Abstract
To achieve strong adhesion to their neighbors and sustain stress and tension, epithelial cells develop many different specialized adhesive structures. Breakdown of these structures occurs during tumor progression, with the development of a fibroblastic morphology characteristic of metastatic cells. During Ras transformation, Rac-signaling pathways participate in the disruption of cadherin-dependent adhesion. We show that sustained Rac activation per se is sufficient to disassemble cadherin-mediated contacts in keratinocytes, in a concentration- and time-dependent manner. Cadherin receptors are removed from junctions before integrin receptors, suggesting that pathways activated by Rac can specifically interfere with cadherin function. We mapped an important region for disruption of junctions to the putative second effector domain of the Rac protein. Interestingly, although this region overlaps the domain necessary to induce lamellipodia, we demonstrate that the disassembly of cadherin complexes is a new Rac activity, distinct from Rac-dependent lamellipodia formation. Because Rac activity is also necessary for migration, Rac is a good candidate to coordinately regulate cell-cell and cell-substratum adhesion during tumorigenesis.
INTRODUCTION
Cell-cell adhesion is an essential feature of epithelia that ensures their polarized status and therefore their differentiation and physiological function. During tumorigenesis, the breakdown of intercellular adhesion has two main consequences: loss of epithelial characteristics and, as dedifferentiation proceeds, increased migration and metastasis of the dissociated cells. Cell-cell adhesion receptors of the cadherin family have been implicated in these cellular processes. First, it is well established that cadherin receptors play an important role in the development and maintenance of the differentiated epithelial phenotype during organogenesis and adult life (reviewed by Gumbiner, 1996). Second, cadherins participate in the contact inhibition of growth shown by nonimmortalized cells (St Croix et al., 1998) and alterations in cadherin function are frequently found, and have a causal role, during tumor progression (Perl et al., 1998).
Cadherins are transmembrane proteins that promote calcium-dependent intercellular adhesion between cells containing the same type of receptor (homophilic binding; Gumbiner, 1996). At the intracellular side, cadherin molecules associate with cytoplasmic proteins known collectively as catenins. At the surface of individual cells, it is believed that cadherin complexes are found as dimers in a lateral association mediated by their extracellular domains (Shapiro et al., 1995; Brieher et al., 1996; Nagar et al., 1996; Yap et al., 1997). Dimers from two opposing cells interact in an antiparallel manner (adhesive association) to form the structural unit of cadherin-mediated cell-cell adhesion (Chitaev and Troyanovsky, 1998). This adhesive interaction requires and is stabilized by extracellular calcium ions and, at the cytoplasmic side, by the association of cadherin receptors with the catenins and actin cytoskeleton (reviewed by Kemler, 1993; Yap et al., 1997; Chitaev and Troyanovsky, 1998). Although nonadhesive cadherin complexes can weakly interact with the cytoskeleton (Sako et al. 1998), the cytoskeletal interaction is greatly enhanced during the formation of cell-cell adhesion, by the clustering of the adhesive complexes at contact sites (reviewed by Kemler, 1993; Brieher et al., 1996; Yap et al., 1997; Chitaev and Troyanovsky, 1998).
Over the past few years, much effort has been put into understanding how cadherin function adhesion is regulated from the cytoplasm. Recently, we and others have demonstrated that cadherin-mediated adhesion requires the activity of the cytosolic proteins of the Rho family of small GTPases (Braga et al., 1997, 1999; Hordijk et al., 1997; Takaishi et al., 1997; Zhong et al., 1997). They belong to the Ras superfamily of small GTPases, proteins whose function is regulated depending on the type of guanine nucleotide bound (reviewed by Van Aelst and D'Souza-Schorey, 1997). When GTP is associated, the small GTPases are in an activated form and competent for signaling. Upon GTP hydrolysis and liberation of phosphate, the small GTPases are inactivated, in a cycle that is tightly modulated by regulatory proteins (Van Aelst and D'Souza-Schorey, 1997). The output signal is dependent on the amount of time that GTP remains associated as well as the localization of the GTP-bound protein within the cell (brought about by GDP-GTP exchange factors or GEF) (Bokoch et al., 1994; Michiels et al., 1997).
The RHO subfamily members, Rho, Rac, and Cdc42, participate in a variety of cellular processes primarily involving actin cytoskeleton reorganization, such as cell-cell adhesion, cell-extracellular matrix adhesion, cytokinesis, and cell motility (Van Aelst and D'Souza-Schorey, 1997). Rho is involved in cell contractility and stress fiber formation, whereas Rac drives actin polymerization and formation of lamellipodia (Ridley and Hall; 1992; Ridley et al., 1992). In epithelial cells, the activity of small GTPases is required both for the formation of new cadherin-mediated contacts and for the maintenance of stable junctions (Braga et al., 1997, 1999; Hordijk et al., 1997; Takaishi et al., 1997; Zhong et al., 1997). Rho and Rac effects on cadherin receptors are modulated by the maturation of the junctions and the cellular context in which the cadherin molecule is expressed (Braga et al., 1999).
In simple epithelial cells such as Madin-Darby canine kidney (MDCK), exogenously expressed myc-tagged Rho and Rac localize at sites of cell-cell adhesion (Adamson et al., 1992; Takaishi et al., 1995, 1997; Jou and Nelson, 1998). Moreover, proteins that can interact with the small GTPase Rac can also localize at intercellular junctions, but the functional significance of their localization is not known (reviewed by Van Aelst and D'Souza Schorey, 1997; Hordijk et al., 1997; Kuroda et al., 1998). In MDCK cells, Rac activation correlates with an increased staining of cadherin receptors and actin at cell-cell borders, suggesting that Rac may strengthen cadherin-dependent adhesion (Hordijk et al., 1997; Takaishi et al., 1997).
However, although Rac function is necessary for cadherin-dependent adhesion, there is evidence in the literature that Rac can play a role during tumor progression. Rac activation is required for the full transformed phenotype induced by oncogenes such as Tiam-1, Ras, and Mas (Habets et al., 1994; Khosravi-Far et al., 1995; Qiu et al., 1995; van Leeuwen et al., 1995; Roux et al., 1997; Zohn et al., 1998). In addition, it has been shown that Rac activation can promote invasion of carcinoma and lymphoma cell lines (Habets et al., 1994; Keely et al., 1997; Shaw et al., 1997). Although Rac clearly participates in cell migration, the question remains of how to reconcile its role in migration with the “strengthening” effect on cell-cell contacts.
In this article, we investigated in more detail the effects of Rac activation on the stability of cadherin receptors in human keratinocytes. Rac activation does not lead to increased levels of cadherin staining at the keratinocyte junctions, contrary to what has been shown for MDCK cells. Moreover, our results suggest that sustained Rac activation can specifically remove cadherin receptors from newly formed and stable cell-cell contacts in a concentration- and time-dependent manner. Interestingly, although the Rac-dependent loss of cadherin function was accompanied by changes in cell shape and protusion formation, we demonstrate that this is a new Rac activity, distinct from its reported cytoskeletal role in lamellipodia formation.
MATERIALS AND METHODS
Cells
Normal human keratinocytes (strain Kb, passages 3 to 7) were cultured on a mitomycin C-treated monolayer of 3T3 fibroblasts at 37°C and 5% CO2 as reported previously (Rheinwald, 1989). Cells were routinely cultured in standard medium (DMEM:F-12 medium, 1:3 mixture; Imperial Laboratories, Hampshire, United Kingdom) containing 1.8 mM calcium ions and supplements as described, but with 5% fetal calf serum. Cultures grown in the absence of calcium-dependent cell-cell contacts used the same medium formulation as described above, but with 0.1 mM calcium ions and serum depleted of divalent ions by treatment with Chelex-100 resin (Bio-Rad, Richmond, CA; Hodivala and Watt, 1994). HaCat cells (immortalized, nontumorigenic human keratinocytes) were a kind gift from N. Fusenig, Deutches Krebsforschungszentrum, Heidelberg, Germany (Ryle et al., 1989). In experiments in which the calcium switch was performed, HaCat cells were transferred to low-calcium medium (1–2 days after plating) and cultured until confluence as described above. Swiss 3T3 cells were routinely cultured as described previously (Ridley and Hall, 1992; Ridley et al., 1992); cells were allowed to reach confluence and become quiescent for 6–10 d before seeding onto coverslips.
Antibodies
E-cadherin staining was performed using either ECCD-2 antibody (rat monoclonal) (Hirai et al., 1989) or HECD-1 (mouse monoclonal; gift from M. Takeichi, Kyoto University, Japan; Shimoyama et al., 1989). Integrin labeling was done using the anti-β1 integrin antibody P5D2 (mouse monoclonal) (Dittel et al., 1993). The other monoclonal antibody used was anti-myc (mouse monoclonal 9E10). Secondary antibodies were bought from Jackson ImmunoResearch Laboratories, West Grove, PA (Stratech Scientific, Luton, United Kingdom): indodicarbocyanine (Cy5)-conjugated donkey anti-mouse IgG; fluorescein isothiocyanate-conjugated goat anti-mouse IgG and FITC-conjugated donkey anti-rat IgG. FITC-phalloidin was purchased from Sigma (Poole, United Kingdom).
Mutagenesis and Subcloning
Point mutations were introduced into constitutively active Rac (L61Rac) by polymerase chain reaction (PCR) with 5′ primers and 3′ primers containing the respective alanine-alanine substitutions in the putative second effector domain as follows: A147 A148 (lys147 glu148 converted to ala147 ala148, respectively; or using single letter code; KE to AA); A162 A163 (glu162 arg163 to ala162 ala163, respectively; QR to AA); A166 A167 (lys166 thr167 to ala166 ala167, respectively; KT to AA), and A170 A171 (asp170 glu171 to ala170 ala171, respectively; DE to AA). The PCR fragments were then subcloned as NcoI-EcoRI inserts into pGEX-2T-L61Rac. All L61Rac mutants were fully sequenced using the Stratagene kit.
L61Rac second effector domain mutants were PCR amplified and subcloned into the EcoRI/BamHI sites of the yeast two hybrid vector pYTH9. The constructs were sequenced to confirm that the Rac sequence was fused in frame with the sequence encoding the GAL4 DNA-binding domain. To create pACTII-NIQGAP2, the sequence corresponding to amino acids 711-1579 of IQGAP2 was amplified using the primers GTG CTA CAT CAT CAT CGG AAG AG and CCT TGA TTG GAG ACT TGA CC and subcloned into the NcoI-BamHI site of the GAL4-activation domain vector pACTII. Rac targets (ROK-α, MLK2, MLK3, and PAK) and RhoGAP subcloned into pACTII vector were a kind gift from Alan Hall (Aspenstrom and Olson, 1995; Nagata et al., 1998).
Recombinant Proteins
Recombinant proteins were purified as glutathione S-transferase-fusion proteins from Escherichia coli by using glutathione beads, thrombin cleaved (unless otherwise stated), dialysed, and concentrated essentially as described (Ridley et al., 1992). The protein concentration of each batch was determined by bicinchoninic acid assay (Pierce, Rockford, IL), by using bovine serum albumin as standard, and the purity of the preparation was evaluated by separation in SDS-PAGE followed by Coomassie blue staining. Biological activity was determined beforehand in fibroblasts and keratinocytes as reported (Ridley and Hall, 1992; Ridley et al., 1992; Braga et al., 1997).
Recombinant proteins used were as follows: C3 transferase (used at 0.1 mg/ml), constitutively active forms of Rac (L61Rac, 4 mg/ml), Rho (L63Rho, 3.76 mg/ml), or H-Ras (V12Ras, 3.77 mg/ml). RacRho chimeras used were as follows: Rac73Rho (3.35 mg/ml), Rac126Rho (0.53 mg/ml), Rac143Rho (0.43 mg/ml), and Rac175Rho (2.39 mg/ml). L61Rac second effector domain mutant recombinant proteins were also prepared (see above for details): A147 A148 (0.89 mg/ml), A162 A163 (2.26 mg/ml), A166 A167 (2.91 mg/ml), and A170 A171 (3.57 mg/ml). In addition to GST, the following proteins were used uncleaved: RhoGAP (1.84 mg/ml); ROK-α (GBD, GTPase binding domain only, 4.44 mg/ml; gift from David Drechsel, Heidelberg, Germany; Burbelo et al., 1995); PAK (GBD only, 4.42 mg/ml; Sander et al., 1998); MLK2 (leucine-zipper and GBD domain; Nagata et al., 1998); and POSH (GBD only, 2 mg/ml, kind gift from Anne Bishop (MRC-LMCB, London, UK); Tapon et al., 1998).
Microinjection
Microinjection was performed essentially as described (Braga et al., 1997). Confluent patches of keratinocytes grown in the absence of contacts were microinjected with the different recombinant proteins mixed with Dextran Texas-Red (Molecular Probes, Eugene, OR) to visualize the injected patches. Within 5 to 15 min after injection, cells were transferred to standard medium to induce calcium-dependent cell-cell contacts for additional 1 to 5 h. Alternatively, medium-sized colonies of keratinocytes cultured in standard medium (mature junctions) were injected with distinct recombinant proteins and incubated for different amounts of time in the same medium. Swiss 3T3 cells were seeded onto coverslips subconfluent and prepared for microinjection as reported (Puls et al., 1999). After microinjection, cells were incubated for 15 to 30 min in the same medium.
Recombinant proteins were injected either neat or at the stated dilutions to better assess their effects on junction disassembly in keratinocytes or lamellipodia formation in Swiss 3T3 cells. Quantification of the effects of Rac mutants on cadherin-mediated adhesion was performed using the following criteria. Patches containing three or more cell-cell borders with perturbed cadherin staining between at least two different injected cells were scored and expressed as a percentage of the total number of microinjected patches. Between 30 and 50 patches (containing 4 to 10 cells each) were analyzed for any given mutant. Quantification of lamellipodium formation in Swiss 3T3 cells is expressed as the percentage of injected cells with lamellipodia/ruffles. Between 60 and 180 injected cells (Swiss 3T3) were scored for each recombinant protein tested. Statistical analysis was performed using Student's t test, assuming unequal variances. Activated Rac DNA (L61Rac-pRK5myc; Lamarche et al., 1996) was microinjected into the nucleus of HaCat cells grown in low-calcium medium. After 2 h of expression, cells were transferred to standard calcium medium to induce junction formation for 4 h. Activated H-Ras (V12 Ras-pRK5myc) and dominant-negative Rac (N17Rac-pRK5myc) were injected in HaCat cells grown in standard medium (mature junctions) and incubated for 5 h. DNA was injected at 0.1 mg/ml.
Immunofluorescence
Cells were fixed in 3% paraformaldehyde for 10 min at room temperature, permeabilized, and stained as described previously (Braga et al., 1997). In some experiments, cells were extracted with CSK buffer containing 0.5% Triton X-100 for 10 min at room temperature before fixation (Braga et al., 1995). Single labeling for E-cadherin was performed by using the mouse monoclonal HECD-1 and FITC-conjugated anti-mouse IgG. Double labeling for cadherins and integrins was performed by sequential incubation with rat anti-E-cadherin monoclonal (ECCD-2), FITC-conjugated anti-rat IgG, followed by mouse anti-1 integrin antibody (P5D2), and Cy5-conjugated anti-mouse IgG. Stainining for myc-tagged proteins was performed using the mouse monoclonal 9E10 and Cy5-conjugated anti-mouse IgG. Filamentous actin in Swiss 3T3 cells was labeled with FITC-phalloidin. Confocal images were obtained (1-μm slices) at the plane in which the majority of cadherin staining in the injected patch was found and processed as reported (Braga et al., 1999). For the Dextran-Texas Red image, the optical section is taken at a different plane (usually a few microns below) to show that the cells are still touching each other and not retracted at the end of the experiment.
Slot Blots
Fusion proteins were immobilized onto PVDF membranes (Millipore, Bedford, MA) by using a slot blot apparatus (Hoeffer, San Francisco, CA). Equal amounts of L61Rac and L61Rac containing additional mutations in the second effector region (A162 A163, and A170 A171) were loaded with radioactive GTP ([γ-32P]GTP, 6000 Ci/mmol; NEN-DuPont, Boston, MA) and allowed to interact with the immobilized proteins as described (Lamarche et al., 1996).
Yeast Two-Hybrid Interactions
Integrated yeast strains were created containing L61Rac and the L61Rac second effector domain mutants fused to the GAL-4 DBD (Aspenstrom and Olson, 1995). Yeast strains were transformed with cDNAs encoding various Rac binding partners in a GAL4-activation domain vector: pACTII-RhoGAP, pACTII-PAK, pACTα-ROK-α (GTPase binding domain only), pACTII-IQGAP2, pACTII-MLK2, and pACTII-MLK3 (Nagata et al., 1998). Interactions were assayed by testing growth of colonies in the presence of 3-amino-1,2,4-triazole and by filter-lift β-galactosidase assay (Aspenstrom and Olson, 1995). IQGAP2 interactions were tested on 10 mM 3AT plates, as the association with activated Rac was barely detectable at the standard concentration used for the other targets (25 mM).
Mammalian Cell Transfections and c-Jun NH2-Terminal Kinase 1(JNK1) Activation Assay
Cos-7 cells were transfected by DEAE-dextran method as described (Lamarche et al., 1996). Plasmid amounts per 10-cm Petri dish were as follows: 5 μg of pCMV-FLAG-JNK1 with 1 μg each of pRK5myc, pRK5myc-RacL61, or the various RacL61 mutants. Twenty-four hours later, transfected cells were serum-starved for 16 h before lysis in 25 mM HEPES (pH 7.6), 1% (vol/vol) Triton X-100, 1% (wt/vol) sodium deoxycholate, 0.1% (wt/vol) SDS, 0.3 M NaCl, 50 mM NaF, 0.1 mM vanadate, 5 mM EDTA, 5 mM EGTA, 40 mM sodium pyrophosphate, and protease inhibitors. To quantitate the amount of JNK1 present in each experiment, one-tenth of each lysate was loaded onto 15% SDS-PAGE and transferred to nitrocellulose membrane. Flag-tagged JNK1 was visualized with an anti-FLAG monoclonal antibody (Sigma) and 0.1 Ci/ml protein A-125I and quantitated by phosphorimage analysis. An equal amount of JNK1 protein was loaded onto 7.5% SDS-PAGE and transferred to nitrocellulose membrane. Activated JNK1 was determined with an antiphospho-JNK1 (Thr 183/Thr185) monoclonal antibody (New England Biolabs, Beverly, MA) and protein A-125I and revealed by autoradiography. The relative levels of activated JNK1 were determined by phosphorimage analysis. To determine the amount of JNK1 in each lane, the membrane was stripped in 100 mM 2-mercaptoethanol, 2% SDS, 62.5 mM Tris-HCl, pH 6.7, at 50°C for 30 min and incubated with an anti-FLAG antibody and revealed by chemiluminescence.
RESULTS
A Role for Rac during Tumorigenesis
As suggested by experiments in MDCK cells, Rac activation may strengthen cadherin-dependent contacts because it induces an increased localization of the receptors and actin at junctions (Hordijk et al., 1997; Takaishi et al., 1997). To test this possibility in normal keratinocytes, L61Rac was microinjected into cells grown in standard medium (mature contacts) and incubated for 2 h (0.5 mg/ml, Figure 1, a and b). Under these conditions, the concentration of cadherin receptors at cell-cell contacts (Figure 1, a and b) and their detergent solubility (Figure 1, c and d) were not increased in the injected keratinocytes compared with neighboring cells.
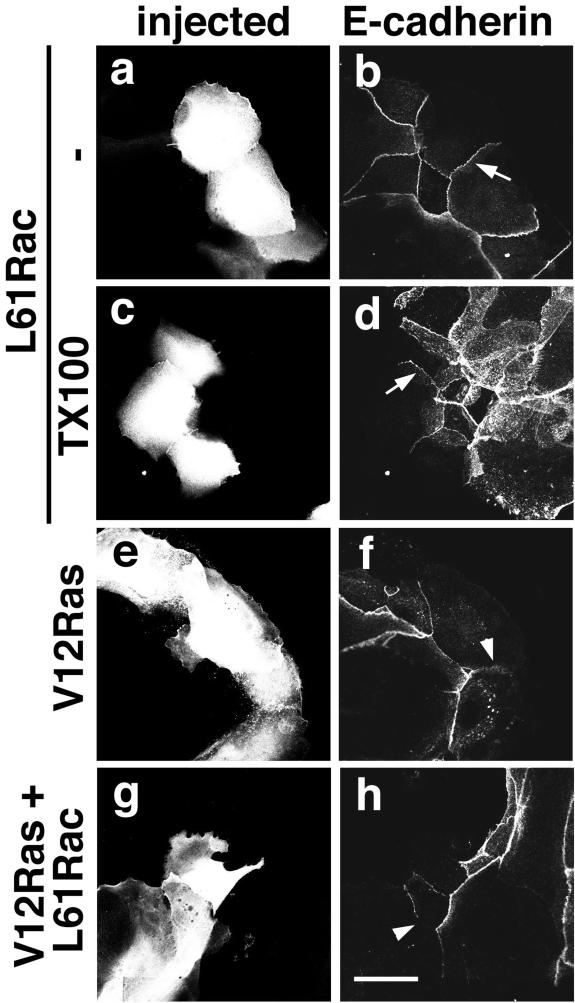
Figure 1.
Rac activation does not strengthen cadherin-dependent adhesion in keratinocytes. Cells containing mature intercellular junctions were microinjected with constitutively active Rac (L61Rac, 0.5 mg/ml). Activated Rac was injected either alone (a–d), or in combination with activated H-Ras (V12Ras + L61Rac, g and h). Controls show keratinocytes injected with H-Ras alone (V12Ras, e and f). After 2 h of incubation, cells were fixed and stained for E-cadherin (b, d, f, and h). In c and d, keratinocytes were preextracted with Triton X-100 before fixation (see MATERIALS AND METHODS). Microinjected patches of cells are seen in a, c, e, and g. Arrowheads (f and h) show absence of cadherin staining at junctions. Arrows (b and d) point to similar levels of cadherin staining in microinjected cells. Bar, 50 μm.
In addition, our results indicate that Rac activation was not sufficient to protect cadherin receptors from different destabilizing effects. One such stimulus is the expression of oncogenic Ras (V12H-Ras), which in keratinocytes interferes with stable cell-cell adhesion (Figure 1, e and f; our unpublished results; Espada et al., 1999). Controls showed that when injected alone, H-Ras disrupted cadherin adhesion within 2 h (Figure 1, e and f). This effect was not changed or delayed by coinjection of activated Rac (Figure 1, g and h). Similar results were observed if junctions were perturbed by inhibition of endogenous Rho (our unpublished results; Braga et al., 1997; Jou and Nelson, 1998). Our data support the conclusion that Rac activation does not increase the localization of cadherin receptors at junctions in normal human keratinocytes as shown in MDCK cell lines.
If Rac is not providing a protective effect for the keratinocyte junctions, what are the consequences of Rac activation? We asked whether Rac-signaling pathways could contribute to the destabilization of cell-cell adhesion seen during oncogenic transformation. Expression of activated H-Ras (V12Ras) in HaCat cells, a keratinocyte cell line, disrupted cadherin adhesion (Figure 2, d–f) similarly to what was observed in normal human keratinocytes (Figure 1, e and f). A dominant-negative form of Rac was expressed in HaCats to levels not high enough to perturb junctions (N17Rac, Figure 2, a–c). When N17Rac was coexpressed with activated Ras (V12Ras), a reduction in the signaling from Rac was sufficient to restore cadherin localization at cell-cell contacts (Figure 2, g–i). In contrast, inhibition of other pathways such as phosphoinositide 3-OH kinase (PI3 kinase) and mitogen-activated protein kinase (MAPK) during Ras activation in normal keratinocytes could only partially rescue the localization of cadherins at junctions (our unpublished results).
Figure 2.
Rac signaling pathways contribute to the destabilization of cadherin receptors after Ras activation. HaCat cells were microinjected with an expression vector containing dominant-negative Rac (N17Rac, a–c), oncogenic Ras (V12Ras, d–f), or both together (g–i). After 5 h, cells were fixed and double labeled for the myc tag (b, e, and h) and E-cadherin (c, f, and i). Injected patches were identified by coinjection of Dextran-Texas Red (a, d, and g). Arrows (c and i) point to the presence of cadherin staining at junctions; arrowhead (f) shows the absence of cadherin staining between two expressing cells. Bar, 50 μm.
Rac Activation Specifically Perturbs Cadherin-dependent Cell-Cell Contacts
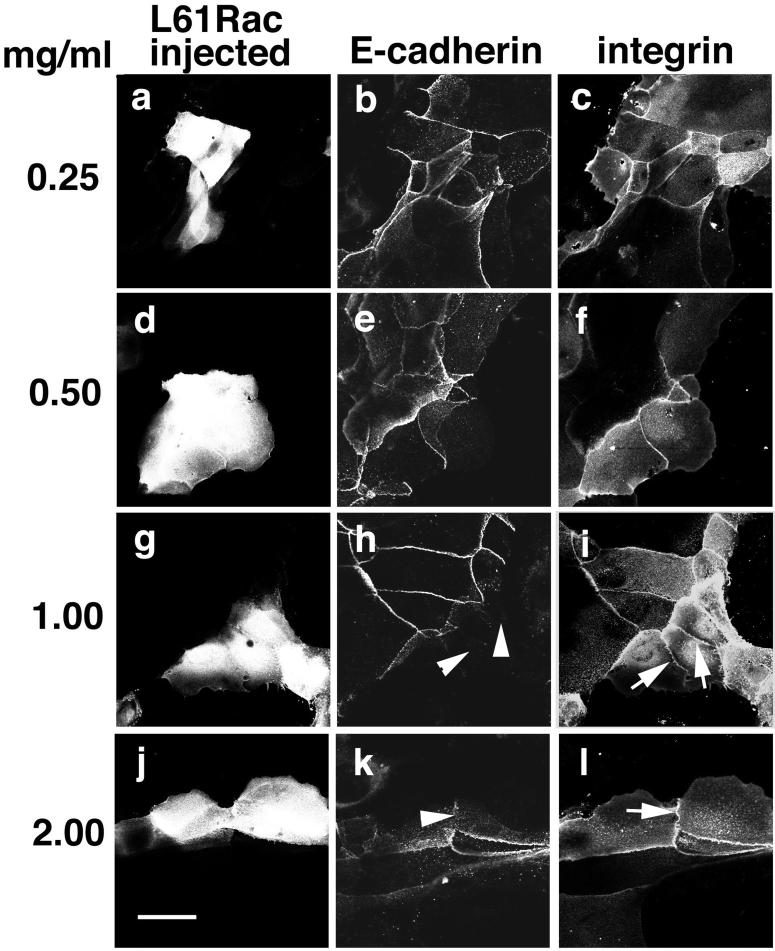
The above-mentioned results suggested that Rac can be activated during transformation and its activity may contribute to destabilization of junctions. We next addressed two questions: 1) whether Rac activation per se was sufficient to disrupt cadherin-dependent adhesion in human keratinocytes, and 2) whether Rac could specifically interfere with cadherin-mediated adhesion. Keratinocytes grown in standard medium (mature junctions) were microinjected with different concentrations of the same batch of activated Rac (L61Rac, 0.25–2.0 mg/ml, Figure 3) and incubated for 2 h. Cells were then fixed and double labeled for E-cadherin (Figure 3, b, e, h, and k) and β1-integrins (Figure 3, c, f, i, and l). With increasing concentrations of activated Rac, cadherin receptors were selectively removed from intercellular junctions (arrowheads, Figure 3), whereas localization of integrins remained unchanged over 2 h (arrows, Figure 3). Our data suggest that Rac activation can specifically destabilize cadherin receptors from mature cell-cell junctions in normal keratinocytes, because it had no significant effect on the localization of integrin receptors.
Figure 3.
Increased Rac activation perturbs the stability of cadherin receptors in mature junctions. Keratinocytes grown in standard medium (mature cell-cell contacts) were microinjected with different concentrations of constitutively active Rac (L61Rac) as follows: 0.25 mg/ml (a–c), 0.5 mg/ml (d–f), 1.0 mg/ml (g–i), and 2.0 mg/ml (j–l). After a 2-h incubation in the same medium, cell were fixed and double labeled for E-cadherin (b, e, h, and k) and integrins (c, f, i, and l). Injected cells are seen in a, d, g, and j. Arrows (i and l) show integrin staining at junctions; arrowheads (h and k) show absence of cadherin receptors at junctions. Bar, 50 μm.
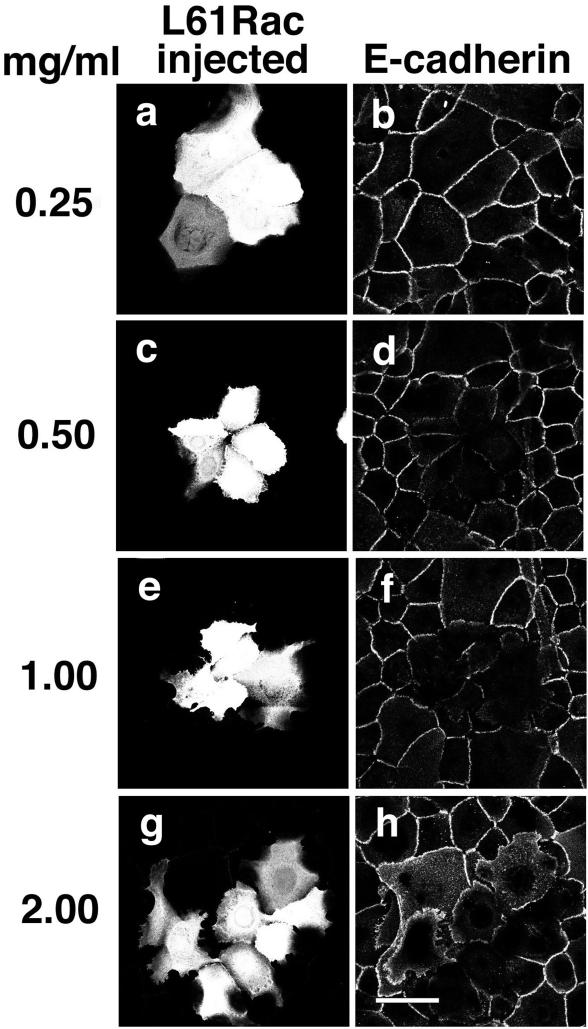
Activation of Rac Is Sufficient to Perturb Cadherin-dependent Adhesion in Human Keratinocytes
We have previously shown that newly formed junctions are more sensitive to the effects of the small GTPases (Braga et al., 1999). The above-mentioned results with Rac activation were also confirmed during induction of intercellular junctions. Keratinocytes grown in low-calcium medium were microinjected with different dilutions of the same batch of activated Rac (L61Rac, 0.25–2 mg/ml, Figure 4). Cell-cell adhesion was induced for 2 h and cells were labeled for E-cadherin (Figure 4, b, d, f, and h). Our results showed that at lower concentration, L61Rac activity was compatible with stable cell-cell contacts (0.25 mg/ml, Figure 4, a and b; Braga et al., 1997). However, increasing amounts of activated Rac clearly perturbed cadherin localization at junctions and cell morphology (0.5–2.0 mg/ml, Figure 4, c–h), with a concomitant formation of protusions and lamellae (Figure 4, e–h). Thus, lower concentrations of active Rac disrupted newly formed junctions (0.5 mg/ml) compared with the amount necessary to perturb mature junctions (1 mg/ml, Figure 3).
Figure 4.
Increasing concentrations of constitutively active Rac (L61Rac) perturb newly formed cell-cell adhesion and epithelial cell shape. Different concentrations of activated Rac (L61Rac) were microinjected into keratinocytes in the absence of intercellular contacts as follows: 0.25 mg/ml (a and b), 0.5 mg/ml (c and d), 1.0 mg/ml (e and f), and 2.0 mg/ml (g and h). Calcium-dependent cell-cell adhesion was induced for 2 h; cells were then fixed and labeled for E-cadherin, followed by an FITC-conjugated anti-mouse IgG (b, d, f, and h). Injected cells are seen in a, c, e, and g. Bar, 50 μm.
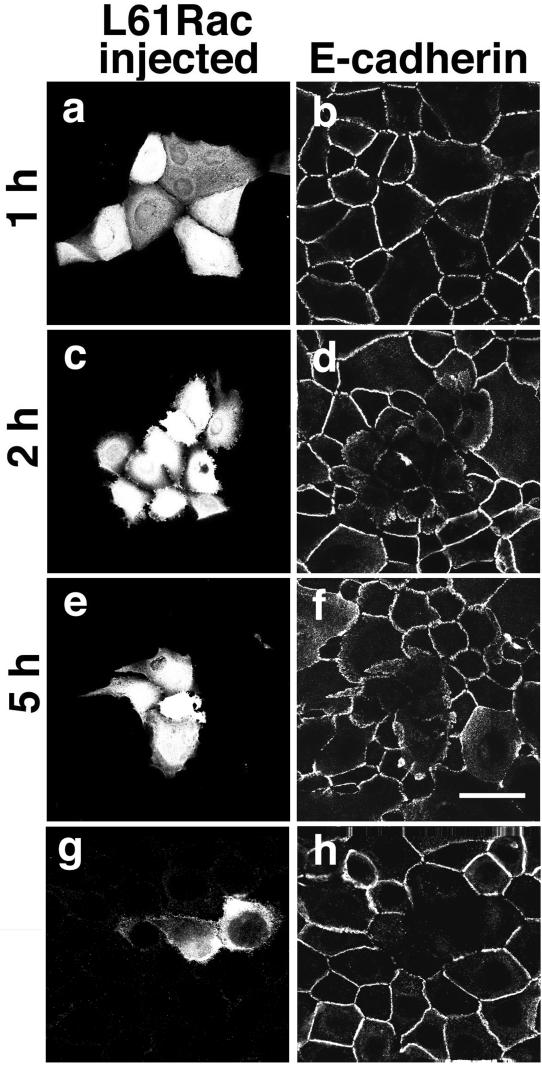
A time course was also performed by microinjecting L61Rac at a given concentration (0.5 mg/ml) into keratinocytes without cell-cell contacts, and transferring the cells to standard medium to induce intercellular adhesion for 1 to 5 h (Figure 5). Although a shorter incubation did not affect the cadherin staining or cell morphology (1 h, Figure 5, a and b), prolonged incubation after Rac activation interfered with both (2 and 5 h, Figure 5, c–f). These results are consistent with the data shown in Figure 4, and suggest that they did not result from toxicity of the more concentrated L61Rac protein solutions injected into keratinocytes.
Figure 5.
Sustained Rac activation interferes with the stability of newly formed cadherin-dependent contacts. Constitutively active Rac (L61Rac, 0.5 mg/ml) was microinjected into cells grown without contacts, and cadherin-dependent cell-cell adhesion was induced for 1 h (a and b), 2 h (c and d), or 5 h (e and f). Alternatively, L61Rac-pRK5myc expression vector was injected into the nucleus of HaCat cells (g and h), and after a 2-h expression, cell-cell contacts were induced for further 4 h. Cells were fixed and labeled for E-cadherin (b, d, f, and h) as stated in Figure 2; injected patches of cells are visualized in a, c, e, and g. In g, cells are labeled for the myc-tag epitope. Bar, 75 μm for g and h; 50 μm for all the other images.
To confirm the disruptive effect of activated Rac on junctions and exclude the contribution of any bacterial protein contaminants, we performed microinjections of L61Rac DNA into the nucleus. We were unable to obtain good expression levels when normal keratinocytes were used, despite testing a variety of different expression vectors. Instead, we used HaCat cells, a human keratinocyte cell line. After induction of cell-cell contacts for 4 h (total expression time 6 h), we observed a qualitative disruption of cadherin adhesion (Figure 5, g and h) as seen in primary keratinocytes (see also Figures 4, c–f, and 5, e and f). This result can be obtained with microinjection of either recombinant proteins or DNA encoding activated Rac in HaCat cells. Taken together, our data indicate that sustained Rac activation during junction formation resulted in changes in cell shape and E-cadherin localization in a time- and concentration-dependent manner.
Although the L61Rac effects on cadherin adhesion in mature junctions were similar to those observed when new cell-cell contacts were established, epithelial cell shape was not significantly perturbed when keratinocytes have stable junctions (Figures 3, j–l, and 4, g and h) and higher levels of active Rac were required to disrupt the intercellular contacts (Figures 3, d and f, and 4, c and d). The same differences were observed in HaCat cells: although formation of junctions was readily affected by expression of active Rac, it was more difficult to disrupt mature contacts (our unpublished observations). The reasons for the distinct effects of Rac on mature junctions versus newly formed junctions are not clear.
Rac Domain Important for Disruption of Cadherin-dependent Contacts
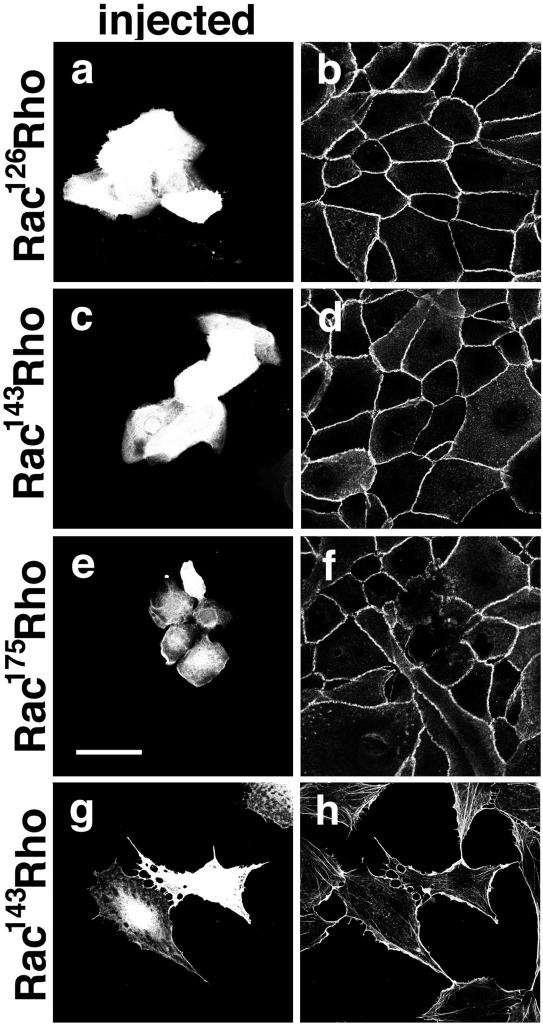
We next determined which domain in the Rac protein is required for its inhibitory activity on cadherin function. Different chimeric molecules containing an activated Rac N-terminal domain and Rho C-terminal domain were microinjected into keratinocytes or Swiss 3T3 fibroblasts (summarized in Figure 6 a; Diekmann et al., 1995; Kwong et al., 1995; Nisimoto et al., 1997). To demonstrate the purity of the microinjected proteins used in this study, the different recombinant proteins are shown in Figure 6b. After injection into keratinocytes without cell-cell contacts, intercellular junctions were induced for 4 h, and cells stained for E-cadherin (Figure 7, b, d, and f). As opposed to full-length activated Rac (0.5 mg/ml, Figure 5, e–h), activated Rho did not cause significant disruption of intercellular junctions at the concentration tested (0.5 mg/ml, our unpublished results). Rac73Rho (3.35 mg/ml, our unpublished results), Rac126Rho (0.53 mg/ml, Figure 7, a and b) and Rac143Rho (0.43 mg/ml; Figure 7, c and d) had no significant effect on cell shape or the localization of cadherin receptors. In contrast, Rac175Rho (2.39 mg/ml, Figure 7, e and f) interfered with cadherin stability and induced formation of lamellae in a similar manner to constitutively active, full-length Rac (0.5 mg/ml, Figure 5, e–h). Rac175Rho diluted to 1 mg/ml showed the same disruptive effect (our unpublished results). These results suggest that the Rac sequence between amino acid residues 143 and 175 was necessary to perturb cell-cell contacts.
Figure 6.
Mapping the Rac domain important for the disruption of cadherin-dependent adhesion. (a) Comparison of the Rac domains relevant for disruption of cadherin-mediated contacts in keratinocytes and lamella formation in Swiss 3T3 cells (Diekmann et al., 1995; data not shown). Rac protein (□), Rho protein (▪), and the small GTPase functional domains are represented: N-terminal effector domain 1, insert region, and the C-terminal effector domain 2. Chimeras containing portions of the Rac sequence (□) and Rho sequence (▪) are also shown: Rac73Rho (3.35 mg/ml); Rac126Rho (0.53 mg/ml); Rac143Rho (0.43 mg/ml); Rac175Rho (2.39 mg/ml) (see text for details). Activated Rac and Rho were tested at 0.5 mg/ml. (b) Recombinant proteins used in this study were separated in SDS-PAGE and visualized by Coomassie blue staining. Molecular weight markers are shown on the left (top to bottom): 36.5, 26, and 20 kDa.
Figure 7.
Different chimeric RacRho molecules were injected into keratinocytes grown in the absence of cell-cell contacts, and calcium-dependent adhesion was induced for 4 h (a–f). The following proteins were injected: Rac126Rho (a and b); Rac143Rho (c, d, g, and h); and Rac175Rho (e and f). Injected cells (a, c, e, and g) and E-cadherin staining (b, d, and f) are visualized. In g and h, Rac143Rho was injected into Swiss 3T3 fibroblasts and cells were stained for filamentous actin (h). Bar, 50 μm.
The chimeric molecules were also evaluated for their ability to induce ruffles by injection into serum starved Swiss 3T3 cells seeded onto fibronectin coverslips for 2 days (Puls et al., 1999). Under these conditions, Rac175Rho induced ruffles and lamellipodia as previously reported (Diekmann et al., 1995). However Rac143Rho was also able to promote lamellipodia formation (Figure 7, g and h; our unpublished results). A previous study did not demonstrate ruffling activity for Rac143Rho (Diekmann et al., 1995). It is not clear why, but we attribute this difference to the culturing conditions, which may affect the nature of the response. In the former study, microinjection was performed on freshly plated, serum-starved fibroblasts (2 h, Diekmann et al., 1995). In our study, we used cells seeded 48 h before in serum-free medium supplemented with 1/50 dilution of conditioned medium (Puls et al., 1999). It is also possible that the protein batch used in our study is more active than the batch used before. The data are summarized in Figure 6a and, taken together, indicate that the Rac domains responsible for lamella formation and cadherin disruption did not overlap completely.
Two Distinct Pathways?
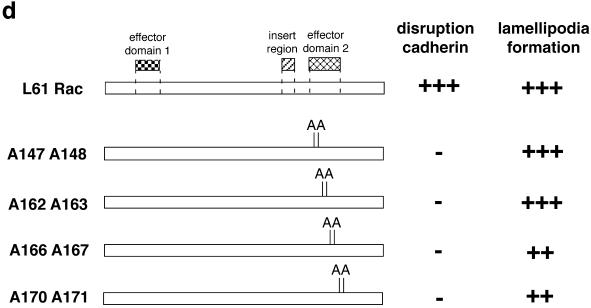
We confirmed the above-mentioned results by an alternative approach, site-directed mutagenesis. This approach allowed us 1) to dissociate between the Rac-induced lamellipodia activity and the Rac-dependent perturbation of cadherin adhesion, and 2) to map the important domain more precisely between amino acids 143 and 175. Mutations were introduced into L61Rac at different positions: A147 A148 (single letter code KE to AA); A162 A163 (QR to AA); A166 A167 (KT to AA); and A170 A171 (DE to AA) (see MATERIALS AND METHODS for details). These mutants were tested for their ability to disrupt cadherin-dependent contacts in keratinocytes or to induce lamella formation in Swiss 3T3 cells and representative pictures are shown in Figure 8. During formation of intercellular adhesion in keratinocytes, all the mutants showed qualitatively the same phenotype (but see below): no changes in cell morphology or significant decrease in cadherin staining at cell-cell borders in keratinocytes (Figure 8, a–d, and our unpublished results). Interestingly, induction of lamellipodia was not impaired in any of the mutants (as assessed by actin staining in fibroblasts, Figure 8, e–h; our unpublished results).
Figure 8.
Second effector domain analysis with respect to disruption of cadherin-dependent adhesion and lamella formation. Different positions within the putative second effector domain were mutated to alanine to generate four mutants in a constitutively active Rac background. Similar results were obtained for all mutants, and representative pictures are shown for the mutants A162 A163 (a, b, e, and f) and A170 A171 (c, d, g, and h). To evaluate disruption of cadherin function in keratinocytes (a–d), cell-cell contacts were induced for 4–5 h after microinjection, and cells were then fixed and stained for E-cadherin (b and d). The same mutants were also analyzed for their ability to induce formation of lamellae/ruffles in Swiss 3T3 cells, after staining with FITC-phalloidin (e–h). Bar, 50 μm.
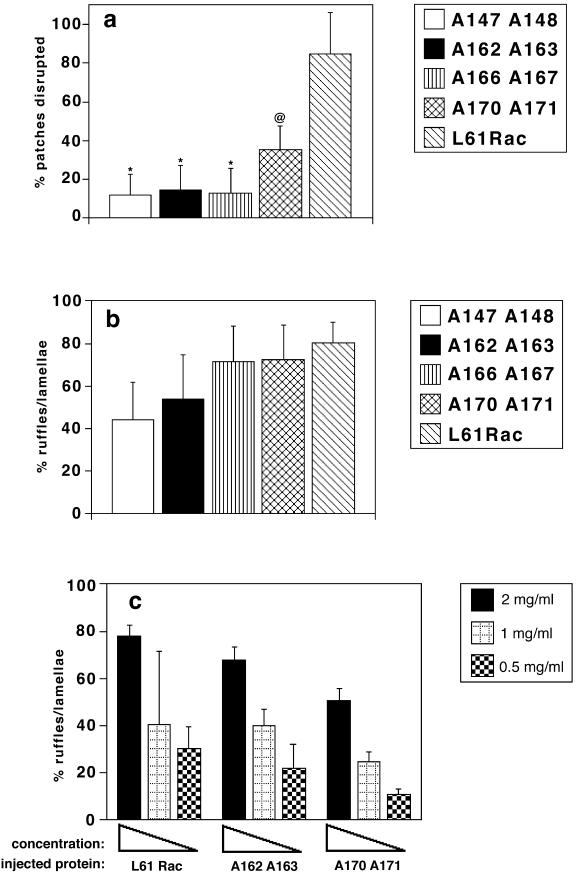
The quantification of these effects is shown in Figure 9. Control L61Rac at 0.5 mg/ml disrupted newly formed junctions in ∼85% of the injected patches after 4–5 h of incubation (Figure 9a). On the other hand, when L61Rac second effector domain mutants were injected at concentrations between 0.9 to 2.9 mg/ml, cadherin-dependent contacts were perturbed in only 10% of injected patches (Student's t test, p < 0.005). The mutant A170 A171 (3.5 mg/ml) was the exception, showing perturbed junctions in 35% of the patches (Student's t test, p < 0.01; Figure 9a). On the other hand, all mutants were able to induce lamellipodia to a similar extent as L61Rac (at 2 mg/ml), and no significant difference was detected (Figure 9b; Student's t test). Further experiments using dilutions of two of the mutants, A162 A163 and A170 A171, revealed that their ruffling activity could be titrated down in a similar pattern to L61Rac (Figure 9c). These results are summarized in Figure 9d and taken together suggest that disruption of cadherin adhesion and lamella formation are two independent activities triggered by Rac.
Figure 9.
Characterization of the Rac second effector domain mutants. (a) Quantification of the effects of the Rac mutants on cadherin-dependent adhesion. Patches of keratinocytes microinjected with the different mutants were scored for the presence of perturbed cadherin staining at intercellular junctions and expressed as a percentage of the total number of patches (see MATERIALS AND METHODS). (b) Quantification of the lamella-inducing activity of Rac mutants. Swiss 3T3 cells were injected with the different proteins and scored as a percentage of cells showing ruffles/lamellae. (c) Titration of lamellipodia formation induced by L61Rac, A162 A163, and A170 A171. The same amount of recombinant protein (2, 1, or 0.5 mg/ml) was injected into Swiss 3T3 cells and the percentage of injected cells with ruffles/lamellae were scored. (d) Summary of Rac mutants' ability to perturb cadherin adhesion in keratinocytes or induce lamellipodia in fibroblasts. A diagram representing constitutively active Rac containing the relevant domains (effector domains 1 and 2, insert domain), and the different mutants generated in the second effector domain. Unless otherwise stated, in the microinjection experiments mutants were used at the following concentrations: A147 A148, 0.89 mg/ml; A162 A163, 2.26 mg/ml; A166 A167, 2.91 mg/ml; and A170 A171, 3.57 mg/ml. L61Rac was tested at 0.5 mg/ml in keratinocytes and at 2 mg/ml in Swiss 3T3 cells. ∗p < 0.005; @p < 0.01 (Student's t test). Results are the mean of at least three independent experiments; Figure 9c shows the mean of at least two experiments for each concentration of distinct mutants. Error bars represent SD.
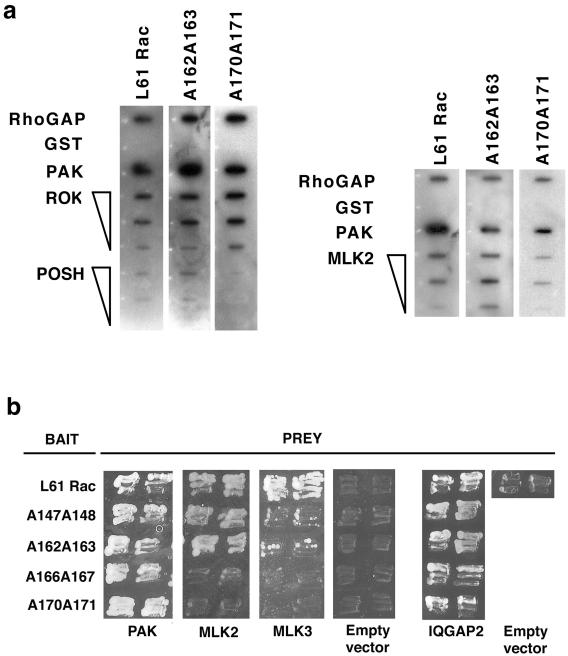
To assess whether the Rac mutants could interact with known Rac targets, in vitro binding assays were performed using recombinant proteins. Fusion proteins containing the GTPase binding domain of known Rac targets were immobilized onto membranes and probed with radioactively labeled Rac or the second effector domain mutants (Figure 10 a; Burbelo et al., 1995; reviewed by Van Aelst and D'Souza Schorey, 1997). In addition, interactions were tested by yeast two-hybrid technique and evaluated by growth on 3AT plates (Figure 10b and Table 1) or β-galactosidase filter assay (our unpublished results). Both techniques produced similar results: no binding was detected to the negative controls (GST, Figure 10a; empty vector, Figure 10b). All GTPases interacted similarly with RhoGAP, PAK, ROK-α, and IQGAP2 (Figure 10; our unpublished observation). Two Rac mutants (A147 A148 and A162 A163) were able to interact with POSH and MLK2. The only target that showed limited binding to all 4-s effector mutants is MLK3 (Table 1). In addition, with the exception of A170 A171, all other Rac mutants were able to activate the JNK kinase pathway (Table 1; our unpublished results). Because of the similar properties of L61Rac and second effector mutants, we concluded that these mutations did not affect the overall shape and activity of the mutants. Instead, the double alanine mutations interfered with the interaction with a particular subset of target(s) (among them MLK3).
Figure 10.
Binding properties of Rac mutants with known Rac targets. (a) In vitro interaction: L61Rac, A162 A163, and A170 A171 were labeled with radioactive GTP and allowed to interact with different Rac-binding proteins: GST-RhoGAP, GST-PAK, GST-ROK-α, GST-POSH, and GST-MLK2. Different amounts of recombinant protein were spotted onto the membranes (10, 5, or 1 μg). GST was used as a negative control; positive (RhoGAP and PAK) and negative controls were used at 10 μg. (b) Yeast two-hybrid interaction by using L61Rac or the second effector domain mutants as baits and the following targets as prey: PAK, MLK2, MLK3, and IQGAP2. Empty vector was used as a negative control. ∗, IQGAP interaction was tested at lower concentration of 3AT (10 mM) because the association with activated Rac was barely detectable with the standard concentration used for the other targets (25 mM). A summary of the yeast two-hybrid results with all the targets tested is shown in Table 1.
Table 1.
Characterization of the Rac second effector mutants
| Assay | Mutants
|
||||
|---|---|---|---|---|---|
| L61Rac | A147 A148 | A162 A163 | A166 A167 | A170 A171 | |
| JNK activation | + | + | + | + | − |
| Target binding | |||||
| RhoGAP | + | + | + | + | + |
| PAK | + | + | + | + | + |
| ROK-α | + | + | + | + | + |
| MLK2 | + | + | + | − | − |
| MLK3 | + | +/− | +/− | − | − |
| IQGAP2 | + | + | + | + | + |
| Empty vector | − | − | − | − | − |
L61Rac and the double alanine Rac mutants were evaluated for activation of the JNK pathway and their binding with distinct targets in the yeast two-hybrid assay. The Rac targets tested are RhoGAP, PAK, ROK-α, MLK2, MLK3 and IQGAP2: (−), negative; (+), positive; (+/−), weakly positive as revealed by growth in 3AT plates (see MATERIALS AND METHODS for details). Empty vector was used as a negative control.
DISCUSSION
In this article, we identified Rac as a key regulator of cadherin-mediated adhesion in human keratinocytes. Our major findings are as follows: Rac-signaling pathways contribute to the destabilization of cadherin receptors at junctions during Ras transformation in keratinocytes. During epithelial tumorigenesis, sustained levels of Rac activation can be achieved in vivo (Mira et al., 2000) and we demonstrate that Rac activation is sufficient to specifically disrupt cadherin-dependent adhesion. In addition, we demonstrate that perturbation of cell-cell contacts is a new Rac activity, distinct from lamellipodia formation. We mapped the putative second effector domain of Rac as an important domain for disruption of cadherin receptor localization, and produced mutants that can be useful tools to identify putative Rac targets. These results are discussed below.
Transfection of activated Rac in MDCK cells induces enhanced levels of cadherin and actin at junctions (Hordijk et al., 1997; Takaishi et al., 1997). In addition, transfection of Tiam-1, a Rac activator, can cause a reversion of the fibroblastoid morphology of Ras-transformed in MDCK (Hordijk et al., 1997; Sander et al., 1998). Contrary to the above-mentioned reports, Rac activation in normal human keratinocytes does not result in an increased localization or stability of cadherin receptors at cell-cell contacts. Our results are consistent with published data that Rac activation promotes cell-cell adhesion breakdown and migration of different carcinoma cells (Keely et al., 1997). In other epithelial cell lines, Rac activation also plays a role in scattering after distinct stimuli such as growth factor stimulation or integrin engagement (Takaishi et al., 1994; Ridley et al., 1995; Shaw et al., 1997; Potempa and Ridley, 1998; Gimond et al., 1999).
This controversy found in the literature might reflect the distinct cellular context, methodology used or different levels of Rac activation achieved. It is also conceivable that activation of Rac-dependent pathways by a constitutively active form (L61Rac) or via Tiam-1 might differ, because the latter also provides a localization signal (Sander et al., 1998; this work). More experimental work is necessary to understand and reconcile the distinct phenotypes produced by Rac activation in different cell types.
Nevertheless, it is clear that in keratinocytes, sustained Rac activation does not alter the detergent solubility of the receptors nor the amount of actin recruited to junctions (our unpublished results). If Rac activation is not promoting the localization of cadherin receptors to junctions, what are the consequences of Rac activity? In keratinocytes, when junction stability is challenged by either Rho inhibition (our unpublished results) or H-Ras activation, coinjection of actived Rac cannot protect cadherin receptors from these destabilizing stimuli (Braga et al., 1997). Indeed, Rac activation is necessary for the disassembly of cadherin contacts induced by the oncogene H-Ras. These results are also in agreement with published data, in which Rac activation contributes to the H-Ras-dependent perturbation of cell-cell contacts in breast cancer cell lines (Quillan, 1999). In keratinocytes, we demonstrate that inhibition of Rac signaling pathways prevents the Ras-dependent perturbation of cell-cell adhesion, whereas blocking PI3 kinase and mitogen-activated protein kinase pathways have only a partial effect (our unpublished results).
Rac Can Specifically Destabilize Cadherin-dependent Adhesion
A previous report has shown activation of Rac 3 in breast cancer cells, suggesting that Rac activation during tumorigenesis might be a widespread process (Mira et al., 2000). Together with our results, the above-mentioned results demonstrate that activation of Rac can occur after transformation and that this pathway may contribute to the Ras-dependent disassembly of junctions. Moreover, our data show that Rac activation is sufficient to disassemble cadherin-dependent contacts in a time- and concentration-dependent manner in human keratinocytes. Activated Rac specifically interferes with the localization of cadherin but not integrin receptors in the time frame examined. Disruption of cadherin adhesion is observed in both newly formed and mature junctions, but it is more clearly seen during the induction of cell-cell adhesion (see RESULTS). However, formation of new cell-cell contacts is not prevented by activated Rac, but rather the stability of cadherin receptors at intercellular contacts is compromised (after 1 h).
Our results that Rac activation may promote junction disassembly are intriguing. It is possible that, as for other biological stimuli, the cellular response to Rac activation may follow a bell-shaped curve: too little Rac is inhibitory to junctions as is too much Rac activity. We think that the Rac destabilization of junctions is unlikely to be a nonspecific effect of bacterial contaminants or overexpression for the following reasons: 1) the same effect is obtained by expression of activated Rac DNA in HaCat cells; 2) microinjection of distinct proteins at much higher concentration cannot disrupt cell-cell contacts; and 3) injected keratinocytes do not show any signs of apoptosis (i.e. annexin V staining, our unpublished results). In addition, there is specificity in the response because cadherin molecules are removed from junctions before other cell-cell adhesion receptors.
Rac activation perturbs cadherin contacts with a concomitant change in cell shape, including formation of lamellae/protusions and a clear conversion to a more fibroblastic morphology. Although lamellae are also present upon Rac activation in keratinocytes containing mature junctions, the change in cell shape is not observed over a 2-h incubation. Interestingly, levels of Rac activation that disrupt newly formed cadherin contacts very efficiently (0.5 mg/ml, 85% of injected patches) can only induce ruffling in 30% of injected fibroblasts.
Both lamella formation and cadherin adhesion require actin polymerization dependent on Rac activity (Machesky and Hall, 1997; Braga et al., 1999). Because our data suggest that lamella formation may antagonize cadherin-mediated adhesion, two possibilities can be envisaged. First, induction of lamellipodia may cause the destabilization of cadherin receptors at junctions, or second, lamella formation and perturbation of cadherin adhesion may be two independent activities triggered by Rac. Our results support the latter possibility because we are able to dissect these two Rac activities.
Important Domain in the Rac Molecule for Interfering with Cadherin-dependent Contacts
Previous work has identified three functional domains in the Rho subfamily of small GTPases: the N-terminal effector domain, an insert region, and a putative C-terminal effector domain (Diekmann et al., 1995; Joseph and Pick, 1995; Kwong et al., 1995; Nisimoto et al., 1997). We restricted an important region for destabilizing intercellular junctions to the putative second effector domain of Rac, between amino acids 143 and 175. This domain overlaps with, but is not identical to, the domain necessary to induce lamellae (Diekmann et al., 1995; our unpublished results). In addition, by mutating specific amino acids within residues 143 and 175 of activated Rac, we obtained mutants that are impaired in their ability to perturb cadherin adhesion in keratinocytes, but are still able to promote ruffling and lamellipodia in Swiss 3T3 cells.
Characterization and quantification of these effects indicate that the second effector domain mutants (A147 A148, A162 A163, A166 A167, and A170 A171) showed around fourfold reduction in the destabilization of cell-cell adhesion compared with L61Rac, in spite of being microinjected at much higher concentration (2- to 8-fold more concentrated). We think it unlikely that the double alanine mutations interfere with the overall stability or activity of the Rac molecule for the following reasons. First, like constitutively active Rac, all second effector domain mutants were able to induce lamellae. Normalization of their concentrations and microinjection of dilutions produced a proportional decline in their ability to induce lamellae, as for L61Rac. Second, their GTP binding ability was not significantly perturbed (our unpublished results). Finally, the mutants can associate with RhoGAP and other Rac targets such as PAK, ROK-α, and IQGAP2 to a similar extent as activated Rac (reviewed by Van Aelst and D'Souza-Schorey, 1997).
To our knowledge, this is the first report showing extensive mutagenesis analysis of the putative second effector domain. This domain forms an exposed loop in the Rac three-dimensional structure, suggesting good access for target interaction (Hirshberg et al., 1997). Interestingly, the binding of the small GTPases Rac and Cdc42 to distinct targets also requires and is stabilized by residues within the second effector domain (Abdul-Manan et al., 1999, Mott et al., 1999; Tolias et al., 2000).
In an attempt to investigate the mechanism by which Rac activation may disturb cadherin function, preliminary results show that new protein synthesis is not required. The process does not involve down-regulation of Rho (Izawa et al., 1998, Rottner et al., 1999; Sander et al., 1999; van Leeuwen et al., 1999; Zondag et al., 2000) nor does Rac activation interfere with the recycling compartment in keratinocytes (our unpublished results; Lamaze et al., 1996). Together with our analyses of putative Rac targets, these results suggest that Rac is able to activate specific pathways that perturb the stability of cadherin receptors at the keratinocyte junction.
If this hypothesis is true, the Rac second effector domain mutants can be useful tools to identify which pathway is important for junction disassembly. Two possibilities can be envisaged. First, the mutants may display a reduced binding to specific targets. Alternatively, the binding interactions may be the same, but the ability to activate the target is compromised. We began to test these two possibilities with known Rac targets. We found that at least activation of the JNK pathway is not affected by the double alanine mutations in the second effector domain. By screening Rac targets for their ability to differentially interact with activated Rac and the second effector mutants, we identified MLK3 (mixed lineage kinase 3) as a putative effector candidate (Burbelo et al., 1995; Nagata et al., 1998; Hartkamp et al., 1999) because it shows reduced binding to all mutants tested. We are currently performing experiments to address the question of whether MLK3 activation per se is sufficient to disturb cadherin-dependent adhesion.
In summary, we demonstrate that Rac is a key regulator of cadherin-dependent cell-cell contacts as sustained Rac activation is sufficient to destabilize normal keratinocyte junctions. An important question that remains to be addressed experimentally is the threshold level of Rac activation that is necessary to perturb cell adhesion during tumor progression. However, it is conceivable other pathways triggered by oncogenes may cooperate with Rac to promote cytoskeletal changes and junction breakdown. Because Rac plays an important role in cell migration, our study sheds light on the biological problem of how cells are able to integrate cell-cell and cell-substratum adhesion during tumorigenesis. Moreover, our data suggest that downstream signaling pathways activated by Rac could be potential therapeutical targets for preventing cell-cell disassembly.
ACKNOWLEDGMENTS
We thank Alan Hall for continuous support and encouragement. We also thank M. Takeichi, D. Kwiatkowiski, and Anne Bishop for generous gifts of antibodies and recombinant proteins; J. Collard, H. Daub, and David Dreschel for plasmids; N. Fusenig for sending cell lines; and Axel Puls and Lars Kjoller for Swiss 3T3 cells and advice on how to grow them. M.B. is in the Medical Research Council Graduate Program at the Medical Research Council Laboratory for Molecular Cell Biology. V.M.M.B is supported by Cancer Research Campaign. N.L.-V. is a Junior Scholar from Fonds de la Recherche en Santé du Québec.
REFERENCES
- Abdul-Manan N, Aghazadeh B, Liu GA, Majumdar A, Ouerfelli O, Siminovitch KA, Rosen MK. Structure of Cdc42 in complex with the GTPase-binding domain of the ‘Wiskott-Aldrich syndrome’ protein. Nature. 1999;399:379–383. doi: 10.1038/20726. [DOI] [PubMed] [Google Scholar]
- Adamson P, Paterson HF, Hall A. Intracellular localization of the p21rho proteins. J Cell Biol. 1992;119:617–627. doi: 10.1083/jcb.119.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspenstrom P, Olson MF. Yeast two-hybrid system to detect protein-protein interactions with Rho GTPases. Methods Enzymol. 1995;256:228–241. doi: 10.1016/0076-6879(95)56027-0. [DOI] [PubMed] [Google Scholar]
- Bokoch GM, Bohl BP, Chuang T. Guanine nucleotide exchange regulates membrane translocation of Rac/Rho GTP-binding proteins. J Biol Chem. 1994;269:31674–31679. [PubMed] [Google Scholar]
- Braga VMM, Del Maschio A, Machesky L, Dejana E. Regulation of cadherin function by Rho and Rac: Modulation by junction maturation and cellular context. Mol Biol Cell. 1999;10:9–22. doi: 10.1091/mbc.10.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga VMM, Hodivala KJ, Watt FM. Calcium-induced changes in distribution and solubility of cadherins and their associated cytoplasmic proteins in human keratinocytes. Cell Adhes Commun. 1995;3:201–215. doi: 10.3109/15419069509081287. [DOI] [PubMed] [Google Scholar]
- Braga VMM, Machesky LM, Hall A, Hotchin NA. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell-cell contacts. J Cell Biol. 1997;137:1421–1431. doi: 10.1083/jcb.137.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brieher WM, Yap AS, Gumbiner BM. Lateral dimerization is required for the homophilic binding activity of C-cadherin. J Cell Biol. 1996;135:487–496. doi: 10.1083/jcb.135.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbelo PD, Drechsel D, Hall A. A conserved binding motif defines numerous candidate target proteins for both Cdc42 and Rac GTPases. J Biol Chem. 1995;270:29071–29074. doi: 10.1074/jbc.270.49.29071. [DOI] [PubMed] [Google Scholar]
- Chitaev NA, Troyanovsky SM. Adhesive but not lateral E-cadherin complexes require calcium and catenins for their formation. J Cell Biol. 1998;142:837–846. doi: 10.1083/jcb.142.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekmann D, Nobes CD, Burbelo PD, Abo A, Hall A. Rac GTPase interacts with GAPs and target proteins through multiple effector sites. EMBO J. 1995;14:5297–5305. doi: 10.1002/j.1460-2075.1995.tb00214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittel BN, McCarthy JB, Wayner EA, LeBien TW. Regulation of human B-cell precursor adhesion to bone marrow stromal cells by cytokines that exert opposing effects on the expression of vascular cell adhesion molecule-1 (VCAM-1) Blood. 1993;81:2272–2282. [PubMed] [Google Scholar]
- Espada J, Perez-Moreno M, Braga VMM, Rodriquez-Viciana P, Cano A. H-Ras activation promotes cytoplasmic accumulation and phosphoinositide 3-OH kinase association of β-catenin in epidermal keratinocytes. J Cell Biol, 1999;146:967–980. doi: 10.1083/jcb.146.5.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimond C, van der Flier A, van Delft A, Brackebusch C, Kuikman I, Collard JG, Fassler R, Sonnenberg A. Induction of cell scattering by expression of β1 integrins in β1-deficient epithelial cells requires activation of members of the Rho family of GTPases and down regulation of cadherin and catenin function. J Cell Biol. 1999;147:1325–1340. doi: 10.1083/jcb.147.6.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- Habets GGM, Scholtes EHM, Zuydgeest D, van der Kammen RA, Stam JC, Berns A, Collard JG. Identification of an invasion-inducing gene, Tiam-1, that encodes a protein with homology to GDP-GTP exchangers for Rho-like proteins. Cell. 1994;77:537–549. doi: 10.1016/0092-8674(94)90216-x. [DOI] [PubMed] [Google Scholar]
- Hartkamp J, Troppmair J, Rapp UR. The JNK/SAPK activator mixed lineage kinase 3 (MLK3) transform NIH 3T3 cells in a MEK-dependent fashion. Cancer Res. 1999;59:2195–2202. [PubMed] [Google Scholar]
- Hirai Y, Nose A, Kobayashi S, Takeichi M. Expression and role of E- and P-cadherin adhesion molecules in embryonic histogenesis. II. Skin morphogenesis. Development. 1989;105:271–277. doi: 10.1242/dev.105.2.271. [DOI] [PubMed] [Google Scholar]
- Hirshberg M, Stockley RW, Dodson G, Webb MR. The crystal structure of human rac1, a member of the rho-family complexed with a GTP analogue. Nat Struct Biol. 1997;4:147–151. doi: 10.1038/nsb0297-147. [DOI] [PubMed] [Google Scholar]
- Hodivala KJ, Watt FM. Evidence that cadherins play a role in the downregulation of integrin expression that occurs during keratinocyte terminal differentiation. J Cell Biol. 1994;124:589–600. doi: 10.1083/jcb.124.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hordijk PL, ten Klooster JP, van der Kammen RA, Michiels F, Oomen LC, Collard JG. Inhibition of invasion of epithelial cells by Tiam 1-Rac signaling. Science. 1997;278:1464–1467. doi: 10.1126/science.278.5342.1464. [DOI] [PubMed] [Google Scholar]
- Izawa I, Amano M, Chihara K, Yamamoto T, Kaibuchi K. Possible involvement of the inactivation of the Rho-Rho-kinase pathways in oncogenic Ras-induced transformation. Oncogene. 1998;17:2863–2871. doi: 10.1038/sj.onc.1202213. [DOI] [PubMed] [Google Scholar]
- Joseph G, Pick E. “Peptide walking” is a novel method for mapping functional domains in proteins. Its application to the Rac1-dependent activation of the NAPDH oxidase. J Biol Chem. 1995;270:29079–29082. doi: 10.1074/jbc.270.49.29079. [DOI] [PubMed] [Google Scholar]
- Jou TS, Nelson WJ. Effects of regulated expression of mutant RhoA and Rac1 small GTPases on the development of epithelial (MDCK) cell polarity. J Cell Biol. 1998;142:85–100. doi: 10.1083/jcb.142.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keely PJ, Westwick JK, Whitehead IP, Der CJ, Parise LV. Cdc42 and Rac1 induce integrin-mediated cell motility and invasiveness through PI(3)K. Nature. 1997;390:632–636. doi: 10.1038/37656. [DOI] [PubMed] [Google Scholar]
- Kemler R. From cadherins to catenins: cytoplasmic protein interactions and regulation of cell adhesion. Trends Genet. 1993;9:317–321. doi: 10.1016/0168-9525(93)90250-l. [DOI] [PubMed] [Google Scholar]
- Khosravi-Far R, Solski PA, Clark GJ, Kinch MS, Der CJ. Activation of the Rac1, RhoA, and mitogen-activated protein kinases is required for Ras transformation. Mol Cell Biol. 1995;15:6443–6453. doi: 10.1128/mcb.15.11.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda S, Fukata M, Nakagawa M, Fujii K, Nakamura N, Ookubo T, Izawa I, Nagase T, Nomura N, Tani H, Shoji I, Matsuura Y, Yonehara S, Kaibuchi K. Role of IQGAP1, a target of the small GTPases Cdc42 and Rac1, in regulation of E-cadherin mediated cell-cell adhesion. Science. 1998;281:832–835. doi: 10.1126/science.281.5378.832. [DOI] [PubMed] [Google Scholar]
- Kwong CH, Adams AG, Leto TL. Characterization of the effector-specifying domain of Rac involved in NADPH oxidase activation. J Biol Chem. 1995;270:19868–19872. doi: 10.1074/jbc.270.34.19868. [DOI] [PubMed] [Google Scholar]
- Lamarche N, Tapon N, Stowers L, Burbelo PD, Aspenstrom P, Bridges T, Chant J, Hall A. Rac and CDC42 induce actin polymerization and G1 cell cycle progression independently of p65 and the JNK/SAPK MAP kinase cascade. Cell. 1996;87:519–529. doi: 10.1016/s0092-8674(00)81371-9. [DOI] [PubMed] [Google Scholar]
- Lamaze C, Chuang TH, Terlecky LJ, Bokoch GM, Schmid SL. Regulation of receptor-mediated endocytosis by Rho and Rac. Nature. 1996;382:177–179. doi: 10.1038/382177a0. [DOI] [PubMed] [Google Scholar]
- Machesky LM, Hall A. Role of actin polymerization and adhesion to extracellular matrix in Rac- and Rho-induced cytoskeletal reorganization. J Cell Biol. 1997;138:913–926. doi: 10.1083/jcb.138.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michiels F, Stam JC, Hordijk PL, van der Kammen RA, Ruuls-Van Stalle L, Feltkamp CA, Collard JG. Regulated membrane localization of Tiam1, mediated by the NH2-terminal plekstrin homology domain, is required for Rac-dependent membrane ruffling and c-Jun NH2-terminal kinase activation. J Cell Biol. 1997;137:387–398. doi: 10.1083/jcb.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mira J-P, Bernard V, Groffen J, Sanders LC, Knaus UG. Endogenous, hyperactive Rac3. controls proliferation of breast cancer cells by a p21-activated kinase-dependent pathway. Proc Natl Acad Sci USA. 2000;97:185–189. doi: 10.1073/pnas.97.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott HR, Owen D, Nietlispach D, Lowe PN, Manser E, Lim L, Laue ED. Structure of the small G protein Cdc42 bound to the GTPase-binding domain of ACK. Nature. 1999;399:384–388. doi: 10.1038/20732. [DOI] [PubMed] [Google Scholar]
- Nagar B, Overduin M, Ikura M, Rini JM. Structural basis of calcium-induced E-cadherin rigidification and dimerization. Nature. 1996;380:360–364. doi: 10.1038/380360a0. [DOI] [PubMed] [Google Scholar]
- Nagata K-I, Puls A, Futter C, Aspenstrom P, Schaefer E, Nakata T, Hirokawa N, Hall A. The MAP kinase kinase MLK2 co-localizes with activated JNK along microtubules and associates with kinesin superfamily motor KIF3. EMBO J. 1998;17:149–158. doi: 10.1093/emboj/17.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisimoto Y, Freeman JLR, Motalebi SA, Hirshberg M, Lambeth JD. Rac binding to p67phox. Structural basis for interactions of the Rac1 effector region and insert region with components of the respiratory burst oxidase. J Biol Chem. 1997;272:18834–18841. doi: 10.1074/jbc.272.30.18834. [DOI] [PubMed] [Google Scholar]
- Perl A, Wilgenbus P, Dahl U, Semb H, Christofori G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature. 1998;392:190–193. doi: 10.1038/32433. [DOI] [PubMed] [Google Scholar]
- Potempa S, Ridley AJ. Activation of both MAP kinase and phosphatidylinositide 3-kinase by Ras is required for hepatocyte growth factor/scatter factor-induced adherens junction disassembly. Mol Biol Cell. 1998;9:2185–2200. doi: 10.1091/mbc.9.8.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puls A, Eliopoulus AG, Nobes CD, Bridges T, Young LS, Hall A. Activation of the small GTPase Cdc42 by the inflammatory cytokines TNF-α, IL-1 and by epstein-barr virus transforming protein LMP-1. J Cell Sci. 1999;112:2983–2992. doi: 10.1242/jcs.112.17.2983. [DOI] [PubMed] [Google Scholar]
- Qiu RG, Chen J, Kim D, McCormick F, Symons M. An essential role for Rac in Ras transformation. Nature. 1995;374:457–459. doi: 10.1038/374457a0. [DOI] [PubMed] [Google Scholar]
- Quillan MP. Rac regulates the stability of adherens junction and its components, thus affecting epithelial cell differentiation and transformation. Oncogene. 1999;18:6434–6442. doi: 10.1038/sj.onc.1203026. [DOI] [PubMed] [Google Scholar]
- Rheinwald JG. Methods for clonal growth and serial cultivation of normal human epidermal keratinocytes and mesothelial cells. In: Baserga R, editor. Cell Growth and Division. A Practical Approach. Oxford: IRL Press; 1989. pp. 81–94. [Google Scholar]
- Ridley AJ, Comoglio PM, Hall A. Regulation of scatter factor/hepatocyte growth factor responses by Ras, Rac and Rho in MDCK cells. Mol Cell Biol. 1995;15:1110–1122. doi: 10.1128/mcb.15.2.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein Rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein Rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Rottner K, Hall A, Small JV. Interplay between Rac and Rho in the control of substrate contact dynamics. Curr Biol. 1999;9:640–648. doi: 10.1016/s0960-9822(99)80286-3. [DOI] [PubMed] [Google Scholar]
- Roux P, Gauthier-Rouviere C, Doucet-Brutin S, Fort P. The small GTPases Cdc42Hs, Rac1 and RhoG delineate Raf-independent pathways that cooperate to transform NIH3T3 cells. Curr Biol. 1997;7:629–637. doi: 10.1016/s0960-9822(06)00289-2. [DOI] [PubMed] [Google Scholar]
- Ryle CM, Breitkreutz D, Stark HJ, Leigh IM, Steinert PM, Roop D, Fusenig NE. Density-dependent modulation of synthesis of keratins 1 and 10 in the human keratinocyte line HACAT and in Ras-transfected tumorigenic clones. Differentiation. 1989;40:42–54. doi: 10.1111/j.1432-0436.1989.tb00812.x. [DOI] [PubMed] [Google Scholar]
- Sako Y, Nagafuchi N, Tsukita S, Takeichi M, Kusumi A. Cytoplasmic regulation of the movement of E-cadherin on the free cell surface as studied by optical tweezers and single particle tracking: corraling and tethering by the membrane skeleton. J Cell Biol. 1998;140:1227–1240. doi: 10.1083/jcb.140.5.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander EE, van Delft S, Klooster JP, Reid T, van der Kammen RA, Michaelis F, Collard JG. Matrix-dependent Tiam/Rac-1 signaling in epithelial cells promotes either cell-cell adhesion or cell migration and is regulated by phosphatidylinositol 3-kinase. J Cell Biol. 1998;143:1385–1398. doi: 10.1083/jcb.143.5.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander EE, Klooster JP, van Delft S, van der Kammen RA, Collard JG. Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J Cell Biol. 1999;147:1009–1021. doi: 10.1083/jcb.147.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro L, Fannon AM, Kwong PD, Thompson A, Lehman MS, Grubel G, Legrand J-F, Als-Nielsen J, Colman DR, Hendrickson WA. Structural basis of cell-cell adhesion by cadherins. Nature. 1995;374:327–337. doi: 10.1038/374327a0. [DOI] [PubMed] [Google Scholar]
- Shaw LM, Rabinovitz I, Wang HHF, Toler A, Mercurio AM. Activation of phosphoinositide 3-OH kinase by the α6β4 integrin promotes carcinoma invasion. Cell. 1997;91:949–960. doi: 10.1016/s0092-8674(00)80486-9. [DOI] [PubMed] [Google Scholar]
- Shimoyama Y, Hirohashi S, Hirano S, Nogushi M, Shimosato Y, Takeichi M, Abe O. Cadherin cell-adhesion molecules in human epithelial tissues and carcinomas. Cancer Res. 1989;49:2128–2133. [PubMed] [Google Scholar]
- St Croix B, Sheehan C, Rak JW, Florenes VA, Slingerland JM, Kerbel RS. E-cadherin-dependent growth suppression is mediated by the cyclin-dependent kinase inhibitor p 27KIP1. J Cell Biol. 1998;142:557–571. doi: 10.1083/jcb.142.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaishi K, Sasaki T, Kameyama T, Tsukita S, Tsukita S, Takai Y. Translocation of activated Rho from the cytoplasm to membrane ruffling area, cell-cell adhesion sites and cleavage furrows. Oncogene. 1995;11:39–48. [PubMed] [Google Scholar]
- Takaishi K, Sasaki T, Kato M, Yamoshi W, Kuroda S, Nakamura T, Takeichi M, Takai Y. Involvement of Rho p21 small GTP-binding protein and its regulator in the HGF-induced cell motility. Oncogene. 1994;9:273–279. [PubMed] [Google Scholar]
- Takaishi K, Sasaki T, Kotani H, Nishioka H, Takai Y. Regulation of cell-cell adhesion by Rac and Rho small G proteins in MDCK cells. J Cell Biol. 1997;139:1047–1059. doi: 10.1083/jcb.139.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapon N, Nagata K, Lamarche N, Hall A. A new Rac target POSH is an SH3-containing scaffold protein involved in the JNK and NF-B signaling pathways. EMBO J. 1998;17:1395–1404. doi: 10.1093/emboj/17.5.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolias KF, Hartwig JH, Ishihara H, Shibasaki Y, Cantley LC, Carpenter CL. Type Iα phosphatidylinositol-4-phosphate 5 kinase mediates Rac-dependent actin assembly. Curr Biol. 2000;10:153–156. doi: 10.1016/s0960-9822(00)00315-8. [DOI] [PubMed] [Google Scholar]
- Van Aelst L, D'Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- van Leeuwen FN, van Delft S, Kain HE, van der Kamme RA, Collard JG. Rac regulates phosphorylation of the myosin-II heavy chain, actomyosin disassembly and cell spreading. Nat Cell Biol. 1999;1:242–248. doi: 10.1038/12068. [DOI] [PubMed] [Google Scholar]
- van Leeuwen FN, Van der Kammen R, Habets GGM, Collard JG. Oncogenic activity of Tiam-1 and Rac-1 in NIH3T3 cells. Oncogene. 1995;11:2215–2221. [PubMed] [Google Scholar]
- Yap AS, Brieher WM, Pruschy M, Gumbiner BM. Lateral clustering of adhesive ectodomain: a fundamental determinant of cadherin function. Curr Biol. 1997;7:308–315. doi: 10.1016/s0960-9822(06)00154-0. [DOI] [PubMed] [Google Scholar]
- Zondag GCM, Evers EE, den Klooster JP, Janssen L, van der Kammen RA, Collard JG. Oncogenic Ras downregulates Rac activity, which leads to increased Rho activity and epithelial-mesenchymal transition. J Cell Biol. 2000;149:775–781. doi: 10.1083/jcb.149.4.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong C, Kinch MS, Burridge K. Rho-stimulated contractility contributes to the fibroblastic phenotype of Ras-transformed epithelial cells. Mol Biol Cell. 1997;8:2329–2344. doi: 10.1091/mbc.8.11.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohn IE, Symons M, Chrzanowska-Wodnicka M, Westwick JK, Der CJ. Mas oncogene signaling and transformation require the small GTP-binding protein Rac. Mol Cell Biol. 1998;18:1225–1235. doi: 10.1128/mcb.18.3.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]