Abstract
Bartonella endocarditis is a severe disease for which blood cultures frequently remain negative. We tested three PCR assays by using specimens of serum sampled early during the disease from 43 patients diagnosed in our laboratory as having Bartonella endocarditis on the basis of serological, culture, and/or valvular molecular detection. We tested a two-step nested PCR (TSN-PCR), a one-step nested PCR (OSN-PCR) with a regular thermal cycler, and a one-step nested PCR with the LightCycler (LCN-PCR). These assays were performed with primers derived from the riboflavin synthase-encoding gene ribC, never before amplified in our laboratory. Due to contamination of negative controls, the results of the TSN-PCR were not interpretable, and this technique was no longer considered. The LCN-PCR had a specificity of 100% and a sensitivity of 58.1%, higher than those of the OSN-PCR (18.6%; P < 0.01) and prolonged blood culturing (7.1%; P < 0.01). The LCN-PCR results correlated strictly with those of other direct diagnostic tests, when available, and identified the causative species for six patients previously diagnosed on the basis of serological analysis only. The efficacy of the LCN-PCR was not influenced by antibiotics (P = 0.96) but was altered by prolonged storage of serum specimens at −20°C (P = 0.04). Overall, the LCN-PCR is specific and more sensitive than traditional methods (i.e., culturing and/or PCR with EDTA-treated blood). It can easily be applied to the diagnosis of patients with suspected Bartonella endocarditis, especially when only serum is available.
Among the 19 currently recognized Bartonella species, 7 have been implicated in human diseases (17). Of these, four have been recognized as causative agents of blood culture-negative endocarditis in humans: Bartonella quintana, Bartonella henselae, Bartonella elizabethae, and Bartonella vinsonii subsp. berkhoffii (32). Bartonella endocarditis represents 3% of all cases of endocarditis and thus is as frequent as Coxiella burnetii endocarditis (4). To date, 113 proven cases have been reported in the international literature, including 101 by our group (1, 3, 5-8, 10, 12-16, 18, 23-25, 27, 29, 32-34). B. quintana and B. henselae are responsible for most of these cases (10), with only one case each of B. elizabethae and B. vinsonii subsp. berkhoffii endocarditis reported to date (6, 32). Bartonella endocarditis is associated with risk factors already identified for B. quintana and B. henselae. B. quintana occurs more often in homeless people and/or people with alcoholism who are in contact with body lice and who have no known valvular disease, while B. henselae occurs in patients who have contact with cats and/or cat fleas and who have previously diagnosed valvular disease (10).
Usually, the microbiological diagnosis of infective endocarditis relies on direct evidence of the causative agent, with blood culturing being the most commonly used method. Unfortunately, culture methods have demonstrated poor sensitivity for Bartonella endocarditis due to the fastidious nature of these bacteria, especially when culturing is used for specimens from patients already treated with antimicrobial agents (20). In contrast, immunohistochemical analysis (21) and PCR amplification from valvular biopsy specimens (10) are the most useful tools, but material for these tests is not always available. Recently, Fournier et al. (11) demonstrated that indirect immunofluorescence has a positive predictive value for Bartonella infection of 95% for an immunoglobulin G (IgG) titer to B. henselae and/or B. quintana of ≥1:800 in patients with endocarditis. However, due to cross-reactions among bartonellae, indirect immunofluorescence cannot reliably identify the causative species (11).
The development of an approach for identifying bacterial DNA in serum, which is easily obtained from patients and may be stored frozen for long periods, is of interest. However, the usual one-step PCR amplification from such a specimen may not achieve sufficient sensitivity (10). In recent studies, in order to increase the detection threshold, we developed for rickettsial diseases a nested PCR assay. To avoid contamination by PCR products issued from previous amplifications, this nested PCR was carried out with primers targeting short, single-use gene fragments, a technique previously described as “suicide PCR” (26). In this assay, we used primers never used before in our laboratory, and we did not include any positive control, in order to avoid contamination. This technique was successfully applied to serum specimens from patients with rickettsial infections (28, 30).
In the present study, we evaluated a similar nested PCR performed directly on serum for the diagnosis of Bartonella endocarditis. For this purpose, we compared two tests done with a conventional thermal cycler: a two-step nested PCR (TSN-PCR) and a one-step nested PCR (OSN-PCR), in which we combined the primary and secondary amplifications without opening the reaction tubes. In addition, we implemented the same nested PCR with the real-time LightCycler PCR system (Roche Diagnostics, Mannheim, Germany), hereafter referred to as LightCycler nested PCR (LCN-PCR). The sensitivities of the nested PCR assays were compared with those of other diagnostic tests. The riboflavin synthase-encoding gene (ribC), which has been shown to differentiate Bartonella species (2) and which had never been used before in our laboratory, was a good candidate for the detection and identification of Bartonella in clinical specimens.
MATERIALS AND METHODS
Study design and case definition.
We performed TSN-PCR, OSN-PCR, and LCN-PCR for patients with proven Bartonella endocarditis and from whom at least 200 μl of serum was available. Some of the serum samples had been thawed and refrozen several times, in particular, those stored for longer periods, but the exact number of thawing-freezing cycles was not available.
Definite endocarditis was defined according to the modified Duke criteria for the diagnosis of infective endocarditis (22). Definite Bartonella infection was diagnosed when a Bartonella species was recovered by culturing or PCR amplification from valvular biopsy specimens as previously described (10) and/or when patients exhibited an IgG antibody titer to B. quintana or B. henselae of >1:800 (11). We compared the sensitivities of all blood-based assays, except for serological analysis, which was used as a diagnostic criterion.
In order to estimate the specificities of the TSN-PCR, LCN-PCR, and OSN-PCR assays, we also tested 100 patients with endocarditis caused by other microorganisms. These patients had a median age of 63 years, 72 were male, and 28 were female. These control patients were infected as follows: 20 with Streptococcus viridans, 18 with Streptococcus bovis, 16 with Staphylococcus aureus, 10 with Escherichia coli, 9 with Enterococcus faecalis, 9 with C. burnetii, 5 with coagulase-negative staphylococci, 3 with Streptococcus pneumoniae, 2 with Neisseria sicca, 1 with Streptococcus agalactiae, 1 with Gemella morbillorum, 1 with Actinobacillus actinomycetemcomitans, 1 with Enterobacter cloacae, 1 with Klebsiella oxytoca, 1 with Stenotrophomonas maltophilia, 1 with Haemophilus aphrophilus, and 1 with Haemophilus paraphrophilus. We did not have serum from patients with endocarditis caused by Brucella, which is one of the bacterial genera that causes endocarditis and that is most closely related to Bartonella.
Questionnaire.
A standardized questionnaire was completed for each patient with Bartonella endocarditis. Recorded data included those used in the diagnostic score of the Duke Endocarditis Service (22); previous antibiotic therapy; valvular surgery; and the presence of environmental exposure factors, such as homelessness, chronic alcoholism (>100 g of alcohol per day for more than 1 year), presence of body lice, immunodeficiency, and contact with cats and/or cat fleas.
Serological analysis and culturing.
All tested serum specimens were kept frozen at −20°C until further analysis. We used serum sampled early following the diagnosis of endocarditis, i.e., within 1 month. Serological analysis by microimmunofluorescence (MIF) and blood and valvular biopsy specimen culturing was carried out as previously reported (10, 19). Some of the serum samples had been thawed and refrozen several times for previous serological studies.
Molecular methods. (i) DNA extraction.
Total genomic DNA was extracted from serum samples by using a QIAamp blood kit (Qiagen, Hilden, Germany) as described by the manufacturer. Two hundred microliters of serum was used. Fifty microliters of elution buffer was used to resuspend the DNA. Genomic DNA was stored at 4°C until used as a template in PCR assays.
(ii) PCR amplification.
For all PCR assays, DNA samples were processed carefully to avoid the risk of cross-contamination. DNA extraction, mixture preparation, and PCR were performed in different rooms to prevent PCR carryover contamination.
(iii) TSN-PCR assay.
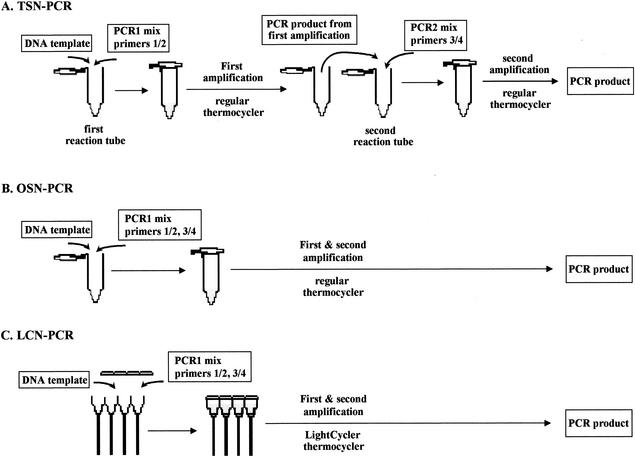
We conducted the TSN-PCR with a PTC-200 automated thermal cycler (MJ Research, Waltham, Mass.) (Fig. 1A) and Elongase DNA polymerase (Gibco-BRL, Cergy Pontoise, France). For the primary amplification, the 25-μl reaction mixture consisted of the following (final concentrations): primers ZRib1F (5′-CGGATATCGGTTGTGTTGAA-3′) and ZRib1R (5′-CATCAATRTGACCAGAAACCA-3′), purchased from Eurogentec (Seraing, Belgium) (0.5 pmol μl−1 each); deoxynucleoside triphosphates (dATP, dCTP, dGTP, and dTTP) (0.2 mM each); 1 μl of Elongase buffer A (Gibco-BRL); 4 μl of Elongase buffer B (Gibco-BRL); 0.6 μl of Elongase enzyme mix (Gibco-BRL); 2.5 μl of the DNA preparation; and sterile water. The amplification was performed under the following conditions: an initial 4 min of denaturation at 94°C was followed by 44 cycles of denaturation for 30 s at 94°C, annealing for 30 s at 57°C, and extension for 45 s at 68°C. The amplification was completed by keeping the sample at 68°C for 10 min to allow complete extension of the PCR products. Two microliters of primary amplification product was used as a template for the secondary amplification with internal primers Zrib2F (5′-GCATCAATTGCGTGTTCA-3′) and Zrib2R (5′-CCCATTTCATCACCCAAT-3′) and the conditions described above, except for an annealing temperature of 52°C. PCR products from the second amplification were separated by electrophoresis on 1% agarose gels, visualized by staining with ethidium bromide, and then purified by using a QIAquick PCR purification kit (Qiagen) as described by the manufacturer.
FIG. 1.
PCR assays used in this study. (A and B) PCR assays performed with a conventional thermal cycler were TSN-PCR, where the amplicon of the first PCR was used as a template for the second PCR (A), and OSN-PCR, where two rounds of PCR cycles occurred in the same tube (B). (C) The PCR assay performed with the LightCycler machine was LCN-PCR, where two rounds of PCR cycles occurred in the same capillary.
(iv) OSN-PCR assay with a standard PCR machine.
We combined the primary and secondary PCR steps of the TSN-PCR assay into a single PCR (Fig. 1B). We used a PTC-200 thermal cycler and Elongase DNA polymerase. The 25-μl reaction mixture consisted of the following (final concentrations): primers (1 pmol μl−1 each), deoxynucleoside triphosphates (dATP, dCTP, dGTP, and dTTP) (0.4 mM each), 2 μl of Elongase buffer A, 8 μl of Elongase buffer B, 1.2 μl of Elongase enzyme mix, 2.5 μl of the DNA preparation, and sterile water. The amplification was performed as described above with 35 cycles for the primary PCR and 33 cycles for the secondary PCR. The amplification was completed by keeping the sample at 68°C for 10 min to allow complete extension of the PCR products.
(v) LCN-PCR assay.
We used a LightCycler thermal cycler, which allows real-time detection of amplified DNA with respect to the number of cycles. In order to determine the optimal amplification conditions for each of the two PCR amplifications, we conducted the primary PCR assay with three negative controls only: DNA from a patient with proven C. burnetii endocarditis and two sterile water samples. Each 20 μl-reaction mixture consisted of 2 μl of DNA Master SYBR Green (DNA Master SYBR Green I kit [Roche Diagnostics]), 2.4 μl of 3 mM MgCl2, 1 μl each of primers ZRib1F and ZRib1R at 0.5 μM, 11.6 μl of sterile distilled water, and 2 μl of DNA. Following initial denaturation at 95°C for 8 min, we used 40 cycles of denaturation at 95°C for 15 s (temperature transition, 20°C/s), annealing at 57°C for 5 s, and extension at 72°C for 18 s, with fluorescence acquisition in the single mode. The amplification was completed by keeping the sample at 68°C for 10 min to allow complete extension of the PCR products. The secondary PCR assay was applied to the three negative controls and incorporated internal primers Zrib2F and Zrib2R under the conditions described above, except for an annealing temperature of 52°C. We observed nonspecific amplification from one negative control at cycle 36 of the primary assay and at cycle 34 of the secondary assay. Subsequently, we decided to limit the number of cycles to 35 for the primary amplification and 33 for the secondary amplification. Using these conditions, we observed no nonspecific amplification from the same negative controls.
Next, we combined both amplifications in a single test for our patients' serum specimens (Fig. 1C). For each sample, we prepared a reaction mixture containing 4 μl of DNA Master SYBR Green; 4.8 μl of 3 mM MgCl2; 1 μl of each of the primers ZRib1F, ZRib1R, ZRib2F, and ZRib2R at 0.5 μM; 5.2 μl of sterile distilled water; and 2 μl of DNA. Following initial denaturation at 95°C for 8 min, our rapid nested PCR program consisted of 35 cycles of denaturation at 95°C for 15 s (temperature transition, 20°C/s), annealing at 57°C for 5 s, and extension at 72°C for 18 s followed by 33 cycles of denaturation at 95°C for 15 s (temperature transition, 20°C/s), annealing at 52°C for 5 s, and extension at 72°C for 18 s. The amplification was completed by keeping the sample at 68°C for 10 min to allow complete extension of the PCR products.
As the LightCycler was not able to interpret the results from the nested PCR, amplicons from the second amplification were separated by electrophoresis and then purified as described above.
(vi) Sequencing of PCR products and sequence analysis.
PCR products were sequenced in both directions by using a d-Rhodamine terminator cycle sequencing ready reaction kit (Perkin-Elmer, Coignieres, France) as described by the manufacturer. Sequencing products were resolved by using an ABI 3100 automated sequencer (Perkin-Elmer). Sequence analysis was performed with the software package ABI Prism DNA Sequencing Analysis Software version 3.0 (Perkin-Elmer), and a multisequence alignment was made with CLUSTAL W software, version 1.81 (35).
Statistical analysis.
The sensitivity of each procedure for the diagnosis of Bartonella endocarditis was determined by dividing the number of true positive results by the number of tested patients with definite Bartonella endocarditis (as defined above). Sensitivities were compared by using the χ2 test. The specificities of the OSN-PCR and LCN-PCR assays were calculated by dividing the number of true negative results by the number of negative controls. The posttest probability was inferred from the observed sensitivities, assuming a 99% specificity. The χ2 and Mann-Whitney tests were used to assess situations more likely to benefit the LCN-PCR assay. The variables evaluated were age, sex, presence of vegetations, preexisting valvular disease, alcoholism, homelessness, contact with cats and/or cat fleas, a fever of >38°C, and antibiotic therapy before serum sampling. To estimate the influence of the duration of storage of serum samples at −20°C before PCR testing on the sensitivity of the LCN-PCR assay, we compared the mean ages of serum specimens by using Student's t test. STATA software (version 7.0; Stata Corporation, College Station, Tex.) was used for analysis.
RESULTS
Patients and negative controls.
Between January 1995 and April 2002, we diagnosed 105 patients as having Bartonella endocarditis, 59 on the basis of culturing and/or PCR and 46 on the basis of an IgG titer of ≥1:800 (11). For 43 of these patients (36 males and 7 females; median age, 50 years), 200 μl of serum was available. Twenty-five had PCR- and/or culture-based evidence of Bartonella infection (20 infected with B. quintana and 5 infected with B. henselae), and the remaining 18 had blood culture-negative endocarditis and exhibited an IgG antibody titer to Bartonella species of ≥1:800 (Table 1). As PCR amplification from EDTA-treated blood was performed for only 10 patients, we did not include the results of this test in our comparative study. Of the 43 patients with Bartonella endocarditis, 93% presented with valvular vegetations and 88% presented with fever. Fifty-three percent were known to have previously diagnosed valvular disease, and 67% underwent valvular surgery. Antibiotic treatment was begun before serum sampling in 69% of cases. Risk factors for Bartonella infection included homelessness in 30%, contact with body lice in 17%, alcoholism in 48%, contact with cats in 36%, and contact with cat fleas in 12.5% of cases. Serum specimens were stored for a median of 4 years before testing (range, 1 month to 18 years).
TABLE 1.
Results of various diagnostic tests used for 43 patients with Bartonella endocarditis
| Patient (reference)b | IgG titer determined by MIF assay for:
|
Species detected bya:
|
Proven diagnosis | ||||||
|---|---|---|---|---|---|---|---|---|---|
| B. henselae | B. quintana | Specific blood culture | PCR on EDTA-treated blood | Valvular biopsy specimen culture | PCR on valvular biopsy specimen | OSN-PCR on serum | LCN-PCR on serum | ||
| 1 (27) | 3,200 | 3,200 | − | − | − | B. quintana | B. quintana | B. quintana | B. quintana |
| 2 (27) | 1,600 | 800 | ND | − | − | − | − | − | |
| 3 (27) | 3,200 | 3,200 | − | ND | ND | ND | − | − | |
| 4 (27) | 1,600 | 400 | ND | − | − | ND | − | − | |
| 5 (27) | 51,200 | 6,400 | − | − | − | B. quintana | − | B. quintana | B. quintana |
| 6 (10) | 6,400 | 25,600 | − | − | ND | ND | − | − | |
| 7 (10) | 400 | 50 | ND | ND | B. quintana | B. quintana | − | − | B. quintana |
| 8 (10) | 1,600 | 100 | − | − | ND | ND | − | − | |
| 9 (10) | 200 | 400 | B. quintana | ND | − | B. quintana | − | B. quintana | B. quintana |
| 10 (10) | 400 | 12,800 | ND | ND | ND | ND | − | B. quintana | B. quintana |
| 11 (10) | 6,400 | 6,400 | ND | ND | ND | ND | − | − | |
| 12 (10) | 200 | 12,800 | − | − | − | B. quintana | B. quintana | B. quintana | B. quintana |
| 13 (10) | 1,600 | 800 | − | B. quintana | B. quintana | B. quintana | B. quintana | B. quintana | B. quintana |
| 14 (10) | 3,200 | 3,200 | − | ND | B. quintana | B. quintana | − | − | B. quintana |
| 15 (10) | 800 | 1,600 | − | ND | B. quintana | B. quintana | − | B. quintana | B. quintana |
| 16 (10) | 400 | 400 | − | ND | − | B. quintana | − | − | B. quintana |
| 17 (10) | 3,200 | 1,600 | ND | ND | ND | B. henselae | − | − | B. henselae |
| 18 (10) | 400 | 800 | − | ND | ND | ND | − | − | |
| 19 (10) | 6,400 | 3,200 | ND | ND | ND | ND | − | B. quintana | B. quintana |
| 20 (10) | 1,600 | 800 | − | ND | ND | B. henselae | B. henselae | B. henselae | B. henselae |
| 21 (10) | 800 | 1,600 | − | ND | ND | B. henselae | − | B. henselae | B. henselae |
| 22 (10) | 1,600 | 1,600 | − | ND | B. quintana | B. quintana | − | − | B. quintana |
| 23 (10) | 3,200 | 1,600 | B. henselae | B. henselae | B. henselae | B. henselae | − | − | B. henselae |
| 24 (10) | 1,600 | 1,600 | − | ND | ND | B. quintana | − | B. quintana | B. quintana |
| 25 (10) | 800 | 800 | ND | ND | ND | ND | − | − | |
| 26 (10) | 1,600 | 3,200 | ND | ND | ND | B. quintana | − | B. quintana | B. quintana |
| 27 (10) | 1,600 | 800 | ND | ND | B. quintana | B. quintana | B. quintana | B. quintana | B. quintana |
| 28 (10) | 3,200 | 1,600 | ND | ND | ND | B. quintana | − | B. quintana | B. quintana |
| 29 (10) | 3,200 | 3,200 | − | ND | − | B. quintana | − | B. quintana | B. quintana |
| 30 (10) | 200 | 800 | − | ND | ND | B. quintana | B. quintana | B. quintana | B. quintana |
| 31 (10) | 400 | 800 | − | ND | B. quintana | B. quintana | B. quintana | B. quintana | B. quintana |
| 32 (10) | 200 | 800 | − | ND | ND | B. quintana | − | B. quintana | B. quintana |
| 33 | 1,600 | 1,600 | − | ND | ND | ND | − | − | |
| 34 | 800 | 800 | − | ND | ND | ND | − | − | |
| 35 | 800 | 800 | − | ND | B. quintana | B. quintana | − | B. quintana | B. quintana |
| 36 | 1,600 | 1,600 | − | ND | ND | ND | − | − | |
| 37 | 3,200 | 800 | − | ND | ND | ND | B. henselae | B. henselae | B. henselae |
| 38 | 800 | 800 | ND | ND | − | B. quintana | − | B. quintana | B. quintana |
| 39 | 6,400 | 6,400 | − | − | ND | ND | − | B. henselae | B. henselae |
| 40 | 1,600 | 1,600 | − | ND | ND | B. henselae | − | B. henselae | B. henselae |
| 41 | 800 | 800 | ND | ND | ND | ND | − | B. henselae | B. henselae |
| 42 | 1,600 | 1,600 | ND | ND | − | ND | − | B. henselae | B. henselae |
| 43 | 800 | 1,600 | ND | ND | ND | ND | − | − | |
−, negative result; ND, not determined.
Patients 33 to 43 are from this study.
PCR results. (i) TSN-PCR.
The TSN-PCR assay yielded positive results for all tested samples, including those from patients and negative controls. Thus, we concluded that these samples were contaminated, and we did not evaluate this technique any further.
(ii) OSN-PCR.
Samples from eight patients exhibited positive results in the OSN-PCR assay (Table 1). Sequences obtained from PCR products were 100% identical to that of B. quintana (GenBank accession no. AJ236917) for six patients, whose samples were all found positive by LCN-PCR, and to that of B. henselae (GenBank accession no. AJ132928) for two patients, whose samples were both found positive by LCN-PCR as well (Table 1). Samples from all negative controls exhibited negative results.
(iii) LCN-PCR.
Samples from 25 patients exhibited positive LCN-PCR results (Table 1). Sequences obtained from PCR products were 100% identical to that of B. quintana for 18 patients and to that of B. henselae for 7 patients. Samples from all 100 negative controls exhibited negative results. The infecting species was not previously identified for 2 of the 18 patients with B. quintana infection and for 4 of the 7 patients infected with B. henselae. For the remaining 19 patients, the identified infecting species matched the results of previous tests (Table 1). The two patients for whom our technique identified for the first time B. quintana infection had a known valvular disease, and one had alcoholism, but neither was homeless or reported contact with body lice. Of the four patients for whom only LCN-PCR identified B. henselae infection, all reported contact with cats, one reported contact with cat fleas, two had a known valvular disease, and one was undergoing long-term corticoid therapy for multiple sclerosis.
Sensitivity and specificity of LCN-PCR.
For the diagnosis of Bartonella endocarditis, LCN-PCR had a specificity of 100% and a sensitivity of 58.1% (25 of 43 patients). The proportion of positive LCN-PCR results was inversely related to the mean duration of storage of serum specimens at −20°C—2.9 years for LCN-PCR-positive samples and 5.1 years for negative samples (P = 0.04). Thus, the sensitivity was 85.7% (6 of 7 patients) for LCN-PCR performed on serum stored for less than 1 year. This sensitivity decreased to 62.5% (5 of 8 patients), 53.3% (8 of 15), and 46.1% (6 of 13) for LCN-PCR performed on serum stored for ≥1 to 3 years, ≥3 to 5 years, and ≥5 years, respectively. None of the other evaluated variables was significantly associated with the proportion of positive LCN-PCR results for patients with Bartonella endocarditis (data not shown). In particular, antibiotic therapy before serum sampling was not associated with decreased sensitivity; sensitivities were 59.3% (16 of 27 patients) in the presence of antibiotic and 58.3% (7 of 12) in its absence (P = 0.96).
Comparison of LCN-PCR with other diagnostic methods.
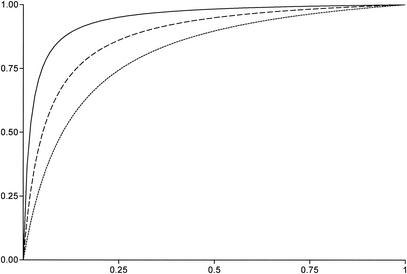
The sensitivity of LCN-PCR (58.1%; 25 of 43 patients) was higher than that of OSN-PCR (18.6%; 8 of 43) (P < 0.01). The sensitivity of LCN-PCR (60.7%; 17 of 28 patients) was also higher than that of prolonged blood culturing (7.1%; 2 of 28) (P < 0.01). Consequently, the area under the receiver operating characteristic curve was larger for LCN-PCR than for OSN-PCR and prolonged blood culturing (Fig. 2). The sensitivity of LCN-PCR (50%; 5 of 10 patients) was slightly higher than that of PCR amplification from EDTA-treated blood (20%; 2 of 10) (P = 0.35). Similarly, the sensitivity of LCN-PCR (63.2%; 12 of 19 patients) was also slightly higher than that of valvular biopsy specimen culturing (47.4%; 9 of 19) (P = 0.33). In contrast, the sensitivity of LCN-PCR (73.1%; 19 of 26 patients) was lower than that of PCR amplification from valvular biopsy specimens (96.1%; 25 of 26) (P = 0.05). However, serum samples from patients with negative LCN-PCR results but positive results for PCR amplification from valvular biopsy specimens had been kept frozen for more than 3 years.
FIG. 2.
Pre- and posttest probabilities of Bartonella endocarditis. A specificity of 99.9% was used for all tests (all positive results were confirmed by sequencing). Dotted line, blood culture; dashed line, OSN-PCR; solid line, LCN-PCR.
DISCUSSION
In the present report, we describe DNA detection methods for the diagnosis of Bartonella endocarditis. Our purpose was to investigate the effectiveness of PCR-based assays carried out on patients' serum specimens. Serum is one of the most easily obtained human samples and, when sampled early in the evolution of a systemic disease, is likely to contain DNA copies of systemic pathogens (28). Due to the presence of inhibitors in blood and to the small amount of bacterial DNA present in serum, especially in patients already treated with antibiotics, we designed three nested PCR assays to increase the sensitivity of PCR. A nested PCR approach was shown to be effective for the diagnosis of rickettsial diseases and plague (26, 28, 30). A major concern with nested PCR amplification is contamination, which may be lateral (i.e., contamination caused by PCR products amplified in other tubes in the same assay) or vertical (i.e., contamination caused by amplicons from previous PCR assays).
To prevent vertical contamination, we used a suicide PCR assay incorporating primers targeting a gene (ribC) never before amplified in our laboratory (2). In a first assay, we carried out a conventional nested PCR with two successive amplifications in a standard thermal cycler. Unfortunately, in this assay, all specimens, including negative controls, were positive. Lateral contamination by aerosols containing amplicons from positive samples probably occurred after the first PCR when the tubes were opened before the second PCR. This result emphasizes the high risk of contamination of conventional PCR assays.
To prevent such lateral contamination, we designed an OSN-PCR assay that was performed in a different room. In this assay, the two primer pairs had different hybridization temperatures and the reaction tubes were not opened during the entire amplification process. Using one of the LightCycler advantages, i.e., the ability to monitor the PCR cycle number before the onset of amplification, we designed an LCN-PCR assay that enabled us to amplify Bartonella DNA and detect contaminant amplification. We limited the number of cycles in each of the two PCR assays to prevent the risk of nonspecific amplification. Independently, a similar technique was recently reported for the detection of RNA from hepatitis C virus (31). In parallel, we investigated the usefulness of the technique with a standard thermal cycler machine. We observed no contamination of the negative controls during LCN-PCR or OSN-PCR.
Overall, LCN-PCR exhibited a sensitivity of 58.1% and was more sensitive than OSN-PCR. This result may be related to differences in PCR reagent quality and enzyme stability and the short time needed to reach the annealing temperature, which reduces the nonspecific annealing of primers. The sensitivity of LCN-PCR was affected by the duration of serum storage at −20°C (P = 0.04), likely through the progressive degradation of frozen DNA. In addition to their longest storage times, the oldest serum specimens included in our study had been thawed and refrozen repeatedly for various tests performed in our laboratory, including MIF, cross-absorption, and Western blotting. Repeated thawing of frozen samples may have played a role in the observed decrease in sensitivity, as previously documented (9). In contrast, LCN-PCR was not influenced by the administration of antibiotics. LCN-PCR was more sensitive than blood culturing and valve sample culturing. However, LCN-PCR performed on serum was less sensitive (19 of 26 patients) than PCR performed on valve samples (25 of 26). The fact that the serum samples obtained from six patients whose valve samples were found positive only by PCR had been stored for more than 3 years may partially explain this difference in sensitivity. Using OSN-PCR, we amplified Bartonella species from samples from eight patients, including two for whom no direct evidence of a causative agent had been obtained by other techniques. LCN-PCR detected Bartonella species in samples from 25 patients. For 6 of these 25 patients, the infecting species had not been identified by other direct diagnostic tests, demonstrating the utility of this technique.
The ribC-derived LCN-PCR, with a one-step procedure in the LightCycler thermal cycler, prevents amplicon carryover. We have demonstrated that this technique can be carried out on serum specimens, especially before valvular surgery, and is not affected by antimicrobial treatment. Its sensitivity is higher when performed on serum samples kept frozen for short periods. In conclusion, LCN-PCR is a valuable tool that may shorten the delay in the diagnosis of Bartonella endocarditis. As Bartonella endocarditis represents 3% of cases of infective endocarditis, we propose that this technique should be applied to patients with blood culture-negative endocarditis and those with both unexplained fever and elevated titers of antibodies to Bartonella species. Moreover, this technique may be useful for other systemic Bartonella infections, in particular, chronic bacteremia, bacillary angiomatosis, peliosis hepatis, and cat scratch disease with visceral involvement.
Acknowledgments
We thank Kelly Johnston for reviewing the manuscript.
We acknowledge funding for Zaher Zeaiter from Fondation pour la Recherche Médicale.
REFERENCES
- 1.Baorto, E., R. M. Payne, L. N. Slater, F. Lopez, D. A. Relman, K. W. Min, and J. W. St. Geme. 1998. Culture-negative endocarditis caused by Bartonella henselae. J. Pediatr. 132:1051-1054. [DOI] [PubMed] [Google Scholar]
- 2.Bereswill, S., S. Hinkelmann, M. Kist, and A. Sander. 1999. Molecular analysis of riboflavin synthesis genes in Bartonella henselae and use of the ribC gene for differentiation of Bartonella species by PCR. J. Clin. Microbiol. 37:3159-3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breathnach, A. S., J. M. Hoare, and S. J. Eykyn. 1997. Culture-negative endocarditis: contribution of bartonella infections. Heart 77:474-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brouqui, P., and D. Raoult. 2001. Endocarditis due to rare and fastidious bacteria. Clin. Microbiol. Rev. 14:177-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruneel, F., J. D'Estanque, P. E. Fournier, G. Arlet, M. Thuong, M. Wolff, J. P. Bedos, S. Lariven, and B. Regnier. 1998. Isolated right-sided Bartonella quintana endocarditis in an immunocompetent adult. Scand. J. Infect. Dis. 30:424-425. [DOI] [PubMed] [Google Scholar]
- 6.Daly, J. S., M. G. Worthington, D. J. Brenner, W. C. Moss, D. G. Hollis, R. S. Weyant, A. G. Steigerwalt, R. E. Weaver, M. I. Daneshvar, and S. P. O'Connor. 1993. Rochalimaea elizabethae sp. nov. isolated from a patient with endocarditis. J. Clin. Microbiol. 31:872-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drancourt, M., R. J. Birtles, G. Chaumentin, F. Vandenesch, J. Etienne, and D. Raoult. 1996. New serotype of Bartonella henselae in endocarditis and cat-scratch disease. Lancet 347:441-443. [DOI] [PubMed] [Google Scholar]
- 8.Drancourt, M., J. L. Mainardi, P. Brouqui, F. Vandenesch, A. Carta, F. Lehnert, J. Etienne, F. Goldstein, J. Acar, and D. Raoult. 1995. Bartonella (Rochalimaea) quintana endocarditis in three homeless men. N. Engl. J. Med. 332:419-423. [DOI] [PubMed] [Google Scholar]
- 9.Farnert, A., A. P. Arez, A. T. Correia, A. Bjoekman, G. Snounou, and V. Do Rosario. 1999. Sampling and storage of blood and the detection of malaria parasites by polymerase chain reaction. Trans. R. Soc. Trop. Med. Hyg. 93:50-53. [DOI] [PubMed] [Google Scholar]
- 10.Fournier, P. E., H. Lelievre, S. J. Eykyn, J. L. Mainardi, T. J. Marrie, F. Bruneel, C. Roure, J. Nash, D. Clave, E. James, C. Benoit-Lemercier, L. Deforges, H. Tissot-Dupont, and D. Raoult. 2001. Epidemiologic and clinical characteristics of Bartonella quintana and Bartonella henselae endocarditis: a study of 48 patients. Medicine (Baltimore) 80:245-251. [DOI] [PubMed] [Google Scholar]
- 11.Fournier, P. E., J. L. Mainardi, and D. Raoult. 2002. Value of microimmunofluorescence for the diagnosis and follow-up of Bartonella endocarditis. Clin. Diagn. Lab. Immunol. 9:795-801. [DOI] [PMC free article] [PubMed]
- 12.Grand, A., M. Celard, R. el Belghiti, W. Ghabdam, G. de Gevigney, A. Dabboura, C. Besnard, K. Ouanes, J. F. Huret, and P. Fichter. 2001. Subacute infectious endocarditis due to the agent of cat scratch fever: Bartonella henselae. Arch. Mal. Coeur. Vaiss. 94:157-161. [PubMed] [Google Scholar]
- 13.Guyot, A., A. Bakhai, N. Fry, J. Merritt, H. Malnick, and T. Harrison. 1999. Culture-positive Bartonella quintana endocarditis. Eur. J. Clin. Microbiol. Infect. Dis. 18:145-147. [DOI] [PubMed] [Google Scholar]
- 14.Hadfield, T. L., R. Warren, M. Kass, E. Brun, and C. Levy. 1993. Endocarditis caused by Rochalimaea henselae. Hum. Pathol. 24:1140-1141. [DOI] [PubMed] [Google Scholar]
- 15.Holmes, A. H., T. C. Greenough, G. J. Balady, R. L. Regnery, B. E. Anderson, J. C. Oikeane, J. D. Fonger, and E. L. McCrone. 1995. Bartonella henselae endocarditis in an immunocompetent adult. Clin. Infect. Dis. 21:1004-1007. [DOI] [PubMed] [Google Scholar]
- 16.Jacoby, G. A., C. M. Hay, R. B. Colvin, and B. D. Walker. 1997. A 38-year-old man with digital clubbing, low-grade fever, and a murmur—Bartonella endocarditis, probably due to Bartonella quintana. N. Engl. J. Med. 336:205-210. [DOI] [PubMed] [Google Scholar]
- 17.Jacomo, V., P. J. Kelly, and D. Raoult. 2002. Natural history of Bartonella infections (an exception to Koch's postulate). Clin. Diagn. Lab. Immunol. 9:8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jalava, J., P. Kotilainen, S. Nikkari, M. Skurnik, E. Vanttinen, O. P. Lehtonen, E. Eerola, and P. Toivanen. 1995. Use of the polymerase chain reaction and DNA sequencing for detection of Bartonella quintana in the aortic valve of a patient with culture-negative infective endocarditis. Clin. Infect. Dis. 21:891-896. [DOI] [PubMed] [Google Scholar]
- 19.La Scola, B., and D. Raoult. 1996. Serological cross reactions between Bartonella quintana, Bartonella henselae, and Coxiella burnetii. J. Clin. Microbiol. 34:2270-2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.La Scola, B., and D. Raoult. 1999. Culture of Bartonella quintana and Bartonella henselae from human samples: a 5-year experience (1993 to 1998). J. Clin. Microbiol. 37:1899-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lepidi, H., P. E. Fournier, and D. Raoult. 2000. Quantitative analysis of valvular lesions during Bartonella endocarditis. A case control study. Am. J. Clin. Pathol. 114:880-889. [DOI] [PubMed]
- 22.Li, J. S., D. J. Sexton, N. Mick, R. Nettles, V. G. J. Fowler, T. Ryan, T. Bashore, and G. R. Corey. 2000. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin. Infect. Dis. 30:633-638. [DOI] [PubMed] [Google Scholar]
- 23.Mainardi, J. L., M. Drancourt, J. M. Roland, J. L. Gestin, D. Raoult, J. F. Acar, and F. W. Goldstein. 1996. Bartonella (Rochalimaea) quintana endocarditis in an Algerian farmer. Clin. Microbiol. Infect. 1:275-276. [DOI] [PubMed] [Google Scholar]
- 24.Patel, R., J. O. Newell, G. W. Procop, and D. H. Persing. 1999. Use of polymerase chain reaction for citrate synthase gene to diagnose Bartonella quintana endocarditis. Am. J. Clin. Pathol. 112:36-40. [DOI] [PubMed] [Google Scholar]
- 25.Piroth, L., P. Menecier, and J. P. Kisterman. 1996. Negative blood culture endocarditis: search for the intracellular germ. Presse Med. 25:1348.. [PubMed] [Google Scholar]
- 26.Raoult, D., G. Aboudharam, E. Crubezy, G. Larrouy, B. Ludes, and M. Drancourt. 2000. Molecular identification by “suicide PCR”of Yersinia pestis as the agent of Medieval Black Death. Proc. Natl. Acad. Sci. USA 97:12800-12803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raoult, D., P. E. Fournier, M. Drancourt, T. J. Marrie, J. Etienne, J. Cosserat, P. Cacoub, Y. Poinsignon, P. Leclercq, and A. M. Sefton. 1996. Diagnosis of 22 new cases of Bartonella endocarditis. Ann. Intern. Med. 125:646-652. [DOI] [PubMed] [Google Scholar]
- 28.Raoult, D., P. E. Fournier, F. Fenollar, M. Jensenius, T. Prioe, J. J. de Pina, G. Caruso, N. Jones, H. Laferl, J. E. Rosenblatt, and T. J. Marrie. 2001. Rickettsia africae, a tick-borne pathogen in travelers to sub-Saharan Africa. N. Engl. J. Med. 344:1504-1510. [DOI] [PubMed] [Google Scholar]
- 29.Raoult, D., P. E. Fournier, F. Vandenesch, J. L. Mainardi, S. J. Eykyn, J. Nash, E. James, C. Benoit-Lemercier, and T. J. Marrie. Outcome and treatment of Bartonella endocarditis. Arch. Intern. Med., in press. [DOI] [PubMed]
- 30.Raoult, D., A. Lakos, F. Fenollar, J. Beytout, P. Brouqui, and P. E. Fournier. 2002. Spotless rickettsiosis caused by Rickettsia slovaca and associated with Dermacentor ticks. Clin. Infect. Dis. 34:1331-1336. [DOI] [PubMed] [Google Scholar]
- 31.Ratge, D., B. Scheiblhuber, O. Landt, J. Berg, and C. Knabbe. 2002. Two-round rapid-cycle RT-PCR in single closed capillaries increases the sensitivity of HCV RNA detection and avoids amplicon carry-over. J. Clin. Virol. 24:161-172. [DOI] [PubMed] [Google Scholar]
- 32.Roux, V., S. J. Eykyn, S. Wyllie, and D. Raoult. 1999. First report of Bartonella vinsonii subsp. berkhoffii as an agent of afebrile blood culture-negative endocarditis in man. J. Clin. Microbiol. 38:1698-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spach, D. H., K. P. Callis, D. S. Paauw, Y. B. Houze, F. D. Schoenknecht, D. F. Welch, H. Rosen, and D. J. Brenner. 1993. Endocarditis caused by Rochalimaea quintana in a patient infected with human immunodeficiency virus. J. Clin. Microbiol. 31:692-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spach, D. H., A. S. Kanter, N. A. Daniels, D. J. Nowowiejski, A. M. Larson, R. A. Schmidt, B. Swaminathan, and D. J. Brenner. 1995. Bartonella (Rochalimaea) species as a cause of apparent “culture-negative”endocarditis. Clin. Infect. Dis. 20:1044-1047. [DOI] [PubMed] [Google Scholar]
- 35.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]