Abstract
Rapid detection of drug resistance in Mycobacterium tuberculosis is essential for efficient treatment and control of this pathogen. The amplification refractory mutation system (ARMS) was used to detect mutations in the rifampin resistance-determining region of the rpoB gene. A total of 39 rifampin-resistant M. tuberculosis isolates in Shanghai were analyzed by this assay, resulting in 92.3% sensitivity (36 of 39) and 87.2% concordance (34 of 39) relative to DNA sequencing, by which 41 mutations of 11 different types, including 9 point mutations and 2 deletions, were identified in the rpoB gene. The most frequent mutations were those associated with codon 531 (21 of 39 [53.8%]) and codon 526 (9 of 39 [23.1%]). The results suggest that the ARMS assay is rapid and simple to implement and could be performed for detection of rifampin resistance in M. tuberculosis to complement conventional culture-based methods.
Tuberculosis (TB), though curable, still remains a major public health concern worldwide. According to the report of the World Health Organization, about one-third of the world's population (1.86 billion people) are infected with Mycobacterium tuberculosis and are at risk of having the infection develop into clinical TB. Approximately 8 million new cases occur each year, resulting in 3 million deaths around the world (15, 19). It has been estimated that among 6 million active TB patients at present, the disease causes 250,000 deaths every year in China (28). Furthermore, control of TB has been further complicated by the emergence of multidrug-resistant (MDR) M. tuberculosis strains and the human immunodeficiency virus epidemic (4, 9).
Rifampin, introduced in 1971, has proven to be one of the most potent antituberculosis agents (2). Rifampin is an effective bactericidal against M. tuberculosis, interacting with DNA-dependent RNA polymerase to inhibit transcription and elongation of RNA (11, 12), and the use of this drug has greatly shortened the duration of chemotherapy. The molecular mechanism of rifampin resistance in M. tuberculosis was first characterized in 1993 (21). Ninety-six percent of rifampin-resistant (Rifr) M. tuberculosis strains possess genetic alterations within an 81-bp rifampin resistance-determining region (RRDR) in the rpoB gene (16, 18), corresponding to codons 507 to533 (Escherichia coli numbering system). In addition, rifampin resistance can be assumed to be a surrogate marker for MDR TB, since more than 90% of Rifr isolates are also isoniazid resistant (6).
Early diagnosis of TB and rapid detection of rifampin resistance are essential for efficient treatment and control of M. tuberculosis. However, culture-based methods for detection of M. tuberculosis infection and testing of drug susceptibility usually take more than 1 month. Although several different genotypic methods, such as PCR single-strand conformational length polymorphism (21, 22), dideoxy fingerprinting (7), heteroduplex analysis (26), and DNA sequencing (13), have been used for analysis of rpoB gene muations associated with rifampin resistance, these procedures are labor-intensive and time-consuming. In the present study, a simple, rapid, and reliable method, the amplification refractory mutation system (ARMS), was developed to detect mutations in the rpoB genes of M. tuberculosis isolates. ARMS is a general technique for the analysis of any point mutation or small deletion (8, 17) and has already been used for the detection of several genetic polymorphisms including α1-antitrypsin deficiency (17), CFTR gene mutation (8), apolipoprotein E genotypes (5), and K-ras mutation (3).
The objectives of this study were to evaluate an assay for identification of rifampin resistance based on ARMS PCR amplification of the rpoB gene and to identify rpoB mutations associated with rifampin resistance in a panel of M. tuberculosis strains isolated in Shanghai.
MATERIALS AND METHODS
M. tuberculosis strains and drug susceptibility testing.
All clinical isolates originated from the Shanghai Lung Hospital and were grown on Löwenstein-Jensen medium. Rifampin resistance testing was performed by the absolute concentration method described by Kim and Hong (14).
Primer design and ARMS PCR protocol.
The rationale of ARMS PCR is that a single nucleotide mismatch at the 3′-OH extremity of the annealed forward primer renders Taq DNA polymerase unable to extend the primer in the PCR under appropriate conditions (8, 17). Thus, the absence of the specific PCR product, with a positive result for the internal control, reveals a deviation from the wild-type DNA sequence. An additional deliberate mismatch adjacent to the 3′-OH terminus of the ARMS primer was introduced in order to enhance discrimination between normal and mutant alleles. Sequences of the ARMS primers for mutation detection are shown in Fig. 1. Other primers used are as follows: control forward primer, 5′-CGAATATCTGGTCCGCTTGC-3′ (positions 2090 to 2109; GenBank accession no. L27989); common reverse primer, 5′-GTCGACCACCTTGCGGTACG-3′ (positions 2627 to 2608). In each PCR, one ARMS primer and the common reverse primer were used for mutation detection, which generated a short PCR product from the wild-type gene but failed to amplify from a mutant allele with a corresponding mutation at the location covered by the mismatch positions on the ARMS primer. A control forward primer that is expected to anneal efficiently to all alleles was used in conjunction with the common reverse primer to generate a longer PCR product as an internal control.
FIG. 1.
Comparison of DNA sequences of rpoB genes in Rifs and Rifr M. tuberculosis isolates. The mutated nucleotides of Rifr isolates are boldfaced. ARMS primers used in this study are shown above and below the sequences. The line with the arrow shows that PCR can be performed well, whereas the line with the dot shows that the ARMS primer is refractory to extension by Taq DNA polymerase. Underlined letters indicate the nucleotide alterations introduced to enhance the 3′ mismatch effect. Codon numbers are assigned on the basis of alignment of the translated E. coli rpoB sequence with a portion of the translated M. tuberculosis sequence and are not the positions of the actual M. tuberculosis rpoB codons.
PCR amplification.
Bacterial suspensions containing approximately 105 bacteria in 100 μl of distilled water were prepared from M. tuberculosis isolates grown on Löwenstein-Jensen slants for 3 to 4 weeks and then treated with 100 μl of a 10% suspension of Chelex 100 as described previously (23). For each PCR, 5 μl of a supernatant containing genomic DNA as a template was added to a final volume of 50 μl containing 0.10 μM control forward primer, 0.15 μM ARMS primer, 0.20 μM common reverse primer, 50 mM KCl, 10 mM Tris-HCl (pH 8.3), 1.2 mM MgCl2, 200 μM deoxynucleoside triphosphates, and 2.5 U of Taq DNA polymerase (MBI Fermentas, Vilnius, Lithuania). The reaction was carried out in a DNA Thermolyne (Gene-Cycler; Bio-Rad, Richmond, Calif.) with the following program: initial denaturation at 94°C for 5 min, followed by 35 cycles of denaturation at 94°C for 35 s, annealing at 56°C for 35 s, and extension at 72°C for 35 s, with an additional extension step at 72°C for 10 min. PCR products were analyzed on a 1.5% agarose gel in 1× TAE buffer (40 mM Tris-acetate, 1 mM EDTA [pH 8.0]) stained with ethidium bromide, and visualized under UV light. Each isolate was employed in three single PCRs with three different primer sets; then the PCR products were analyzed by agarose gel electrophoresis at the same time. All amplifications were repeated at least twice.
DNA sequencing of the rpoB gene.
The RRDR of the rpoB gene was sequenced after PCR amplification in order to analyze the mutations associated with rifampin resistance. PCR was performed by using another forward primer (5′-TGGTCGCCGCGATCAAG-3′) and the common reverse primer (5′-GTCGACCACCTTGCGGTACG-3′) to generate a 296-bp fragment from nucleotide 2332 to 2627 (GenBank accession no. L27989); then the amplification products were directly sequenced on an ABI Prism model 3700 DNA sequencer (Applied Biosystems, Foster City, Calif.) by Shanghai GeneCore Biotechnologies Co., Ltd.
Nucleotide sequence accession numbers.
The new alleles found in this study have been deposited in GenBank under accession no. AF532616 and AF532617.
RESULTS
Evaluation of the ARMS assay for analysis of rpoB gene mutations.
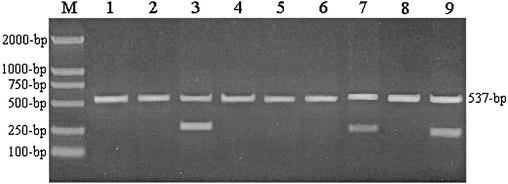
Each ARMS primer is complementary to the corresponding sequence of the wild-type gene except for one additional deliberate mismatch at the fourth nucleotide from the 3′-OH terminus of the primer. However, there are two mismatched nucleotides at the 3′ end between the ARMS primer and the mutant allele. A single mismatch at the fourth nucleotide from the 3′ terminus of the ARMS primer has little influence on the yield of PCR products, whereas the mismatch at the 3′-OH extremity of the primer is refractory to extension by the Taq DNA polymerase so that the yield of product from the mutant allele is not detectable (8, 17). ARMS PCR products of some strains with typical mutations in the RRDR of the rpoB gene are shown in Fig. 2.
FIG. 2.
Agarose gel electrophoresis of ARMS PCR products of the M. tuberculosis rpoB gene. Lanes 1, 2, 4, 5, 6, and 8 show that only the 537-bp internal control PCR product was generated from the strains with a D516V, D516G, H526Y, H526D, H526N, or S531L mutation in the RRDR of the rpoB gene, respectively. Lanes 3, 7, and 9 show that the wild-type strain (H37Rv) without corresponding mutations generated two fragments (537 and 261 bp with the ARMS-516 primer, 537 and 230 bp with the ARMS-526 primer, and 537 and 216 bp with the ARMS-531 primer). Lane M, DNA molecular weight standard (DNA Marker DL-2000 [TaKaRa Biotechnology, Dalian, China]).
A total of 45 clinical isolates were analyzed by the ARMS assay; the results indicated that all 6 Rifs strains including H37Rv showed no changes in the rpoB gene. Among 39 Rifr isolates, 36 were found to have mutations in the rpoB gene: 21 had mutations at codon 531, 9 had mutations at codon 526, and 6 had mutations at codon 516. No mutation was detected in the other three Rifr strains (Table 1).
TABLE 1.
Rapid detection of rpoB gene mutations of M. tuberculosis isolates in Shanghai by ARMS compared with DNA sequencing resultsa
| Strain no.a | Rifampin resistanceb | Mutated codon(s)
|
|
|---|---|---|---|
| ARMS assay | DNA sequencing | ||
| FK002 | R | Ser531 | 531 TCG→TTG |
| FK003 | R | Ser531 | 531 TCG→TTG |
| FK004 | R | Ser531 | 531 TCG→TTG |
| FK006 | R | Ser531 | 531 TCG→TTG |
| FK012 | R | Ser531 | 531 TCG→TTG |
| FK014 | R | Ser531 | 531 TCG→TTG |
| FK019 | R | Ser531 | 531 TCG→TTG |
| FK029 | R | Ser531 | 531 TCG→TTG |
| FK035 | R | Ser531 | 531 TCG→TTG |
| FK098 | R | Ser531 | 531 TCG→TTG |
| FK099 | R | Ser531 | 531 TCG→TTG |
| FK135 | R | Ser531 | 531 TCG→TTG |
| FK153 | R | Ser531 | 531 TCG→TTG |
| FK156 | R | Ser531 | 531 TCG→TTG |
| FK025 | R | Ser531 | 531 TCG→TTG |
| FK031 | R | Ser531 | 531 TCG→TTG |
| FK136 | R | Ser531 | 531 TCG→TTG |
| FK141 | R | Ser531 | 531 TCG→TTG |
| FK117 | R | Ser531 | 531 TCG→TTG |
| FK149 | R | Ser531 | 531 TCG→TTG |
| FK159 | R | Ser531 | 531 TCG→TTG |
| FK001 | R | His526 | 526 CAC→TAC |
| FK030 | R | His526 | 526 CAC→TAC |
| FK033 | R | His526 | 526 CAC→TAC |
| FK043 | R | His526 | 526 CAC→TAC |
| FK114 | R | His526 | 526 CAC→TAC |
| FK010 | R | His526 | 526 CAC→GAC |
| FK011 | R | His526 | 526 CAC→GAC |
| FK115 | R | His526 | 526 CAC→GAC |
| FK022 | R | His526 | 526 CAC→AAC |
| FK158 | R | Asp516 | 516 GAC→GTC |
| FK008 | R | Asp516 | 516 GAC→GGC |
| 511 CTG→CCG | |||
| FK140 | R | Asp516 | 516 GAC→GGC |
| 511 CTG→CCG | |||
| FK154 | R | Asp516 | 516 GAC→GGC |
| 511 CTG→CCG | |||
| FK026 | R | Asp516 | 516-520 deletionc |
| FK119 | R | Asp516 | 514-515 deletionc |
| FK133 | R | WTd | 513 CAA→CTA |
| FK160 | R | WT | 533 CTG→CCG |
| FK007 | R | WT | No mutations |
| Co.020 | S | WT | NDe |
| Co.032 | S | WT | ND |
| Co.137 | S | WT | ND |
| Co.139 | S | WT | ND |
| Co.166 | S | WT | No mutations |
| H37Rv | S | WT | ND |
Co., control.
R, resistant; S, susceptible.
New allele found in this study.
WT, wild type.
ND, not done.
Mutations in the rpoB gene of Rifr M. tuberculosis isolates in Shanghai.
DNA sequencing analysis of the 39 Rifr isolates showed that 33 strains have a single mutation, 3 strains have double mutations, and 2 strains have gene deletions in the 81-bp RRDR of the rpoB gene. One strain was identified as having no mutation, although it tested repeatedly as a Rifr isolate (Table 1). A total of 41 mutations of 11 different types, including 9 point mutations and 2 deletions, were identified (Table 3).
TABLE 3.
Sequence analysis of rpoB genes found in RifrM. tuberculosis strains in Shanghai
| Mutated codon | Mutation | Amino acid change | No. (%) of mutated sites |
|---|---|---|---|
| 531 | TCG→TTG | Ser→Leu | 21 (51.2) |
| 526 | CAC→TAC | His→Tyr | 5 (12.2) |
| CAC→GAC | His→Asp | 3 (7.3) | |
| CAC→AAC | His→Asn | 1 (2.4) | |
| 516 | GAC→GTC | Asp→Val | 1 (2.4) |
| GAC→GGCa | Asp→Gly | 3 (7.3) | |
| 511 | CTG→CCGa | Leu→Pro | 3 (7.3) |
| 513 | CAA→CTA | Gln→Leu | 1 (2.4) |
| 533 | CTG→CCG | Leu→Pro | 1 (2.4) |
| 514-515 | TTC ATG deletionb | 1 (2.4) | |
| 516-520 | GAC CAG AAC AAC CCG deletionc | 1 (2.4) |
DISCUSSION
It has been known that ARMS is a simple and rapid method used to detect gene mutations in many research fields. The mechanism of this system is that oligonucleotides which are complementary to a given DNA sequence except for a mismatch at their 3′-OH residue will not function as primers in PCR under appropriate conditions because of the absence of 3′-exonucleolytic proofreading activity associated with Taq DNA polymerase (8, 17).
In this study, an ARMS assay for detection of rifampin resistance in M. tuberculosis was successfully developed to analyze 39 Rifr isolates in Shanghai. Thirty-six out of 39 (92.3%) Rifr strains were detected as having genetic alternations in one of the three most common codons: codon 531, 526, or 516. Furthermore, the ARMS protocol in this study differs from those in other reports (5, 8, 17). The ARMS assays reported previously were designed to amplify the mutated gene regions and to detect specific nucleotide changes at the corresponding particular positions, but the ARMS assay as described here was directed against the wild-type gene rather than a mutant allele. Targeting the wild-type sequence is a more comprehensive tactic than targeting a particular mutation. For example, three different kinds of mutations at codon 526 were detected in this study. In contrast, an ARMS assay with a primer targeting one certain codon 526 mutation could analyze only the corresponding allele (data not shown). A second, 537-bp internal control fragment was generated in all reactions in order to avoid false-negative results. A further deliberate mismatch close to the 3′-OH end of the ARMS primer was employed to enhance specificity (5, 8, 17).
The RRDR of the rpoB gene was sequenced after PCR amplification to analyze the mutations associated with rifampin resistance and to verify the detection results of the ARMS assay. The most frequent mutations were found at codons 531, 526, and 516, with frequencies of 53.8, 23.1, and 10.3%, respectively. Similar results have been reported in other papers (10, 20, 24, 27). Among all mutations, S531L (TCG→TTG) occurred at the highest frequency, 51.2%. Three different types of mutations (H526Y, H526D, and H526N) were seen at codon 526 (Table 3). Though the D516V mutation (GAC→GTC) occurred at frequencies of 37.9% in Hungarian isolates (1) and 13.3% in Asian isolates (10), a very low frequency of this mutation, only 2.4%, was found in this investigation. However, D516G (GAC→GGC) combined with the L511P change (CTG→CCG) occurred at a relatively high frequency (7.3%). It is noteworthy that all the mutations mentioned above were identified by the ARMS assay, resulting in an 87.2% concordance between the ARMS results and the results of phenotypic rifampin susceptibility testing and genetic DNA sequencing (Table 2). Two isolates with deletions at codons 514 and 515 or codons 516 to 520, and three isolates with double mutations (D516V and L511P), were also detected by the ARMS-516 primer, resulting in a 92.3% sensitivity. Three Rifr isolates which were demonstrated repeatedly to be rifampin resistant by conventional susceptibility testing gave false wild-type results by the ARMS assay due to limitations of the methodology: either their mutations are outside the detection range (Q513L and L533P) or no mutation exists in the RRDR of the rpoB gene even though the isolate (strain FK007) is resistant to rifampin. Similar observations have also been reported by others (13, 20-22, 24, 27), which suggests that mutations beyond the 81-bp region of the rpoB gene or the existence of at least one additional molecular mechanism may be involved in the rifampin resistance of M. tuberculosis.
TABLE 2.
Sensitivity of the ARMS assay and concordance with DNA sequencing
| Type of isolate (n) | No. detected by ARMS | Sensitivity of ARMS (%) | Concordance with DNA sequencing (%) |
|---|---|---|---|
| Rifs (6) | 6 | 100 | ND |
| Rifr (39) | 36 | 92.3 (36/39) | 87.2 (34/39) |
The ARMS assay described here may be used for rapid detection of the mutations in the rpoB gene associated with the rifampin resistance of M. tuberculosis, although the method has limitations as well. For example, (i) this assay can detect only the existence of mutations, not their nature, so it cannot be a substitute for DNA sequencing analysis, and (ii) it is impossible to detect 100% of rpoB gene mutations, since more than 40 types of mutations covering about 20 codons are involved in the RRDR of M. tuberculosis (16, 18). However, the sensitivity of this assay is 92.3%, which is relatively high and roughly equivalent to those of the line probe assay (89.7%) (1) and the mismatch RNA/RNA assay (93.8%) (25). Furthermore, compared with those methods, the ARMS assay is more convenient, less expensive, and easier to perform, since it utilizes only commonly available reagents and equipment. The entire procedure, including genome DNA extraction, ARMS PCR amplification, and agarose gel electrophoresis, can be finished within 1 day. In addition, if a biotin-labeled common probe and enzyme immunosorbent assay are introduced to analyze the ARMS PCR products, a large number of clinical samples will be detected at the same time. Therefore, the application of such a rapid and simple method for detection of the rifampin resistance of M. tuberculosis would be potentially valuable for efficient treatment and control of M. tuberculosis.
REFERENCES
- 1.Bártfai, Z., Á. Somoskövi, C. Ködmön, N. Szabó, E. Puskás, L. Kosztolányi, E. Faragó, J. Mester, L. M. Parsons, and M. Salfinger. 2001. Molecular characterization of rifampin-resistant isolates of Mycobacterium tuberculosis from Hungary by DNA sequencing and the line probe assay. J. Clin. Microbiol. 39:3736-3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bass, J. B., Jr., L. S. Farer, P. C. Hopewell, R. O'Brien, R. F. Jacobs, F. Ruben, D. E. Snider, Jr., and G. Thornton. 1994. Treatment of tuberculosis and tuberculosis infection in adults and children. Am. J. Respir. Crit. Care Med. 149:1359-1374. [DOI] [PubMed] [Google Scholar]
- 3.Carpenter, K. M., L. G. Durrant, K. Morgan, D. Bennett, J. D. Hardcastle, and N. A. Kalsheker. 1996. Greater frequency of K-ras Val-12 mutation in colorectal cancer as detected with sensitive methods. Clin. Chem. 42:904-909. [PubMed] [Google Scholar]
- 4.Cohn, D. L., F. Bustreo, and M. C. Raviglione. 1997. Drug-resistant tuberculosis: review of the worldwide situation and the WHO/IUATLD Global Surveillance Project. Clin. Infect. Dis. 24:S121-S130. [DOI] [PubMed] [Google Scholar]
- 5.Donohoe, G. G., A. Salomaki, T. Lehtimaki, K. Pulkki, and V. Kairisto. 1999. Rapid identification of apolipoprotein E genotypes by multiplex amplification refractory mutation system PCR and capillary gel electrophoresis. Clin. Chem. 45:143-146. [PubMed] [Google Scholar]
- 6.Drobniewski, F. A., and S. M. Wilson. 1998. The rapid diagnosis of isoniazid and rifampin resistance in Mycobacterium tuberculosis—a molecular story. J. Med. Microbiol. 47:189-196. [DOI] [PubMed] [Google Scholar]
- 7.Felmlee, T. A., Q. Liu, A. C. Whelen, D. Williams, S. S. Sommer, and D. H. Persing. 1995. Genotypic detection of Mycobacterium tuberculosis rifampin resistance: comparison of single-strand conformation polymorphism and dideoxy fingerprinting. J. Clin. Microbiol. 33:1617-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrie, R. M., M. J. Schwarz, N. H. Robertson, S. Vaudin, M. Super, G. Malone, and S. Little. 1992. Development, multiplexing, and application of ARMS tests for common mutations in the CFTR gene. Am. J. Hum. Genet. 51:251-262. [PMC free article] [PubMed] [Google Scholar]
- 9.Frieden, T. R., L. F. Sherman, K. L. Maw, P. I. Fujiwara, J. T. Crawford, B. Nivin, V. Sharp, D. Hewlett, Jr., K. Brudney, D. Alland, and B. N. Kreisworth. 1996. A multi-institutional outbreak of highly drug-resistant tuberculosis: epidemiology and clinical outcomes. JAMA 276:1229-1235. [PubMed] [Google Scholar]
- 10.Hirano, K., C. Abe, and M. Takahashi. 1999. Mutations in the rpoB gene of rifampin-resistant Mycobacterium tuberculosis strains isolated mostly in Asian countries and their rapid detection by line probe assay. J. Clin. Microbiol. 37:2663-2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin, D. J., and C. A. Gross. 1988. Mapping and sequencing of mutations in the Escherichia coli rpoB gene that lead to rifampicin resistance. J. Mol. Biol. 202:45-58. [DOI] [PubMed] [Google Scholar]
- 12.Jin, D. J., and C. A. Gross. 1989. Characterization of the pleiotropic phenotypes of rifampin-resistant rpoB mutants of Escherichia coli. J. Bacteriol. 171:5229-5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kapur, V., L.-L. Li, S. Iordanescu, M. R. Hamrick, A. Wanger, B. N. Kreiswirth, and J. M. Musser. 1994. Characterization by automated DNA sequencing of mutations in the gene (rpoB) encoding the RNA polymerase βsubunit in rifampin-resistant Mycobacterium tuberculosis strains from New York City and Texas. J. Clin. Microbiol. 32:1095-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim, S. J., and Y. P. Hong. 1992. Drug resistance of Mycobacterium tuberculosis in Korea. Tuber. Lung Dis. 73:219-224. [DOI] [PubMed] [Google Scholar]
- 15.Kochi, A. 1991. The global tuberculosis situation and the new control strategy of the World Health Organization. Tubercle 72:1-6. [DOI] [PubMed] [Google Scholar]
- 16.Musser, J. M. 1995. Antimicrobial agent resistance in mycobacteria: molecular genetic insights. Clin. Microbiol. Rev. 8:496-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newton, C. R., A. Graham, L. E. Heptinstall, S. J. Powell, C. Summers, N. Kalsheker, J. C. Smith, and A. F. Markham. 1989. Analysis of any point mutation in DNA. The amplification refractory mutation system (ARMS). Nucleic Acids Res. 17:2503-2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramaswamy, S., and J. M. Musser. 1998. Molecular genetic basis of antimicrobial agent resistance in Mycobacterium tuberculosis: 1998 update. Tuber. Lung Dis. 79:3-29. [DOI] [PubMed] [Google Scholar]
- 19.Raviglione, M. C., D. E. Sinder, and A. Kochi. 1995. Global epidemiology of tuberculosis: morbidity and mortality of a worldwide epidemic. JAMA 273:220-226. [PubMed] [Google Scholar]
- 20.Schilke, K., K. Weyer, G. Bretzel, B. Amthor, J. Brandt, V. Sticht-Groh, P. B. Fourie, and W. H. Haas. 1999. Universal pattern of rpoB gene mutations among multidrug-resistant isolates of Mycobacterium tuberculosis complex from Africa. Int. J. Tuberc. Lung Dis. 3:620-626. [PubMed] [Google Scholar]
- 21.Telenti, A., P. Imboden, F. Marchesi, D. Lowrie, S. T. Cole, M. J. Colston, L. Matter, K. Schoolfer, and T. Bodmer. 1993. Detection of rifampicin-resistant mutations in Mycobacterium tuberculosis. Lancet 341:647-650. [DOI] [PubMed] [Google Scholar]
- 22.Telenti, A., P. Imboden, F. Marchesi, T. Schmidheini, and T. Bodmer. 1993. Direct, automated detection of rifampin-resistant Mycobacterium tuberculosis by polymerase chain reaction and single-strand conformation polymorphism analysis. Antimicrob. Agents Chemother. 37:2054-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torres, M. J., A. Criado, J. C. Palomares, and J. Aznar. 2000. Use of real-time PCR and fluorimetry for rapid detection of rifampin and isoniazid resistance-associated mutations in Mycobacterium tuberculosis. J. Clin. Microbiol. 38:3194-3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valim, A. R., M. L. Rossetti, M. O. Ribeiro, and A. Zaha. 2000. Mutations in the rpoB gene of multidrug-resistant Mycobacterium tuberculosis isolates from Brazil. J. Clin. Microbiol. 38:3119-3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watterson, S. A., S. M. Wilson, M. D. Yates, and F. A. Drobniewski. 1998. Comparison of three molecular assays for rapid detection of rifampin resistance in Mycobacterium tuberculosis. J. Clin. Microbiol. 36:1969-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams, D. L., L. Spring, M. Salfinger, T. P. Gillis, and D. H. Persing. 1998. Evaluation of polymerase chain reaction-based universal heteroduplex generator assay for direct detection of rifampin susceptibility of Mycobacterium tuberculosis from sputum specimens. Clin. Infect. Dis. 26:446-450. [DOI] [PubMed] [Google Scholar]
- 27.Yuen, L. K., D. Leslie, and P. J. Coloe. 1999. Bacteriological and molecular analysis of rifampin-resistant Mycobacterium tuberculosis strains isolated in Australia. J. Clin. Microbiol. 37:3844-3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhuang, Y. 2000. Prospect, p. 60-61. In D. Zhang, Y. Zhuang, and G. Jin (ed.), Modern tuberculosis. People's Military Medical Press, Beijing, China.