Abstract
We describe two cases in Brazil of human subcutaneous infections due to Phaeoacremonium spp. The first case was caused by Phaeoacremonium aleophilum. The patient presented with a unique fistulized nodule on the left ankle. The fungus was detected by direct microscopic examination and was isolated repeatedly from material collected from the lesion. This is the first reported case of human infection caused by this fungus. The second case was caused by Phaeoacremonium rubrigenum. The patient presented with multiple nodules around the left ankle and foot. The fungus was detected by direct examination of pus and histological sections of the nodules. It was repeatedly isolated from the clinical specimens. This is the second reported case of human infection caused by this species.
CASE REPORTS
Case 1.
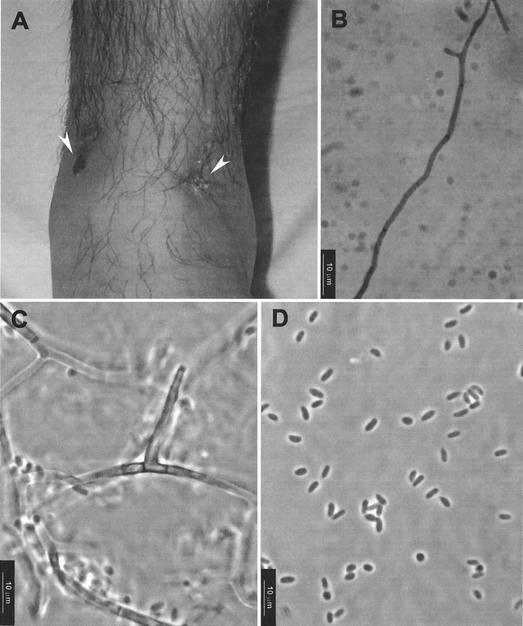
The patient was a 19-year-old male Brazilian clerk residing in the interior of the Rio Grande do Sul State, Brazil. He reported the presence of a painful nodule on the surface of his left ankle but had no history of recent injury in the affected area. The nodule was excised on six occasions, but it was not studied histologically or microbiologically. On examination in June 2001, a mycological study of the pus collected from a fistula close to the nodule was performed (Fig. 1A). The patient was otherwise in good physical condition and had no remarkable past medical history. Routine laboratory examination showed no abnormal findings. A direct microscopic examination of a potassium hydroxide preparation of the collected material was negative. However, cultures on Sabouraud dextrose agar (Oxoid, Basingstoke, England) at 35 to 37°C were positive. After 5 days of incubation, numerous whitish colonies with the same morphology were present. The fungus was tentatively identified as an Acremonium sp. Two more samples of pus were collected a week apart, and the same fungus grew again. Direct examination of the last collected material showed the presence of a few hyaline, septate hyphae (Fig. 1B). An X ray of the affected region demonstrated no bone or joint involvement. The nodule was surgically removed, and the patient received itraconazole (100 mg/day) for 2 months. The patient was then considered cured. Only two healed spots were present in the areas previously occupied by the nodules and the fistula.
FIG. 1.
(A) Nodular lesion (left arrowhead) and fistula (right arrowhead) on the anterior face of the left ankle; (B) potassium hydroxide wet preparation of pus showing a septate hyphae; (C and D) conidiogenous cell and conidia of P. aleophilum FMR 7682.
Case 2.
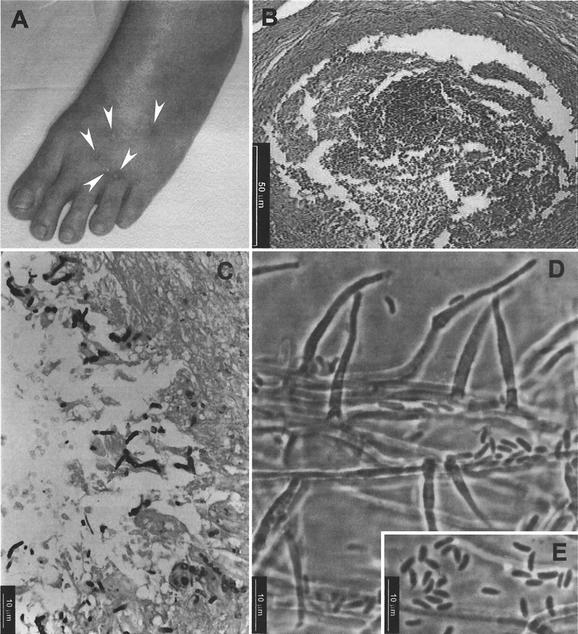
The patient was a 55-year-old Brazilian white male residing in the interior of the Rio Grande do Sul State. He reported nonpainful multiple nodules on his left ankle and foot, with spontaneous drainage of pus. The nodules had appeared 8 months earlier. The patient self-administered ampicillin, cephalexin, and ciprofloxacin, though without medical prescription, but his condition did not improve. There was no recent history of injury to the ankle. Past medical history revealed that he had suffered from hypertension. Eight years before the case study, he had received a renal transplant, for which he had been placed on immunosuppressive therapy with cyclosporine (100 mg/day), prednisone (15 mg/day) and azathioprine (150 mg/day). Otherwise, the patient was in good condition. On examination in October 1999, multiple subcutaneous nodules (0.8 to 1 cm in diameter) with fibroelastic consistency and without fluctuation were observed around the left ankle and in the dorsal region of the left foot (Fig. 2A). The overlying epidermis was almost normal; neither peripheral redness nor local heat was observed. The nodules were surgically excised. Direct microscopic examination of the pus revealed the presence of hyaline, branched, and septate hyphae. Portions of various removed nodules were fixed in 10% formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin, periodic acid-Schiff stain, and Grocott-Gomori methanamine silver and by Fontana-Masson procedures. The exudate was homogenized in a sterile saline solution and used for microbiological analyses. Several parts of the suspension were cultured by standard techniques in media routinely used for aerobic and anaerobic bacterial isolation and on Sabouraud dextrose agar (Oxoid) incorporating chloramphenicol (0.05%) with and without cycloheximide (0.04%) for fungal isolation. All cultures were incubated at 35 to 37°C in the dark. Histological examination demonstrated the presence of a chronically inflamed fistula characterized by an extensive granulomatous reaction with central necrosis (Fig. 2B). Sections of the stained tissue showed numerous septate, hyphal fragments of various lengths and with diameters measuring 2 to 3.5 μm (Fig. 2C). Fontana-Masson staining revealed the absence of melanin on the hyphae. Bacteriological cultures were negative, but those for fungi were positive. Multiple dark-colored colonies, apparently belonging to the same species, grew in all Sabouraud petri dishes. The fungus was tentatively identified as an Acremonium sp.
FIG. 2.
(A) Nodular lesions (arrowheads) on the dorsal region of the left foot; (B) hematoxylin and eosin stain from the nodular tissue showing an abscess with central necrosis and sparse inflammatory cells; (C) Grocott-Gomori methanamine silver stain showing hyphal elements; (D and E) conidiogenous cells and conidia of P. rubrigenum FMR 7681.
In January 2000, treatment with itraconazole (200 mg/day) was initiated. Treatment continued for 5 months, but the nodules reappeared. In August 2000, the nodules were surgically excised once more and itraconazole was replaced with terbinafine (500 mg/day), which was administered for 6 months. The nodules reappeared and, in February 2001, terbinafine was replaced with fluconazole (300 mg/week). However, the patient showed some impairment of hepatic function, which was probably caused by fluconazole or azathioprine. Fluconazole was therefore suspended, and the azathioprine dosage was reduced. Amphotericin B was not administered because of the patient's previous renal transplant and the nephrotoxicity of this drug. The hepatic function of the patient improved, but no fungal treatment was given. In July 2001, the nodules persisted (15 nodules were counted) and a new biopsy showed the presence of granulomas with histiocytes in the patient's dermis and subcutaneous tissue, with foci of fat necrosis. Throughout the tissue there were numerous, short, thick-walled, contorted, irregular, and sparsely ramified hyphae. Cultures from the biopsy material were again positive for the same fungus.
Cultures obtained from both cases were referred to the Faculty of Medicine of the Rovira i Virgili University in Reus, Tarragona, Spain, to be identified and to determine the antifungal susceptibility.
Mycological study and diagnosis.
Two isolates (FMR 7682 from case 1 and FMR 7681 from case 2) were subcultured on potato dextrose agar (PDA; Difco Laboratories, Detroit, Mich.), malt extract agar (MEA; Difco), and homemade oatmeal agar (OA; 30 g of oat flakes, 1 g of MgSO4 · 7H2O, 1.5 g of KH2PO4, 15 g of agar, 1,000 ml of tap water) and were incubated in darkness at 25, 37, and 40°C. We determined the microscopic characteristics of the isolate by making wet mounts with lactic acid, which we then examined by light microscopy. Based on preliminary microscopic examination, both isolates belonged to the genus Phaeoacremonium.
FMR 7682 (case 1).
The colonies on PDA at 25°C attained a diameter of 27 to 28 mm after 14 days. They were more or less cottony, honey colored to brownish gray, and radially folded, with reverse sides that were irregularly pigmented brown. The macroscopic features on MEA were very similar to those on PDA, except that the fungus grew more slowly (16 to 17 mm in diameter after 14 days). On OA, the fungus grew faster, attaining a diameter of 44 mm after 14 days. Its colonies were cottony and pale brownish gray, and their reverse sides were colorless. A typical diffusing yellow pigment was produced in the three culture media. At 37°C the colonies of the fungus attained a diameter of 19 to 21 mm after 14 days, and at 40°C the growth was nil.
Microscopically, vegetative hyphae were subhyaline or pale brown, usually had verruculose walls, and measured 1.5 to 3 μm in width. The conidiophores were very simple and often reduced to conidiogenous cells which were subhyaline, subcylindrical, or subulate, 4 to 23 μm long by 1.5 to 2.5 μm wide at the base, and tapered toward a 1- to 1.5-μm-wide apex (Fig. 1C). The conidiogenous cells had a narrow funnel-shaped collarette. The conidia were unicellular, hyaline, predominantly cylindrical, and 3 to 6 μm long by 1.5 to 2.5 μm wide (Fig. 1D). The morphological features described above, especially the predominance of cylindrical conidia and the production of a diffusing yellow pigment, are shared by two Phaeoacremomium species, P. aleophilum and P. viticola. However, the ability to grow at 37°C, the absence of bluish-red tinges in the colonies, and the conidial size (narrower in P. viticola) allowed us to identify FMR 7682 as P. aleophilum.
FMR 7681 (case 2).
The colonies grown on PDA at 25°C attained a diameter of 31 to 32 mm after 14 days. They were lanose to fasciculate, radially folded, pale brown to grayish brown at the center, and cream colored toward the periphery, and their reverses were reddish brown. On MEA, the colonies grew more slowly, reaching 18 to 20 mm in diameter after 14 days. They were radially folded, brownish gray, and slightly cottony and had thin fascicles at the center but were cream colored and velvety toward the periphery. The macroscopic features of the colonies on OA were similar to those of colonies on PDA, but the fungus grew faster, reaching a diameter of 40 to 41 mm after 14 days. No diffusible pigment was produced in any medium. The fungus grew slowly at 37°C, attaining a diameter of 17 to 18 mm after 14 days, and there was no growth at 40°C.
Microscopically, vegetative hyphae were pale brown, ranged from 1.5 to 4 μm in width, and were often rough walled. The conidiophores bore one or two conidiogenous cells. The conidiogenous cells were subhyaline to pale brown and had smooth or verruculose walls. They were subcylindrical and 4.5 to 25 μm long by 1.5 to 3 μm wide, and they ended in a funnel-shaped collarette (Fig. 2D). The conidia were unicellular, hyaline, ellipsoidal or allantoid, and 2.5 to 5 μm long by 1.5 to 2 μm wide and formed slimy heads at the apices of the conidiogenous cells (Fig. 2E). On the basis of the above-described characteristics, we identified FMR 7681 as P. rubrigenum. We confirmed this by comparing our clinical isolate with a reference strain of the same species (CBS 498.94). The morphological features of both strains were practically identical. The only difference was that FMR 7681 showed colonies with a reddish reverse only when it was grown on PDA.
Living cultures of the two isolates from both clinical cases were deposited in the Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands (P. aleophilum CBS 110034 and P. rubrigenum CBS 110033), and in the Institute of Hygiene and Epidemiology, Brussels, Belgium (P. aleophilum IHEM 19078 and P. rubrigenum IHEM 19079).
Antifungal susceptibility testing.
We tested the two strains to determine their susceptibilities to seven antifungal drugs (amphotericin B, itraconazole, ketoconazole, fluconazole, terbinafine, ravuconazole, and voriconazole) (Table 1). We did the tests with a previously described microdilution method (12), mainly according to the guidelines of the National Committee for Clinical Laboratory Standards, with RPMI 1640 medium (buffered to pH 7.0 with 0.165 M morpholinepropanesulfonic acid), an inoculum of 4.5 × 103 to 5.1 × 103 CFU/ml, an incubation temperature of 32 to 35°C, an incubation time of 72 h, and an additive drug dilution procedure. MICs were defined as the lowest concentration at which no growth occurred. The results of susceptibility testing for both isolates were very similar. The lowest MICs were those of voriconazole and ravuconazole. The two species involved had not been tested before, so we were not able to compare our in vitro results with those of other authors.
TABLE 1.
Antifungal susceptibilities of the two clinical isolates of Phaeoacremonium spp.
| Isolateb | MICa (μg/ml)
|
||||||
|---|---|---|---|---|---|---|---|
| AMB | FCZ | ITRZ | KCZ | RCZ | TF | VCZ | |
| P. aleophilum FMR 7682 | 2 | 8 | >16 | 4 | 1 | 2 | 1 |
| P. rubrigenum FMR 7681 | 2 | 8 | 8 | 2 | 0.5 | 2 | 1 |
AMB, amphotericin B; FCZ, fluconazole; ITRA, itraconazole; KCZ, ketoconazole; RCZ, ravuconazole; TF, terbinafine; VCZ, voriconazole.
FMR, Faculty of Medicine in Reus, Spain.
Subcutaneous fungal infections are local, primary infections of the subcutis that originate a leukocytic response and usually appear as cystic abscesses or granulomas. Numerous species have been involved in these processes. They are often darkly pigmented fungi, saprobes that are commonly found in the environment. The infection usually follows traumatic implantation of fungal elements from contaminated soil, thorns, or wood splinters. The etiological agent is present in the clinical specimens as more or less darkly pigmented, septate, and irregular hyphae or yeastlike elements. Isolation on culture is needed to identify the causative fungus (7, 14). These infections are distributed worldwide, although their occurrence in tropical, subtropical, and temperate climates is considerably higher. There is an increased risk to patients with debilitating disease or immune deficiency.
Common, darkly pigmented fungi that cause subcutaneous infections are species of Alternaria, Exophiala, Phialophora, Phaeoacremonium, and Pyrenochaeta, among others. We present the first authenticated case of human infection caused by P. aleophilum and only the second case of human infection caused by P. rubrigenum. In the former, the fungus was detected, although very scarcely, by direct examination, and in the latter, it was detected by both direct examination and analysis of histological sections. In both cases, the fungi were repeatedly recovered from culture.
The genus Phaeoacremonium was recently proposed by Crous et al. (1) based on the species Phialophora parasitica. This is a well-known pathogen, which was considered the type species of the genus. At present, the Phaeoacremonium genus comprises four more species: P. aleophilum, P. inflatipes, P. rubrigenum, and P. viticola (3).
Phaeoacremonium is morphologically similar to Acremonium, and the two can easily be confused by inexperienced clinical microbiologists. Phaeoacremonium is different mainly because of its darkly pigmented colonies and the conspicuous collarettes of its conidiogenous cells. P. aleophilum has so far been known to grow only on the stems and roots of Vitis vinifera in Italy and Yugoslavia (1). Surprisingly, despite the infrequent occurrence of the fungus in nature, we have found it infecting humans on a different continent from that where it was first described. This species differs from the other Phaeoacremonium opportunistic species, i.e., P. parasiticum, P. inflatipes, and P. rubrigenum (2), mainly by the honey color of its colonies, the production of a diffusing yellow pigment on agar, and the predominance of cylindrical conidia. The other species in this paper, P. rubrigenum, is only slightly better known, as this is the second reported case of human infection from it. The two cases of infection attributed to this species are very similar; both are subcutaneous infections of the foot. The first case was described in Japan in 1999 by Matsui et al.; just as in our case, that patient did not recall a preceding trauma at the infected site (9). In both cases, the probable risk factor for the infection was long-term corticosteroid and azothioprine treatment. Other similarities between the two cases were that the lesions were excised twice and that the patients relapsed despite treatment with fluconazole, which had been administered after the failure of itraconazole. The other two Phaeoacremonium pathogenic species mentioned above are P. parasiticum and P. inflatipes. The better known is the former, which is usually a plant pathogen but has also been reported in several cases as an etiological agent of subcutaneous, deep, and disseminated human infections (2, 4, 6). P. inflatipes has been reported in only two cases of subcutaneous infections (5, 11).
A recent molecular study (3) based on the analysis of the β-tubulin gene and intertransgenic spacer region sequences has shown that P. rubrigenum and P. parasiticum are very similar genetically. They are also very similar morphologically. The main distinctive features of P. rubrigenum are the reddish color of the reverse sides of its colonies and its reduced growth at 35 to 37°C.
The two clinical isolates in this study have shown poor in vitro susceptibilities to the seven antifungals tested, including itraconazole, which is often used in the treatment of subcutaneous infections caused by darkly pigmented fungi (5, 13). In such infections, timely surgical intervention is an effective part of successful treatment (7). Itraconazole was used in both cases and failed to resolve the infection. The MICs of this drug for both isolates were high (>16 μg/ml for P. aleophilium and 8 μg/ml for P. rubrigenum). The high MICs seem to correlate with the clinical outcome, at least in the second case, where repeated removal of nodules together with different antifungal treatments was unable to resolve the infection. In the first case, these procedures were successful, so it is difficult to determine the role of itraconazole. In our study, ravuconazole and voriconazole were the most active drugs in vitro. Other authors (2) have also noticed the poor in vitro activities of the fungicidal drugs against P. parasiticum, although voriconazole has shown in vitro activity (10).
It is difficult to explain how the two patients acquired their infections because they did not report any type of injury. Generally, the people that show fungal subcutaneous infections are rural workers in daily contact with soil, thorns, wood slivers, or other trauma-causing objects. It is possible that our patients suffered microtraumas of which they were unaware and that very small wooden splinters or some other organic material was introduced into the skin. The patients were otherwise healthy. It seems unlikely, therefore, that defective host defenses were responsible for the persistence of their infections. However, in the case of the renal transplant patient, there is a possibility that antirejection therapy could have played a role. Because penetrating slivers are common and phaeomycotic cysts are rare, it is suspected that the retention of a contaminated sliver is necessary to cause the lesions (15). The presence of wooden slivers in the lesions has been reported in some cases of subcutaneous infections (8). There are also numerous published cases in which no foreign bodies were observed. However, it is likely that a more generous sampling and more careful examination of the pus would have revealed splinters in more cases (7). Totally excising the nodules is usually an uncomplicated way of healing a patient with such conditions, but with our patients this procedure was unsuccessful. In one patient, the nodule had to be removed several times before the infection was cured, and in the other, the infection was not resolved because multiple nodules were present and the eradication of all the infected tissue was probably never achieved.
REFERENCES
- 1.Crous, P. W., W. Gams, M. J. Wingfield, and P. J. van Wyk. 1996. Phaeoacremonium gen. nov. associated with wilt and decline diseases of woody hosts and human infections. Mycologia 88:786-796. [Google Scholar]
- 2.De Hoog, G. S., J. Guarro, J. Gené, and M. J. Figueras. 2000. Atlas of clinical fungi, 2nd ed. Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands.
- 3.Dupont, J., W. Laloui, S. Magnin, P. Larignon, and M. F. Roquebert. 2000. Phaeoacremonium viticola, a new species associated with Esca disease of grapevine in France. Mycologia 92:499-504. [Google Scholar]
- 4.Fincher, R. M. E., J. F. Fisher, A. A. Padhye, L. Ajello, and J. C. M. Steele. 1988. Subcutaneous phaeohyphomycotic abscess caused by Phialophora parasitica in a renal allograft recipient. J. Med. Vet. Mycol. 26:311-314. [PubMed] [Google Scholar]
- 5.Hood, S. V., C. B. Moore, J. S. Cheesbrough, A. Mene, and D. W. Denning. 1997. Atypical eumycetoma caused by Phialophora parasitica successfully treated with itraconazole and flucytosine. Br. J. Dermatol. 136:953-956. [PubMed] [Google Scholar]
- 6.Kitamura, K., T. Mochizuki, H. Ishizaki, S. Udagawa, and R. Fukushiro. 2000. Phaeomycotic cyst caused by Phaeoacremonium parasiticum. Jpn. J. Med. Mycol. 41:90-95. (In Japanese.) [DOI] [PubMed] [Google Scholar]
- 7.Kwon-Chung, K. J., and J. E. Bennett. 1992. Medical mycology. Lea & Febiger, Philadelphia, Pa.
- 8.Markham, W. D., R. D. Key, A. A. Padhye, and L. Ajello. 1990. Phaeohyphomycotic cyst caused by Tetraploa aristata. J. Med. Vet. Mycol. 28:147-150. [PubMed] [Google Scholar]
- 9.Matsui, T., K. Nishimoto, S. Udagawa, H. Ishihara, and T. Ono. 1999. Subcutaneous phaeohyphomycosis caused by Phaeoacremonium rubrigenum in an immunosuppressed patient. Jpn. J. Med. Mycol. 40:99-102. [DOI] [PubMed] [Google Scholar]
- 10.McGinnis, M. R., and L. Pasarell. 1998. In vitro testing of susceptibilities of filamentous ascomycetes to voriconazole, itraconazole, and amphotericin B, with consideration of phylogenetic implications. J. Clin. Microbiol. 36:2353-2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Padhye, A. A., M. S. Davis, D. Baer, A. Reddick, K. K. Sinha, and J. Ott. 1998. Phaeohyphomycosis caused by Phaeoacremonium inflatipes. J. Clin. Microbiol. 36:2763-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pujol, I., J. Guarro, C. Llop, L. Soler, and J. Fernández-Ballart. 1996. Comparison study of broth macrodilution and microdilution antifungal susceptibility tests for the filamentous fungi. Antimicrob. Agents Chemother. 40:2106-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richardson, M. D., and M. Kokki. 2001. Therapeutic guidelines in systemic fungal infections. Current Medical Literature Ltd., London, United Kingdom.
- 14.Rippon, J. W. 1988. Medical mycology. The pathogenic fungi and the pathogenic actinomycetes, 3rd ed. Saunders, Philadelphia, Pa.
- 15.Ziefer, A., and D. H. Connor. 1980. Phaeomycotic cyst: a clinico-pathologic study of twenty-five patients. Am. J. Trop. Med. Hyg. 29:901-911. [PubMed] [Google Scholar]