Abstract
Recombinant listeriolysin O and internalin A were used as antigens in an enzyme-linked immunosorbent assay (ELISA) for the specific detection of anti-Listeria monocytogenes antibodies in cattle. The results showed sensitivities and specificities of 82 and 92%, respectively, for the listeriolysin O ELISA, and 100 and 90%, respectively, for the internalin A ELISA, respectively. The test may be useful for the confirmation of listeria-related abortions and mastitis but does not seem to be indicated for use in the diagnosis of listeria-related encephalitis in cattle. A representative sample of 1,652 serum samples from the healthy dairy cattle population in Switzerland was tested by both ELISAs. The results showed that 11% of the healthy dairy cows in Switzerland simultaneously presented antibodies toward listeriolysin O and internalin A, and 48% of the farms had one or several animals simultaneously positive by assays with both antigens. Multivariable analysis at the farm level confirmed that feeding of silage represents a significant risk factor for a positive listeria serology. Detailed analysis identified corn silage but not grass silage as the major factor in this association. Cattle breed and hygiene on the farm were also identified as significant factors associated with the serological status of farms. In conclusion, the results of the study show that internalin A is a promising new antigen for use in listeria serology and that specific anti-L. monocytogenes antibodies are found in a significant proportion of healthy dairy cows in Switzerland.
Listeria monocytogenes is a ubiquitous pathogen that leads to severe diseases in humans (7). Listeriosis is also a well-known disease of animals, particularly of ruminants, in which it is often associated with the consumption of poor-quality silage. Listeriosis in ruminants occurs mainly in the form of encephalitis, abortion, stillbirth, and mastitis; but healthy L. monocytogenes carriers are not uncommon (21, 37, 40). Infections in humans are usually of food-borne origin, and dairy products rank among the most frequent food items implicated in listeriosis outbreaks (12). Furthermore, clinical L. monocytogenes isolates from humans and ruminants present many similarities and often belong to the same genetic lineages (8). Thus, ruminants and their environment may represent an important source of food contamination and infections for humans. Serology would be a useful tool for epidemiological studies aimed at clarifying the role of cattle in the epidemiology of human listeriosis. However, the use of serology for the study of listeriosis has been hampered in the past by the rather poor performances of the available tests (7, 16). Research on the pathogenesis of listeriosis has identified many virulence factors specific for L. monocytogenes which could serve as antigens for new improved serological tests. The best known among them is listeriolysin O (28), a toxin involved in the intracellular spread of L. monocytogenes (14). Several studies in human medicine have demonstrated the potential of this toxin as an antigen for the serological diagnosis of listeriosis in humans (6, 18). Similar studies have shown that small ruminants develop detectable anti-listeriolysin O antibody titers during L. monocytogenes infections (2, 24, 25, 26, 29). Calves orally infected with L. monocytogenes (1, 3, 4, 5) and dairy cows with intramammary L. monocytogenes infections (9, 10, 11) also produce significant humoral responses toward listeriolysin O. The only major disadvantage of this antigen is its potential cross-reactivity with antibodies directed against related toxins produced by bacterial species other than L. monocytogenes (17). Attempts to use more specific truncated forms of the listeriolysin O protein have been made, but either these antigens may be difficult to produce or their use may result in a significant loss of sensitivity (17). Internalin A is another virulence factor of L. monocytogenes expressed at the bacterial cell surface and involved in the internalization of the microorganism into host cells (14, 15). It could possibly represent an interesting antigen for serological tests. When used concomitantly with listeriolysin O, internalin A may also help to increase the specificities of serological tests for the diagnosis of L. monocytogenes infections.
The aim of the present work was to develop an enzyme-linked immunosorbent assay (ELISA) with recombinant listeriolysin O and internalin A as antigens specific for the detection of L. monocytogenes infections. This test was subsequently used with a representative collection of sera from dairy cows in Switzerland in order to assess the frequency of subclinical L. monocytogenes infections in these animals and to identify risk factors associated with these infections at the farm level.
MATERIALS AND METHODS
Cloning of listeriolysin O and internalin A.
Listeriolysin O (amino acids 26 to 529) (28) and internalin A (amino acids 2 to 710) (15) were cloned by PCR with DNA from a representative L. monocytogenes serovar 4b isolate (isolate LL195) from the Swiss listeriosis epidemic of 1983 to 1987 (8). Primers HLY1 (5′-GGGGGATCCGATGCATCTGCATTCAATAA-3′), HLY2 (5′-TGAGCTGCAGTTATTCGATTGGATTATCTA-3′), INL1 (5′-GGGGGATCCAAGACGGTCTTAGGAAAAAC-3′), and INL2 (5′-TGAGCTGCAGTGAAGCTTCTTTTGAATTAT-3′) were used for the amplifications, in which 40 amplification cycles of 1 min at 94°C, 1 min at 55°C, and 3 min at 72°C, followed by a final extension at 72°C for 10 min, were used. The BamHI and PstI restriction sites included in the primers were used for directional cloning of the amplified fragments into the pQE9 plasmid vector (Qiagen, Hilden, Germany) by standard protocols (35). The final constructs were transformed into Escherichia coli strain M15(pREP4).
Antigen production.
The bacterial clones were grown at 30°C in Luria-Bertani broth containing 100 μg of ampicillin per ml and 25 μg of kanamycin per ml. Antigen production was induced by addition of 500 μg of isopropyl-β-d-thiogalactoside per ml and further incubation at 30°C for 4 h. The antigens were then purified under denaturing condition (8 M urea) with nickel-nitrilotriacetic acid resin (Qiagen) according to the recommendations of the manufacturer. The concentration of the antigens produced was measured with a protein assay dye reagent (Bio-Rad, Hercules, Calif.) and a bovine gamma globulin standard (Bio-Rad) according to the instructions of the manufacturer. The purities of the antigens were controlled by polyacrylamide gel electrophoresis and Coomassie blue staining, and the specificities of the serological reactions were controlled by immunoblotting with sera from cows with bacteriologically proven L. monocytogenes infections.
Bovine sera and epidemiological data.
A total of 1,652 serum samples representative of the healthy dairy cow population in Switzerland (13, 38) were used for the present study. A total of 1,547 of these serum samples originated from 107 farms for which epidemiological data were available (13, 38) and were used to detect risk factors associated with a positive listeria serology at the univariable level. The factors used for the latter analysis are listed in Table 1. A subset of 1,359 serum samples from animals for which complete data records for all the variables under investigation were available was used for a logistic regression analysis at the farm level. The number of dairy cows per farm ranged from 5 to 40 (median, 15 dairy cows). Between 4 and 36 serum samples (median, 14 serum samples) were tested per farm, and these corresponded to sera from 50 to 100% (median, 94%) of the animals present on each farm. Silage of diverse types (grass, 57%; corn, 45%; beet pulp, 14%; beet leaves, 1%) was fed to the dairy cows on 65 (61%) of the farms. A single type of silage was fed to the animals on 17% of the farms, whereas two or more different types of silage were fed to the animals on 44% of the farms.
TABLE 1.
Associations between independent variables and positive listeria serology at the farm levela
| Variable and description | Testb | P | OR (95% CI) |
|---|---|---|---|
| Farm and farmer | |||
| Herd size (no. of cows) | W | 0.172 | |
| Major breed on farmc,d | C | 0.025 | NAe |
| Sheep and/or goats on farm (yes or no) | C | 0.994 | |
| No. of workers on farm | C | 0.472<6Tc> | |
| Percent employment on farm for milk productiond | W | 0.043 | NA |
| Formal training as farmer (yes or no) | C | 0.524 | |
| Continuous education (yes or no) | C | 0.327 | |
| Animal environment and hygiene | |||
| Time in pasture (no. of days/yr) | W | 0.429 | |
| Tethering (yes or no) | C | 0.483 | |
| Stands for animals (long or short) | C | 0.117 | |
| Type of floor (rubber or other)d | C | 0.063 | 0.4 (0.2-1.1) |
| Type of bedding (straw or other) | C | 0.408 | |
| Cow trainer (yes or-sometimes or never)d | C | 0.055 | 0.4 (0.2-1.0) |
| Udder health | |||
| Milking system (no bucket or bucket) | C | 0.970 | |
| Teat dipping (yes or no) | C | 0.346 | |
| Systematic dry off with antibiotics (yes or no) | C | 0.444 | |
| Drying off with antibiotics only for mastitis (yes or no) | C | 0.805 | |
| Regular California mastitis testing (yes or no) | C | 0.950 | |
| Feed | |||
| Feeding of silage in general (yes or no)d | C | 0.000 | 4.7 (2.0-11.0) |
| Feeding of corn silage (yes or no) | C | 0.000 | 7.0 (3.0-16.4) |
| Feeding of grass silage (yes or no) | C | 0.014 | 2.7 (1.2-6.0) |
| Feeding of beet pulp silage (yes or no) | C | 0.087 | 2.7 (0.8-8.4) |
| Feeding of dry grass pellets (yes or no) | C | 0.622 | |
| Feeding of dry corn pellets (yes or no)d | C | 0.027 | 0.4 (0.2-0.9) |
| Feeding of cereal mix (yes or no) | C | 0.637 | |
| Feeding of malt (yes or no) | F | 0.595 | |
| Feeding of pressed beet pulp (yes or no) | F | 0.498 | |
| Feeding of fresh beet (yes or no)d | C | 0.009 | 0.3 (0.1-0.7) |
| Feeding of potatoes (yes or no) | C | 0.318 | |
| Feeding of dairy feed supplement (yes or no) | C | 0.618 | |
| Feeding of metabolic supplement (yes or no) | F | 0.184 | |
| Feeding of calcium and vitamin mix (yes or no) | C | 0.902 | |
| Feeding of propylene glycol as supplement (yes or no)d | C | 0.055 | 0.4 (0.2-1.0) |
The serological status of a farm was used for the analysis; a farm was considered positive if at least one animal was serologically positive for both listeriolysin O and internalin A simultaneously.
W, Wilcoxon rank sum test; C, chi-square test; F, Fisher exact test.
For farms (n = 4) with more than one breed, the major breed was taken into account for the analysis.
Factors used to start the backward elimination and forward inclusion procedures for the logistic regression models.
NA, not applicable.
In addition, 11 serum samples from animals with bacteriologically proven cases of listeriosis (1 serum sample from an animal with encephalitis, 4 serum samples from animals with abortions, 6 serum samples from animals with subclinical mastitis; see Table 2) and 38 serum samples from 31 additional animals with clinically suspected cases of cerebral listeriosis (32) registered at the clinics of the veterinary schools of Bern and Zurich, Switzerland, between December 1996 and February 2000 were tested.
TABLE 2.
Serological results obtained with sera from 11 animals with bacteriologically proven listeriosis
| Animal | Clinical form | No. of daysa | Intensity of reactionb
|
|
|---|---|---|---|---|
| Listeriolysin O | Internalin A | |||
| A | Cerebral | 2 | 0.33 (positive) | 0.20 (positive) |
| B | Abortion | 0 | 0.09 (negative) | 1.10 (positive) |
| C | Abortion | 1 | 0.03 (negative) | 0.23 (positive) |
| D | Abortion | 36 | 1.15 (positive) | 1.39 (positive) |
| E | Abortion | 71 | 1.39 (positive) | 1.30 (positive) |
| 113 | 1.46 (positive) | 1.28 (positive) | ||
| 244 | 1.38 (positive) | 1.30 (positive) | ||
| 420 | 1.17 (positive) | 1.23 (positive) | ||
| F | Subclinical mastitis | 133 | 0.63 (positive) | 0.54 (positive) |
| G | Subclinical mastitis | 133 | 0.69 (positive) | 0.29 (positive) |
| H | Subclinical mastitis | 133 | 0.86 (positive) | 0.16 (positive) |
| I | Subclinical mastitis | 133 | 0.31 (positive) | 0.55 (positive) |
| J | Subclinical mastitis | 133 | 0.39 (positive) | 0.42 (positive) |
| K | Subclinical mastitis | 133 | 0.30 (positive) | 0.25 (positive) |
Number of days between diagnosis and blood sampling.
The intensity of the reaction in comparison to that for a constant positive control, which had an intermediate intensity. The cutoff values for listeriolysin O and internalin A were 0.30 and 0.15, respectively.
ELISA.
Microtiter plates (type F flat-bottom ELISA plates; Petra-Labortechnik, Chur, Switzerland) were coated overnight at 4°C with 100-μl antigen solutions (2 μg/ml) in 0.1 M carbonate buffer (pH 9.6). After the plates were washed (0.9% NaCl, 0.05% Tween 20), blocked for 2 h at room temperature with BLOTTO-Tween buffer (19), and given a second washing, 100 μl of each serum sample that had been diluted 1/200 in BLOTTO-Tween was incubated in the wells for 3 h at room temperature. After the plates were washed, 100 μl of monoclonal anti-bovine immunoglobulin G alkaline phosphatase conjugate (Sigma, St. Louis, Mo.) diluted 1/20,000 in 50 mM Tris-150 mM NaCl-0. 05% Tween 20 (pH 7.5) was added to each well and the plate was incubated for 90 min at room temperature. After the plate was washed, the enzymatic reaction was developed by adding 100 μl of alkaline phosphatase substrate solution (Bio-Rad). The optical density at 405 nm was measured after 20 to 30 min on a Dynatech MR 5000 ELISA reader (Dynex Technologies, Chantilly, Va.). All serum samples were tested in duplicate. The results were all expressed in proportion to the results for a positive control with an intermediate intensity after subtraction of the results for a blank reaction without serum.
Statistical methods.
In order to ensure the specificity of the results, animals were considered positive only if they had positive serological results by both ELISAs with each of the antigens. Animals positive by only one ELISA or negative by both ELISAs were considered serologically negative. The statistical analyses were performed with Statistix (version 7) software for Windows (Analytical Software, Tallahassee, Fla.) and Number Cruncher Statistical Systems (NCSS) 2001 software (NCSS, Kaysville, Utah). Depending on the structures of the variables, the Wilcoxon rank sum test, the chi-square test, and Fisher's exact test was used for the univariable analysis. The logistic regression analyses were performed with NCSS 2001 software by using both a backward procedure and a forward procedure. Only the variables with significance levels of 10% or less were used at the start of these analyses. The thresholds for exclusion or inclusion in the final models were set at 5%. Interactions between the variables remaining in the final models were tested by separately adding single interaction terms into the model.
RESULTS
Repeatability and cutoff values.
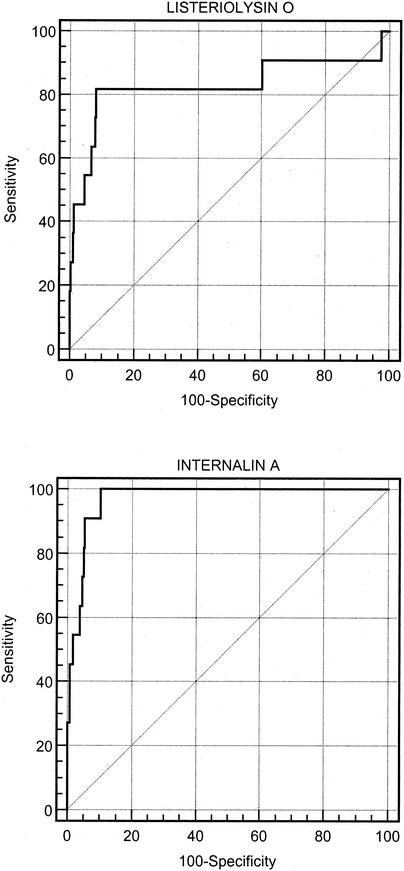
The coefficients of variation obtained with both antigens with one weakly positive serum sample and one strongly positive serum sample by repeated testing (n = 46) ranged between 7 and 12%. A receiver operating characteristic (ROC) curve analysis was performed to define the optimal cutoff values for the 11 serum samples from the dairy cows with bacteriologically proven listeriosis and the 580 serum samples from dairy cows not fed silage and representative of the healthy Swiss dairy cow population (Fig. 1). The areas under the curve were 0.83 (95% confidence interval [CI] = 0.80 to 0.86) and 0.97 (95% CI = 0.95 to 0.98) for listeriolysin O and internalin A, respectively. The optimal cutoff values defined for listeriolysin O and internalin A that minimized the numbers of misclassifications were 0.30 and 0.15, respectively. The estimated sensitivities and specificities were 81.8% (95% CI = 48.2 to 97.2%) and 91.9% (95% CI = 89.4 to 94.0%), respectively, for listeriolysin O, and 100% (95% CI = 100 to 100%) and 89.8% (95% CI = 87.1 to 92.2%), respectively, for internalin A. When the two tests were used in a serial testing procedure to increase the specificity, the resulting sensitivity and specificity were 81.8% (95% CI = 48.2 to 97.7%) and 97.1% (95% CI = 95.3 to 98.3%), respectively.
FIG. 1.
ROC curves for the listeriolysin O and internalin A ELISAs obtained with sera from 11 animals with bacteriologically proven cases of listeriosis and 580 serum samples from the healthy dairy cow population in Switzerland not fed any kind of silage.
Clinical listeriosis infections.
The serological results obtained for the 11 animals with bacteriologically proven listeriosis are presented in Table 2. A positive serology was obtained with internalin A for the serum samples from all four animals with abortions but was obtained with listeriolysin O only for the two serum samples collected from the animals several weeks after the abortions and not those collected at the time of abortion. A strongly positive serological result by tests with both antigens persisted for more than 1 year for one animal (Table 2, animal E). Positive results of variable intensity were obtained with the two antigens when sera from animals with subclinical listeria-related mastitis were tested. Only weakly positive results were obtained with the acute-phase serum from an animal with a bacteriologically proven case of listeria encephalitis.
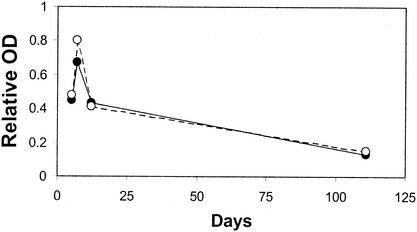
Five and 10 of 31 animals with clinical suspicion of listeria-related encephalitis gave positive results by assays with the listeriolysin O and internalin A antigens, respectively. The five listeriolysin O-positive serum samples were all simultaneously positive by the assay with the internalin A antigen. The brains of four animals with suspicion of listeria-related encephalitis were examined bacteriologically, and L. monocytogenes was isolated from only one of them. This animal was serologically weakly positive for both listeriolysin O and internalin A (Table 2, animal A), whereas the other three bacteriologically negative animals were all clearly serologically negative by the assays with both antigens at the time of entrance into the clinic (maximum values for listeriolysin O and internalin A, 0.02 and 0.05, respectively). The sera from the 31 animals (only the first serum sample collected from each animal was used) were not more frequently positive than the 1,652 serum samples from the healthy dairy cow population (P values, 0.60 for listeriolysin O and 0.11 for internalin A by Fisher's exact test and the chi-square test, respectively). However, the animals with encephalitis had significantly higher values by assays with the internalin A antigen than the healthy dairy cow population when the quantitative results of the ELISA were compared (P = 0.037 by the Wilcoxon rank sum test). The results obtained with successive serum samples from one of the animals for which there was a clinical suspicion of listeria-related encephalitis and that was successfully treated with massive doses of penicillin showed a short but clear increase in antibody titers toward both antigens followed by a rapid decrease in antibody titers toward both antigens (Fig. 2).
FIG. 2.
Serological follow-up of an animal with clinical suspicion of listeria-related encephalitis with successful treatment. White circles, results obtained with listeriolysin O; black circles, results obtained with internalin A. Days represent the number of days after the occurrence of the first neurological signs. The relative optical density is expressed in proportion to that for a constant positive control with intermediate intensity.
Risk factors associated with a positive serology at the farm level.
Among the 1,652 serum samples tested, 276 (16.7%), 423 (25.6%), and 182 (11.0%) were positive for listeriolysin O, internalin A, and both antigens simultaneously, respectively. Seventy-nine (70.5%), 82 (73.2%), and 54 (48.2%) of the farms had one or more animals positive for listeriolysin O, internalin A, and both antigens, respectively. A strong association was visible between the results obtained by the assays with the two antigens both at the animal level (odds ratio [OR] = 9.1; 95% CI = 6.9 to 12.1) and at the farm level (OR = 6.7; 95% CI = 2.7 to 16.8). The within-farm prevalence for simultaneous listeriolysin O and internalin A positivity varied from 0 to 65% (mean, 10%; median, 0%; third quartile, 14%).
The results of the univariable analysis and the statistical tests used are reported in Table 1. Thirty-three, 44, and 60% of the animals on farms with the Brown Swiss breed, the Holstein breed, and the Red Holstein or Simmental breed were positive, respectively. Animals on farms in which most of the work was dedicated to milk production rather than to other occupations were significantly less likely to be positive.
In addition, the forward and the backward procedures for the logistic regression analysis ended with the same multivariable model (Table 3, model 1). In the multivariable analysis the feeding of silage was confirmed to be a major risk factor. Two protective factors were identified. The first one was the Brown Swiss breed, and the second was the presence of a rubber mat on the floor. Among the variables used for the multivariable analyses, a strong correlation (OR = 11; P < 0.0001)—and therefore, some colinearity—was observed between the type of floor on which the animals were kept (rubber mat or other) and the use of a cow trainer (an electrical device used to force the animals to step back when urinating and defecating, thus keeping the bedding area and the animals clean). Thus, a second set of backward and forward analyses was performed after the “type of floor” was deleted from the starting variables list. Both the forward and the backward procedures ended with the same model with the three variables “silage,” “Brown Swiss breed,” and “cow trainer” (Table 3, model 2). Similarly to the use of rubber mats, the use of a cow trainer was identified as a significant protective factor. No significant interaction between the variables remaining in the final models was detected.
TABLE 3.
Variables associated with serological results in the logistic regression modela
| Variable | Model 1
|
Model 2
|
||
|---|---|---|---|---|
| Coefficient (P) | OR (95% CI) | Coefficient (P) | OR (95% CI) | |
| Constant | 0.57 (0.386) | 0.05 (0.916) | ||
| Feeding of silage | 1.87 (<0.001) | 6.51 (2.38-17.80) | 1.70 (<0.001) | 5.48 (2.12-14.17) |
| Brown Swiss breed | −1.29 (0.005) | 0.28 (0.11-0.69) | −1.32 (0.005) | 0.27 (0.11-0.66) |
| Rubber mat | −1.59 (0.024) | 0.20 (0.05-0.81) | ||
| Cow trainer | −1.03 (0.040) | 0.36 (0.13-0.95) | ||
Model 1, 75% of outcomes correctly predicted, the chi-square for likelihood ratio test of 27.34 (P < 0.001); model 2, 72% of outcomes correctly predicted, chi-square for likelihood ratio test of 25.96 (P < 0.001).
Type of silage.
Since grass and corn silage are used together on 41% of the farms, we used a Mantel-Haenszel procedure to separate the effects of each one and to check whether only one type of silage or both types of silage represent significant risk factors. No heterogeneity was detected between the strata. However, the corrected OR remained significant (OR = 7.86; P < 0.0001) only for corn silage and not for grass silage (OR = 0.76; P = 0.64). This lack of a significant association between grass silage and listeria serology on the one hand and the strong association between corn silage and listeria serology on the other was confirmed by running new backward and forward multivariate procedures after splitting of the variable silage into its three major components (corn silage, grass silage, and other types of silage). Only corn silage remained a significant risk factor (Table 4) at the end of this procedure.
TABLE 4.
Variables associated with serological results in the logistic regression model after splitting the variable “silage” into its different componentsa
| Variable | Model 1
|
Model 2
|
||
|---|---|---|---|---|
| Coefficient (P) | OR (95% CI) | Coefficient (P) | OR (95% CI) | |
| Constant | −0.92 (0.022) | 0.208 (0.668) | ||
| Feeding of corn silage | 2.12 (<0.001) | 8.35 (1.29-19.08) | 2.088 (<0.001) | 8.07 (3.08-21.12) |
| Brown Swiss breed | −1.03 (0.033) | 0.36 (0.14-0.92) | −1.085 (0.024) | 0.34 (0.13-0.86) |
| Rubber mat | −1.60 (0.020) | 0.20 (0.05-0.77) | ||
| Cow trainer | −1.219 (0.021) | 0.30 (0.10-0.83) | ||
Model 1, 72% of outcomes correctly predicted, chi-square for likelihood ratio test of 33.34 (P < 0.001); model 2, 74% of outcomes correctly predicted, chi-square for likelihood ratio test of 33.14 (P < 0.001).
DISCUSSION
L. monocytogenes is widespread in the environment, and a significant proportion of animals sporadically shed the organism in their feces or milk (10, 21, 23, 37, 40). Thus, many animals in the healthy dairy cattle population are expected to present antibodies toward L. monocytogenes, and a specific L. monocytogenes-negative control population is very difficult to obtain under field conditions. However, feeding of silage of poor quality has been identified in the past as the major source of L. monocytogenes infections in ruminants. Therefore, we chose to use animals of the healthy dairy cattle population not fed with any kind of silage as negative controls to set the cutoff values for our ELISA. Despite the limitations associated with this strategy, our two antigens performed well in the ELISA. The results obtained with recombinant listeriolysin O and internalin A were strongly correlated both at the animal level and at the farm level, thus giving credence to the specificity and validity of the positive serological reactions observed. As visible in the ROC curve analysis, the internalin A ELISA seemed to perform better than the listeriolysin O ELISA. Therefore, recombinant internalin A certainly represents a promising antigen for listeriosis serology.
Our results show that, similarly to anti-listeriolysin O antibodies (9, 10, 11), anti-internalin A antibodies are present in the sera of dairy cows suffering from subclinical listeria-related mastitis. Since L. monocytogenes is not excreted continuously in the milk of infected cows (10, 39), milk serology with internalin A is likely to represent an interesting diagnostic tool for such cases (11). High levels of specific anti-internalin A antibodies were also present in the sera of our animals with listeria-related abortions. They seemed to appear earlier than the anti-listeriolysin O antibodies and to persist for extended periods of time. Unfortunately, the sensitivity of our ELISA seems to be insufficient for simple use as a confirmatory diagnostic test in cases of listeria-related encephalitis. This finding is in agreement with the results of other researchers, who suggested that the humoral response in ruminants with encephalitis is poor (27). However, monitoring of a successfully treated animal showed that a clear but only short-lived elevation of titers of specific antilisteria antibodies of the immunoglobulin G subclass took place a few days after the appearance of the first clinical signs. This strongly suggests that, as for other infectious diseases, the aggressive antimicrobial treatment used in cases of listeria-related encephalitis limits the immune response of the host. Thus, treatment of the animals studied may have diminished their antilisteria humoral responses. In addition, only a very few of the suspected listeria-related encephalitis cases studied here could be confirmed by bacteriology and/or histology, and some of them may represent encephalitis of other etiologies. Therefore, the apparent low sensitivities of our tests for cases of encephalitis are not entirely surprising. The performances of the internalin A and listeriolysin O ELISAs for the diagnosis of listeria-related encephalitis in cattle could certainly be improved by monitoring the specific immune responses of the animals shortly after the onset of clinical signs.
When only the animals with elevated titers by the ELISAs with both antigens simultaneously were considered positive, approximately 11% of the dairy cows in Switzerland were found to be positive. Since antibodies are expected to persist in animals even after cessation of the antigenic stimulus, the seroprevalence observed here is in agreement with the slightly lower prevalence of L. monocytogenes in feces from dairy cattle obtained in other studies (22, 40). Interestingly, none of the variables related to control of udder health showed any association with the serological results. This finding is in agreement with the relative rarity of listeria-related mastitis (23).
Feeding of silage (particularly low-quality silage) has been known for a long time to be a risk factor for listeriosis (27). Our results identifying silage as a major risk factor for seropositivity were therefore expected. However, our analysis correcting for confounding factors evidenced differences in the risk associated with the different types of silage. We could demonstrate a very strong association (OR ≥ 8) between feeding of corn silage and seropositivity, but no association was found between consumption of grass silage and seropositivity when we corrected for confounding factors. Concerns about the safety of corn silage with regard to the presence of L. monocytogenes have recently been expressed (34). Even high-quality corn silage with a pH below 4.0 may contain L. monocytogenes, and in an outbreak of sheep listeriosis, the source of contamination was traced to cross contamination of food by corn silage and not directly to the grass silage fed to the animals (42). The findings presented in the previous reports together with our findings strongly suggest that corn silage may be more frequently or more heavily contaminated with L. monocytogenes and present a greater risk of infection than grass silage. Feeding of a few other feeds and feed additives was associated with serological status at the univariable level but did not reach significance in the multivariable analysis. These factors (feeding of dry corn pellets, fresh beets, metabolic supplements, and propylene glycol) may possibly be associated with particular conditions in the digestive tracts of the animals. Similarly to what has been suggested for verotoxigenic E. coli (33), such factors (particularly those aimed at controlling the fatty acid and carbohydrate contents of the rumen to avoid metabolic diseases) may indirectly affect the presence and/or survival of L. monocytogenes in the digestive tracts of cattle. Further studies on this topic will be necessary to confirm this hypothesis.
Dairy farms in Switzerland usually have only one cattle breed and are affiliated with specific breeders' associations with different geographical distributions and possibly different management practices. Consequently, the level of exposure to L. monocytogenes may vary between breeds, thus giving a likely explanation for the different levels of seropositivity between breeds observed here. Heritable host factors have been shown to play a significant role in susceptibility and the immune response to infectious agents (20, 30). The level of the humoral response following exposure to specific antigens in particular has been shown to be a heritable characteristic (41, 43). Therefore, similarly to the situation observed in cattle for susceptibility to trypanosomiasis (31), breed-associated genetic factors may also play a role in the immune response and possibly in the susceptibility of cattle to L. monocytogenes. Further studies on this topic are clearly warranted.
Two management factors (the use of rubber mats and the use of a cow trainer) were found to be significantly associated with serological results. Rubber mats and cow trainers help to keep the animals and their direct environment clean. Therefore, both of these factors may have a direct effect in reducing the risk of contamination of dairy cows with L. monocytogenes from the environment. They are probably also markers for hygiene in general at the farm level and for the awareness of the farmer about this criterion. A lack of hygiene and cleanliness has already been shown by others (36) to be an important factor associated with the contamination of milk with L. monocytogenes. The present study suggests that hygiene plays a role not only in the contamination of milk with this agent during or after milking but also in the general exposure of cows to this agent. Thus, the improvement of hygiene in the animal environment may represent a major factor in the control of L. monocytogenes contamination of milk at the preharvest level as well as at the harvest level.
In conclusion, our work shows that internalin A represents a promising new tool for listeriosis serology. We could also show differences between types of silage with regard to their potential role in the exposure of cattle to L. monocytogenes. Our results strongly suggest that not only the type of feed consumed but also hygiene practices may play an important role in reducing the level of exposure of the animals to this important agent of zoonosis. Finally, our results suggest that genetic factors may play a role in the immune response or in the susceptibility of cattle toward L. monocytogenes.
Acknowledgments
We thank J. Martig, U. Braun, M. Meylan, D. Camenzind, and the veterinary practitioners who helped us with the collection of sera from animals with clinical listeriosis. We thank K. Stärk for giving us access to the serum collection and epidemiological data for the healthy dairy cattle population. We also thank S. McEwen for reading of the manuscript and useful suggestions.
This project was supported by a grant from the Swiss Veterinary Office.
REFERENCES
- 1.Baetz, A. L., and I. V. Wesley. 1995. Detection of anti-listeriolysin O in dairy cattle experimentally infected with Listeria monocytogenes. J. Vet. Diagn. Investig. 7:82-86. [DOI] [PubMed] [Google Scholar]
- 2.Baetz, A. L., I. V. Wesley, and M. G. Stevens. 1996. The use of listeriolysin O in an ELISA, a skin test and a lymphocyte blastogenesis assay on sheep experimentally infected with Listeria monocytogenes, Listeria ivanovii or Listeria innocua. Vet. Microbiol. 51:151-159. [DOI] [PubMed] [Google Scholar]
- 3.Barbuddhe, S. B., S. V. S. Malik, S. P. Choudhary, and L. K. Gupta. 1998. Kinetics of interferon-gamma production and its comparison with anti-listeriolysin O detection in experimental bovine listeriosis. Vet. Res. Commun. 22:505-516. [DOI] [PubMed] [Google Scholar]
- 4.Barbuddhe, S. B., S. V. S. Malik, S. Bhatnagar, and L. K. Gupta. 1999. Cytotoxic T-cell, delayed type hypersensitive and listeriolysin O responses in experimental bovine listeriosis. Vet. Microbiol. 64:333-341. [DOI] [PubMed] [Google Scholar]
- 5.Barbuddhe, S. B., S. V. S. Malik, and L. K. Gupta. 2000. Kinetics of antibody production and clinical profiles of calves experimentally infected with Listeria monocytogenes. J. Vet. Med. Ser. B 47:497-502. [DOI] [PubMed] [Google Scholar]
- 6.Berche, P., K. A. Reich, M. Bonnichon, J. L. Beretti, C. Geoffroy, J. Raveneau, P. Cossart, J. L. Gaillard, P. Geslin, H. Kreis, and M. Veron. 1990. Detection of anti-listeriolysin O for serodiagnosis of human listeriosis. Lancet 335:624-627. [DOI] [PubMed] [Google Scholar]
- 7.Bille, J., J. Rocourt, and B. Swaminathan. 1999. Listeria, Erysipelothrix, and Kurthia, p. 346-356. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. ASM Press, Washington, D.C.
- 8.Boerlin, P., E. Bannerman, T. Jemmi, and J. Bille. 1996. Subtyping Listeria monocytogenes isolates genetically related to the Swiss epidemic clone. J. Clin. Microbiol. 34:2148-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bourry, A., and B. Poutrel. 1996. Bovine mastitis caused by Listeria monocytogenes: kinetics of antibody responses in serum and milk after experimental infection. J. Dairy Sci. 79:2189-2195. [DOI] [PubMed] [Google Scholar]
- 10.Bourry, A., B. Poutrel, and J. Rocourt. 1995. Bovine mastitis caused by Listeria monocytogenes: characteristics of natural and experimental infections. J. Med. Microbiol. 43:125-132. [DOI] [PubMed] [Google Scholar]
- 11.Bourry, A., T. Cochard, and B. Poutrel. 1997. Serological diagnosis of bovine, caprine, and ovine mastitis caused by Listeria monocytogenes by using an enzyme-linked immunosorbent assay. J. Clin. Microbiol. 35:1606-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farber, J. M., and P. I. Peterkin. 1991. Listeria monocytogenes, a food-borne pathogen. Microbiol. Rev. 55:476-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frei, C., P. P. Frei, K. D. C. Stärk, D. U. Pfeiffer, and U. Kihm. 1997. The production system and disease incidence in a national random longitudinal study of Swiss dairy herds. Prev. Vet. Med. 32:1-21. [DOI] [PubMed] [Google Scholar]
- 14.Fsihi, H., P. Steffen, and P. Cossart. 2001. Listeria monocytogenes, p. 751-803. In E. A. Groisman (ed.), Principles of bacterial pathogenesis. Academic Press, Inc., San Diego, Calif.
- 15.Gaillard, J. L., P. Berche, C. Frehel, E. Gouin, and P. Cossart. 1991. Entry of Listeria monocytogenes into cells is mediated by internalin, a repeat protein reminiscent of surface antigens from gram-positive cocci. Cell 65:1127-1141. [DOI] [PubMed] [Google Scholar]
- 16.Gellin, B. G., and C. V. Broome. 1989. Listeriosis. JAMA 261:1313-1320. [PubMed] [Google Scholar]
- 17.Gholizadeh, Y., C. Poyart, M. Juvin, J. L. Beretti, J. Croizé, P. Berche, and J. L. Gaillard. 1996. Serodiagnosis of listeriosis based upon detection of antibodies against recombinant truncated forms of listeriolysin O. J. Clin. Microbiol. 34:1391-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gholizadeh, Y., M. Juvin, J. L. Beretti, P. Berche, and J. L. Gaillard. 1997. Culture-negative listeriosis of the central nervous system diagnosed by detection of antibodies to listeriolysin O. Eur. J. Clin. Microbiol. Infect. Dis. 16:176-178. [DOI] [PubMed] [Google Scholar]
- 19.Harlow, E., and D. Lane. 1988. Antibodies, a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 20.Hill, A. V. 1998. The immunogenetics of human infectious diseases. Annu. Rev. Immunol. 16:593-617. [DOI] [PubMed] [Google Scholar]
- 21.Husu, J. R. 1990. Epidemiological studies on the occurrence of Listeria monocytogenes in the feces of dairy cattle. Zentbl. Veterinärmed. Sect. B 37:276-282. [DOI] [PubMed] [Google Scholar]
- 22.Iida, T., M. Kanzaki, T. Maruyama, S. Inoue, and C. Kaneuchi. 1991. Prevalence of Listeria monocytogenes in intestinal contents of healthy animals in Japan. J. Vet. Sci. 53:873-875. [DOI] [PubMed] [Google Scholar]
- 23.Jensen, N. E., F. M. Aarestrup, J. Jensen, and H. C. Wegener. 1996. Listeria monocytogenes in bovine mastitis. Possible implication for human health. Int. J. Food Microbiol. 32:209-216. [DOI] [PubMed] [Google Scholar]
- 24.Lhopital, S., J. Marly, P. Pardon, and P. Berche. 1993. Kinetics of antibody production against listeriolysin O in sheep with listeriosis. J. Clin. Microbiol. 31:1537-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Low, J. C., and W. Donachie. 1991. Clinical and serum antibody responses of lambs to infection by Listeria monocytogenes. Res. Vet. Sci. 51:185-192. [DOI] [PubMed] [Google Scholar]
- 26.Low, J. C., R. C. Davies, and W. Donachie. 1992. Purification of listeriolysin O and development of an immunoassay for diagnosis of listeric infections in sheep. J. Clin. Microbiol. 30:2705-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Low, J. C., and W. Donachie. 1997. A review of Listeria monocytogenes and listeriosis. Vet. J. 153:9-29. [DOI] [PubMed] [Google Scholar]
- 28.Mengaud, J., M.-F. Vincente, J. Chenevert, J. M. Pereira, C. Geoffroy, B. Gicquel-Sanzey, F. Vaquero, J. C. Perez-Diaz, and P. Cossart. 1988. Expression in Escherichia coli and sequence analysis of the listeriolysin O determinant of Listeria monocytogenes. Infect. Immun. 56:766-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miettinen, A., and J. Husu. 1991. Antibodies to listeriolysin O reflect the acquired resistance to Listeria monocytogenes in experimentally infected goats. FEMS Microbiol. Lett. 77:181-186. [DOI] [PubMed] [Google Scholar]
- 30.Morris, C. A. 2000. Genetics of susceptibility in cattle and sheep, p. 343-355. In R. F. E. Axford, S. C. Nicholas, and J. B. Owen (ed.), Breeding for disease resistance in farm animals. CAB International, Wallingford, Oxon, United Kingdom.
- 31.Naessens, J., A. J. Teale, and M. Sileghem. 2002. Identification of mechanisms of natural resistance to African trypanosiomasis in cattle. Vet. Immunol. Immunopathol. 87:187-194. [DOI] [PubMed] [Google Scholar]
- 32.Radostits, O. M., C. G. Gay, D. C. Blood, and K. W. Hinchcliff. 1999. Veterinary medicine, a textbook of the diseases of cattle, sheep, pigs, goats and horses. The W. B. Saunders Company Ltd., London, United Kingdom.
- 33.Russell, J. B., F. Diez-Gonzalez, and G. N. Jarvis. 2000. Invited review: effect of diet shifts on Escherichia coli in cattle. J. Dairy Sci. 83:863-873. [DOI] [PubMed] [Google Scholar]
- 34.Ryser, E. T., S. M. Arimi, and C. W. Donnelly. 1997. Effects of pH on distribution of listeria ribotypes in corn, hay, and grass silage. Appl. Environ. Microbiol. 63:3695-3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 36.Sanaa, M., B. Poutrel, J. L. Menard, and F. Serieys. 1993. Risk factors associated with contamination of raw milk by Listeria monocytogenes in dairy farms. J. Dairy Sci. 76:2891-2898. [DOI] [PubMed] [Google Scholar]
- 37.Skovgaard, N., and C. A. Morgen. 1988. Detection of Listeria spp. in faeces from animals, in feeds, and in raw foods of animal origin. Int. J. Food Microbiol. 6:229-242. [DOI] [PubMed] [Google Scholar]
- 38.Stärk, K. D. C., C. Frei-Stäheli, P. P. Frei, D. U. Pfeiffer, J. Danuser, L. Audigé, J. Nicolet, M. Strasser, B. Gottstein, and U. Kihm. 1997. Häufigkeit und Kosten von Gesundheitsproblemen bei Schweizer Milchkühen und deren Kälber (1993-1994). Schweiz. Arch. Tierheilkd. 139:343-353. [PubMed] [Google Scholar]
- 39.Stephan, R., D. Senczek, C. Müller, and C. Feusi. 2000. Isolierung von Listeria spp. und Aspergillus fumigatus—zwei Fallberichte aus der Mastitisdiagnostik. Schweiz. Arch. Tierheilkd. 142:387-390. [PubMed] [Google Scholar]
- 40.Unnerstad, H., A. Romell, H. Ericsson, M. L. Danielsson-Tham, and W. Tham. 2000. Listeria monocytogenes in faeces from clinically healthy dairy cows in Sweden. Acta Vet. Scand. 41:167-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wagter, L. C., B. A. Mallard, B. N. Wilkie, K. E. Leslie, P. J. Boettcher, and J. C. M. Dekkers. 1999. A quantitative approach to classifying Holstein cows based on antibody responsiveness and its relationship to peripartum mastitis occurrence. J. Dairy Sci. 83:488-498. [DOI] [PubMed] [Google Scholar]
- 42.Wiedmann, M., T. Arvik, J. L. Bruce, J. Neubauer, F. del Piero, M. C. Smith, J. Hurley, H. O. Mohammed, and C. A. Batt. 1997. Investigation of a listeriosis epizootic in sheep in New York State. Am. J. Vet. Res. 58:733-737. [PubMed] [Google Scholar]
- 43.Wilkie, B., and B. Mallard. 1999. Selection for high immune response: an alternative approach to animal health maintenance. Vet. Immunol. Immunopathol. 72:231-235. [DOI] [PubMed] [Google Scholar]