.Banerjeei SK, Zoubine MN, Sarkar DK, Weston AP, Shah JH, Campbell DR. 2-Methoxyestradiol blocks estrogen-induced rat pituitary tumor growth and tumor angiogenesis: possible role of vascular endothelial growth factor. Anticancer Res. 2000;20:2641–2645. [PubMed] [Google Scholar]
- 15.Lippert TH, Adlercreutz H, Berger MR, Seeger H, Elger W, Mueck AO. Effect of 2-methoxyestradiol on the growth of methyl-nitroso-urea (MNU)-induced rat mammary carcinoma. J Steroid Biochem Mol Biol. 2003;84:51–56. doi: 10.1016/s0960-0760(02)00268-6. [DOI] [PubMed] [Google Scholar]
- 16.Lavallee TM, Zhan XH, Johnson MS, Herbstritt CJ, Swartz G, Williams MS, Hembrough WA, Green SJ, Pribluda VS. 2-Methoxyestradiol up-regulates death receptor 5 and induces apoptosis through activation of the extrinsic pathway. Cancer Res. 2003;63:468–475. [PubMed] [Google Scholar]
- 17.Pribluda VS, Gubish ER, Jr, Lavallee TM, Treston A, Swartz GM, Green SJ. 2-Methoxyestradiol: an endogenous antiangiogenic and antiproliferative drug candidate. Cancer Metastasis Rev. 2000;19:173–179. doi: 10.1023/a:1026543018478. [DOI] [PubMed] [Google Scholar]
- 18.Basu A, Haldar S. Identification of a novel Bcl-xL phosphorylation site regulating the sensitivity of taxol- or 2-methoxyestradiol- induced apoptosis. FEBS Lett. 2003;538:41–47. doi: 10.1016/s0014-5793(03)00131-5. [DOI] [PubMed] [Google Scholar]
- 19.Qanungo S, Basu A, Das M, Haldar S. 2-Methoxyestradiol induces mitochondria dependent apoptotic signaling in pancreatic cancer cells. Oncogene. 2002;21:4149–4157. doi: 10.1038/sj.onc.1205508. [DOI] [PubMed] [Google Scholar]
- 20.Golab J, Nowis D, Skrzycki M, Czeczot H, Baranczyk-Kuzma A, Wilczynski GM, Makowski M, Mroz P, Kozar K, Kaminski R, Jalili A, Kopec' M, Grzela T, Jakobisiak M. Antitumor effects of photodynamic therapy are potentiated by 2-methoxyestradiol. A superoxide dismutase inhibitor. J Biol Chem. 2003;278:407–414. doi: 10.1074/jbc.M209125200. [DOI] [PubMed] [Google Scholar]
- 21.Chauhan D, Catley L, Hideshima T, Li G, Leblanc R, Gupta D, Sattler M, Richardson P, Schlossman RL, Podar K, Weller E, Munshi N, Anderson KC. 2-Methoxyestradiol overcomes drug resistance in multiple myeloma cells. Blood. 2002;100:2187–2194. doi: 10.1182/blood-2002-02-0376. [DOI] [PubMed] [Google Scholar]
- 22.Banerjee S, Saxena N, Sengupta K, Banerjee SK. 17Alphaestradiol-induced VEGF-A expression in rat pituitary tumor cells is mediated through ER independent but PI3K-Akt dependent signaling pathway. Biochem Biophys Res Commun. 2003;300:209–215. doi: 10.1016/s0006-291x(02)02830-9. [DOI] [PubMed] [Google Scholar]
- 23.Zoubine MN, Banerjee S, Saxena NK, Campbell DR, Banerjee SK. WISP-2: a serum-inducible gene differentially expressed in human normal breast epithelial cells and in MCF-7 breast tumor cells. Biochem Biophys Res Commun. 2001;282:421–425. doi: 10.1006/bbrc.2001.4584. [DOI] [PubMed] [Google Scholar]
- 24.Zhu BT, Conney AH. Functional role of estrogen metabolism in target cells: review and perspectives. Carcinogenesis. 1998;19:1–27. doi: 10.1093/carcin/19.1.1. [DOI] [PubMed] [Google Scholar]
- 25.Fajardo I, Quesada AR, Nunez DCI, Sanchez-Jimenez F, Medina MA. A comparative study of the effects of genistein and 2-methoxyestradiol on the proteolytic balance and tumour cell proliferation. Br J Cancer. 1999;80:17–24. doi: 10.1038/sj.bjc.6690315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cockerill GW, Gamble JR, Vadas MA. Angiogenesis: models and modulators. Int Rev Cytol. 1995;159:113–160. doi: 10.1016/s0074-7696(08)62106-3. [DOI] [PubMed] [Google Scholar]
- 27.Rooman I, Schuit F, Bouwens L. Effect of vascular endothelial growth factor on growth and differentiation of pancreatic ductal epithelium. Lab Invest. 1997;76:225–232. [PubMed] [Google Scholar]
- 28.Oberg-Welsh C, Sandler S, Andersson A, Welsh M. Effects of vascular endothelial growth factor on pancreatic duct cell replication and the insulin production of fetal islet-like cell clusters in vitro. Mol Cell Endocrinol. 1997;126:125–132. doi: 10.1016/s0303-7207(96)03977-9. [DOI] [PubMed] [Google Scholar]
- 29.Tian X, Song S, Wu J, Meng L, Dong Z, Shou C. Vascular endothelial growth factor: acting as an autocrine growth factor for human gastric adenocarcinoma cell MGC803. Biochem Biophys Res Commun. 2001;286:505–512. doi: 10.1006/bbrc.2001.5409. [DOI] [PubMed] [Google Scholar]
- 30.Mabjeesh NJ, Escuin D, Lavallee TM, Pribluda VS, Swartz GM, Johnson MS, Willard MT, Zhong H, Simons JW, Giannakakou P. 2-ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell. 2003;3:363–375. doi: 10.1016/s1535-6108(03)00077-1. [DOI] [PubMed] [Google Scholar]
- 31.Sweeney CJ, Miller KD, Sissons SE, Nozaki S, Heilman DK, Shen J, Sledge GW., Jr The antiangiogenic property of docetaxel is synergistic with a recombinant humanized monoclonal antibody against vascular endothelial growth factor or 2-methoxyestradiol but antagonized by endothelial growth factors. Cancer Res. 2001;61:3369–3372. [PubMed] [Google Scholar]
- 32.Agani F, Kirsch DG, Friedman SL, Kastan MB, Semenza GL. p53 does not repress hypoxia-induced transcription of the vascular endothelial growth factor gene. Cancer Res. 1997;57:4474–4477. [PubMed] [Google Scholar]
- 33.Banerjee SK, Sarkar DK, Weston AP, De A, Campbell DR. Over expression of vascular endothelial growth factor and its receptor during the development of estrogen-induced rat pituitary tumors may mediate estrogen-initiated tumor angiogenesis. Carcinogenesis. 1997;18:1155–1161. doi: 10.1093/carcin/18.6.1155. [DOI] [PubMed] [Google Scholar]
- 34.Bachelder RE, Crago A, Chung J, Wendt MA, Shaw LM, Robinson G, Mercurio AM. Vascular endothelial growth factor is an autocrine survival factor for neuropilin-expressing breast carcinoma cells. Cancer Res. 2001;61:5736–5740. [PubMed] [Google Scholar]
- 35.von Marschall Z, Cramer T, Hocker M, Burde R, Plath T, Schirner M, Heidenreich R, Breier G, Riecken EO, Wiedenmann B, Rosewicz S. De novo expression of vascular endothelial growth factor in human pancreatic cancer: evidence for an autocrine mitogenic loop. Gastroenterology. 2000;119:1358–1372. doi: 10.1053/gast.2000.19578. [DOI] [PubMed] [Google Scholar]
- 36.Mueller MD, Vigne JL, Minchenko A, Lebovic DI, Leitman DC, Taylor RN. Regulation of vascular endothelial growth factor (VEGF) gene transcription by estrogen receptors alpha and beta. Proc Natl Acad Sci USA. 2000;97:10972–10977. doi: 10.1073/pnas.200377097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sengupta K, Banerjee S, Saxena N, Banerjee SK. Estradiolinduced vascular endothelial growth factor-A expression in breast tumor cells is biphasic and regulated by estrogen receptor-alpha dependent pathway. Int J Oncol. 2003;22:609–614. [PubMed] [Google Scholar]
- 38.Folkman J, Shing Y. Angiogenesis. J Biol Chem. 1992;267:10931–10934. [PubMed] [Google Scholar]
- 39.Neufeld G, Cohen T, Gitay-Goren H, Poltorak Z, Tessler S, Sharon R, Gengrinovitch S, Levi BZ. Similarities and differences between the vascular endothelial growth factor (VEGF) splice variants. Cancer Metastasis Rev. 1996;15:153–158. doi: 10.1007/BF00437467. [DOI] [PubMed] [Google Scholar]
- 40.Brown LF, Berse B, Jackman RW, Tognazzi K, Manseau EJ, Senger DR, Dvorak Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in adenocarcinomas of the gastrointestinal tract. Cancer Res. 1993;53:4727–4735. [PubMed] [Google Scholar]
- 41.Brooks PC. Cell adhesion molecules in angiogenesis. Cancer Metastasis Rev. 1996;15:187–194. doi: 10.1007/BF00437471. [DOI] [PubMed] [Google Scholar]