Abstract
Since the mid-1980s, there has been a resurgence of severe forms of invasive group A streptococcal (GAS) disease in many Western countries. In Hong Kong, a similar increase has also been observed in recent years. One hundred seven GAS isolates collected from 1995 to 1998 from individuals with necrotizing fasciitis, toxic shock syndrome, meningitis, or other type of bacteremic sepsis (invasive group, n = 24) as well as from individuals with minor skin and throat infections (noninvasive group, n = 83) were characterized through serologic and/or emm sequence typing. Thirty-two M protein gene sequence types were identified. Types M1, M4, and M12 were the most prevalent in both the invasive group and the noninvasive group; together they accounted for 70.8 and 37.3% of the isolates, respectively. No clear pattern of skin and throat infection M types was observed. Type M1 was overrepresented in the invasive and pharyngeal isolates. The same pulsed-field gel electrophoresis pattern was shared by most invasive and all pharyngeal M1 isolates. Overall, resistance to erythromycin (32%) and tetracycline (53%) was high, but M1 isolates were significantly less likely to have resistance to either antimicrobial agent than non-M1 isolates. One novel emm sequence type, stHK, was identified in an isolate from a patient with necrotizing fasciitis. Minor emm gene sequence alterations were noted for 31 isolates, and for 13 of these isolates, deletion, insertion, or point mutations were seen in the hypervariable 50 N-terminal residues.
The M protein is an important virulence determinant in the pathogenesis of both suppurative and nonsuppurative diseases due to group A streptococci (GAS). Approximately 150 different M protein gene sequence types have been documented (14). Some types have seemed to be associated with certain patterns of disease more frequently than others. Historically, types such as M1, M2, M3, M4, M12, M15, M49, M55, M56, M59, M60, and M61 have been associated with poststreptococcal glomerulonephritis, while types M5, M6, M18, M19, and M24 have been linked to rheumatic fever (4, 5, 17). More recently, types M1 and M3 were also epidemiologically associated with the resurgence of some severe forms of GAS infections, such as toxic shock syndrome and necrotizing fasciitis (23, 25). However, in the absence of type distribution data for concurrent control strains from the healthy population, the true propensity of any M type to cause a specific clinical manifestation still remains controversial (21). Furthermore, studies that have described the distribution of GAS types have been done mainly in Western countries. There have been few data from Asia, where the epidemiology of GAS may be different (16, 20, 28, 33). Studies from Malaysia and Thailand, where rheumatic fever is endemic, have indeed reported a different GAS type distribution in the region (16, 20, 28, 33).
In this study, we report the result of an analysis of GAS isolates obtained from patients with severe systemic infections in Hong Kong. As controls, GAS isolates obtained over approximately the same period from patients with minor upper respiratory tract or skin infections were also studied.
MATERIALS AND METHODS
Bacterial strains.
A total of 113 isolates of GAS obtained from clinical specimens submitted to four hospital-based laboratories in Hong Kong from 1995 to 1998 were studied. Six were later confirmed to be repeat isolates from the same patients, giving 107 isolates to be included in the analysis in this study (15). The sources of the specimens included hospitalized patients as well as outpatients attending emergency rooms, specialty outpatient clinics, and a university health clinic. The isolates could be separated into two clinical categories.
Isolates in group I (invasive group) were from 24 patients with severe systemic GAS infections, including necrotizing fasciitis (n = 7), toxic shock syndrome (n = 2), meningitis (n = 2), and bacteremic sepsis (n = 13). Isolates in group II (noninvasive group) were from 83 patients with infections of the skin, including impetigo and minor wound infections (n = 52), throat (n = 21), or other sites (e.g., eye, vagina, and urethra) (n = 10). The 83 noninvasive isolates were chosen randomly from all of the positive isolations from nonsterile sites during the period. The total number of isolates from nonsterile sites was estimated to be about 600. The distribution of the isolates by group, year of isolation, and specimen type is shown in Table 1.
TABLE 1.
Sources of 107 GAS from Hong Kong
| Parameter | No. of isolates
|
|
|---|---|---|
| Invasive (n = 24) | Noninvasive (n = 83) | |
| Yr of isolation | ||
| 1995 | 5 | 7 |
| 1996 | 6 | 25 |
| 1997 | 6 | 27 |
| 1998 | 7 | 24 |
| Patient age (yr) | ||
| ≤12 | 3 | 26 |
| 13-64 | 13 | 48 |
| ≥65 | 8 | 9 |
| Source of isolation | ||
| Blood | 16 | 0 |
| Cerebrospinal fluid | 2 | 0 |
| Tissue or other body fluids | 6 | 0 |
| Throat | 0 | 21 |
| Skin | 0 | 52 |
| Other | 0 | 10 |
All isolates showed beta-hemolysis on horse blood agar, agglutinated with GAS-specific antiserum, and were susceptible to 0.04-U bacitracin disks. All isolates were stored at −70°C in commercial cryopreservative beads until testing.
Typing of isolates.
All isolates were characterized by T-antigen agglutination pattern and by M type and/or opacity factor (OF) type by using previously described methods. T-antigen typing sera from all recognized T antigens, 37 M typing sera, and 24 OF typing sera were available from the World Health Organization (WHO) Collaborating Center for Reference and Research on Streptococci at the University of Minnesota (20). The following M typing antisera were used: types 1 to 6, 8, 12, 14, 15, 17 to 19, 23 to 26, 29 to 33, 36 to 41, 43, 47, 49, 51 to 53, and 55 to 57. The OF typing antisera used were as follows: types 2, 4, 9, 11, 22, 25, 28, 48, 49, 58 to 64, 66, 68, 73, 75 to 78, and 81.
Bacteria that were nontypeable in the serological analysis and all M1 isolates were subjected to emm sequencing by using a method described at the Centers for Disease Control and Prevention (CDC) website (http://www.cdc.gov/ncidod/biotech/strep/strepindex.html). Primer 1 ([TATT(C/G)GCTTAGAAAATTAA]) and primer 2 (GCAAGTTCTTCAGCTTGTTT) were used for amplification of the emm gene, and the product was used as the template for sequencing with primer emmseq2 (TATTCGCTTAGAAAATTAAAAACAGG). Nucleotide sequencing of 450 to 700 bases from the beginning of emmseq2 was performed by using the Big Dye dideoxynucleotide chain termination method with an ABI 377 genetic analyzer (Perkin-Elmer, Foster City, Calif.). For amplification of emm, the cycling parameters were 94°C for 15 s, 46°C for 30 s, and 72°C for 1 min 15 s for the first 10 cycles and then 94°C for 15 s, 46°C for 30 s, and 72°C for 1 min 15 s (with a 10-s increment for each of the subsequent 19 cycles) for the subsequent 20 cycles. For sequencing, the cycling parameters were 96°C for 30 s, 55°C for 10 s, and 60°C for 4 min. The BLAST 2 program (National Center for Biotechnology Information; available at http://www.ncbi.nlm.nih.gov/BLAST/and at http://www.cdc.gov/ncidod/biotech/strep/strepblast.htm) was used to compare the DNA sequence with published sequences in the GenBank and CDC databases. A sequence was considered to be a given emm type if it had ≥95% identity over the first 160 nucleotides (counting from the beginning of the sequence obtained with emmseq2), allowing for one alteration of the reading frame of up to seven codons (13). This sequence includes 70 bases coding for the signal peptide and 90 bases coding for the first 30 amino acids of the mature protein. Alleles were designated on the basis of any changes in the deduced hypervariable N-terminal 50 amino acids of the processed M protein compared to the product of the M type reference strain emm sequence.
Serotyping was performed at the WHO Collaborating Center for Reference and Research on Streptococci at the University of Minnesota, and emm sequence typing was conducted at the Centre of Infection, The University of Hong Kong. The nucleotide sequence of the novel emm sequence type stHK was independently verified at the WHO Collaborating Center for Reference and Research on Streptococci at the University of Minnesota and at the CDC. For our analysis, the indicated type represents that obtained by M serotyping, anti-OF (AOF) serotyping, and/or emm sequence typing. Although there are documented examples in which AOF types are divergent from actual M types, we believe that the observed consistency of T-antigen pattern within AOF type, the correlation of T-antigen pattern-AOF type combinations with corresponding historical T-antigen pattern-M-type combinations, and the confirmation by emm sequence typing of at least one isolate of each AOF type make such a lack of correlation unlikely in this study (3).
Macrorestriction and PFGE.
Sixteen M1 isolates were selected for analysis by pulsed-field gel electrophoresis (PFGE). The protocol was modified from one described previously (31). Briefly, bacterial cells were grown in Todd-Hewitt broth and then lysed with lysozyme and lysostaphin. The blocks were digested overnight at 30°C with restriction enzyme SmaI (20 U/100 μl; Amersham Biosciences). The digested blocks were electrophoresed at 6 V/cm in 1% molecular-grade, biology-grade agarose by using a Bio-Rad CHEF DRII tank for 26 h at 14°C. Ramping times were 10 to 35 s.
Susceptibility testing and PCR for erythromycin resistance determinants.
Susceptibilities to erythromycin, tetracycline, and chloramphenicol were determined by Kirby-Bauer disk diffusion (CO2 incubation). MICs were determined by the broth microdilution method (ambient air incubation) for isolates resistant to chloramphenicol and intermediately resistant to erythromycin. All results were interpreted according to NCCLS criteria (26). For the identification of erythromycin resistance genes (ermA, ermB, ermC, ermTR, and mef), PCR was performed with published primers as described previously (12).
Statistical analysis.
The chi-square or Fisher's exact test was used for statistical analysis. A P value of <0.05 was consider significant. The SPSS 10.0 statistical package (SPSS Hong Kong Ltd., Hong Kong) was used for all analyses.
Nucleotide sequence accession numbers.
The emm gene sequence data can be found in GenBank under the following accession numbers: AY046085 (stHK), AY147175 (emm14.3), AY147176 (emm1.2.b), AY147177 to AY147180 (emm58.5), AY147181 (emm1.16), AY147182 (emm93.1), AY147183 (emm64.3), AY147184 (emm75.1), AY147185 (emm39.1), AY147854 (emm1.17), and AY147855 (emm58.4).
RESULTS
Distribution of M types.
A total of 32 M types were identified in the 107 isolates (Table 2). Fourteen of these M types were not covered by the typing antisera, including one isolate (SP01) with the novel emm sequence type stHK. In descending order of frequency, the prevalent types (with a frequency of >3% in the population) were M12 (20.6%), M1 (15.0%), M58 (10.3%), M4 (9.3%), M53 (4.7%), and M49 (4.7%). Three M types (M1, M4, and M12) were the most prevalent in isolates from both the invasive group and the noninvasive group. These three M types together accounted for 70.8 and 37.3% of the invasive and noninvasive isolates, respectively. While M58 was common (13.3%) in the noninvasive isolates, this M type was absent from the invasive isolates. Type M1 was more frequent in the invasive isolates than in the noninvasive isolates overall (8 of 24 versus 8 of 83; P = 0.004) but was present at similar frequencies in the invasive and throat isolates (8 of 24 versus 5 of 21; P = 0.5).
TABLE 2.
Distribution of streptococcal types according to type of infection
| M/AOF/emm typea | No. (%) of isolates
|
||
|---|---|---|---|
| Invasive | Noninvasive | Total | |
| M/emm1 | 8 (33.3) | 8 (9.6) | 16 (15.0) |
| M/AOF/emm4 | 3 (12.5) | 7 (8.4) | 10 (9.3) |
| emm8 | 1 (1.2) | 1 (0.9) | |
| emm11 | 1 (4.2) | 1 (1.2) | 2 (1.9) |
| M12 | 6 (25.0) | 16 (19.3) | 22 (20.6) |
| emm14 | 1 (1.2) | 1 (0.9) | |
| M18 | 1 (1.2) | 1 (0.9) | |
| AOF/emm22 | 1 (4.2) | 2 (2.4) | 3 (2.8) |
| emm25 | 2 (2.4) | 2 (1.9) | |
| emm27 | 1 (1.2) | 1 (0.9) | |
| AOF/emm28 | 2 (2.4) | 2 (1.9) | |
| emm39 | 1 (4.2) | 1 (0.9) | |
| emm42 | 1 (1.2) | 1 (0.9) | |
| AOF/emm49 | 5 (6.0) | 5 (4.7) | |
| M/emm53 | 5 (6.0) | 5 (4.7) | |
| AOF/emm58 | 11 (13.2) | 11 (10.3) | |
| emm63 | 1 (1.2) | 1 (0.9) | |
| emm64 | 1 (1.2) | 1 (0.9) | |
| emm71 | 1 (4.2) | 1 (0.9) | |
| emm74 | 1 (1.2) | 1 (0.9) | |
| AOF/emm75 | 2 (2.4) | 2 (1.9) | |
| AOF/emm77 | 3 (3.6) | 3 (2.8) | |
| emm81 | 1 (4.2) | 1 (0.9) | |
| emm87 | 2 (2.4) | 2 (1.9) | |
| AOF/emm89 | 1 (1.2) | 1 (0.9) | |
| emm93 | 1 (1.2) | 1 (0.9) | |
| emm95 | 1 (4.2) | 1 (0.9) | |
| emm101 | 2 (2.4) | 2 (1.9) | |
| emm102 | 2 (2.4) | 2 (1.9) | |
| emm103 | 2 (2.4) | 2 (1.9) | |
| emm112 | 1 (1.2) | 1 (0.9) | |
| emm-stHK | 1 (4.2) | 1 (0.9) | |
| Total | 24 | 83 | 107 |
Types were obtained through one or more of the techniques of serological M typing, AOF typing, and/or emm gene sequence typing.
A total of 10 different M types were found in the 24 invasive isolates (Table 3). Of the 11 patients with the most severe forms of infection (necrotizing fasciitis, toxic shock syndrome, and meningitis), 6 had type M1 and 1 had type M12. In the noninvasive group, a total of 27 different M types were identified for throat and skin isolates. The most common types (such as M1, M12, M49, M58, and M77) could be found in both skin and throat isolates. On the other hand, some types (such as M4, M53, and M58) were found more frequently in skin isolates. Statistically, only type M1 was found significantly more frequently in throat isolates than in skin isolates (P = 0.02).
TABLE 3.
Distribution of GAS M types according to source of isolate
| M type | No. of isolates from the indicated sourcea:
|
||||||
|---|---|---|---|---|---|---|---|
| Invasive
|
Noninvasive
|
||||||
| NF | TSS | Meningitis | Bacteremic sepsis | Throat | Skin | Other mucosal sites | |
| 1 | 4 | 1 | 1 | 2 | 5 | 2 | 1 |
| 4 | 3 | 6 | 1 | ||||
| 12 | 1 | 5 | 4 | 7 | 5 | ||
| 22 | 1 | 1 | 1 | ||||
| 49 | 2 | 2 | 1 | ||||
| 53 | 4 | 1 | |||||
| 58 | 1 | 10 | |||||
| 77 | 1 | 2 | |||||
| Otherb | 3 | 1 | 2 | 7 | 18 | 1 | |
| Total | 7 | 2 | 2 | 13 | 21 | 52 | 10 |
NF, necrotizing fasciitis; TSS, toxic shock syndrome. Bacteremic sepsis includes skin and soft tissue infections (n = 4), pneumonia (n = 1), and primary bacteremia (n = 8).
Number of isolates for other M types: NF, 1 for M39, 1 for M95, and 1 for stHK; meningitis, 1 for M71; bacteremic sepsis, 1 for M11 and 1 for M81; throat, 1 for M8, 1 for M14, 1 for M18, 1 for M63, 1 for M75, 1 for M93, and 1 for M101; skin, 1 for M11, 2 for M25, 1 for M27, 1 for M28, 1 for M42, 1 for M64, 1 for M74, 1 for M75, 2 for M87, 1 for M89, 1 for M101, 2 for M102, 2 for M103, and 1 for M112; and other mucosal sites, 1 for M28.
Sixteen isolates in the present collection belonged to type M1, and all were analyzed by PFGE. Eight were isolated from patients with invasive infections (four with necrotizing fasciitis, two with primary bacteremia, one with meningitis, and one with toxic shock syndrome). The other eight isolates were obtained from patients with uncomplicated pharyngitis (n =5), skin infections (n =2), and throat colonization (n =1). After macrorestriction with SmaI, 15 of the isolates produced indistinguishable PFGE patterns. The single remaining isolate, from a patient with toxic shock syndrome, produced a similar pattern with a three-band difference.
Sequence analysis.
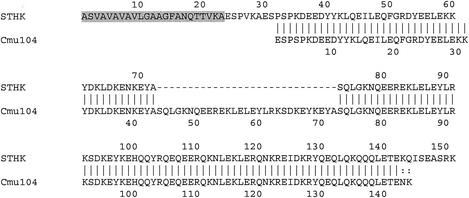
The M protein genes of the 58 nonserotypeable isolates and the 16 M1 isolates were sequenced. One isolate (SP01) from a patient with necrotizing fasciitis had a novel emm sequence type designated stHK. From a BLAST search, it was found that stHK shared 78% sequence identity with the previously described cmu104 (GenBank accession number AF091805). stHK differs from cmu104 in the presence of an additional six residues at the N terminus of the mature protein and a 29-amino-acid deletion (Fig. 1). Even though strain SP01 was OF negative, the emm stHK sequence is most similar to corresponding sequences from OF-positive isolates, so it was expected that this strain would contain the sof gene (3). Although the presence of sof is a prerequisite for the OF-positive phenotype, its presence does not always correlate with an OF-positive phenotype. The remaining 73 isolates all had >95% homology to CDC reference emm sequences over the first 160 bases.
FIG. 1.
Alignment of deduced amino acid sequences of STHK and Cmu104. The predicted partial signal peptide of STHK is shaded. Dashes indicate a 29-residue deletion in STHK.
In 31 of the 74 isolates, one or more allelic variations were detected (Table 4); 13 of these isolates displayed deletion, insertion, or point mutations in the hypervariable 50 N-terminal residues relative to M type reference strains. Eleven of these variant 50-residue sequences were not in the GenBank and CDC databases. The remaining 42 isolates all had DNA sequences totally identical to the corresponding reference emm sequences.
TABLE 4.
Allelic variations in 31 GAS isolates
| emm sequencea | No. of isolates with variations/ total no.b | Amino acid variation(s)c |
|---|---|---|
| emm1 | 10/15 | D77N |
| emm1.16 | 1/15 | N29T |
| emm1.17 | 1/15 | D31S |
| emm1.2.b | 1/1 | K40E |
| emm14.3 | 1/1 | G18D + E22K + K23N + A25G + V35T + A65V + T81Ad |
| emm39.1 | 1/1 | I9R + E10Q + E11K + K14E + Q31E + G45E + K52E + N53K + V54L + K67E |
| emm53 | 1/5 | D87E + N110D |
| emm58.4 | 1/11 | D20G |
| emm58.5 | 4/11 | L10F + T42A |
| emm64.3 | 1/1 | R2K + G6R + A9D + N16S + R29K + K38E + D45G + N55K + N57K + K61E + K62N + N64K + E66D + V78D + V83A + E86N + D117N |
| emm71 | 1/1 | A57T |
| emm74 | 1/1 | E67K |
| emm75.1 | 1/2 | R31K |
| emm77 | 1/3 | S112I |
| emm93.1 | 1/1 | Y10H + G29D + N39D + K73R |
| emm101 | 1/2 | One in-frame deletion of 13 residues (ELERLKNERHDHD) from positions 80 to 92 |
| emm102 | 1/2 | L67H + L83Q + L94Q |
| emm102 | 1/2 | T102A |
| emm103 | 1/2 | In-frame insertion of 7 residues (QEERQKN) between positions 102 and 103 |
Alleles (indicated by numeral following decimal point) are based on changes in the predicted hypervariable N-terminal 50 residues of the processed M proteins compared to CDC reference sequences for the products of emm1, emm1.2, emm14, emm39, emm58, emm64, emm75, and emm93. Alleles emm14.3 and emm75.1, which have been seen within the United States, were in the CDC database prior to this study (from pharyngeal and invasive isolates, respectively). Other alterations outside of this 50-residue segment are also indicated.
Total number of isolates with the corresponding emm type. Except for emm53, emm58, and emm75 isolates, all isolates with the indicated emm type were sequenced. A total of 4 of 5 emm53 isolates, 10 of 11 emm58 isolates, and 1 of 2 emm75 isolates were sequenced.
The number indicates the position of the amino acid in the mature protein. All strains with allelic variations were sequenced two or three times.
The amino acid sequences of the emm14.3 product and the product of emm14 (reference sequence) diverge significantly after mature protein residue 93. However, this region of the Hong Kong emm14.3 sequence has complete homology to the corresponding region (mature protein residue 101) of the reference emm14.3 sequence in the CDC database.
Antimicrobial susceptibility.
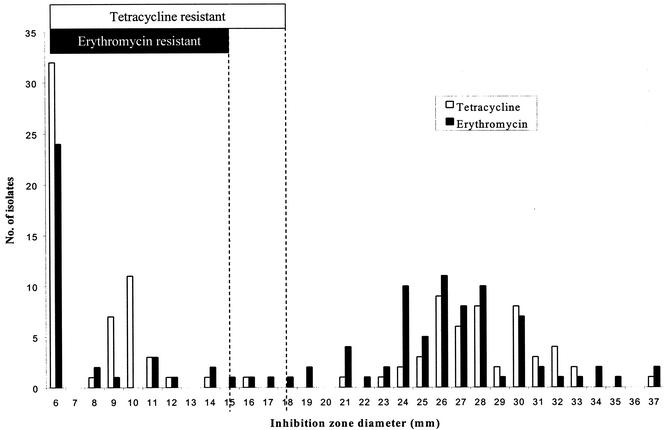
Overall, 4.7% of isolates (5 of 107) were intermediately resistant and 31.8% of isolates (34 of 107) were fully resistant to erythromycin, as determined by the disk diffusion method. The five erythromycin-intermediate isolates were examined further by determination of the MICs. The erythromycin MICs for all five isolates were in the resistant range (MIC, 1 to 4 μg/ml). For tetracycline, 0.9% of isolates (1 of 107) were intermediate and 53.3% of isolates (57 of 107) were resistant. One isolate (S2) was resistant to chloramphenicol; the MIC was 32 μg/ml. Most of the erythromycin- and tetracycline-resistant isolates grew to the edges of the disks (Fig. 2). Twenty-eight percent were resistant to both erythromycin and tetracycline.
FIG. 2.
Distribution of inhibition zone diameters for erythromycin and tetracycline. The NCCLS interpretive breakpoints (sensitive, intermediate, and resistant) were as follows: for erythromycin, ≥21, 16 to 20, and ≤15 mm; and for tetracycline, ≥23, 19 to 22, and ≤18 mm. The broken lines indicate the cutoff for resistance.
The proportions of antibiotic-resistant isolates were similar in different age groups and in the invasive and noninvasive isolates. Resistance to erythromycin and tetracycline was found in 18 and 25 different M types, respectively. Interestingly, M1 isolates were significantly less likely than isolates of all other types to be resistant to either erythromycin (1 of 16 versus 38 of 91; P = 0.005) or tetracycline (2 of 16 versus 55 of 91; P < 0.001). PCR analysis for erythromycin resistance genes was performed for the 39 erythromycin-resistant isolates. Of these, 17 isolates had the ermTR gene alone, 14 had the mef gene alone, and 2 had both the ermTR gene and the mef gene. The remaining six isolates had the ermB gene alone. The five isolate that had intermediate results in the disk diffusion test all possessed the ermTR gene.
DISCUSSION
This study found that the types (M1, M4, and M12) that were the most prevalent in isolates from patients with invasive disease were also commonly found in skin or pharyngeal isolates. Despite this finding, type M1 was disproportionately represented in invasive and pharyngeal isolates, as has been reported elsewhere (8, 17, 24, 25, 30). Although type M3 has been reported to be prevalent in the United States, Canada, and other countries (2, 6, 9, 11, 17), particularly for invasive isolates, this M type was not observed in the present study. Previous studies suggested that a virulent M1 clone was responsible for the majority of severe GAS infections that have occurred since the mid-1980s (8, 24, 25, 30). On the other hand, Kiska et al. showed that the major invasive types, M1 and M3, were equally prevalent in pharyngeal isolates (23). Furthermore, the M1 and M3 isolates causing invasive infections had PFGE patterns that were identical to those of concurrent pharyngeal isolates (23). In a recent study involving a comparison of a large number of invasive isolates with control strains, there was again no evidence of an association between a particular clone and invasive infection (18). These published results are in agreement with our observations that most invasive M1 isolates and noninvasive M1 isolates shared identical PFGE profiles. On the other hand, a reduction in the prevalence of M1 isolates in patients with uncomplicated pharyngitis was apparently temporally associated with a reduction in the clinical manifestations of severe GAS infections (21).
In Hong Kong, a previous study suggested that there was also an increase in severe forms of GAS infection during the mid-1990s (32). Meningitis, necrotizing fasciitis, and toxic shock syndrome due to GAS were almost unknown in the preceding 10 to 15 years. We postulate that the recent appearance of these severe forms of disease in this region is probably a reflection of changes in the epidemiology of the prevalent M types. The rapid introduction of new strains into a population is well documented (1). Therefore, the introduction and dissemination of streptococcal strains with enhanced virulence potential are plausible explanations for this increase in severe forms of infection and are compatible with the disproportionate representation of type M1 in the throat and invasive isolates. However, we have no definite proof that such introduction and dissemination have occurred (27). As suggested by Kiska et al., pharyngeal infections may have served as a reservoir for virulent GAS clones (23).
The overall rate of erythromycin resistance in our GAS isolates was high—32%—compared with reported rates of 2.1 to 4.6% in Canada (12, 34), 2.6% in the United States (19), and 8.6% in Finland (29). Our findings are similar to the overall rate in Italy (10)—25.9%. Similarly, we found a high rate of resistance to tetracycline—53%—compared to 4% in the United States (19). The high rates of resistance that we found in Hong Kong are probably a reflection of the high level of antibiotic usage in the community, which has also brought about high macrolide and tetracycline resistance rates in pneumococci (7). Interestingly, antibiotic resistance seems to be more common in non-M1 isolates than in M1 isolates. Only 6.3 and 12.5% of M1 isolates were not susceptible to erythromycin and tetracycline, respectively, compared to 41.8 and 61.5%, respectively, of non-M1 isolates (P value for both, <0.005).
One novel emm sequence type, stHK, was found in an isolate from a patient with necrotizing fasciitis. This sequence type differs from cmu104, described previously, at two regions in the 5′ hypervariable region. In the absence of additional 5′ sequences from cmu104, it is impossible to determine whether the six-residue difference at the N terminus is an omission error or a genuine deletion.
Allelic variations in the M proteins of our isolates, compared to reference sequences, were predominantly due to single base substitutions, with several new alleles representing substitutions in the 50 N-terminal residues. Only a single novel sequence type was found in this study. This study also showed that the GAS type distribution in Hong Kong differs from those in Thailand (28) and Malaysia (16). In the latter two countries, acute rheumatic fever is still an important health problem, but in Hong Kong, the disease has been seen very rarely in the last 20 years. Nonetheless, the serotype distribution in any population is in constant flux (1, 22). Besides serotype distribution, GAS disease epidemiology is also subject to influence from ethnic, cultural, and socioeconomic factors. If there are “rheumatogenic” streptococci, it is certainly possible, or even likely, that they are different from streptococci seen in the United States and Europe. Additional studies similar to those reported here with isolates from patients with rheumatic fever and from controls are warranted.
Acknowledgments
This study was supported by a grant from the Committee on Research and Conference Grants, The University of Hong Kong.
We thank Frankie K. H. Chow and Terence K. M. Cheung for excellent technical support.
REFERENCES
- 1.Anthony, B. F., E. L. Kaplan, L. W. Wannamaker, and S. S. Chapman. 1976. The dynamics of streptococcal infections in a defined population of children: serotypes associated with skin and respiratory infections. Am. J. Epidemiol. 104:652-666. [DOI] [PubMed] [Google Scholar]
- 2.Beall, B., R. Facklam, T. Hoenes, and B. Schwartz. 1997. Survey of emm gene sequences and T-antigen types from systemic Streptococcus pyogenes infection isolates collected in San Francisco, California; Atlanta, Georgia; and Connecticut in 1994 and 1995. J. Clin. Microbiol. 35:1231-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beall, B., G. Gherardi, M. Lovgren, R. R. Facklam, B. A. Forwick, and G. J. Tyrrell. 2000. emm and sof gene sequence variation in relation to serological typing of opacity-factor-positive group A streptococci. Microbiology 146:1195-1209. [DOI] [PubMed] [Google Scholar]
- 4.Bisno, A. L. 1991. Group A streptococcal infections and acute rheumatic fever. N. Engl. J. Med. 325:783-793. [DOI] [PubMed] [Google Scholar]
- 5.Bisno, A. L. 2001. Nonsuppurative poststreptococcal sequelae: rheumatic fever and glomerulonephritis, p. 2117-2127. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases. Churchill Livingstone, Philadelphia, Pa.
- 6.Chelsom, J., A. Halstensen, T. Haga, and E. A. Hoiby. 1994. Necrotising fasciitis due to group A streptococci in western Norway: incidence and clinical features. Lancet 344:1111-1115. [DOI] [PubMed] [Google Scholar]
- 7.Chiu, S. S., P. L. Ho, F. K. Chow, K. Y. Yuen, and Y. L. Lau. 2001. Nasopharyngeal carriage of antimicrobial-resistant Streptococcus pneumoniae among young children attending 79 kindergartens and day care centers in Hong Kong. Antimicrob. Agents Chemother. 45:2765-2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cleary, P. P., E. L. Kaplan, J. P. Handley, A. Wlazlo, M. H. Kim, A. R. Hauser, and P. M. Schlievert. 1992. Clonal basis for resurgence of serious Streptococcus pyogenes disease in the 1980s. Lancet 339:518-521. [DOI] [PubMed] [Google Scholar]
- 9.Colman, G., A. Tanna, A. Efstratiou, and E. T. Gaworzewska. 1993. The serotypes of Streptococcus pyogenes present in Britain during 1980-1990 and their association with disease. J. Med. Microbiol. 39:165-178. [DOI] [PubMed] [Google Scholar]
- 10.Cornaglia, G., M. Ligozzi, A. Mazzariol, M. Valentini, G. Orefici, R. Fontana, et al. 1996. Rapid increase of resistance to erythromycin and clindamycin in Streptococcus pyogenes in Italy, 1993-1995. Emerg. Infect. Dis. 2:339-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies, H. D., A. McGeer, B. Schwartz, K. Green, D. Cann, A. E. Simor, D. E. Low, et al. 1996. Invasive group A streptococcal infections in Ontario, Canada. N. Engl. J. Med. 335:547-554. [DOI] [PubMed] [Google Scholar]
- 12.De Azavedo, J. C., R. H. Yeung, D. J. Bast, C. L. Duncan, S. B. Borgia, and D. E. Low. 1999. Prevalence and mechanisms of macrolide resistance in clinical isolates of group A streptococci from Ontario, Canada. Antimicrob. Agents Chemother. 43:2144-2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Facklam, R., B. Beall, A. Efstratiou, V. Fischetti, D. Johnson, E. Kaplan, P. Kriz, M. Lovgren, D. Martin, B. Schwartz, A. Totolian, D. Bessen, S. Hollingshead, F. Rubin, J. Scott, and G. Tyrrell. 1999. emm typing and validation of provisional M types for group A streptococci. Emerg. Infect. Dis. 5:247-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Facklam, R. F., D. R. Martin, M. Lovgren, D. R. Johnson, A. Efstratiou, T. A. Thompson, S. Gowan, P. Kriz, G. J. Tyrrell, E. Kaplan, and B. Beall. 2002. Extension of the Lancefield classification for group A streptococci by addition of 22 new M protein gene sequence types from clinical isolates: emm103 to emm124. Clin. Infect. Dis. 34:28-38. [DOI] [PubMed] [Google Scholar]
- 15.Ho, P. L., D. N. C. Tsang, D. R. Johnson, W. M. Tang, T. L. Que, T. K. Ng, and E. L. Kaplan. 2002. Characterization of Streptococcus pyogenes in Hong Kong, p. 87-90. In D. R. Martin and J. R. Tagg (ed.), Streptococci and streptococcal diseases. Proceedings of the XIV Lancefield International Symposium on Streptococci and Streptococcal Diseases. The XIV Organizing Committee, Auckland, New Zealand.
- 16.Jamal, F., S. Pit, D. R. Johnson, and E. L. Kaplan. 1995. Characterization of group A streptococci isolated in Kuala Lumpur, Malaysia. J. Trop. Med. Hyg. 98:343-346. [PubMed] [Google Scholar]
- 17.Johnson, D. R., D. L. Stevens, and E. L. Kaplan. 1992. Epidemiologic analysis of group A streptococcal serotypes associated with severe systemic infections, rheumatic fever, or uncomplicated pharyngitis. J. Infect. Dis. 166:374-382. [DOI] [PubMed] [Google Scholar]
- 18.Johnson, D. R., J. T. Wotton, A. Shet, and E. L. Kaplan. 2002. A comparison of group A streptococci from invasive and uncomplicated infections: are virulent clones responsible for serious streptococcal infections? J. Infect. Dis. 185:1586-1595. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan, E. L., D. R. Johnson, M. C. Del Rosario, and D. L. Horn. 1999. Susceptibility of group A beta-hemolytic streptococci to thirteen antibiotics: examination of 301 strains isolated in the United States between 1994 and 1997. Pediatr. Infect. Dis. J. 18:1069-1072. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan, E. L., D. R. Johnson, P. Nanthapisud, S. Sirilertpanrana, and S. Chumdermpadetsuk. 1992. A comparison of group A streptococcal serotypes isolated from the upper respiratory tract in the USA and Thailand: implications. Bull. W. H. O. 70:433-437. [PMC free article] [PubMed] [Google Scholar]
- 21.Kaplan, E. L., D. R. Johnson, and C. D. Rehder. 1994. Recent changes in group A streptococcal serotypes from uncomplicated pharyngitis: a reflection of the changing epidemiology of severe group A infections? J. Infect. Dis. 170:1346-1347. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan, E. L., J. T. Wotton, and D. R. Johnson. 2001. Dynamic epidemiology of group A streptococcal serotypes associated with pharyngitis. Lancet 358:1334-1337. [DOI] [PubMed] [Google Scholar]
- 23.Kiska, D. L., B. Thiede, J. Caracciolo, M. Jordan, D. Johnson, E. L. Kaplan, R. P. Gruninger, J. A. Lohr, P. H. Gilligan, and F. W. Denny, Jr. 1997. Invasive group A streptococcal infections in North Carolina: epidemiology, clinical features, and genetic and serotype analysis of causative organisms. J. Infect. Dis. 176:992-1000. [DOI] [PubMed] [Google Scholar]
- 24.Martin, D. R., and L. A. Single. 1993. Molecular epidemiology of group A streptococcus M type 1 infections. J. Infect. Dis. 167:1112-1117. [DOI] [PubMed] [Google Scholar]
- 25.Musser, J. M., V. Kapur, J. Szeto, X. Pan, D. S. Swanson, and D. R. Martin. 1995. Genetic diversity and relationships among Streptococcus pyogenes strains expressing serotype M1 protein: recent intercontinental spread of a subclone causing episodes of invasive disease. Infect. Immun. 63:994-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Committee for Clinical Laboratory Standards. 2001. Performance standards for antimicrobial susceptibility testing: 11th informational supplement. M100-S11. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 27.O'Brien, K. L., B. Beall, N. L. Barrett, P. R. Cieslak, A. Reingold, M. M. Farley, R. Danila, E. R. Zell, R. Facklam, B. Schwartz, and A. Schuchat. 2002. Epidemiology of invasive group A streptococcus disease in the United States, 1995-1999. Clin. Infect. Dis. 35:268-276. [DOI] [PubMed] [Google Scholar]
- 28.Pruksakorn, S., N. Sittisombut, C. Phornphutkul, C. Pruksachatkunakorn, M. F. Good, and E. Brandt. 2000. Epidemiological analysis of non-M-typeable group A Streptococcus isolates from a Thai population in northern Thailand. J. Clin. Microbiol. 38:1250-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seppala, H., T. Klaukka, J. Vuopio-Varkila, A. Muotiala, H. Helenius, K. Lager, P. Huovinen, et al. 1997. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. N. Engl. J. Med. 337:441-446. [DOI] [PubMed] [Google Scholar]
- 30.Seppala, H., J. Vuopio-Varkila, M. Osterblad, M. Jahkola, M. Rummukainen, S. E. Holm, and P. Huovinen. 1994. Evaluation of methods for epidemiologic typing of group A streptococci. J. Infect. Dis. 169:519-525. [DOI] [PubMed] [Google Scholar]
- 31.Stanley, J., D. Linton, M. Desai, A. Efstratiou, and R. George. 1995. Molecular subtyping of prevalent M serotypes of Streptococcus pyogenes causing invasive disease. J. Clin. Microbiol. 33:2850-2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang, W. M., P. L. Ho, K. K. Fung, K. Y. Yuen, and J. C. Leong. 2001. Necrotising fasciitis of a limb. J. Bone Jt. Surg. Br. Vol. 83:709-714. [DOI] [PubMed] [Google Scholar]
- 33.Tran, P. O., D. R. Johnson, and E. L. Kaplan. 1994. The presence of M protein in nontypeable group A streptococcal upper respiratory tract isolates from Southeast Asia. J. Infect. Dis. 169:658-661. [DOI] [PubMed] [Google Scholar]
- 34.Weiss, K., J. De Azavedo, C. Restieri, L. A. Galarneau, M. Gourdeau, P. Harvey, J. F. Paradis, K. Salim, and D. E. Low. 2001. Phenotypic and genotypic characterization of macrolide-resistant group A Streptococcus strains in the province of Quebec, Canada. J. Antimicrob. Chemother. 47:345-348. [DOI] [PubMed] [Google Scholar]