Abstract
Enteropathogenic Escherichia coli (EPEC) has been associated with infantile diarrhea and mortality in humans in developing countries. While diarrhea is also a major problem among primates kept in captivity, the role of E. coli is unclear. This study was designed to characterize diarrheagenic E. coli recovered from the feces of 56 New World nonhuman primates, primarily marmosets (Callithrix spp.). Seventeen of the 56 primates had signs of diarrhea and/or enteritis. E. coli recovered from feces from these animals was tested by PCR for genes encoding virulence factors of diarrheagenic E. coli and for patterns of adherence to HeLa cells. In addition, isolates were characterized by the fluorescence actin staining test and by their ability to induce attaching and effacing lesions. PCR for the eae gene was positive in 10 of the 39 (27%) apparently healthy animals and in 8 of the 17 (47%) animals with diarrhea and/or enteritis. Colonies of eae+ E. coli were serotyped and examined by PCR for genes encoding EPEC virulence markers. The eae+ E. coli isolates recovered from both healthy and sick nonhuman primates demonstrated virulence-associated attributes similar to those of EPEC strains implicated in human disease and are designated monkey EPEC. The results presented here indicate that EPEC may be a significant pathogen for nonhuman primates, deserving further investigation. The similarities between the affected animals investigated in this study and human EPEC infections suggest that marmosets may represent an important model for EPEC in humans.
Enteropathogenic Escherichia coli (EPEC) strains are important agents of infantile diarrhea, heading the statistics of infantile mortality in developing countries (35, 52).
In contrast to other diarrheagenic Escherichia, EPEC does not produce any classical protein toxins but induces diarrhea by intimate binding to intestinal cells. Diarrhea is the result of a series of signals triggered by the pathogen-host membrane interaction, which in turn provokes reorganization of the cytoskeleton of the affected cell, involving accumulation of polymerized actin beneath the adherent bacteria, with a consequent loss in microvillus structure and effacement of the intestinal villi (11, 52, 53). The lesion is called attaching and effacing (A/E) and is encoded by genes present in a pathogenicity island of the EPEC chromosome, called the locus of enterocyte effacement (LEE). This region contains genes for the production of an outer membrane protein (intimin) and the translocated intimin receptor (Tir), in addition to genes encoding proteins of a type III secretion system (10, 35, 53). The carboxy-terminal region of intimin varies, resulting in five major subtypes of intimin, designated α, β, γ, δ, and ɛ (1, 38). The most common sites of insertion of the LEE in the bacterial chromosome are the selC and pheU loci, but some strains of EPEC may have the LEE inserted in a third or unknown region (47).
Although not essential for the development of A/E lesions, fimbriae called bundle-forming pili (BFP) encoded by the Escherichia attaching factor (EAF) plasmid promote the localized adherence of bacteria to epithelial cells, facilitating the occurrence of the lesion (7, 13, 35).
E. coli strains which are able to induce the A/E lesion and are Stx negative are considered to be EPEC strains. Those EPEC strains which harbor the EAF plasmid are conventionally designated typical EPEC, and those which lack the plasmid are called atypical EPEC (35). Recently, it has been proposed that demonstration of the production of BFP would be the best way to differentiate typical and atypical EPEC (52). Epidemiological studies have indicated humans as the only natural reservoir of typical EPEC, since the serotypes included in this group have not been recovered from animals. On the other hand, atypical EPEC serotypes have been strongly associated with animal hosts (52).
Typical EPEC strains have been clearly associated with diarrhea in humans (25, 52). Atypical EPEC strains have been discussed by various authors (19, 55, 56) and are considered to be human emerging pathogens (52). With respect to animals, A/E E. coli has been isolated from healthy and diarrheic animals of various species, including pigs (23, 28, 41), cows (2, 17), sheep and goats (5), horses (54), dogs (3, 16, 33), and rabbits (30).
Although enteric diseases, specifically diarrhea, are frequently associated with morbidity and mortality in nonhuman primates in captivity, studies on the role of different agents in these diseases are lacking (20, 32). Thomson and Scheffler (48) reported an outbreak of diarrhea caused by A/E E. coli in marmosets maintained at the Primatology Center. More recently, EPEC was associated with a simian immunodeficiency virus opportunistic infection in rhesus monkeys (27) and with ulcerative colitis in cotton-top tamarins (26).
In addition to their importance in biomedical research, neotropical primates account for one-third of all monkey species known worldwide; therefore, the study of the impact of various pathogens on these populations is extremely valuable, especially in the context of conservation (31).
Thus, the objective of the present study was to characterize diarrheagenic E. coli strains isolated from neotropical primates with or without signs of enteric disease, with special emphasis on typical and atypical EPEC.
MATERIALS AND METHODS
Animals and feces collection.
A total of 56 nonhuman primates were studied after authorization by the Brazilian Institute of Environment (protocol 02027.008961/01-16) and the Biomedical Institute/USP-Ethical Committee for Animal Research (protocol 042/2002). Thirty-two live animals of the genus Callithrix were sampled. Thirty-one of these were apparently healthy and were maintained at the Departamento de Áreas Verdes do Município de São Paulo (DEPAVE), and one animal with diarrhea was obtained from a private breeding colony. The DEPAVE is a center for the screening and recovery of wild animals and receives animals that are captured in public areas of the city of São Paulo, Brazil, and then later referred to zoologic or breeding institutions. The remaining 24 animals were submitted for postmortem examination at the Laboratory of Comparative Pathology of Wild Animals (LAPCOM), Faculdade de Medicina Veterinária e Zootecnia, Universidade de São Paulo. These randomly chosen animals differed in origin (zoo, private breeding colony, and the wild). Sixteen of these animals had nonbloody acute diarrhea before death and/or macroscopic alterations compatible with enteritis as mucosal thickness associated with congestion or hyperemia and inflammatory exudation, while the remaining eight animals had other alterations but none related to intestinal disease. Feces were collected with sterile swabs from the rectum of live animals and the colon of dead animals and were transported to the laboratory in Stuart medium (Stuart CLR medium CE0373; MEUS, Piove di Sacco, Italy).
Bacterial isolation and characterization.
The swabs were streaked onto plates of MacConkey agar, and the plates incubated at 37°C for 24 h. Lactose-positive and lactose-negative colonies were identified biochemically by standard techniques using EPM, MILi, and Simmons citrate (49, 50). One to three colonies from cultures from each animal, selected as potentially diarrheagenic according the PCR for virulence factors, were subcultured on tryptic soy agar and also stored in brain heart infusion agar plus 50% glycerol at −70°C for further utilization. Isolates were serotyped at the Health Canada Laboratory for Food-Borne Zoonoses, Guelph, Ontario, Canada.
Analysis of virulence factors by PCR.
To determine the presence of diarrheagenic E. coli among the selected subcultures, PCR tests were carried out for genes encoding thermolabile toxins (LT-I and LT-II) and thermostable toxins (STa and STb) produced by enterotoxigenic E. coli and Shiga toxins (Stx1 and Stx2) of Shiga toxin-producing E. coli. The following factors were analyzed in order to characterize the EPEC strains: a fragment of the eae gene; intimin subtypes α, β, γ, δ, and ɛ; the bfpA gene; the EAF plasmid; and the LEE insertion site. All isolates were also subjected to PCR assay to characterize enteroaggregative E. coli (EAEC). PCR conditions for the amplification of the above gene sequences were previously described (1, 4, 9, 12, 18, 29, 37, 38, 42, 45, 46, 47). Sequences of the primers used, annealing temperatures, and the sizes of the amplified fragments are shown in Table 1.
TABLE 1.
Primer sequences, annealing temperatures, and sizes of amplified fragments from selected genes of diarrheagenic E. coli
| Gene or virulence factor | Primer | Sequence (5′-3′) | Annealing temp (°C) | Fragment size (bp) | Reference |
|---|---|---|---|---|---|
| LT-I | A | GGC GAC AGA TTA TAC CGT GC | 48 | 696 | 46 |
| B | CCG AAT TCT GTT ATA TAT GTC | ||||
| LT-II | A | AGA TAT AAT GAT GGA TAT GTA TC | 52 | 300 | M. Blanco, personal communication |
| B | TAA CCC TCG AAA TAA ATC TC | ||||
| STa | A | TCT GTA TTA TCT TTC CCC TC | 43 | 186 | 46 |
| B | ATA ACA TCC AGC ACA GGC | ||||
| STb | A | ATC GCA TTT CTT CTT GCA TC | 60 | 172 | 4 |
| B | G GGC GCC AAA GCA TGC TCC | ||||
| Stx1 | A | GAA GAC TCC GTG GGA TTA CG | 55 | 130 | 42 |
| B | AGC GAT GCA GCT ATT AAT AA | ||||
| Stx2 | A | CTT CGG TAT CCT ATT CCC GG | 55 | 478 | 37 |
| C | GGA TGC ATC TCT GGT CAT TG | ||||
| eaeA | A | ACG TTG CAG CAT GGG TAA CTC | 56 | 815 | 12 |
| B | GAT CGG CAA CAG TTT CAC CTG | ||||
| Intimin α | α | CCT TAG GTA AGT TAA GT | 52 | ≅558 | 1 |
| Intimin β | β | TAA GGA TTT TGG GAC CC | 50 | ≅562 | |
| Intimin γ | γ | ACA AAC TTT GGG ATG TTC | 58 | ≅562 | |
| Intimin δ | δ | TAC GGA TTT TGG GGC AT | 52 | ≅563 | |
| Reverse | Ru | TTT ATG TGC AGC CCC CCA T | |||
| Intimin ɛ | SK1 | CCC GAA TTC GGC ACA AGC ATA AGC | 68 | 2608 | 38 |
| LP5 | AGC TCA CTC GTA GAT GAC GGC AAG CG | ||||
| LEE | K913 | CAT CGG CTG GCG GAA GAT AT | 52 | 300 | 47 |
| Insertion in pheU | K914 | CGC TTA AAT CGT GGC GTC | |||
| LEE | K260 | GAG CGA ATA TTC CGA TAT CTG GTT | 60 | 527 | 29 |
| Insertion in selC, intact | K261 | CCT GCA AAT AAA CAC GGC GCA T | |||
| LEE | K260 | GAG CGA ATA TTC CGA TAT CTG GTT | 60 | 418 | 29 |
| Insertion in right juntion of selC | K255 | GGT TGA GTC GAT TGA TCT CTG G | |||
| EAF | A | CAG GGT AAA AGA AAG ATG ATA A | 55 | 397 | 9 |
| B | TAT GGG GAC CAT GTA TTA TCA | ||||
| bfpA | A | AAT GGT GCT TGC GCT TGC TGC | 63 | 326 | 18 |
| B | GCC GCT TTA TCC AAC CTG GTA | ||||
| EAEC | START | CTG GCG AAA GAC TGT ATC AT | 52 | 630 | 45 |
| STOP | CAA TGT ATA GAA ATC CGC TGT T |
Detection of BFP expression.
Western blotting was used to determine the expression of BFP in samples positive for bfpA by PCR, using the following method.
The bacteria were inoculated in Dulbecco's modified Eagle medium and incubated for 18 h at 37°C with shaking. An aliquot of 100 μl of this culture was inoculated into 10 ml of Dulbecco's modified Eagle medium and incubated at 37°C to reach an optical density at 600 of 0.250. The proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis using a 15% polyacrylamide gel and transferred to a nitrocellulose membrane (Hybond-C Extra; Amersham Life Science) by electroblotting. The immunoblots were blocked with 3% bovine serum albumin (Sigma) and reacted with rabbit anti-BFP antibodies. After reaction with alkaline phosphatase-conjugated anti-rabbit immunoglobulin G (Sigma), the BfpA pilin subunit was visualized by the addition of 5-bromo-4-chloro-3-indolylphospate-nitroblue tetrazolium (Sigma Fast tablets).
Adherence pattern and actin aggregation.
The adherence assays were carried out using infected HeLa cell cultures incubated for 3 and 6 h as previously described (6). The same cell lines infected with E. coli isolates and incubated for 6 h were subjected to the fluorescence actin staining (FAS) test (24).
Electron microscopy.
A/E lesions were evaluated at the ultrastructural level by inoculation of HEp-2 monolayer cells grown in flasks with isolates identified as 7a, 7b, 15a, and 15b, as standardized for the adhesion test (6). After 6 h of incubation at 37°C, the cells were washed three times with phosphate-buffered saline and fixed with 2% glutaraldehyde in phosphate buffer for at least 2 h. The cell layer was scraped off, centrifuged at 3,400 × g for 5 min, resuspended in saline, and subjected to two additional centrifugations. The cells were then stained with 1% osmium solution for 24 h and washed three times as described above. The cells were incubated with 0.5% uranyl for 24 h, washed, embedded in melted agar, and kept in the refrigerator for 24 h. After cutting out the agar, 0.5% uranyl was added and the specimens were dehydrated, embedded in resin, and cut into semi- and ultrathin sections 50 to 70 nm thick with a glass knife coupled to a Sorval MT 5000 ultramicrotome. The sections were observed under a Phillips EM201 transmission electron microscope.
Histopathological evaluation.
Fragments of small intestine and colon were collected from all necropsied animals, fixed in 10% buffered formaldehyde solution, and processed by routine paraffin embedding techniques. Sections were stained with hematoxylin-eosin and toluidine blue and observed under an Olympus BMA-50 trinocular light microscope.
Statistics.
The χ2 test was used to verify differences between groups (statistical significance, P < 0.05).
RESULTS
Isolation and characterization of diarrheagenic E. coli.
Monkey EPEC (MEPEC) or EPEC-like strains were the only groups of diarrheagenic E. coli isolated from the fecal samples. These were identified based upon the detection of the eae gene and the absence of genes for Stx1 and Stx2. No E. coli strains harboring gene sequences for LT-I, LT-II, STa, STb, Stx1, and Stx2 toxins were detected in any of the nonhuman primates studied.
Table 2 shows the species, age, gender, duration of captivity, origin, and clinical status of the animals from which MEPEC or EPEC-like strains were isolated. Clinical history and cause of death are demonstrated in Table 3. Eighteen out of 56 (32%) of the animals had E. coli strains carrying the eae gene, including 8 of 17 (47%) with diarrhea and/or enteritis and 10 of 39 (26%) without these signs or alterations (Table 4). Considering only animals positive for MEPEC or EPEC-like strains, the bacterium was isolated from 32% (10 of 39) of the live apparently healthy animals and from one live marmoset with diarrhea. MEPEC or EPEC-like strains were recovered from 44% (7 of 16) of the dead animals with diarrhea and/or enteritis but not from dead animals without diarrhea or enteritis (Table 4).
TABLE 2.
Characteristics of neotropical primates which harbored eae-positive E. coli
| Animal no. | Species | Agea | Gender | Time in captivityb | Source | Clinical status |
|---|---|---|---|---|---|---|
| 1 | Callithrix penicillata | Adult | Female | 2.5 yr | DEPAVE | Alive, healthy |
| 2 | Callithrix jacchus | Adult | Male | 6 mo | DEPAVE | Alive, healthy |
| 3 | Callithrix jacchus | Adult | Male | 1 day | DEPAVE | Alive, healthy |
| 4 | Callithrix penicillata | Adult | Male | 3 mo | DEPAVE | Alive, healthy |
| 5 | Callithrix penicillata | Adult | Male | 3 mo | DEPAVE | Alive, healthy |
| 6 | Callithrix jacchus | Adult | Male | 11 mo | DEPAVE | Alive, healthy |
| 7 | Callithrix jacchus | Young adult | Male | 1.5 mo | DEPAVE | Alive, healthy |
| 8 | Callithrix penicillata | Juvenile | Male | 1 mo | DEPAVE | Alive, healthy |
| 9 | Callithrix jacchus | Adult | Male | 15 days | DEPAVE | Alive, healthy |
| 10 | Callithrix jacchus | Juvenile | Male | 15 days | DEPAVE | Alive, healthy |
| 11 | Callithrix jacchus | Adult | Female | Bornc | Private breeder | Alive, diarrhea |
| 12 | Saguinus fuscicollis | Adult | Female | Born | LAPCOM/Zoo | Dead (diarrhea-enteritis) |
| 13 | Callithrix penicillata | Adult | Male | Born | LAPCOM/Pbe | Dead (diarrhea-enteritis) |
| 14 | Callithrix geoffroyi | Young adult | Female | Born | LAPCOM/Pb | Dead (diarrhea-enteritis) |
| 15 | Callithrix penicillata | Adult | Female | Born | LAPCOM/Pb | Dead (diarrhea-enteritis) |
| 16 | Callithrix jacchus | Adult | Female | Born | LAPCOM/Pb | Dead (diarrhea-enteritis) |
| 17 | Alouatta fusca | Adult | Female | Wildd | LAPCOM | Dead (enteritis) |
| 18 | Callithrix jacchus | Juvenile | Male | Born | LAPCOM/Pb | Dead (enteritis) |
Juvenile, animals between infancy and sexual maturity; young adult to adult, sexually mature.
Duration of residence at DEPAVE.
Born in captivity.
Free ranging.
Pb, Private breeder colony.
TABLE 3.
Clinical history and cause of death of neotropical primates which harbored eae-positive E. coli
| Animal no. | Clinical history | Causes of death |
|---|---|---|
| 12 | Intermittent diarrhea for 30 days; serous, nonbloody diarrhea for 2 days prior to death; wasting disease | Euthanasia, cachexia |
| 13 | Serous-mucous nonbloody diarrhea for 4 days prior to death; wasting disease | Euthanasia, cachexia |
| 14 | Serous-mucous nonbloody diarrhea for 2 days prior to death; wasting disease | Nutritional, cachexia |
| 15 | Serous nonbloody diarrhea for 1 day prior to death; pneumonia | Respiratory, pneumonia |
| 16 | Serous nonbloody diarrhea for 1 day prior to death; wasting disease | Nutritional, cachexia |
| 17 | Free-ranging animal, unknown clinical history; no sign of diarrhea; poor physical condition; dog bites | Traumatism, aggression |
| 18 | Pneumonia | Respiratory, pneumonia |
TABLE 4.
Association of the eae gene with E. coli isolated from neotropical primates that died or survived after having had diarrhea and/or enteritis or having no signs of illness
| Animal status | Animals with diarrhea and/or enteritis
|
Animals without diarrhea and/or enteritis
|
||
|---|---|---|---|---|
| Total no. | No. eae positive | Total no. | No. eae positive | |
| Alive | 1 | 1 | 31 | 10 |
| Dead | 16a | 7 | 8a | 0 |
| Total | 17 | 8 | 39 | 10 |
The percentage of dead animals among animals with diarrhea and/or enteritis was significantly higher than that among animals that had been apparently healthy.
Virulence factors in eae+ stx-lacking E. coli.
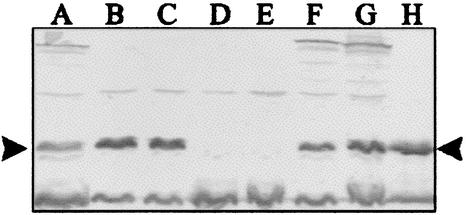
E. coli strains were selected for the determination of intimin subtypes, LEE insertion site, and the presence of bfpA and EAF. Only isolates that showed different phenotypes or genotypes were considered. Among these, PCR showed that four isolates had intimin genes of subtype β, five had intimin genes of subtype α, and 12 were nontypeable (Table 5). With respect to the LEE insertion site, most isolates (13 of 21) had the insertion in selC, with three of them also showing an interrupted pheU. In five isolates, only pheU was interrupted, while in three isolates there was no LEE insertion at either locus (Table 5). The bfpA gene was detected by PCR in 33% (7 of 21) of the isolates, while the EAF plasmid was not detected in any isolate. BFP expression was demonstrated by Western blotting in five of the seven bfpA-positive samples (Table 5; Fig. 1).
TABLE 5.
Characterization of eae-positive E. coli strains isolated from healthy neotropical primates and those with diarrhea and/or enteritis
| Isolatea | bfpA gene | BFP expressionb | EAFc | Intimin subtype | sel C insertion | phe U insertion | Adherence pattern(s)e | FASf | Serotype |
|---|---|---|---|---|---|---|---|---|---|
| 1 | − | − | − | β | − | − | NC | + | O128:H2 |
| 2 | − | − | − | NTd | + | − | LAL, AA | + | O49:H46 |
| 3 | − | − | − | NT | − | − | DA | + | O127:H40 |
| 4a | − | − | − | NT | + | + | NC | +h | O33:NM |
| 4b | − | − | − | NT | + | + | NC | +h | OR:H34 |
| 5 | − | − | − | β | − | + | NC | +h | O167:H9 |
| 6 | − | − | − | NT | + | − | LAL, AA | + | O49:H46 |
| 7a | + | + | − | β | − | + | LA, AA | + | O49:H46 |
| 7b | + | + | − | β | − | + | LA | + | O132:H31 |
| 8 | + | + | − | NT | − | + | LA, AA | + | O132:H31 |
| 12a | − | − | − | NT | − | − | DA | +h | O127:H40 |
| 12b | − | − | − | NT | NAg | + | NC | + | O26:H7 |
| 13a | + | − | − | NT | + | − | LAL, AA | + | O167:H9 |
| 13b | + | − | − | NT | + | − | LAL, AA | + | O167:H6 |
| 15a | + | + | − | α | + | − | LA, AA | + | O142:H6 |
| 15b | + | + | − | α | + | − | LA, AA | + | O167:H9 |
| 15c | − | − | − | α | + | − | LAL, AA | + | O142:H6 |
| 16a | − | − | − | α | + | − | NC | + | O142:H6 |
| 16b | − | − | − | α | + | − | NC | + | O127:H? |
| 17a | − | − | − | NT | + | + | NC | + | O139:H4 |
| 17b | − | − | − | NT | + | − | NC | + | O8:H10 |
Isolates with the same number correspond to the same animal; different letters indicate different isolates.
Tested by Western blotting with BFP antiserum.
The presence of the EAF was determined by PCR.
NT, nontypable.
NC, noncharacteristic; LA, localized adherence; LAL, localized adherence-like; AA, aggregative adherence; DA, diffuse adherence.
Detected by FAS test in HeLa cells (6 h of incubation).
NA, selC not amplified.
Weak reaction.
FIG. 1.
Western blot analysis of EPEC strains harboring bfpA gene. Anti-BFP serum was used at a dilution of 1 in 1,000. Strains were loaded as follows: lane A, strain 8; lane B, strain 15b; lane C, strain 15a; lane D, strain 13b; lane E, strain 13a; lane F, strain 7b; lane G, strain 7a; lane H, strain E2348/69.
Adherence pattern and ability of actin aggregation.
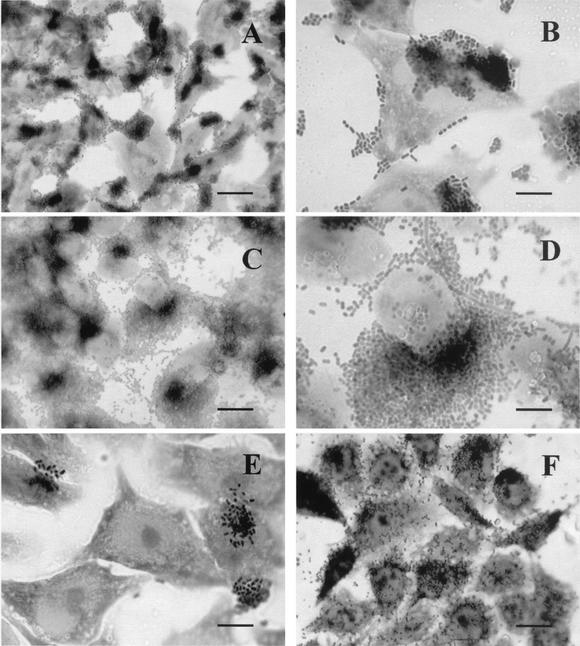
All the isolates adhered to HeLa cells. Most of the isolates in which BFP was expressed (four of five) showed a variation of the typical localized adhesion (LA) pattern. Bacterial microcolonies attached to the cell surface as clusters were accompanied by prominent agglutination of the bacterial cells on the glass coverslip as well as around HeLa cells, typical of the aggregative adherence (AA) phenotype, although the PCR assay for EAEC was negative for all of them (Fig. 2A to D). This phenomenon was observed as soon as 3 h of infection and confirmed at 6 h. The LA-like (LAL) pattern, characterized by the presence of less-compact clusters of bacteria observed only after 6 h of incubation, was verified for 5 of the 21 isolates (Fig. 2E). The diffuse adherence (DA) pattern was detected with two strains (Fig. 2D), and nine isolates had patterns of adherence that were different from any pattern described in the literature and were therefore assigned the designation noncharacteristic. As shown in Table 5, all isolates were able to promote actin accumulation in HeLa cells.
FIG. 2.
HeLa cell adherence assay with E. coli strains isolated from healthy and sick marmosets. Composite adherence pattern showing LA and AA phenotypes is demonstrated as follows: (A and C) strains 15a and 8, respectively (bar = 50 μm); (B and D) strains 15b and 8, respectively (bar = 10 μm). LAL (strain 2) (bar = 10 μm) (E) and DA (strain 3) (bar = 25 μm) (F) patterns are also shown.
Characterization of A/E lesions.
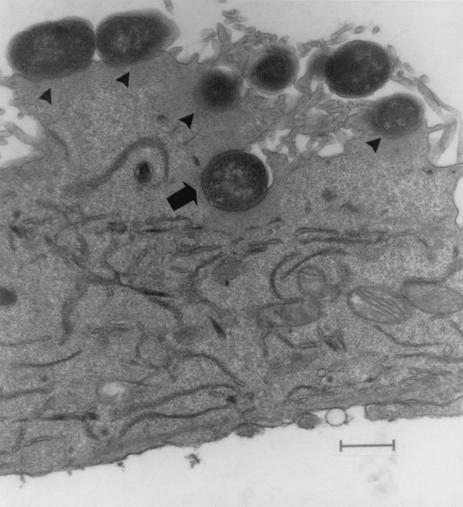
All strains studied by electron microscopy induced A/E lesions which were characterized by tight binding between the bacterial and cell membranes, accompanied by alterations in the cytoskeleton seen by condensation of actin and formation of a pedestal below the binding site. Internalization of bacteria after 6 h of infection was observed for all the isolates (Fig. 3).
FIG. 3.
Electron micrograph of HEp-2 cells after incubation for 6 h at 37°C with E. coli 15a. Note that the E. coli is closely attached to the cell membrane and that there is electron-dense material beneath the adherent bacteria (arrowheads). Some bacteria are partially internalized (arrow). Bar = 2,190 nm.
Serotyping.
The 21 eaeA-positive E. coli strains belonged to 11 serogroups and 13 serotypes (Table 5). O167:H9, O127, and O49:H46 were the most prevalent, identified in three animals each (23%), followed by serotypes O142:H6 and O132:H31 (each identified in two animals [15%]) and O139:H4, O128:H2, O26:H7, O167:H6, O33:NM, OR:H34, and O8:H10 (identified in one animal each). The serotype O167:H9 and the serogroup O127 were isolated from both healthy and sick animals; serogroups O26:H7, O142:H6, O139:H4, and O8:H10 were only identified in animals with enteritis; and serogroups O128:H2, O49:H46, O132:H31, O167:H6, OR:H34, and O33:NM were found only in apparently healthy animals (Tables 2 and 5). Different colonies of serotypes O132:H31, O127:H40, and O142:H6, showed homogeneity regarding the LEE insertion site. With respect to intimin subtype characterization, in addition to isolates of serotype O132:H31 and O49:H46 which included subtype β and untypeable isolates, serotype O167:H9 also demonstrated intimin variation (one isolate characterized as untypeable, one as intimin β, and one as α subtype). The three isolates serotyped as O142:H6 showed the α intimin subtype.
Histopathological evaluation.
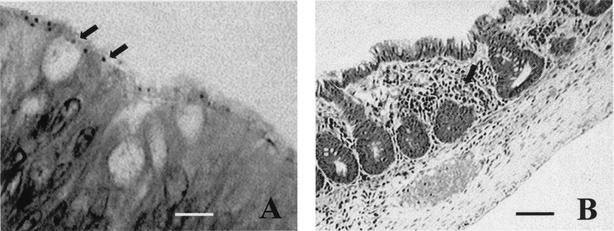
Histopathological evaluation of the colon of animals submitted for necropsy and from which eae+ E. coli was isolated revealed distortion and reduction in crypt size and an inflammatory infiltrate. Such alterations were absent or occurred in a mild form in animals lacking this type of bacteria. The presence of adherent bacteria in some tissue sections of animals with eae+ E. coli was also verified (Fig. 4).
FIG. 4.
Toluidine blue-stained histopathological section of the colon from an animal harboring eae+ E. coli. (A) Presence of adherent bacteria (arrows). Bar = 10 μm. (B) Reduction in crypt size and inflammatory infiltrate (arrow). Bar = 100 μm.
DISCUSSION
Although enteritis is a frequent cause of morbidity and mortality in colonies of nonhuman primates held in captivity, the role of the various agents in this condition is poorly understood (20, 32). In the present study we examined neotropical primates with and without signs of enteric disease for the presence of E. coli with diarrheagenic potential. We observed a significant difference (P = 0.00003) between the percentage of animals that died among those with diarrhea and/or enteritis (94%) compared with the percentage that died from the animals which showed no signs of diarrhea and/or enteritis (21%), indicating that these phenomena (diarrhea and/or enteritis and death) are related, as also reported by others (20, 21).
We did not detect gene sequences that are markers of classical toxigenic E. coli in any of the fecal samples from the individuals studied, nor did we isolate Salmonella spp. or Shigella spp. (data not shown), pathogens frequently implicated in intestinal disease in nonhuman primates (20, 32). The only potential pathogenic E. coli strains we detected were strains that harbored genes for intimin production and lacked genes for Stx1 and Stx2. Also, all strains studied were able to cause the A/E lesion as determined by the FAS test, and confirmed by electron microscopy of cells infected with some of the strains. Since there is a consensus that EPEC strains are defined by the ability to induce A/E lesions combined with the inability to produce Shiga toxins and not only on the basis of O:H antigens (35), the present results permit us to define the E. coli strains isolated here as being EPEC or MEPEC strains.
The EPEC stains were isolated from 47% of the animals with diarrhea and/or enteritis versus only 26% of apparently healthy individuals. Although this difference was not statistically significant, the higher percentage of isolation from sick animals, together with the absence of this pathogen in necropsied animals with no enteritis, suggests that EPEC may be involved in the disease in these primates, although other intestinal pathogens that were not investigated could be involved. This conclusion is supported by the observation of bacteria adhering to intestinal cells of EPEC-positive necropsied animals and is associated with histopathological alterations such as reduced crypt size and presence of an inflammatory infiltrate. The same phenomenon has been observed previously in rhesus monkeys with AIDS infected with EPEC (27).
Outbreaks of hemorrhagic diarrhea in marmosets, opportunistic infections in rhesus monkeys with AIDS, and ulcerative colitis in cotton-top tamarins have been associated with EPEC (26, 27, 48). However, there are no reports in the literature about the characterization of this potential pathogen in apparently healthy animals, as observed in the present study. Adults and juvenile animals may represent important sources of infection for more-susceptible individuals since they live in family groups (32).
In children younger than 1 year there is a strong correlation between diarrhea and the isolation of typical and atypical EPEC (25, 44). With respect to animals, there are speculations about the pathogenic potential of EPEC for different species (2, 3, 5, 16, 23, 28, 41), with their pathogenicity having been well characterized in rabbits, the species used as an experimental model (30). However, some researchers have observed that even strains isolated from sick animals mostly belong to serotypes that differ from those isolated from humans, a fact that has led to use of the term EPEC-like strains (30, 35).
The present results demonstrate that in the case of nonhuman primates, several isolates belong to serogroups and/or serotypes related to those implicated in human disease, such as the traditional EPEC serogroups O127, O128, O142, and O26 (58), with the last one including also enterohemorrhagic E. coli strains (39). Three of the serotypes isolated are related to outbreaks of diarrhea in humans. Serotype O142:H6, isolated from two sick animals, and serotype O128:H2, isolated from a healthy animal, are classified as typical and atypical EPEC serotypes, respectively, and are important agents of children's diarrhea all over the world (8, 14, 51, 52). In contrast, serotype O167:H9, isolated from three animals, although not included among the EPEC serotypes, was characterized as A/E E. coli that caused an outbreak of gastroenteritis involving a large number of school children (36). Strains of serogroup O26, in addition to being a recognized human pathogen, were characterized as EPEC causing hemorrhagic colitis in an outbreak affecting a marmoset colony maintained at the Primatology Center (48).
An important marker of EPEC pathogenicity is the presence of the EAF plasmid which codes for the fimbria denoted BFP, responsible for the LA phenotype characteristic of typical EPEC (35). None of the strains isolated here from nonhuman primates was positive in the PCR assay for EAF, although 7 of 21 were positive for bfpA, and 5 of these 7 expressed this fimbria. Except for an isolate of serotype O142:H6, none of the others that expressed this characteristic belonged to the classical EPEC serotypes (O49:H6, O132:H31, O167:H9, and O167:H6). Serotypes related to children's diarrhea such as the atypical O119:H2 and the typical O142:H6 have been reported as EAF-negative, bfpA-positive EPEC strains in Brazil (51, 52), despite the importance of the EAF plasmid for the regulation of plasmid and chromosomal genes involved in the establishment of the A/E lesion (15, 22). Trabulsi et al. (52) have speculated that the production of BFP may be the best way to differentiate typical and atypical EPEC strains. Thus, the presence of this gene sequence associated with the expression of BFP even in strains not belonging to classical serotypes indicates the virulence potential of these strains isolated from nonhuman primates.
The LA phenotype was identified in all strains expressing BFP, but not in the two bfpA-positive, BFP-negative strains. These two strains showed the LAL phenotype previously reported for strains that had lost the EAF plasmid (40, 44), classified as atypical EPEC strains. In both cases, and in all strains that exhibited the LA phenotype, the AA phenotype was also observed in spite of a negative PCR assay for the gene usually involved in AA. The AA pattern is observed in EAEC, a pathogen emerging worldwide as a cause of persistent diarrhea (34). It is possible that these bacteria isolated from nonhuman primates have a mosaic of characteristics of virulence constructed from the acquisition of genes that code for other fimbrial and/or membrane antigens (34). These genes may have been acquired and disseminated among different groups of animal E. coli strains since a similar phenomenon has been observed in strains from cattle and rabbits (A. Pestana de Castro, personal communication).
The A/E lesion occurs due to genes present in the LEE pathogenicity island, which contains the eae gene that codes for intimin expression (35). Phylogenetic studies have suggested that the site of insertion of this gene complex is directly related to the evolutionary origin of the strain. On this basis, strains characterized as typical EPEC serotypes are included in the same evolutionary branch, where the LEE is inserted into the selC locus of the bacterial chromosome (57), and the intimin is of the α subtype (1). In atypical EPEC serotypes, the LEE may be inserted in the pheU locus or another site (47, 57). All the isolates which had the intimin α subtype had the LEE inserted in selC and had been recovered from animals with enteritis. Three of them were serotyped as O142:H6, and one was characterized as O127:H?, suggesting that the origin of these strains may be similar to that of human strains. The fact that other isolates had the LEE in different loci (pheU, both sites, or none of them) and had intimin that was subtype β or nontypeable supports the idea that the LEE region is a dynamic entity in terms of the transfer of the full cassette or isolated genes (43; J. V. Newman, K. G. Mansfield, and D. B. Schauer, Abstr. 100th Gen. Meet. Am. Soc. Microbiol., abstr. D166, 2000), a fact possibly favoring the appearance of new pathogenic strains (43).
The participation of diarrheagenic E. coli strains, more specifically EPEC, in enteric diseases of nonhuman primates held in captivity may be underestimated. The results reported here indicate that this pathogen may be involved in the signs and symptoms of enteric disease, since the bacteria isolated from animals with enteritis had virulence properties similar to those detected in strains related to human disease. In addition to the impact these bacteria may have on the health of colonies of animals held in captivity, there is also the potential risk of transmission to humans, which characterizes the zoonotic potential of these infections. The isolation of strains with genotypic and phenotypic characteristics similar to those of typical EPEC serotypes indicates that not only humans but also nonhuman primates may represent a natural reservoir and source of infection of these bacteria for both human and nonhuman primates.
Acknowledgments
This work was supported by a grant from the Fundação de Amparo a Pesquisa do Estado de São Paulo and the Conselho Nacional de Pesquisae Desenvolvimento Científico e Tecnológico.
We thank Adriana Joppert and the Departamento de Áreas Verdes do Município de São Paulo for providing live animals for our research as well as the Laboratório de Patologia Comparada de Animais Selvagens and its researchers for access to necropsied animals. We are also indebted to Shirlei Meire Da Silva for her technical assistance with the electron microscopy studies, Maria Natália de Oliveira for her technical help, and Maurício Garcia for statistical analysis of the data.
REFERENCES
- 1.Adu-Bobie, J., G. Frankel, B. Christopher, A. G. Gonçalves, L. R. Trabulsi, G. Douce, S. Knutton, and G. Dougan. 1998. Detection of intimins α, β, γ, and δ, four intimin derivates expressed by attaching and effacing microbial pathogens. J. Clin. Microbiol. 36:663-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aidar, L., A. S. Penteado, L. R. Trabulsi, J. E. Blanco, M. Blanco, J. Blanco, and A. F. Pestana de Castro. 2000. Subtypes of intimin among non-toxigenic Escherichia coli from diarrheic calves in Brazil. Can. J. Vet. Res. 64:15-20. [PMC free article] [PubMed] [Google Scholar]
- 3.Beutin, L. 1999. Escherichia coli as a pathogen in dogs and cats. Vet. Res. 30:285-298. [PubMed] [Google Scholar]
- 4.Blanco, M., J. E. Blanco, H. E. Gonzalez, A. Mora, W. Jansen, T. A. T. Gomes, L. F. Zerbini, T. Yano, A. F. Pestana de Castro, and J. Blanco. 1997. Genes coding for enterotoxins and verotoxins in porcine Escherichia coli strains belonging to different O:K:H serotypes: relationships with toxic phenotypes. J. Clin. Microbiol. 35:2958-2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cid, D., J. A. Ruiz-Santa-Quiteria, I. Marin, R. Sanz, J. A. Orden, R. Amils, and R. De La Fuente. 2001. Association between intimin (eae) and espB gene subtypes in attaching and effacing Escherichia coli strains isolated from diarrhoeic lambs and goat kids. Microbiology 147:2341-2353. [DOI] [PubMed] [Google Scholar]
- 6.Cravioto, A., R. J. Gross, S. M. Scotland, and B. Rowe. 1979. An adhesive factor found in strains of Escherichia coli belonging to the traditional infantile enteropathogenic serotypes. Curr. Microbiol. 3:95-99. [Google Scholar]
- 7.Donnenberg, M. S., and J. B. Kaper. 1992. Enteropathogenic Escherichia coli. Infect. Immun. 60:3953-3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donnenberg, M. S., and T. Whittam. 2001. Pathogenesis and evolution of virulence in enteropathogenic and enterohemorrhagic Escherichia coli. J. Clin. Investig. 107:539-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franke, J., S. Fraake, H. Schmidt, A. Schwarzkopf, L. H. Wieler, G. Baljer, L. Beutin, and H. Karch. 1994. Nucleotide sequence analysis of enteropathogenic Escherichia coli (EPEC) adherence factor probe and development of PCR for rapid detection of EPEC harboring virulence plasmids. J. Clin. Microbiol. 32:2460-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frankel, G., A. D. Phillips, I. Rosenshine, G. Dougan, J. B. Kaper, and S. Knutton. 1998. Enteropathogenic and enterohaemorrhagic Escherichia coli: more subversive elements. Mol. Microbiol. 30:911-921. [DOI] [PubMed] [Google Scholar]
- 11.Frankel, G., A. D. Phillips, L. R. Trabulsi, S. Knutton, G. Dougan, and S. Matthews. 2001. Intimin and the host cell—is it bound to end in Tir(s)? Trends Microbiol. 9:214-218. [DOI] [PubMed] [Google Scholar]
- 12.Gannon, V. P. J., M. Rashed, R. K. King, and T. E. J. Gosteyn. 1993. Detection and characterization of the eae gene of Shiga-like toxin-producing Escherichia coli using polymerase chain reaction. J. Clin. Microbiol. 31:1268-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Girón, J. A., A. S. Y. Ho, and G. K. Schoolnik. 1991. An inducible bundle-forming pilus of enteropathogenic Escherichia coli. Science 254:710-713. [DOI] [PubMed] [Google Scholar]
- 14.Gomes, T. A. T., P. M. Griffin, C. Ivey, L. R. Trabulsi, and S. R. T. S. Ramos. 1996. EPEC infections in São Paulo. Rev. Microbiol. 27:25-33. [Google Scholar]
- 15.Gomez-Duarte, O. G., and J. B. Kaper. 1995. A plasmid-encoded regulatory region activates chromosomal eaeA expression in enteropathogenic Escherichia coli. Infect. Immun. 63:1767-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gouffaux, F., B. China, L. Janssen, and J. Mainil. 2000. Genotypic characterization of enteropathogenic Escherichia coli (EPEC) isolated in Belgium from dogs and cats. Res. Microbiol. 151:865-871. [DOI] [PubMed] [Google Scholar]
- 17.Gouffaux, F., B. China, and J. Mainil. 2001. Organization and in vitro expression of esp genes of the LEE (locus of enterocyte effacement) of bovine enteropathogenic and enterohemorrhagic Escherichia coli. Vet. Microbiol. 83:275-286. [DOI] [PubMed] [Google Scholar]
- 18.Gunzburg, S. T., N. G. Tornieporth, and L. W. Riley. 1995. Identification of enteropathogenic Escherichia coli by PCR-bases detection of the bundle-forming pilus gene. J. Clin. Microbiol. 33:1375-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hedberg, C. W., S. J. Savariano, J. M. Besser, C. J. Paulus, V. M. Thelen, L. J. Myers, D. N. Cameron, T. J. Barrett, J. B. Kaper, and M. T. Osterholm. 1997. An outbreak of foodborne illness caused by Escherichia coli O39:NM, an agent that does not fit into the existing scheme for classifying diarrheogenic E. coli. J. Infect. Dis. 176:1625-1628. [DOI] [PubMed] [Google Scholar]
- 20.Hird, D. W., J. H. Anderson, and J. T. Bielitzki. 1984. Diarrhea in nonhuman primates: a survey of primates colonies for incidence rates and clinical opinion. Lab. Anim. Sci. 34:465-470. [PubMed] [Google Scholar]
- 21.Holmberg, C. A., R. Leininger, E. Wheeldon, D. Slater, R. Henrickson, and J. Anderson. 1982. Clinicopathological studies of gastrointestinal disease in macaques. Vet. Pathol. 19:163-170. [PubMed] [Google Scholar]
- 22.Kaper, J. B., and O. G. Gomez-Duarte. 1997. The per regulator of enteropathogenic Escherichia coli. Mol. Microbiol. 23:179-181. [DOI] [PubMed] [Google Scholar]
- 23.Kim, J., C. Choi, and C. Chae. 2001. Prevalence of eae+ Escherichia coli isolated from pigs with diarrhea. J. Vet. Diagn. Investig. 13:355-356. [DOI] [PubMed] [Google Scholar]
- 24.Knutton, S., T. Baldwin, P. H. Willians, and A. S. Mcneish. 1989. Actin accumulation at sites of adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect. Immun. 57:1290-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levine, M. M., and R. Eldeman. 1984. Enteropathogenic Escherichia coli of classic serotypes associated with infant diarrhea: epidemiology and pathogenesis. Epidemiol. Rev. 6:31-51. [DOI] [PubMed] [Google Scholar]
- 26.Mansfield, K. G., L. Kuei-Chin, X. Dongling, J. Newman, D. Schauer, J. Mackey, A. A. Lackner, and A. Carville. 2001. Enteropathogenic Escherichia coli and ulcerative colitis in cotton-top tamarins (Saguinus oedipus). J. Infect. Dis. 184:803-807. [DOI] [PubMed] [Google Scholar]
- 27.Mansfield, K. G., L. Kuei-Chin, J. Newman, D. Schauer, J. Mackey, A. A. Lackner, and A. Carville. 2001. Identification of enteropathogenic Escherichia coli in simian immunodeficiency virus-infected infant and adult rhesus macaques. J. Clin. Microbiol. 39:971-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martins, M. F., N. M. Martinez-Rossi, A. Ferreira, M. Brocchi, T. A. F. Yano, and W. D. Silveira. 2000. Pathogenic characteristics of Escherichia coli strains isolated from newborn piglets with diarrhea in Brazil. Vet. Microbiol. 76:51-59. [DOI] [PubMed] [Google Scholar]
- 29.Mc Daniel, T. K., K. G. Jarvis, M. S. Donnenberg, and J. B. Kaper. 1995. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc. Natl. Acad. Sci. USA 92:1664-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Milon, A., E. Oswald, and J. De Rycke. 1999. Rabbit EPEC: a model for the study of enteropathogenic Escherichia coli. Vet. Res. 30:203-219. [PubMed] [Google Scholar]
- 31.Mittermeier, R. A. 1986. Primate conservation priorities in the neotropical regions, p. 221-240. In K. Benirschke (ed.), Primates. The road to self-sustaining populations. Springer-Verlag, New York, N.Y.
- 32.Montali, R., and M. Bush. 1999. Diseases of the Callitrichidae, p. 369-376. In M. E. Fowler and R. E. Miller (ed.), Zoo and wild animal medicine. W. B. Saunders Company, Philadelphia, Pa.
- 33.Nakazato, G. 2001. Estudo dos fatores de virulência de amostras de Escherichia coli isoladas de cães com diarréia e normais. Características de intiminas produzidas, detectáveis pela reação em cadeia da polimerase (PCR). M.Sc. thesis. Unicamp, Campinas, Brazil.
- 34.Nataro, J. P., T. Steiner, and R. L. Guerrant. 1998. Enteroaggregative Escherichia coli. Emerg. Infect. Dis. 4:251-261. [DOI] [PMC free article] [PubMed]
- 35.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohtani, Y., M. Kameda, and K. Takamura. 1991. An outbreak of gastroenteritis possibly caused by Escherichia coli O167:H9. Kansenshogaku Zasshi 65:35-39. [DOI] [PubMed] [Google Scholar]
- 37.Olsvik, O., and N. A. Strockbine. 1993. PCR detection of heat-stable, heat-labile, and Shiga-like toxin genes in Escherichia coli, p. 271-276. In D. H. Persing, T. F. Smith, F. C. Tenover, and T. J. White (ed.), Diagnostic molecular microbiology. American Society for Microbiology, Washington, D.C.
- 38.Oswald, O., H. Schmidt, S. Morabito, H. Karch, O. Marchès, and A. Caprioli. 2000. Typing of intimin genes in human and animal enterohemorrhagic and enteropathogenic Escherichia coli: characterization of a new intimin variant. Infect. Immun. 68:64-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peixoto, J. C. C., S. Y. Bando, J. A. G. Ordoñez, B. A. Botelho, L. R. Trabulsi, and C. A. Moreira-Filho. 2001. Genetic differences between Escherichia coli O26 strains isolated in Brazil and other countries. FEMS Microbiol. Lett. 196:239-244. [DOI] [PubMed] [Google Scholar]
- 40.Pelayo, J. S., I. C. A. Scaletsky, M. Z. Pedroso, V. Sperandio, J. A. Girón, G. Frankel, and L. R. Trabulsi. 1999. Virulence properties of atypical EPEC strains. J. Med. Microbiol. 48:41-49. [DOI] [PubMed] [Google Scholar]
- 41.Penteado, A. S., L. Aidar, A. F. Pestana de Castro, A. Yamada, J. R. Andrade, J. Blanco, M. Blanco, and J. E. Blanco. 2001. eae-negative attaching and effacing Escherichia coli from piglets with diarrhea. Res. Microbiol. 152:75-81. [DOI] [PubMed] [Google Scholar]
- 42.Pollard, D. R., W. M. Johnson, H. Lior, S. D. Tyler, and K. R. Rozee. 1990. Rapid and specific detection of verotoxin genes in Escherichia coli by the polymerase chain reaction. J. Clin. Microbiol. 28:540-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sandner, L., L. E. Eguiarte, A. Navarro, A. Cravioto, and V. Souza. 2001. The elements of the locus of enterocyte effacement in human and wild mammal isolates of Escherichia coli: evolution by assemblage or disruption? Microbiology 147:3149-3158. [DOI] [PubMed] [Google Scholar]
- 44.Scaletsky, I. C. A., M. Z. Pedroso, C. A. G. Oliva, R. L. B. Carvalho, M. B. Morais, and U. Fagundes-Neto. 1999. A localized adherence-like pattern as a second pattern of adherence of classic enteropathogenic Escherichia coli to HEp-2 cells that is associated with infantile diarrhea. Infect. Immun. 67:3410-3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmidt, H., C. Knop, S. Franke, S. Aleksic, J. Heesemann, and H. Karch. 1995. Development of PCR for screening of enteroaggregative Escherichia coli. J. Clin. Microbiol. 33:701-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schultsz, H., G. J. Pool, R. van Ketel, B. De Wever, P. Spelman, and J. Dankert. 1994. Detection of enterotoxigenic Escherichia coli in stool samples by using nonradioactively labeled oligonucleotide DNA probes and PCR. J. Clin. Microbiol. 32:2393-2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sperandio, V., J. B. Kaper, M. R. Bortolini, B. C. Neves, R. Keller, and L. R. Trabulsi. 1998. Characterization of the locus of enterocyte effacement (LEE) in different enteropathogenic Escherichia coli (EPEC) and Shiga-toxin producing Escherichia coli (STEC) serotypes. FEMS Microbiol. Lett. 164:133-139. [DOI] [PubMed] [Google Scholar]
- 48.Thomson, J. A., and J. J. Scheffler. 1996. Hemorrhagic typhlocolitis associated with attaching and effacing Escherichia coli in common marmosets. Lab. Anim. Sci. 46:275-279. [PubMed] [Google Scholar]
- 49.Toledo, M. R. F., C. F. Fontes, and L. R. Trabulsi. 1982. MILi—um meio para a realização dos testes de motilidade, indol e lisina descarboxilase. Rev. Microbiol. 13:230-235. [Google Scholar]
- 50.Toledo, M. R. F., C. F. Fontes, and L. R. Trabulsi. 1982. EPM—modificação do meio de Rugai e Araújo para a realização simultÂnea dos testes de produção de gás a partir da glicose, H2S, urease e triptofano desaminase. Rev. Microbiol. 13:309-315. [Google Scholar]
- 51.Trabulsi, L. R., L. C. Campos, T. S. Whittam, T. A. T. Gomes, J. Rodrigues, and A. G. Gonçalves. 1996. Traditional and non-traditional enteropathogenic Escherichia coli serogroups. Rev. Microbiol. 27:1-6. [Google Scholar]
- 52.Trabulsi, L. R., R. Keller, and T. A. T. Gomes. 2002. Typical and atypical enteropathogenic Escherichia coli. Emerg. Infect. Dis. 8:508-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vallance, B. A., and B. B. Finlay. 2000. Exploitation of host cells by enteropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 97:8799-8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Duijkeren, E., A. J. van Asten, and W. Gaastra. 2000. Characterization of Escherichia coli isolated from adult horses with and without enteritis. Vet. Q. 22:162-166. [DOI] [PubMed] [Google Scholar]
- 55.Vieira, M. A. M., J. R. C. Andrade, L. R. Trabulsi, A. C. P. Rosa, A. M. G. Dias, S. R. T. S. Ramos, G. Frankel, and T. A. Gomes. 2001. Phenotypic and genotypic characteristics of Escherichia coli strains of non-enteropathogenic E. coli (EPEC) serogroups that carry eae and lack the EPEC adherence factor and shiga toxin DNA probe sequences. J. Infect. Dis. 183:762-772. [DOI] [PubMed] [Google Scholar]
- 56.Viljanen, M. K. T., T. Peltola, S. Y. T. Junnila, L. Olkkonen, H. Jarvinen, M. Kuistila, and P. Huovinen. 1990. Outbreak of diarrhea due to Escherichia coli O111:B4 in school children and adults: association of Vi antigen-like reactivity. Lancet 336:831-834. [DOI] [PubMed] [Google Scholar]
- 57.Wieler, L. H., T. K. MacDaniel, T. S. Whittam, and J. B. Kaper. 1997. Insertion site of locus of enterocyte effacement in enteropathogenic and enterohemorrhagic Escherichia coli differs in relation to clonal phylogeny of the strains. FEMS Microbiol. Lett. 156:49-53. [DOI] [PubMed] [Google Scholar]
- 58.World Health Organization. 1987. Programme for control of diarrhoeal diseases (CDD/83.3 Rev 1), p. 27. In Manual for laboratory investigation of acute enteric diseases. World Health Organization, Geneva, Switzerland.