Abstract
Several subtypes of Mycobacterium kansasii have been described, but their respective pathogenic roles are not clear. This study investigated the distribution of subtypes and the pathogenicity of M. kansasii strains (n = 191) isolated in Switzerland between 1991 and 1997. Demographic, clinical, and microbiological information was recorded from clinical files. Patients were classified as having an infection according to the criteria of the American Thoracic Society. Subtypes were defined by PCR-restriction enzyme analysis of the hsp65 gene. Subtype 1 comprised 67% of the isolates (n = 128), while subtypes 2 and 3 comprised 21% (n = 40) and 8% (n = 15), respectively. Other subtypes (subtypes 4 and 6 and a new subtype, 7) were recovered from only 4% of patients (n = 8). M. kansasii subtype 1 was considered pathogenic in 81% of patients, while M. kansasii subtype 2 was considered pathogenic in 67% of patients and other subtypes were considered pathogenic in 6% of patients. The majority of patients with M. kansasii subtype 2 were immunocompromised due to the use of corticosteroids (21% of patients) or coinfection with HIV (62.5% of patients). Subtyping M. kansasii may improve clinical management by distinguishing pathogenic from nonpathogenic subtypes.
Mycobacterium kansasii is, after Mycobacterium avium, the mycobacterial species most frequently responsible for disease due to nontuberculous mycobacteria (7). However, little is known about its pathogenicity, mode of transmission, and natural reservoir. M. kansasii is often recovered from tap water and occasionally from river or lake water (12, 16, 17, 24). The isolation of this bacterium from other environmental sources, such as soil and animals, is very rare (1, 2, 18). M. kansasii causes pulmonary disease similar to tuberculosis in immunocompetent patients and pulmonary, extrapulmonary, or disseminated disease in patients with various immunodeficiencies, in particular human immunodeficiency virus (HIV) infection (6, 15, 19). Blood culture isolates, especially those from HIV-infected patients, should always be considered pathogenic (14). However, the isolation of M. kansasii from sputum is not proof of disease, as it may represent a colonization of the respiratory tract (1). The American Thoracic Society has recently proposed diagnostic criteria based on radiographic, clinical, and microbiological data (2).
Phylogenetic and molecular analyses have demonstrated that M. kansasii is a heterogeneous species with several distinct subtypes (1, 18, 21, 22). M. kansasii subtype 1, as defined by polymorphism analysis of the hsp65 gene, is the subtype by far the most frequently isolated from humans; however, it is only rarely isolated from the environment. In contrast, HIV-infected patients seem to be particularly susceptible to infection with M. kansasii subtype 2, which may act as an opportunistic agent (1, 27). Little is known about the pathogenic roles of other subtypes (subtypes 3 to 7), which are generally isolated from environmental sources (1). The aim of the present study was to analyze M. kansasii subtypes isolated from clinical specimens in Switzerland in order to determine the pathogenicity of each subtype.
MATERIALS AND METHODS
M. kansasii isolates.
M. kansasii strains isolated between 1991 and 1997 at six major Swiss laboratories (in Lausanne, Zurich, Bern, Basel, Geneva, and Lugano) were included in a national survey. M. kansasii isolates were identified both biochemically and genetically by using AccuProbe (GenProbe Inc., San Diego, Calif.) for M. kansasii or PCR-restriction enzyme pattern analysis (26). One isolate from each patient was included. M. kansasii isolates that were identified at other Swiss laboratories (n = 23) could not be included in this national survey.
PCR-restriction enzyme pattern analysis of hsp65.
For the purpose of the study, all the strains were retyped at one center (Lausanne) by using PCR and restriction enzyme analysis of the hsp65 gene (20, 26; www.hospvd.ch/prasite). Analysis of the hsp65 gene by PCR-restriction analysis has been widely used for the diagnosis of mycobacterial infections and the characterization of newly described isolates (5, 9, 11, 23). Strains were classified into subtypes according to the restriction fragment length polymorphism patterns observed (1, 21).
Clinical data.
Demographic, clinical, microbiological, and follow-up information was recorded from clinical files by using a standardized anonymous form. Patients were classified as having an infection according to the criteria of the American Thoracic Society (2). M. kansasii isolates collected from a normally sterile tissue (biopsy specimen, blood, or bone marrow) were considered pathogenic. Disseminated infection was defined by the presence of M. kansasii in blood, bone marrow, or simultaneously from three different anatomic sites. Isolates from patients with clinical presentations that could not be classified according to the American Thoracic Society criteria were assessed by an infectious disease specialist (A.T.) and a medical microbiologist (G.P.) as being pathogenic or not according to clinical and follow-up data, without knowledge of the M. kansasii subtype.
Statistical analysis.
Statistical analysis was performed with SPSS software (SPSS version 10.0; SPSS, Chicago, Ill.). Fisher's exact test was used to compare the characteristics of patients with the same subtype of M. kansasii, and the Mann-Whitney test was performed for comparison of median ages and duration of symptoms. Logistic regression was performed to compare the pathogenic roles of the subtypes. The variables included in the multivariate model were as follows: subtype of M. kansasii, HIV status, presence or absence of chronic obstructive pulmonary disease (COPD), smoking habits, and use of corticosteroids.
RESULTS
Distribution of subtypes.
Of the M. kansasii isolates collected in Switzerland between 1991 and 1997, 89% (191 isolates; one per patient) were included in this study. Subtype 1 was the subtype most frequently isolated (n = 128; 67% of all isolates in the study), followed by subtype 2 (n = 40; 21%), subtype 3 (n = 15; 8%), subtype 6 (n = 4; 2%), and subtype 4 (n = 1; 0.5%) (Table 1). Subtypes 1 and 2 were isolated at sites nationwide, whereas subtype 3 was most frequently isolated in the two contiguous areas, Lausanne and Geneva, where it accounted for 19 and 20% of the isolates, respectively. M. kansasii subtype 5 was never detected in our study. However, a novel subtype with a unique restriction pattern, subtype 7, was identified in three patients (2%). BstEII digestion of the PCR products of this subtype resulted in a pattern identical to that for subtypes 2, 3, and 6, while HaeIII digestion gave a unique pattern (Table 2). Subtype 7 was confirmed to belong to the M. kansasii species by sequencing the 16S rRNA gene and the 16S to 23S rRNA spacer region.
TABLE 1.
Distribution of M. kansasii isolates in Switzerland according to subtypea
| Area | No. of isolates | No. (%) classified as subtype:
|
|||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 6 | 7 | ||
| Zurich | 88 | 66 (75) | 15 (17) | 4 (5) | 0 (0) | 0 (0) | 3 (4) |
| Lausanne | 36 | 21 (58) | 7 (19) | 7 (19) | 0 (0) | 1 (3) | 0 (0) |
| Bern | 31 | 21 (68) | 10 (32) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Geneva | 20 | 7 (35) | 6 (30) | 4 (20) | 1 (5) | 2 (10) | 0 (0) |
| Basel | 13 | 11 (85) | 1 (8) | 0 (0) | 0 (0) | 1 (8) | 0 (0) |
| Lugano | 3 | 2 (67) | 1 (33) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Total | 191 | 128 (67) | 40 (21) | 15 (8) | 1 (0.5) | 4 (2) | 3 (2) |
No subtype 5 strains were isolated.
TABLE 2.
Patterns of hsp65 PCR product after restriction enzyme digestion with BstEII and HaeIII
| M. kansasii subtype | BstEII fragment lengths (bp) | HaeIII fragment lengths (bp) |
|---|---|---|
| 1 | 240, 210 | 140, 105, 80 |
| 2 | 240, 135, 85 | 140, 105 |
| 3 | 240, 135, 85 | 140, 95, 70 |
| 4 | 240, 125, 85 | 140, 115, 70 |
| 5 | 325, 125 | 140, 100, 80 |
| 6 | 240, 135, 85 | 140, 105, 70 |
| 7 | 240, 135, 85 | 140, 95, 80 |
The mean number of positive cultures per patient was 1.84 (range, 1 to 12). Of the isolates producing positive cultures, 73% were collected from the respiratory tract. Mycobacteremia was detected in 32.5% of HIV-infected patients, in 2.5% of non-HIV-infected patients, and in 4.5% of patients with unknown HIV serostatus.
Characteristics of patients.
Clinical data were available for 114 of the 191 patients (Table 3). The distribution of M. kansasii subtypes 1 and 2 among the 77 patients for whom no clinical data were available was comparable to that found among the 114 patients for whom data were available. Data for the four patients with M. kansasii subtype 6 were not available.
TABLE 3.
Characteristics of 114 patients from whom M. kansasii was isolateda
| Characteristic(s) | Value for M. kansasii subtype:
|
||
|---|---|---|---|
| 1 (n = 74)b | 2 (n = 24) | 3, 4, and 7 (n = 16) | |
| Demography | |||
| Mean age ± SD (yr) | 45 ± 19.5c | 40.5 ± 16.7c | 76.0 ± 22.3 |
| Male | 46 (62.0) | 15 (62.5) | 11 (68.7) |
| Urban residents | 42 (56.7) | 15 (62.5) | 11 (68.7) |
| Swiss | 54 (73.0) | 20 (83.3) | 11 (68.7) |
| Risk factors | |||
| HIV infection | 23 (31.1)c | 15 (62.5)c | 1 (6.2) |
| Corticoid use | 5 (6.7) | 5 (20.8) | 3 (18.7) |
| Tobacco use | 43 (58.1) | 18 (75.0) | 8 (50.0) |
| COPD | 15 (20.3) | 6 (25.0) | 9 (56.2) |
| Other pulmonary diseases | 45 (60.8) | 16 (66.7) | 9 (56.2) |
| Source of isolated | |||
| Pulmonary | 67 (90.5) | 19 (79.2) | 9 (56.2) |
| Nonpulmonary | 20 (27.0) | 8 (33.3) | 4 (25.0) |
| Disseminated | 17 (23.0)c | 7 (29.2)c | 0 (0.0) |
| Clinical presentation | |||
| Asymptomatic | 4 (5.4) | 2 (8.3) | 3 (18.7) |
| Systemic symptoms | 55 (74.3)c | 18 (75.0)c | 6 (37.5) |
| Respiratory symptoms | 60 (81.1) | 20 (83.3) | 13 (81.2) |
| Median duration of symptoms (wk) | 8c | 6c | 2 |
| Results of chest radiography | |||
| Normal | 9 (12.1) | 1 (4.2) | 0 (0.0) |
| Abnormale | 64 (86.5) | 22 (91.7) | 16 (100.0) |
| Interstitial infiltrate | 40 (54.1) | 8 (33.3) | 6 (37.5) |
| Cavitation | 17 (23.0)c | 2 (8.3) | 0 (0.0) |
| Alveolar infiltrate | 7 (9.5)c | 5 (20.8) | 5 (31.3) |
| Nodular infiltrate | 4 (5.4) | 3 (12.5) | 0 (0.0) |
| Mediastinal enlargement | 4 (5.4) | 4 (16.7) | 1 (6.3) |
| Pleural effusion | 3 (4.1) | 0 (0.0) | 1 (6.3) |
| Other | 3 (4.1) | 1 (4.2) | 3 (18.8) |
| Not available | 1 (1.4) | 1 (4.2) | 0 (0.0) |
| Results of smear examination | |||
| Positive | 24 (32.4)c | 4 (16.7) | 0 (0.0) |
| Negative | 22 (29.7) | 11 (45.8) | 10 (62.5) |
| Not available | 28 (37.8) | 9 (37.5) | 6 (37.5) |
| Antimycobacterial therapy | 53 (71.6)c,f | 10 (41.7)c | 4 (35.0) |
| Outcome | |||
| Cured | 32 (43.2) | 7 (29.2) | 7 (43.7) |
| Treatment failure | 8 (15.1) | 2 (20.0) | 0 (0.0) |
| Death | 35 (47.3)c | 11 (45.8)c | 2 (12.5) |
| Attributed to M. kansasii | 17 (22.9)c | 6 (25.0) | 0 (0.0) |
Numbers in parentheses are percentages.
n, total number of patients.
P is <0.05 compared to values for subtypes 3, 4, and 7.
Patients may have M. kansasii strains isolated from multiple body sites.
Patients may have more than one type of radiologic lesion.
P is <0.05 compared to values for subtype 2.
Patients infected or colonized with M. kansasii subtype 1 were mostly middle-aged men living in an urban environment, 31% of whom were HIV infected. They presented symptoms with a median duration of 2 months. M. kansasii subtype 1 was most frequently isolated from the respiratory tract (in 90.5% of patients), and smear examinations were positive for 24 (52%) of the 46 patients for whom the test was available. A disseminated infection was observed in 23% of the patients. While interstitial infiltrates were the predominant radiographic finding (in 54% of patients), cavitary lesions were observed in 23% of patients. The mortality rate was 47%, and half of the deaths were attributed to M. kansasii infection: 11 (65%) of the 17 patients who died from M. kansasii infections were HIV coinfected, with severe immunosuppression (number of CD4-positive cells, <20/mm3), and 7 (64%) of the 11 had disseminated M. kansasii infections. The remaining six patients, not known to be HIV coinfected, were older (mean age, 69) and presented comorbidities, such as pulmonary neoplasia (1 of 6), chronic pulmonary disease (2 of 6), or corticosteroid treatment (2 of 6). One patient with unknown HIV serostatus had a disseminated M. kansasii infection.
Patients infected or colonized with M. kansasii subtype 2 were on average 40 years old and immunocompromised due to either coinfection with HIV (62.5%) or the use of corticosteroids (21%). M. kansasii subtype 2 was most frequently isolated from the respiratory tract (in 79% of patients), and smear examinations were positive for 4 (27%) of the 15 patients for whom the test was available. The association between HIV infection and M. kansasii subtype 2 was statistically significant (P, <0.009) (Table 3).
Compared to those with subtypes 1 and 2, patients infected or colonized with subtypes 3, 4, and 7 were older (P, <0.001). These patients, the majority of whom were male, had underlying pulmonary disease (100%), but few were infected with HIV (P = 0.001). Among these patients, there were no signs of disseminated disease (P, <0.03) or instances of death attributed to M. kansasii infection. While all presented abnormal chest X-ray findings (triggering the request for culture), none of the abnormalities could be attributed to a M. kansasii infection. None of these patients had positive specimens as determined by smear examination.
Pathogenicity according to subtypes.
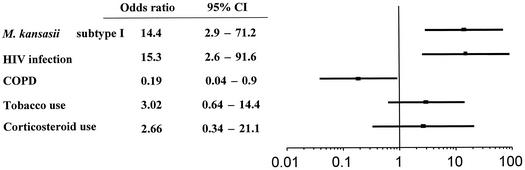
Among the 114 patients, 77 were considered to be infected rather than colonized with M. kansasii. Overall, 60 (81%) of 74 patients with isolates of M. kansasii subtype 1 and 16 (67%) of 24 with isolates of subtype 2 but only 1 (6%) with subtypes 3, 4, and 7 was considered infected according to the criteria of the American Thoracic Society or based on blind evaluation by two independent investigators. In a multivariate analysis, adjusted to take into account HIV infection, COPD, smoking habits, and the use of corticosteroids, M. kansasii subtype 1 was shown to be independently associated with pathogenicity (odds ratio, 14.4; 95% confidence intervals, 2.9 to 71.2 [Fig. 1 ]). M. kansasii subtype 2 was considered pathogenic in 14 (93%) of the 15 HIV-infected patients and in 2 (22%) of the 9 patients that were not seropositive for HIV (P, <0.001). M. kansasii subtypes 3 and 4 were never considered pathogenic, while one patient with subtype 7 was classified as infected.
FIG. 1.
Adjusted odds ratios of having a pathogenic strain of M. kansasii subtype I. Horizontal lines are 95% confidence intervals (95% CI).
DISCUSSION
This national survey documents for the first time, with a large collection of M. kansasii strains, the distribution and pathogenicity of the various M. kansasii subtypes. M. kansasii subtype 1 was the most commonly isolated, followed by subtype 2. While both subtypes were found in all regions of the country, other subtypes had a more diverse geographical distribution. In addition to the six subtypes previously described (21, 26), a novel subtype, subtype 7, was identified in three patients. Differences in local isolation rates for the different subtypes may reflect the existence of different ecosystems or, to a lesser extent, differences in the pretreatment of specimens in clinical laboratories. For example, nalidixic acid added to BACTEC media to control overgrowth of contaminants may compromise the recovery of M. kansasii from some specimens (8).
This study allowed the assignment of the degree of pathogenicity to the various subtypes. It confirms the morbidity and mortality associated with subtype 1 (10) and the role of subtype 2 as an opportunistic pathogen infecting immunosuppressed individuals (27). In HIV-infected patients, M. kansasii is generally identified at the stage of advanced immunodeficiency (mean CD4-positive cell count, <100 cells/mm3) (3, 4, 6, 28). In contrast, clinical infection with other subtypes appears to be exceptional, and the isolation of these subtypes from patients with underlying pulmonary disease suggests that they act as colonizing agents or environmental contaminants. This notion will have to be reassessed by larger epidemiological studies. Further investigation is necessary to elucidate the virulence factors of the M. kansasii subtypes 1 and 2, such as hemolytic activity and the production of phospholipase C or other similar enzymes, possibly related to pathogenicity (13).
Retaining the heterogeneous group of isolates within the M. kansasii taxon might be questioned. Given the differences in genetic structures, pathogenic roles, clinical significances, and environmental distributions and possibly in the routes of transmission (1), it may be speculated that some of the subtypes represent a distinct species of mycobacterium (25). Independent of these considerations, however, the present study underscores that the identification of M. kansasii to the subtype level may be not only an interesting epidemiological tool but also a process relevant to clinical management, as it allows the differentiation of pathogenic from the nonpathogenic subtypes.
Acknowledgments
Support for this work was provided by the Swiss HIV Cohort Study (Swiss National Science Foundation, grant 3345-062041).
We thank Paul Majcherczyk for editorial assistance.
The members of the Swiss HIV Cohort Study are M. Battegay (chairman of the Scientific Board), M.-C. Bernard, E. Bernasconi, H. Bucher, P. Bürgisser, M. Egger, P. Erb, W. Fierz, M. Flepp (chairman of the Clinical and Laboratory Committee), P. Francioli (president of the SHCS, Centre Hospitalier Universitaire Vaudois, CH-1011 Lausanne), H. J. Furrer, M. Gorgievski, H. Günthard, P. Grob, B. Hirschel, C. Kind, T. Klimkait, B. Ledergerber, U. Lauper, M. Opravil, F. Paccaud, G. Pantaleo, L. Perrin, J.-C. Piffaretti, M. Rickenbach (head of Data Center), C. Rudin (chairman of the Mother & Child Substudy), J. Schupbach, A. Telenti, P. Vernazza, T. Wagels, and R. Weber.
REFERENCES
- 1.Alcaide, F., I. Richter, C. Bernasconi, B. Springer, C. Hagenau, R. Schulze-Robbecke, E. Tortoli, R. Martin, E. C. Bottger, and A. Telenti. 1997. Heterogeneity and clonality among isolates of Mycobacterium kansasii: implications for epidemiological and pathogenicity studies. J. Clin. Microbiol. 35:1959-1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Thoracic Society. 1997. Diagnosis and treatment of disease caused by nontuberculous mycobacteria. Am. J. Respir. Crit. Care Med. 156:S1-S25. [DOI] [PubMed]
- 3.Bamberger, D. M., M. R. Driks, M. R. Gupta, M. C. O'Connor, P. M. Jost, R. E. Neihart, D. S. McKinsey, L. A. Moore, and Kansas City AIDS Research Consortium. 1994. Mycobacterium kansasii among patients infected with human immunodeficiency virus in Kansas City. Clin. Infect. Dis. 18:395-400. [DOI] [PubMed] [Google Scholar]
- 4.Bloch, K. C., L. Zwerling, M. J. Pletcher, J. A. Hahn, J. L. Gerberding, S. M. Ostroff, D. J. Vugia, and A. L. Reingold. 1998. Incidence and clinical implications of isolation of Mycobacterium kansasii: results of a 5-year, population-based study. Ann. Intern. Med. 129:698-704. [DOI] [PubMed] [Google Scholar]
- 5.Brunello, F., M. Ligozzi, E. Cristelli, S. Bonora, E. Tortoli, and R. Fontana. 2001. Identification of 54 mycobacterial species by PCR-restriction fragment length polymorphism analysis of the hsp65 gene. J. Clin. Microbiol. 39:2799-2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campo, R. E., and C. E. Campo. 1997. Mycobacterium kansasii disease in patients infected with human immunodeficiency virus. Clin. Infect. Dis. 24:1233-1238. [DOI] [PubMed] [Google Scholar]
- 7.Choudhri, S., J. Manfreda, J. Wolfe, S. Parker, and R. Long. 1995. Clinical significance of nontuberculous mycobacteria isolates in a Canadian tertiary care center. Clin. Infect. Dis. 21:128-133. [DOI] [PubMed] [Google Scholar]
- 8.Conville, P. S., J. W. Andrews, and F. G. Witebsky. 1995. Effect of PANTA on growth of Mycobacterium kansasii in BACTEC 12B medium. J. Clin. Microbiol. 33:2012-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.da Silva Rocha, A., A. M. Werneck Barreto, C. E. Dias Campos, M. Villas-Boas da Silva, L. Fonseca, M. H. Saad, W. M. Degrave, and P. N. Suffys. 2002. Novel allelic variants of mycobacteria isolated in Brazil as determined by PCR-restriction enzyme analysis of hsp65. J. Clin. Microbiol. 40:4191-4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies, P. D. 1994. Infection with Mycobacterium kansasii. Thorax 49:435-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devallois, A., K. S. Goh, and N. Rastogi. 1997. Rapid identification of mycobacteria to species level by PCR-restriction fragment length polymorphism analysis of the hsp65 gene and proposition of an algorithm to differentiate 34 mycobacterial species. J. Clin. Microbiol. 35:2969-2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falkinham, J. O., III. 1996. Epidemiology of infection by nontuberculous mycobacteria. Clin. Microbiol. Rev. 9:177-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomez, A., A. Mve-Obiang, B. Vray, W. Rudnicka, I. C. Shamputa, F. Portaels, W. M. Meyers, P. A. Fonteyne, and L. Realini. 2001. Detection of phospholipase C in nontuberculous mycobacteria and its possible role in hemolytic activity. J. Clin. Microbiol. 39:1396-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greub, G., K. Jaton, V. Beer, G. Prod'hom, and J. Bille. 1998. The detection of mycobacteria in blood cultures using the Bactec system: 6 weeks versus 12 weeks of incubation? routine terminal Ziel-Neelsen? Clin. Microbiol. Infect. 4:401-404. [DOI] [PubMed] [Google Scholar]
- 15.Guay, D. R. 1996. Nontuberculous mycobacterial infections. Ann. Pharmacother. 30:819-830. [DOI] [PubMed] [Google Scholar]
- 16.McSwiggan, D. A., and C. H. Collins. 1974. The isolation of M. kansasii and M. xenopi from water systems. Tubercle 55:291-297. [DOI] [PubMed] [Google Scholar]
- 17.Parenti, D. M., J. S. Symington, J. Keiser, and G. L. Simon. 1995. Mycobacterium kansasii bacteremia in patients infected with human immunodeficiency virus. Clin. Infect. Dis. 21:1001-1003. [DOI] [PubMed] [Google Scholar]
- 18.Picardeau, M., G. Prod'hom, L. Raskine, M. P. LePennec, and V. Vincent. 1997. Genotypic characterization of five subspecies of Mycobacterium kansasii. J. Clin. Microbiol. 35:25-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pintado, V., E. Gomez-Mampaso, P. Martin-Davila, J. Cobo, E. Navas, C. Quereda, J. Fortun, and A. Guerrero. 1999. Mycobacterium kansasii infection in patients infected with the human immunodeficiency virus. Eur. J. Clin. Microbiol. Infect. Dis. 18:582-586. [DOI] [PubMed] [Google Scholar]
- 20.Plikaytis, B. B., B. D. Plikaytis, M. A. Yakrus, W. R. Butler, C. L. Woodley, V. A. Silcox, and T. M. Shinnick. 1992. Differentiation of slowly growing Mycobacterium species, including Mycobacterium tuberculosis, by gene amplification and restriction fragment length polymorphism analysis. J. Clin. Microbiol. 30:1815-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richter, E., S. Niemann, S. Rusch-Gerdes, and S. Hoffner. 1999. Identification of Mycobacterium kansasii by using a DNA probe (AccuProbe) and molecular techniques. J. Clin. Microbiol. 37:964-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ross, B. C., K. Jackson, M. Yang, A. Sievers, and B. Dwyer. 1992. Identification of a genetically distinct subspecies of Mycobacterium kansasii. J. Clin. Microbiol. 30:2930-2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Springer, B., W. K. Wu, T. Bodmer, G. Haase, G. E. Pfyffer, R. M. Kroppenstedt, K. H. Schroder, S. Emler, J. O. Kilburn, P. Kirschner, A. Telenti, M. B. Coyle, and E. C. Bottger. 1996. Isolation and characterization of a unique group of slowly growing mycobacteria: description of Mycobacterium lentiflavum sp. nov. J. Clin. Microbiol. 34:1100-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steadham, J. E. 1980. High-catalase strains of Mycobacterium kansasii isolated from water in Texas. J. Clin. Microbiol. 11:496-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Telenti, A. 1998. More on “what's in a name… ”—pragmatism in mycobacterial taxonomy. Int. J. Tuberc. Lung Dis. 2:182-183. [PubMed] [Google Scholar]
- 26.Telenti, A., F. Marchesi, M. Balz, F. Bally, E. C. Bottger, and T. Bodmer. 1993. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J. Clin. Microbiol. 31:175-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tortoli, E., M. T. Simonetti, C. Lacchini, V. Penati, and P. Urbano. 1994. Tentative evidence of AIDS-associated biotype of Mycobacterium kansasii. J. Clin. Microbiol. 32:1779-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Witzig, R. S., B. A. Fazal, R. M. Mera, D. M. Mushatt, P. M. Dejace, D. L. Greer, and N. E. Hyslop, Jr. 1995. Clinical manifestations and implications of coinfection with Mycobacterium kansasii and human immunodeficiency virus type 1. Clin. Infect. Dis. 21:77-85. [DOI] [PubMed] [Google Scholar]