Abstract
Preterm delivery (PTD) is the leading cause of infant morbidity and mortality in the United States. An epidemiological association between PTD and various bacteria that are part of the vaginal microflora has been reported. No single bacterial species has been identified as being causally associated with PTD, suggesting a multifactorial etiology. Quantitative microbiologic cultures have been used previously to define normal vaginal microflora in a predictive model. These techniques have been applied to vaginal swab cultures from pregnant women in an effort to develop predictive microbiologic models for PTD. Logistic regression analysis with microbiologic information was performed for various risk groups, and the probability of a PTD was calculated for each subject. Four predictive models were generated by using the quantitative microbiologic data. The area under the curve (AUC) for the receiver operating curves ranged from 0.74 to 0.94, with confidence intervals (CI) ranging from 0.62 to 1. The model for the previous PTD risk group with the highest percentage of PTDs had an AUC of 0.91 (CI, 0.79 to 1). It may be possible to predict PTD by using microbiologic risk factors measured once the gestation period has reached the 20-week time point.
The complex microflora of the human vagina is thought to play an important role in both health and disease. Although a great deal of quantitative and qualitative data describing the vaginal microflora are available, it is only recently that statistical models defining normal vaginal microflora have been developed (33). It has also been possible to predict, on the basis of these quantitative microbiologic models, when the vaginal microflora is abnormal (50, 51). Part of the modeling process is to identify microbial risk factors, such as an abnormal vaginal microflora (i.e., vaginal candidiasis or bacterial vaginosis [BV]), that are associated with a specific outcome. The application of these predictive statistical modeling techniques, using microbiologic data, to other outcomes such as preterm births is appealing because women at risk for adverse pregnancy outcomes might then be identified early during their pregnancies. Early identification of women at high risk for an adverse outcome would allow for interventional therapy to be directed only at such women.
Preterm delivery (PTD) is the leading cause of infant morbidity and mortality in the United States (2, 12, 19). Preterm labor, premature rupture of membranes, and low birth weight have been associated with either specific maternal genital tract infections or an altered vaginal microflora during pregnancy (1, 8, 35, 37, 47, 48). Because bacteria are thought to contribute to PTD, substances of bacterial origin thought to act as mediators of PTD have been sought. Organisms implicated in PTD include specific agents, such as Chlamydia trachomatis, Ureaplasma urealyticum, and Bacteroides and yeast species (13, 30, 32, 33, 54, 55). A constellation of organisms representative of those associated with BV have also been cited as having the potential to produce inflammatory mediators, including phospholipases, leading to increased production of prostaglandins (9, 11, 16, 18, 22, 28, 56). Despite the epidemiological association between PTD and an altered vaginal microflora during pregnancy, the actual mechanism(s) by which preterm labor and delivery occur remains to be identified.
Based on these previous observations, interventional therapy has met with variable success (4, 10, 15, 20, 23, 31, 36). Antimicrobial agents directed at specific organisms, such as Ureaplasma, Chlamydia, Gardnerella vaginalis, and obligately anaerobic microorganisms are generally able to eradicate the organism(s) in question but have little effect on pregnancy outcome. Although certain antibiotic regimens have been shown in some studies to decrease the rate of preterm births in specific groups, such as women with BV, the effects were limited to only a subset of women delivering preterm and were not consistently observed. These observations indicate that, while a variety of abnormal microbiologic findings have been noted in women giving birth after a gestation period of less than 37 weeks, there may also be other risk factors or combinations of risk factors not accounted for by these previous microbiologic and clinical studies.
As the first phase of an ongoing clinical trial designed to determine microbiologic risk factors associated with preterm births, we performed quantitative and qualitative analysis of vaginal microbiologic cultures obtained from 229 pregnant subjects (gestation time point, 20 weeks) that were stratified by risk group. Analysis of the data identified several microbiologic risk factors that were used to construct multivariate predictive microbiologic models for PTD. The second phase of this ongoing trial will be to determine how well such predictive models work in identifying women at risk for preterm births.
MATERIALS AND METHODS
Subjects.
Women presenting for their first prenatal care examination at Faculty and Resident Practices, Brigham and Women's Hospital, over a 28-month period were given an enrollment package containing a description of the study. The study nurse identified eligible subjects and approached patients for enrollment during their routine prenatal visits (each visit occurred when the gestation time had reached between 18 and 22 weeks). The study nurse asked each woman if she was interested in participating in the study and answered any questions regarding the clinical study and sampling procedures. Informed consent was obtained using an institutional review board-approved consent form. Any of the following criteria were considered grounds for exclusion at enrollment: young age (i.e., age of less than 15 years); receipt of antimicrobial therapy within 4 weeks of initial sampling; multiple gestation; previous pregnancy loss (after a gestation period of less than 24 weeks) due to sepsis, cervical cerclage, placenta previa, or Rh isoimmunization; or any other obstetric problem or chronic medical condition predisposing to PTD, including chronic hypertension on medication, pregnancy-induced hypertension, insulin-requiring diabetes, renal disease with a baseline creatinine level of >2.0, or autoimmune disease requiring steroids. These criteria were designed to exclude women likely to have medically indicated preterm births. Subjects were placed into one of four risk groups: (i) women with no apparent risks, (ii) women with a prior preterm birth, (iii) women with vaginal bleeding during the current pregnancy, and (iv) women with a prior PTD and vaginal bleeding during the current pregnancy. Group sizes for this phase of the study were based on historic data for preterm births of patients at the Faculty and Resident Practice and prior quantitative microbiologic studies designed to detect quantitative differences (i.e., 0.5 log10 CFU/g of sample) in bacterial populations of the different groups (44, 45), as gauged by the vaginal swab sampling technique.
Determination of gestational age.
Gestational age was determined by using the last menstrual period (LMP) and earliest ultrasound. If the ages determined by using the LMP and the earliest ultrasound were concordant within the defined limits of ultrasound (within 7 days of each other for a gestation period ranging from 0 to 12 weeks, within 10 days of each other for a gestation period ranging from 12 to 20 weeks, within 2 weeks of each other for a gestation period ranging from 20 to 28 weeks, and within 3 weeks of each other for a gestation period of longer than 28 weeks), then the age determined by using the LMP was taken to be correct. If the results were discordant, then the age determined by using the earliest ultrasound was taken to be correct.
Demographic data.
Demographic, behavioral, and obstetric data were collected through the use of a questionnaire administered to each subject by the study nurse. Additional demographic and obstetric information was abstracted from each subject's medical record.
Collection of samples for microbiologic analysis.
Vaginal swab samples were obtained for laboratory-based microbiologic analysis from enrolled subjects in the antenatal clinic at the Center for Women and Newborn, Brigham and Women's Hospital. The study nurse collected the first vaginal swab samples when the gestational age was between 18 and 22 weeks. To ensure patient confidentiality and to minimize possible bias in the interpretation of laboratory-based tests, all samples were labeled with a numeric code before being sent to the laboratory. The key to the code was kept in a locked file, housed at the Center for Women and Newborn. The code was not linked to the microbiologic data until final outcome data and medical record data were abstracted.
Vaginal swab samples were collected by using the duplicate swab sampling technique for quantitative and qualitative microbiology (7). Briefly, the study nurse inserted two sterile cotton swabs simultaneously into the vaginal vault. The swabs were rotated against the upper vaginal walls to achieve saturation and carefully removed. One swab (the preweighed swab) was returned to a sterile preweighed tube, and the other swab was placed into 10.5 ml of prereduced Amies transport medium without charcoal (PML Microbiologicals, Tualatin, Oreg.). Both swabs were transported to the microbiology laboratory for processing within 2 h. The preweighed swab and tube were reweighed, and the difference between the final weight (of swab and tube together) and the preswab weight (of swab and tube together) was recorded as the sample weight. This method has been validated for obtaining sample weight in previous studies (6, 7, 43). Two additional samples were obtained with sterile cotton swabs: one was used for measuring pH, making a smear for Nugent scoring, and detection of volatile amines, and the other swab was used for Trichomonas culture.
Isolation of microorganisms and determination of microbiologic counts.
Upon arrival at the microbiology laboratory (within 2 h of sample collection), the swab for microbiologic analysis was placed into an anaerobic chamber for processing. The swab in transport medium was vigorously vortexed to disperse the sample, and serial decimal dilutions (to 10−5) were made in phosphate buffered saline, pH 7.2. A 0.1-ml aliquot of each dilution was plated onto enrichment or selective agar media, and the aliquot was spread over the surface of the medium with a sterile plastic tube. The media used for recovery of obligate anaerobes included BMB (prereduced brucella base blood agar containing 5% sheep blood, hemin, and menadione) and BMB containing 5% laked sheep blood, 100 μg of kanamycin per ml, and 7.5 μg of vancomycin per ml (PML Microbiologicals). Facultative organisms were isolated with tryptic soy base-5% sheep blood agar, MacConkey's agar, and mannitol-salt agar (all from PML Microbiologicals). Fastidious organisms were isolated on chocolate agar (PML Microbiologicals) (42, 44-46). A7 medium was used for the isolation of Mycoplasma and Ureaplasma species (Northeast Laboratory, Waterville, Maine). Trichomonas organisms were detected with Diamond's medium (PML Microbiologicals). Testing for C. trachomatis is routinely performed for all antenatal subjects in the Clinical Microbiology Laboratory at the Brigham and Women's Hospital with the use of a nucleic acid probe system (GenProbe, San Diego, Calif.).
Identification of microorganisms.
Following incubation under appropriate atmospheric conditions, colonies were enumerated on the various agar media and individual colony types were selected for identification based on colony morphology and Gram stain results. A variety of phenotypic methods, including long-chain fatty acid analysis, short-chain fatty acid analysis, and traditional biochemical testing, were used for identification purposes as described previously (14, 43-46). All counts were recorded as the numbers of log10 CFU per gram of sample.
Determination of H2O2 production.
Following identification, the numerically predominant Lactobacillus isolate for each sample was tested for production of hydrogen peroxide (H2O2) by a modified method described previously (17). The medium included DeMan-Rogosa-Sharpe agar (for detection of lactobacilli; Difco Laboratories, Detroit, Mich.) as the base, and tetra methyl benzidine hydrochloride hydrate (0.25 mg/ml; Sigma, St. Louis, Mo.) and horseradish peroxidase (0.02 mg/liter; Sigma) were added. Each isolate was inoculated onto the prepared plates and incubated in an anaerobic chamber for 2 to 3 days. The plates were removed from the chamber and exposed to ambient air. H2O2 was determined to be present if blue pigment was visible in and around the H2O2-producing colonies.
Diagnosis of BV.
The results of testing by the clinical criteria of Amsel (pH >4.5, presence of an amine odor upon addition of 10% KOH to the swab sample, presence of clue cells detected microscopically in wet mounts, and presence of a thin, homogenous vaginal discharge) were recorded for all subjects (2). In addition, the Nugent Gram stain scoring system was used (this sytem scores the numbers of lactobacilli, gram-negative and gram-variable rods [i.e., Prevotella and G. vaginalis], and curved gram-variable rods [i.e., Mobiluncus]) (26, 40, 41, 53). Unstained smears were obtained at each visit and sent to the microbiology laboratory for Gram staining and scored by two independent evaluators (A.B.O. and M.L.D.).
Statistical methods.
All data were maintained in relational databases created with commercial software (Access 97; Microsoft), with a common sample number linking demographic and outcome data to microbiologic data. Possible microbiologic risk factors were identified by using Fisher's exact test for observational data and the t test for independent means as well as for correlation coefficients for numeric data (Statistica; Softstat, Tulsa, Okla.). Predictive models were generated by stepwise logistic regression analysis. Cross-validations were performed by the leave-one-out procedure.
RESULTS
Demographic information.
A total of 231 women were enrolled in the study; however, 2 subjects were not included in the analysis (1 subject was excluded following an in utero death due to fetal cytomegalovirus infection before the gestation period reached 24 weeks, and the sample from 1 subject was lost in transit to the laboratory). Of the 229 remaining subjects, 48 (21%) were in the vaginal-bleeding-only risk group, 37 (16%) were in the previous-PTD risk group, 19 (8%) were in the previous-PTD-and-vaginal-bleeding risk group, and 125 (55%) were in the no-risk group. A total of 36 (15.7%) spontaneous preterm births occurred during this study. Women in the risk group with a prior preterm birth history (with or without vaginal bleeding) had the highest rate (33.9%) of PTD during this study period (for women with no obvious risk factors, the PTD rate was 8.9% [P < 0.001], and for women with only vaginal bleeding during this pregnancy, the PTD rate was 14.5% [P < 0.03]). The distribution of births by gestational age is shown in Table 1. Subjects ranged in age from less than 20 years old (0.5% of subjects) to more than 40 years old (4.5% of subjects), with 95% of subjects being between the ages of 20 and 39. The mean age for women delivering at less than 37 weeks was 29.8 years (standard deviation, ± 5.7 years), and for women delivering at term, the mean age was 29.9 years (standard deviation, ± 5.6 years). Approximately 55% of subjects had private medical insurance, with the rest being either self-insured or uninsured. A comparison of pregnancy outcomes by insurance status indicated that women who are uninsured or self-insured have a higher rate of preterm births than do women with insurance (22.2 versus 10.7%; P < 0.03). No significant differences in outcome based on ethnic group, Nugent score, parity (nulliparous, multiparous, and prima gravida), smoking history, or alcohol history were noted.
TABLE 1.
Gestational age at birth
| Week | No. of births observed |
|---|---|
| 21 | 1 |
| 24 | 1 |
| 28 | 1 |
| 31 | 1 |
| 33 | 4 |
| 34 | 9 |
| 35 | 6 |
| 36 | 13 |
| 37 | 21 |
| 38 | 43 |
| 39 | 56 |
| 40 | 50 |
| 41 | 20 |
| 42 | 2 |
| 43 | 1 |
| Total | 229 |
Descriptive microbiologic information.
A comparison of frequencies of isolation and mean counts for the major genera comprising the vaginal microflora is shown in Table 2. For the 229 subjects included in this study, 2,059 isolates representing 113 phenotypes were characterized. The mean values for total anaerobic bacterium counts, total aerobic bacterium counts, vaginal pH, and Nugent scores were similar for women delivering at less than 37 weeks and for those delivering at term. No statistically significant differences between the PTD group and the term delivery group were noted in frequencies of isolation and mean counts for the major microbiologic categories. The frequencies of isolation and mean total counts for G. vaginalis, Prevotella spp., Peptostreptococcus spp., Mycoplasma hominis, and Ureaplama urealyticum were similar for women delivering at less than 37 weeks and those delivering at term. Trichomonas sp. strains were isolated from two subjects (0.1%). Neither subject delivered preterm.
TABLE 2.
Vaginal microflora summarya
| Organism(s) | Value for gestation time of:
|
|||
|---|---|---|---|---|
| <37 weeks
|
≥37 weeks
|
|||
| Frequency of isolationb | No. of organismsc | Frequency of isolationb | No. of organisms | |
| All anaerobes | 8.33 ± 1.47 | 8.38 ± 1.57 | ||
| All aerobes | 7.24 ± 2.21 | 7.12 ± 2.29 | ||
| Corynebacterium spp. | 26/36 | 4.50 ± 0.87 | 212/193 | 4.66 ± 0.88 |
| Lactobacillus sp.d | 75/36 | 7.82 ± 1.43 | 377/193 | 7.99 ± 1.30 |
| H2O2 positive | 40/36 | 7.69 ± 1.50 | 195/193 | 8.25 ± 1.21e |
| H2O2 negative | 25/36 | 7.49 ± 1.42 | 119/193 | 7.60 ± 1.36 |
| Gardnerella vaginalis | 13/36 | 8.78 ± 1.30 | 55/193 | 8.90 ± 1.28 |
| Prevotella spp. | 44/36 | 5.98 ± 1.62 | 177/193 | 5.72 ± 1.80 |
| Prevotella bivia | 8/36 | 5.83 ± 1.50 | 42/193 | 5.53 ± 1.50 |
| Peptostreptococcus spp. | 32/36 | 5.30 ± 1.40 | 134/193 | 5.44 ± 1.43 |
| Peptostreptococcus assacharolyticus | 16/36 | 5.23 ± 1.35 | 55/193 | 5.05 ± 1.26 |
| Peptostreptococcus magnus | 10/36 | 5.23 ± 1.40 | 44/193 | 5.41 ± 1.55 |
| Peptostreptococcus prevotii | 2/36 | 5.70 ± 0.40 | 23/193 | 6.13 ± 1.08 |
| Staphylococcus spp. | 43/36 | 4.14 ± 0.69 | 226/193 | 4.18 ± 0.70 |
| Streptococcus spp. | 37/36 | 5.62 ± 1.66 | 111/193 | 4.99 ± 1.32 |
| Mycoplasma hominis | 7/36 | 6.15 ± 0.81 | 36/193 | 6.03 ± 1.02 |
| Ureaplasma urealyticum | 5/36 | 6.26 ± 0.88 | 45/193 | 5.86 ± 0.73 |
Vaginal pH and Nugent scores were as follows (values are means ± standard deviations): for a gestation period of <37 weeks, 4.76 ± 0.78 and 2.8 ± 2.75, respectively, and for a gestation period of >37 weeks, 4.74 ± 0.79 and 2.45 ± 2.60, respectively.
Values are numbers of observations/numbers of subjects.
Values are log10 CFU/gram of sample ± standard deviations.
A total of 10 strains in the preterm group and 63 strains in the term group were not characterized for H2O2 production.
P < 0.01 when compared to value for gestation period of <37 weeks.
Evaluation of the multiple isolates of lactobacilli encountered during this study revealed that women who were simultaneously harboring strains that produced H2O2 and strains that did not produce H2O2 were much more likely to have PTDs. While no statistical difference was noted for women harboring either strains that produced H2O2 or strains that did not produce H2O2 (10 of 33 women who had PTDs and 59 of 154 women who had at-term deliveries [P > 0.40] for strains producing H2O2; 5 of 33 women who had PTDs and 42 of 154 women who had at-term deliveries [P > 0.18] for strains not producing H2O2), it was noted that a significantly higher percentage of women harboring both strain types had PTDs (15 of 33 women versus 39 of 154 women [P < 0.03]). (Results of testing for H2O2 production were not available for 3 women who had PTDs or for 39 women who had at-term deliveries.) In addition, the total number of H2O2-producing lactobacilli was significantly lower in women who had PTDs than in those who had at-term deliveries (mean ± standard error of the mean, 7.69 ± 0.24 versus 8.25 ± 0.09 log10 CFU/g of sample; P < 0.01).
Predictive statistical model for PTD.
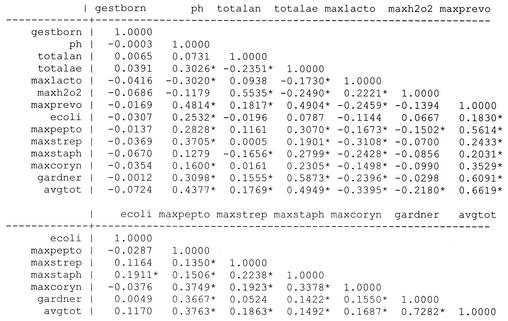
A correlation matrix containing the major microbiologic members of the vaginal microflora was constructed as a preliminary step in developing statistical models for preterm birth (Fig. 1). There was a strong overall correlation between the total aerobic bacterium counts and the average total bacterium count (avgtot) (r = 0.49; P < 0.01). An even stronger correlation existed between these two variables for the subset of women delivering after a gestation period of less than 37 weeks (r = 0.62; P < 0.01). The maximum counts of H2O2-producing lactobacilli and total anaerobic bacteria were noted to show a significant correlation (r = 0.55; P < 0.01). An even stronger correlation existed between these two variables for women delivering after a gestation period of less than 37 weeks (r = 0.61; P < 0.01). Vaginal pH levels had a positive correlation with the total aerobic counts (r = 0.30; P < 0.01) and a negative correlation with the total counts for Lactobacillus spp. (r = −0.30; P < 0.01) (as the pH decreased, the count of lactobacilli increased).
FIG. 1.
Correlation matrix for all subjects. ∗, significance at P value of <0.05. Abbreviations: gestborn, gestational age at birth; ph, pH; totalan, all anaerobes; totalae, all aerobes; maxlacto, maximum count of Lactobacillus spp.; maxh2o2, maximum count of H2O2-producing Lactobacillus spp.; maxprevo, maximum count of Prevotella spp.; ecoli, Escherichia coli count; maxpepto, maximum count of Peptostreptococcus spp.; maxstrep, maximum count of Strepotococcus spp.; maxstaph, maximum count of Staphylococcus spp.; maxcoryn, maximum count of Corynebacterium spp.; gardner, Gardnerella sp. count; avgtot, average total number of bacteria.
For women in different risk groups, we examined whether PTD might be related to somewhat different microbial profiles. Therefore, we considered separate models for women with and without prior PTDs.
For the women who had had a prior PTD, the rate of preterm birth was 37.8% for the period of this study. For these women, we considered two models: model 1 included women who had no vaginal bleeding during the current pregnancy, and model 2 included women with or without vaginal bleeding during the current pregnancy.
On the other hand, for women who had not previously had a PTD, the rate of preterm birth for women with vaginal bleeding was 14.5% and for women with no obvious risk factor the rate of preterm birth was 8.9%. Hence, these two groups were combined and models 3 and 4 were instead constructed according to the presence or absence of H2O2-positive Lactobacillus spp., as follows: model 3 included women with H2O2-positive Lactobacilus spp. present, and model 4 included women from whom H2O2-positive Lactobacilus spp. were absent.
Models 2, 3, and 4 are based on mutually exclusive groups of patients. Model 1 is a subset of Model 2 that focused on women without vaginal bleeding during the current pregnancy. A total of 206 (90%) of the 229 study subjects were evaluated by one of the models. Subjects with missing data were not evaluated.
For the previous-PTD risk group, the model was constructed as shown below, using numerous iterations to find the best fit. If p is taken to be the probability of PTD, then logit (p) can be calculated as follows:
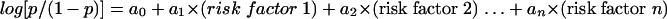 |
(1) |
where a1…an are estimated regression coefficients. If the maximum counts for H2O2-producing lactobacilli were not available, then the maximum counts for Lactobacillus spp. were used in the regression.
One measure for the overall performance of the predictive logistic regression model is the area under the receiver operating curve (AUC). Subjects with predicted probability values above a specified threshold value were considered to be likely to have a PTD during the current pregnancy. The threshold probability was chosen so that the sensitivity and specificity values of the predictive model could be optimized.
For women with a prior PTD but without vaginal bleeding during the current pregnancy, the logistic regression function for model 1 is as follows: logit (p) = 20.86 − 1.32 × pH − 2.61 × total anaerobic count + 0.58 × maximum count of H2O2-positive lactobacilli + 0.70 × maximum count of Peptostreptococcus spp. Using this model, the predicted probability (p) is a number between 0 and 1. The area under the curve (AUC) has a value of 0.94, with a confidence interval (CI) of 0.79 to 1. Cross-validation by the leave-one-out procedure was performed, and the results were relatively stable. Comparison of the results predicted on the basis of the logistic regression model to the outcomes actually observed indicated that the predictive logistic model with a cutoff probability of 0.3 yielded a sensitivity of 88.9% and a specificity of 89.5% in predicting which subjects would have PTDs. The microbiologic risk factors included in the regression equation and the AUC measures for the four models are shown in Table 3. Predicted probabilities and actual outcomes for model 1 based on subjects with a prior PTD are shown in Table 4.
TABLE 3.
Regression models by risk groups
| Risk group characteristic(s) | m/na | Regression model | AUC | CI for AUC |
|---|---|---|---|---|
| Previous PTD but no vaginal bleeding | 9/28 | logit (p) = 20.86 − 1.33 × pH −2.6 × total anaerobic count + 0.58 × maximum H2O2-positive Lactobacillus sp. count + 0.70 × maximum Peptostreptococcus sp. count | 0.91 | 0.79-1.00 |
| Previous PTD with or without vaginal bleeding | 11/32 | logit (p) = 27.35 − 3.27 × pH + 1.92 × bothb − 2.22 × total anaerobic bacterium count + 0.64 × maximum H2O2-positive Lactobacillus sp. count + 0.22 × maximum Peptostreptococcus sp. count + 0.21 × Gardnerella sp. count − 0.32 × maximum Staphylococcus sp. count | 0.82 | 0.65-0.98 |
| Bleeding or no obvious risks; H2O2-positive lactobacilli present | 15/139 | logit (p) = 2.05 − 0.75 × total anaerobic bacterium count − 0.48 × total aerobic bacterium count + 0.29 × maximum H2O2-positive Lactobacillus sp. count + 0.21 × maximum Staphylococcus sp. count + 0.27 × maximum Corynebacterium sp. count + 0.34 × maximum Gardnerella sp. count | 0.74 | 0.62-0.87 |
| Bleeding or no obvious risks; H2O2-positive lactobacilli absent | 2/35 | logit (p) = −81.14 − 0.45 × total aerobic bacterium count + 8.72 × maximum Lactobacillus sp. count + 0.74 × maximum Streptococcus sp. count | 0.94 | 0.81-1.00 |
m, number of PTD cases; n, number of subjects with all covariates.
both, indicator variable for having both vaginal bleeding and previous PTD.
TABLE 4.
Probability calculations and actual outcomes for women with a previous PTDa
| Subject | Probability of PTD | Outcome |
|---|---|---|
| 1 | 0.0002 | Term |
| 2 | 0.0155 | Term |
| 3 | 0.0393 | Term |
| 4 | 0.0559 | Term |
| 5 | 0.0655 | Term |
| 6 | 0.0734 | Term |
| 7 | 0.0759 | Term |
| 8 | 0.0779 | Term |
| 9 | 0.0835 | Term |
| 10 | 0.0853 | Term |
| 11 | 0.1102 | Term |
| 12 | 0.1149 | Term |
| 13 | 0.1336 | Term |
| 14 | 0.1766 | Term |
| 15 | 0.2378 | Term |
| 16 | 0.2378 | Term |
| 17 | 0.2881 | Term |
| 18 | 0.6093 | Term∗b |
| 19 | 0.9414 | Term∗b |
| 20 | 0.1988 | Preterm∗b |
| 21 | 0.3204 | Preterm |
| 22 | 0.5244 | Preterm |
| 23 | 0.5604 | Preterm |
| 24 | 0.6063 | Preterm |
| 25 | 0.7116 | Preterm |
| 26 | 0.7941 | Preterm |
| 27 | 0.9221 | Preterm |
| 28 | 0.9422 | Preterm |
Data shown are for 28 of 36 subjects without any missing data.
∗, probability calculation with a cutoff of 0.3 varies from actual outcome. All three subjects thus denoted had total anaerobic counts that were significantly higher than those observed for the rest of the group (P < 0.034).
DISCUSSION
Previous studies conducted in our laboratory have extensively characterized the vaginal microflora of overtly healthy women (42, 44, 45). Based on the accumulated data from these earlier studies, a predictive model for normal vaginal microflora was constructed and tested (34). Prospective use of this model to predict an abnormal vaginal microflora has proven to be a practical application of these methods (49-52). The present study was an attempt to apply similar microbiologic and statistical methods to the prediction of preterm births, based on quantitative analysis of the vaginal microflora analysis after 20 weeks of gestation.
Our previous studies have demonstrated that, for any given bacterial phenotype, the range of concentrations varies widely from subject to subject as well as for the same subject sampled longitudinally. This may account for the difficulty in correlating the presence or absence of any specific species or total bacterial counts with an outcome such as preterm birth. It was noted during the development of a statistical model for normal vaginal microflora that the most predictive models included multiple bacterial risk factors. In the present study, we elected to use only microbiologic data or vaginal pH levels as risk factors for predicting PTD. Although a variety of demographic data for each subject were available, only the risk group was considered during construction of the predictive models. This approach allows for the use of observed microbiologic data in the absence of other confounding variables, such as a history of smoking and alcohol use, that have been suggested to influence pregnancy outcome. Subsequent statistical modeling has included both demographic data (such as age, ethnic group, insurance status, history of smoking and/or alcohol use), and parity. While these models may be more complete in that they use all of the available data, the AUC for these models is not significantly better than that obtained when only microbiologic data are used (unpublished data).
Among the organisms most commonly present as vaginal microflora are members of the genus Lactobacillus. Lactobacillus acidophilus is often reported to be the member of this genus most commonly isolated from vaginal secretions. Most of these previous reports have relied exclusively on conventional methods of phenotyping. However, recent studies using 16S rRNA-based ribotyping indicate that Lactobacillus crispatus, Lactobacillus jensenii, and Lactobacillus gasseri are the species most commonly present as part of the vaginal microflora (3). The role of lactobacilli within the vaginal environment is generally regarded as beneficial, with the production of lactic acid being responsible, in part, for the low pH of the vagina. This low pH is inhibitory for many potentially pathogenic organisms that also colonize the vaginal environment in low concentrations. More recently, it has been reported that the production of H2O2 by some members of the genus Lactobacillus is also associated with a healthy vaginal environment (3, 17, 25, 27). The inhibitory effect in vitro of H2O2 for organisms not producing catalase is well known. With regard to the vaginal microflora, the inhibitory effect of H2O2 for G. vaginalis has been documented, although the effect of H2O2 within the vaginal environment for other non-catalase-producing species, including anaerobic streptococci and other obligate anaerobes, is not known. Women with BV have decreased colonization rates for H2O2-producing strains of lactobacilli and increased colonization rates for non-H2O2-producing strains. It has also been reported that non-H2O2-producing strains of lactobacilli are present as part of the normal vaginal microflora (21, 24, 25, 29). In many women, both H2O2- and non-H2O2-producing strains can be isolated. While the presence or absence of H2O2- and non-H2O2-producing strains has been reported with respect to pregnancy outcome, this is the first attempt to use such information to develop a predictive model for PTD. Of particular note during the construction of the predictive models is the central role for both the type and total numbers of lactobacilli present as part of the vaginal microflora. The observation that women harboring both H2O2-producing and non-H2O2-producing Lactobacillus strains are at increased risk for PTD is a clear indication that this genus is an important microbiologic risk factor for predicting outcome. Although the characterization of lactobacilli in this initial phase of the study included only genus designation and H2O2 production, our continuing study includes characterization of isolates by restriction fragment length polymorphism and pulsed-field gel electrophoresis analyses, species-level identification by 16S ribosomal DNA-based sequencing, and testing for the presence of lactocin genes by highly specific molecular probes. Further characterization of these strains by molecular techniques may provide additional useful information.
Many gram-positive organisms produce small peptides that are antibacterial in nature, variously called bacteriocins, lantibiotics, and lactocins. In the case of lactobacilli, such substances are often directed at suppressing growth of closely related species. It has been reported, for example, that a bacteriocin produced by L. acidophilus is directed at inhibition of Lactobacillus debrueckii, a closely related species (5). The characteristics of these small peptide inhibitors produced by lactobacilli are remarkably similar among gram-positive species and often contain posttranslational modifications of specific amino acids (38). Some of these compounds, such as lactocin S, can act as both bacteriocin and as cytolysin (38). While lactobacilli may be useful markers for a healthy vaginal environment, the possibility that specific strains or combinations of strains are harmful should not be ignored, particularly in light of their ability to produce substances capable of inhibiting other normal vaginal microflora organisms. The elevated vaginal pH associated with pregnancy, coupled with the presence of multiple strains of lactobacilli, may provide an ideal environment for production of bacteriocins. It has also been reported that a bacteriocin similar to those produced by lactobacilli is capable of inducing cellular injury (39) via the generation of superoxide anions. Among the genera present as part of the vaginal microflora, Lactobacillus is almost unique in that it has never been explored as a possible mediator of tissue inflammation.
The reliability of the models defined here to prospectively predict PTD is currently being evaluated in an ongoing clinical trial. If the models are able to prospectively predict preterm births reliably, the ability to use targeted interventional therapy only for those women at risk for preterm birth becomes a genuine possibility. The ability to construct these models using only microbiologic data suggests a primary role for bacteria in the etiology of preterm births. Understanding the mechanisms by which bacteria contribute to PTD is the basis for our continued research efforts.
Acknowledgments
This research was supported by grant RO1 HD35667 from the Institute of Child Health and Human Development, National Institutes of Health.
A.B.O., M.-L.L., E.L., M.L.D., and R.E.T. are members of the Microbiology and Prematurity Study Group. Other participants include Robin Ross, Andrea DuBois, Linda Steele, Dorothy Pender, and Amy Cohen.
REFERENCES
- 1.Abele-Horn, M., J. Peters, O. Genzel-Boroviczeny, C. Wolff, A. Zimmermann, and W. Gottschling. 1997. Vaginal Ureaplasma urealyticum colonization: influence on pregnancy outcome and neonatal morbidity. Infection 25:286-291. [DOI] [PubMed]
- 2.Amsel, R., P. A. Totten, C. A. Spiegel, K. C. Chen, D. Eschenbach, and K. K. Holmes. 1983. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am. J. Med. 74:14-22. [DOI] [PubMed] [Google Scholar]
- 3.Antonio, M. A., S. E. Hawes, and S. L. Hillier. 1999. The identification of vaginal Lactobacillus species and the demographic and microbiologic characteristics of women colonized by these species. J. Infect. Dis. 180:1950-1956. [DOI] [PubMed] [Google Scholar]
- 4.Antsaklis, A., G. Daskalakis, S. Michalas, and D. Aravantinos. 1997. Erythromycin treatment for subclinical Ureaplasma urealyticum infection in preterm labor. Fetal Diagn. Ther. 12:89-92. [DOI] [PubMed] [Google Scholar]
- 5.Barefoot, S. F., and T. R. Klaenhammer. 1983. Detection and activity of lactacin B, a bacteriocin produced by Lactobacillus acidophilus. Appl. Environ. Microbiol. 45:1808-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartlett, J. G., N. E. Moon, P. R. Goldstein, B. Goren, A. B. Onderdonk, and B. F. Polk. 1978. Cervical and vaginal bacterial flora: ecologic niches in the female lower genital tract. Am. J. Obstet. Gynecol. 130:658-661. [DOI] [PubMed] [Google Scholar]
- 7.Bartlett, J. G., A. B. Onderdonk, E. Drude, C. Goldstein, M. Anderka, S. Alpert, and W. M. McCormack. 1977. Quantitative bacteriology of the vaginal flora. J. Infect. Dis. 136:271-277. [DOI] [PubMed] [Google Scholar]
- 8.Bergstrom, S., and A. Libombo. 1995. Low birthweight and post partum endometritis-myometritis. Acta Obstet. Gynecol. Scand. 74:611-613. [DOI] [PubMed] [Google Scholar]
- 9.Briselden, A. M., B. J. Moncla, C. E. Stevens, and S. L. Hillier. 1992. Sialidases (neuraminidases) in bacterial vaginosis and bacterial vaginosis-associated microflora. J. Clin. Microbiol. 30:663-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carey, J. C., M. A. Klebanoff, J. C. Hauth, S. L. Hillier, E. A. Thom, J. M. Ernest, R. P. Heine, R. P. Nugent, M. L. Fischer, K. J. Leveno, R. Wapner, M. Varner, et al. 2000. Metronidazole to prevent preterm delivery in pregnant women with asymptomatic bacterial vaginosis. N. Engl. J. Med. 342:534-540. [DOI] [PubMed] [Google Scholar]
- 11.Chaim, W., M. Mazor, and J. R. Leiberman. 1997. The relationship between bacterial vaginosis and preterm birth. A review. Arch. Gynecol. Obstet. 259:51-58. [DOI] [PubMed] [Google Scholar]
- 12.Chambers, S., J. C. Pons, A. Richard, M. Chiesa, J. Bouyer, and E. Papiernik. 1991. Vaginal infections, cervical ripening and preterm delivery. Eur. J. Obstet. Gynecol. Reprod. Biol. 38:103-108. [DOI] [PubMed] [Google Scholar]
- 13.Cotch, M. F., S. L. Hillier, R. S. Gibbs, D. A. Eschenbach, et al. 1998. Epidemiology and outcomes associated with moderate to heavy Candida colonization during pregnancy. Am. J. Obstet. Gynecol. 178:374-380. [DOI] [PubMed] [Google Scholar]
- 14.Delaney, M. L., A. M. DuBois, and A. B. Onderdonk. 1995. Qualitative and quantitative assessment of vaginal microflora following the use of cotton tampons for two or twelve hours. Microecol. Ther. 23:18-15.
- 15.Duff, P., M. L. Lee, S. L. Hillier, L. M. Herd, M. A. Krohn, and D. A. Eschenbach. 1991. Amoxicillin treatment of bacterial vaginosis during pregnancy. Obstet. Gynecol. 77:431-435. [PubMed] [Google Scholar]
- 16.Eschenbach, D. A. 1994. Vaginitis including bacterial vaginosis. Curr. Opin. Obstet. Gynecol. 6:389-391. [PubMed] [Google Scholar]
- 17.Eschenbach, D. A., P. R. Davick, B. L. Williams, S. J. Klebanoff, K. Young-Smith, C. M. Critchlow, and K. K. Holmes. 1989. Prevalence of hydrogen peroxide-producing Lactobacillus species in normal women and women with bacterial vaginosis. J. Clin. Microbiol. 27:251-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flynn, C. A., A. L. Helwig, and L. N. Meurer. 1999. Bacterial vaginosis in pregnancy and the risk of prematurity: a meta-analysis. J. Fam. Pract. 48:885-892. [PubMed] [Google Scholar]
- 19.Garbaciak, J. A., Jr. 1992. Prematurity prevention: who is at risk? Clin. Perinatol. 19:275-289. [PubMed] [Google Scholar]
- 20.Hauth, J. C., R. L. Goldenberg, W. W. Andrews, M. B. DuBard, and R. L. Copper. 1995. Reduced incidence of preterm delivery with metronidazole and erythromycin in women with bacterial vaginosis. N. Engl. J. Med. 333:1732-1736. [DOI] [PubMed] [Google Scholar]
- 21.Hawes, S. E., S. L. Hillier, J. Benedetti, C. E. Stevens, L. A. Koutsky, P. Wolner-Hanssen, and K. K. Holmes. 1996. Hydrogen peroxide-producing lactobacilli and acquisition of vaginal infections. J. Infect. Dis. 174:1058-1063. [DOI] [PubMed] [Google Scholar]
- 22.Hay, P. E., D. J. Morgan, C. A. Ison, S. A. Bhide, M. Romney, P. McKenzie, J. Pearson, R. F. Lamont, and D. Taylor-Robinson. 1994. A longitudinal study of bacterial vaginosis during pregnancy. Br. J. Obstet. Gynaecol. 101:1048-1053. [DOI] [PubMed] [Google Scholar]
- 23.Hillier, S., M. A. Krohn, D. H. Watts, P. Wolner-Hanssen, and D. Eschenbach. 1990. Microbiologic efficacy of intravaginal clindamycin cream for the treatment of bacterial vaginosis. Obstet. Gynecol. 76:407-413. [PubMed] [Google Scholar]
- 24.Hillier, S. L., M. A. Krohn, E. Cassen, T. R. Easterling, L. K. Rabe, and D. A. Eschenbach. 1995. The role of bacterial vaginosis and vaginal bacteria in amniotic fluid infection in women in preterm labor with intact fetal membranes. Clin. Infect. Dis. 20(Suppl. 2):S276-S278. [DOI] [PubMed]
- 25.Hillier, S. L., M. A. Krohn, S. J. Klebanoff, and D. A. Eschenbach. 1992. The relationship of hydrogen peroxide-producing lactobacilli to bacterial vaginosis and genital microflora in pregnant women. Obstet. Gynecol. 79:369-373. [DOI] [PubMed] [Google Scholar]
- 26.Hillier, S. L., M. A. Krohn, R. P. Nugent, R. S. Gibbs, et al. 1992. Characteristics of three vaginal flora patterns assessed by gram stain among pregnant women. Am. J. Obstet. Gynecol. 166:938-944. [DOI] [PubMed] [Google Scholar]
- 27.Hillier, S. L., M. A. Krohn, L. K. Rabe, S. J. Klebanoff, and D. A. Eschenbach. 1993. The normal vaginal flora, H2O2-producing lactobacilli, and bacterial vaginosis in pregnant women. Clin. Infect. Dis. 16(Suppl. 4):S273-S281. [DOI] [PubMed]
- 28.Hillier, S. L., R. P. Nugent, D. A. Eschenbach, M. A. Krohn, R. S. Gibbs, D. H. Martin, M. F. Cotch, R. Edelman, J. G. Pastorek II, A. V. Rao, et al. 1995. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. N. Engl. J. Med. 333:1737-1742. [DOI] [PubMed] [Google Scholar]
- 29.Holst, E., A. R. Goffeng, and B. Andersch. 1994. Bacterial vaginosis and vaginal microorganisms in idiopathic premature labor and association with pregnancy outcome. J. Clin. Microbiol. 32:176-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ismail, M. A., G. Pridjian, J. U. Hibbard, C. Harth, and A. A. Moawad. 1992. Significance of positive cervical cultures for Chlamydia trachomatis in patients with preterm premature rupture of membranes. Am. J. Perinatol. 9:368-370. [DOI] [PubMed] [Google Scholar]
- 31.Joesoef, M. R., S. L. Hillier, G. Wiknjosastro, H. Sumampouw, M. Linnan, W. Norojono, A. Idajadi, and B. Utomo. 1995. Intravaginal clindamycin treatment for bacterial vaginosis: effects on preterm delivery and low birth weight. Am. J. Obstet. Gynecol. 173:1527-1531. [DOI] [PubMed] [Google Scholar]
- 32.Kimpen, J., E. Bosmans, J. Janssen, J. Lambrechts, A. Van Hoof, J. Gielen, H. Vandeput, and A. Van Waes. 1986. Screening for Ureaplasma urealyticum infections in the neonate and the association with prematurity. Eur. J. Obstet. Gynecol. Reprod. Biol. 22:53-60. [DOI] [PubMed] [Google Scholar]
- 33.Lamont, R. F., D. Taylor-Robinson, J. S. Wigglesworth, P. M. Furr, R. T. Evans, and M. G. Elder. 1987. The role of mycoplasmas, ureaplasmas and chlamydiae in the genital tract of women presenting in spontaneous early preterm labour. J. Med. Microbiol. 24:253-257. [DOI] [PubMed] [Google Scholar]
- 34.Lee, M.-L., R. A. Ross, M. L. Delaney, and A. B. Onderdonk. 1995. Mathematical modeling of the vaginal microflora. Microecol. Ther. 23:18-21. [Google Scholar]
- 35.McGregor, J. A., J. I. French, D. Lawellin, and J. K. Todd. 1988. Preterm birth and infection: pathogenic possibilities. Am. J. Reprod. Immunol. Microbiol. 16:123-132. [DOI] [PubMed] [Google Scholar]
- 36.McGregor, J. A., J. I. French, R. Richter, M. Vuchetich, V. Bachus, K. Seo, S. Hillier, F. N. Judson, J. McFee, J. Schoonmaker, et al. 1990. Cervicovaginal microflora and pregnancy outcome: results of a double-blind, placebo-controlled trial of erythromycin treatment. Am. J. Obstet. Gynecol. 163:1580-1591. [DOI] [PubMed] [Google Scholar]
- 37.Minkoff, H., A. N. Grunebaum, R. H. Schwarz, J. Feldman, M. Cummings, W. Crombleholme, L. Clark, G. Pringle, and W. M. McCormack. 1984. Risk factors for prematurity and premature rupture of membranes: a prospective study of the vaginal flora in pregnancy. Am. J. Obstet. Gynecol. 150:965-972. [DOI] [PubMed] [Google Scholar]
- 38.Nes, I. F., and J. R. Tagg. 1996. Novel lantibiotics and their pre-peptides. Antonie Leeuwenhoek 69:89-97. [DOI] [PubMed] [Google Scholar]
- 39.Novak, R. M., T. J. Holzer, and C. R. Libertin. 1993. Human neutrophil oxidative response and phagocytic killing of clinical and laboratory strains of Enterococcus faecalis. Diagn. Microbiol. Infect. Dis. 17:1-6. [DOI] [PubMed] [Google Scholar]
- 40.Nugent, R. P., S. L. Hillier, et al. 1992. Mucopurulent cervicitis as a predictor of chlamydial infection and adverse pregnancy outcome. Sex. Transm. Dis. 19:198-202. [DOI] [PubMed] [Google Scholar]
- 41.Nugent, R. P., M. A. Krohn, and S. L. Hillier. 1991. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of Gram stain interpretation. J. Clin. Microbiol. 29:297-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Onderdonk, A. B., M. L. Delaney, G. R. Zamarchi, M. L. Hirsch, A. Munoz, and E. H. Kass. 1989. Normal vaginal microflora during use of various forms of catamenial protection. Rev. Infect. Dis. 11(Suppl. 1):S61-S67. [DOI] [PubMed]
- 43.Onderdonk, A. B., B. F. Polk, N. E. Moon, B. Goren, and J. G. Bartlett. 1977. Methods for quantitative vaginal flora studies. Am. J. Obstet. Gynecol. 128:777-781. [DOI] [PubMed] [Google Scholar]
- 44.Onderdonk, A. B., G. R. Zamarchi, M. L. Rodriguez, M. L. Hirsch, A. Munoz, and E. H. Kass. 1987. Qualitative assessment of vaginal microflora during use of tampons of various compositions. Appl. Environ. Microbiol. 53:2779-2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Onderdonk, A. B., G. R. Zamarchi, M. L. Rodriguez, M. L. Hirsch, A. Munoz, and E. H. Kass. 1987. Quantitative assessment of vaginal microflora during use of tampons of various compositions. Appl. Environ. Microbiol. 53:2774-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Onderdonk, A. B., G. R. Zamarchi, J. A. Walsh, R. D. Mellor, A. Munoz, and E. H. Kass. 1986. Methods for quantitative and qualitative evaluation of vaginal microflora during menstruation. Appl. Environ. Microbiol. 51:333-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Papiernik, E. 1990. Preterm labor, preterm delivery, intrauterine infection, and preterm rupture of membranes. Curr. Opin. Obstet. Gynecol. 2:8-12. [PubMed] [Google Scholar]
- 48.Romero, R., F. Shamma, C. Avila, C. Jimenez, R. Callahan, J. Nores, M. Mazor, C. A. Brekus, and J. C. Hobbins. 1990. Infection and labor. VI. Prevalence, microbiology, and clinical significance of intraamniotic infection in twin gestations with preterm labor. Am. J. Obstet. Gynecol. 163:757-761. [DOI] [PubMed] [Google Scholar]
- 49.Ross, R. A., M. L. Delaney, and A. B. Onderdonk. 1995. Candida albicans in an in vitro model of the vaginal ecosystem. Microecol. Ther. 25:320-323. [Google Scholar]
- 50.Ross, R. A., M.-L. Lee, M. Delaney, and A. Onderdonk. 1995. Use of continuous culture growth systems for modeling vaginal microflora behavior. Microecol. Ther. 23:16-17. [Google Scholar]
- 51.Ross, R. A., M. L. Lee, M. L. Delaney, and A. B. Onderdonk. 1994. Mixed-effect models for predicting microbial interactions in the vaginal ecosystem. J. Clin. Microbiol. 32:871-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ross, R. A., M. L. Lee, and A. B. Onderdonk. 1995. Effect of Candida albicans infection and clotrimazole treatment on vaginal microflora in vitro. Obstet. Gynecol. 86:925-930. [DOI] [PubMed] [Google Scholar]
- 53.Schwebke, J. R., S. L. Hillier, J. D. Sobel, J. A. McGregor, and R. L. Sweet. 1996. Validity of the vaginal gram stain for the diagnosis of bacterial vaginosis. Obstet. Gynecol. 88:573-576. [DOI] [PubMed] [Google Scholar]
- 54.Sweet, R. L., D. V. Landers, C. Walker, and J. Schachter. 1987. Chlamydia trachomatis infection and pregnancy outcome. Am. J. Obstet. Gynecol. 156:824-833. [DOI] [PubMed] [Google Scholar]
- 55.Van Winter, J. T., J. A. Ney, P. L. Ogburn, Jr., and R. V. Johnson. 1994. Preterm labor and congenital candidiasis. A case report. J. Reprod. Med. 39:987-990. [PubMed] [Google Scholar]
- 56.Woodrow, N., and R. F. Lamont. 1998. Bacterial vaginosis: its importance in obstetrics. Hosp. Med. 59:447-450. [PubMed] [Google Scholar]