Abstract
To study the epidemiology of Pseudomonas aeruginosa colonization in a 32-bed burn wound center (BWC), 321 clinical and 45 environmental P. aeruginosa isolates were collected by prospective surveillance culture over a 1-year period and analyzed by serotyping, drug susceptibility testing, and amplified fragment length polymorphism (AFLP) analysis. Among 441 patients treated at the center, 70 (16%) were colonized with P. aeruginosa, including 12 (17%) patients who were colonized on admission and 58 (83%) patients who acquired the organism during their stay. Of the 48 distinct AFLP genotypes found, 21 were found exclusively in the environment, 15 were isolated from individual patients only, and 12 were responsible for the colonization of 57 patients, of which 2 were also isolated from the environment, but secondary to patient carriage. Polyclonal P. aeruginosa colonization with strains of two to four genotypes, often with different antibiotic susceptibility patterns, was observed in 19 patients (27%). Two predominant genotypes were responsible for recurrent outbreaks and the colonization of 42 patients (60% of all colonized patients). The strain with one of those genotypes appeared to be endemic to the BWC and developed multidrug resistance (MDR) at the end of the study period, whereas the strain with the other genotype was antibiotic susceptible but resistant to silver sulfadiazine (SSDr). The MDR strain was found at a higher frequency in sputum samples than the SSDr strain, which showed a higher prevalence in burn wound samples, suggesting that anatomic habitat selection was associated with adaptive resistance to antimicrobial drugs. Repeated and thorough surveys of the hospital environment failed to detect a primary reservoir for any of those genotypes. Cross-acquisition, resulting from insufficient compliance with infection control measures, was the major route of colonization in our BWC. In addition to the AFLP pattern and serotype, analysis of the nucleotide sequences of three (lipo)protein genes (oprI, oprL, and oprD) and the pyoverdine type revealed that all predominant strains except the SSDr strain belonged to recently identified clonal complexes. These successful clones are widespread in nature and therefore predominate in the patient population, in whom variants accumulate drug resistance mechanisms that allow their transmission and persistence in the BWC.
The burn wound represents a site susceptible to opportunistic colonization. Although present techniques of burn wound care have significantly reduced the incidence of infections in patients with burn wounds (45), severely burned patients may still develop life-threatening infections. The situation for patients with Pseudomonas aeruginosa infections is particularly problematic since this organism is inherently resistant to many drug classes and is able to acquire resistance to all effective antimicrobial drugs (16, 31).
On 5 August 1998, an antibiotic-resistant serotype O12 P. aeruginosa strain was isolated from the burn wounds of three patients and the sputum of two patients in the burn wound center (BWC) of the Queen Astrid Military Hospital, Brussels, Belgium. An outbreak of infection due to a P. aeruginosa strain originating from an exogenous source was initially suspected. All patients were meticulously screened for P. aeruginosa, and all isolates were serotyped and genotyped by random amplified polymorphic DNA PCR (RAPD-PCR) (10). RAPD-PCR demonstrated the presence of an epidemic serotype O12 P. aeruginosa strain. An extensive search of the hospital environment failed to identify a reservoir for the epidemic strain. Antibiotic therapy, reinforcement of isolation precautions, and improved environmental decontamination measures failed to eradicate the epidemic strain, which caused multiple outbreaks in the BWC for more than a year. By the end of 1998, the epidemic strain had acquired resistance to all commercially available antibiotics to become truly multidrug resistant (MDR). The epidemic strain disappeared in 1999 when the occupancy rate of the BWC dropped to 10% and the P. aeruginosa colonization rate subsequently dropped to 0%. In the present study, the amplified fragment length polymorphism (AFLP) technique, a highly discriminatory and reproducible genotyping method (50, 58), was applied to perform a detailed analysis of the routes of P. aeruginosa colonization in the BWC. We conducted a retrospective analysis of a selection of 321 clinical and 45 environmental P. aeruginosa isolates collected in the BWC over a period of 1 year (July 1998 to July 1999) by AFLP analysis, drug susceptibility testing (11 antibiotics and 5 topical antibacterial agents), and O serotyping.
MATERIALS AND METHODS
Setting.
The BWC of the Queen Astrid Military Hospital is a referral center for Belgium. The intensive care unit (ICU) has 8 single-bed rooms with an occupancy rate of 80%, 1 admission room, and 3 bathrooms; the medium-care unit (MCU) has 12 double-bed rooms and 2 bathrooms. Each ICU room has its own sink and trolley with equipment for patient care. All bathrooms are equipped with a hydrotherapy facility. Two ICU rooms and one bathroom are equipped with laminar airflow. Hand washing with a detergent skin cleansing solution (Hibiscrub; Zeneca Pharma, Cergy, France) is required between patient contacts; caps, masks, shoe covers, and gloves are required at all times in the patient rooms and bathrooms. Sterile gloves are required for wound care procedures. Equipment that is shared between patients, including hydrotherapy equipment, is disinfected between each use with Ivisol (Shülke und Mayr Benelux, Haarlem, The Netherlands), a phenol derivative-based disinfectant, and Mikrozid (Shülke und Mayr Benelux), an ethanol- and propanol-based disinfectant, according to the guidelines of the manufacturer. Serial samples are taken on admission and every second day (ICU), weekly (MCU), or whenever required for clinical reasons. Blood cultures are performed for patients with clinical signs of septicemia. Samples are taken by classical sampling procedures (18). The patient-to-nurse ratio during the day is 1:1 for the ICU and 2:1 for the MCU. During holidays, less experienced nursing personnel stand in to maintain this ratio. All patients admitted to the ICU and MCU between 22 July 1998 and 24 July 1999 were included in the study.
Treatment of burn patients.
Silver sulfadiazine (1% [SSD]; Flammazine; Solvay, Brussels, Belgium) is applied to the burn wounds on a daily basis. A mixture of 1% SSD and 2.2% cerium nitrate (Flammacerium; Solvay) is applied when the burn wounds are colonized with P. aeruginosa. Mafenide acetate (8.5%; Sulfamylon cream; Queen Astrid Military Hospital, Brussels, Belgium) and 10% povidone-iodine (Isobetadine cream; Asta Medica, Brussels, Belgium) are occasionally used. Patients are bathed every day in filter-sterilized water containing chlorhexidine (Hibidil; Zeneca Pharma). During hydrotherapy, dressings are removed and the burn wounds are cleansed. Used antiseptic solutions and antibacterial creams are discarded and replaced by fresh sterile ones. Burn wounds are surgically excised 1 to 2 weeks after admission. One of the following three combinations of antibiotics was administrated as first-line (empirical) treatment whenever P. aeruginosa infection was suspected: aztreonam-piperacillin, ceftazidime-amikacin, or imipenem-amikacin.
Environmental survey.
Environmental samples (n = 300) were obtained on 10 August, 10 November, and 21 December 1998. Environmental samples included water from faucets, detergents, hand-washing solutions and swabs from sinks, hydrotherapy equipment, respirator drains, toilet bowls, floors, and other damp surfaces with potential for cross-contamination throughout the burn unit. Contact cultures (cetrimide agar; Sanofi Pasteur, Brussels, Belgium) of the hands of the physicians and nurses were also collected. The water feeding the hydrotherapy facilities was sampled every 2 months.
Microbiological analysis.
The bacteria were isolated from clinical samples by standard microbiology procedures (18). P. aeruginosa was identified by the analytical profile index procedure (API 20NE test; bioMérieux, Brussels, Belgium). P. aeruginosa was isolated from environmental swabs inoculated on cetrimide agar (Sanofi Pasteur) and identified by multiplex PCR, based on the amplification of two outer membrane lipoprotein genes, oprI and oprL, as described previously (11). Samples of water (1 liter) and antiseptics (100 ml) were filtered through a 0.45-μm-pore-size filter (Millipore, Brussels, Belgium) and cultured on pyocyanosel agar (bioMérieux). P. aeruginosa isolates were stored in Luria-Bertani broth medium (Gibco-BRL-Life Technologies, Merelbeke, Belgium) containing 50% glycerol at −80°C.
Definitions.
Colonization was defined as the isolation of P. aeruginosa from two consecutive cultures of samples from the same site. Colonization on admission was defined as the isolation of P. aeruginosa within 24 h. Cross-colonization was defined as the isolation of genotypically identical isolates from patients who were in the BWC at the same time.
AFLP analysis.
An ABI 377 automated fluorescence sequencer (Applied Biosystems, Nieuwerkerk a/d Ijssel, The Netherlands) and the AFLP Microbial Fingerprinting kit (Applied Biosystems) were used for AFLP analysis, as detailed in the protocols of the manufacturer. The enzymes used were T4 DNA ligase, EcoRI, and Tru9I (all purchased from Roche Diagnostics, Brussels, Belgium). The primer pair used was EcoRI-0 (6-carboxyfluorescein) and MseI-C. Normalization and fragment sizing were carried out as described previously (42). The criteria of Speijer et al. (53) for differentiation of P. aeruginosa by AFLP analysis were used to interpret the AFLP patterns.
Serotyping.
Isolates were serotyped by slide agglutination according to the international serogrouping schema for P. aeruginosa (30) by using a panel of 16 type O monovalent antisera (Sanofi Pasteur), according to the guidelines of the manufacturer.
Drug susceptibility testing.
All isolates were tested by the classical Bauer-Kirby agar disk diffusion method (2) for susceptibility to the following antibiotics: amikacin, aztreonam, cefepime, ceftazidime, ceftriaxone, ciprofloxacin, imipenem, meropenem, piperacillin, piperacillin-tazobactam, and tobramycin. NCCLS breakpoints (39) were used to determine susceptibility. All discs (Neo-Sensitabs) were purchased from Rosco (Taastrup, Denmark).
Susceptibility to topical antibacterial agents was tested by the agar well diffusion (AWD) method (56). In short, 8-mm holes were made in 4-mm-thick Mueller-Hinton agar plates (Sanofi Pasteur) with a sterile biopsy needle. The surface of the plates was swabbed with approximately 108 bacteria, and the wells were loaded with approximately 0.2 ml of topical agent. Inhibition zones were measured after overnight incubation at 37°C. The following topical agents were tested: 1% SSD (Flammazine; Solvay), 1% SSD plus 2.2% cerium nitrate (Flammacerium; Solvay), 8.5% mafenide acetate (Sulfamylon cream; Queen Astrid Military Hospital), 10% povidone-iodine (Isobetadine cream; Asta Medica), and 0.2% nitrofurazone (Furacine; Norgine, Heverlee, Belgium).
RESULTS
Microbiological analysis.
A total of 366 P. aeruginosa isolates, including 45 environmental isolates, were selected and studied retrospectively. At least one isolate from each colonized site of each colonized patient was selected. The clinical samples included burn wound swab or biopsy (n = 149), sputum (n = 46), throat swab (n = 28), urine (n = 26), nasal swab (n = 24), blood (n = 4), catheter (n = 26), and probe (n = 14) specimens. P. aeruginosa was also recovered from unfiltered tap water (n = 13), sinks (n = 12), plant rhizospheres (n = 5), cleaned bathtubs (n = 3), bedpan-cleaning devices (n = 3), cleaned stretchers from hydrotherapy facilities (n = 2), respirator equipment (n = 2), toilet bowls (n = 2), a cleaned floor (n = 1), water from a clean trolley (n = 1), and chlorhexidine solution (Hibidil; n = 1). All cultures from the hands of the medical personnel were negative for P. aeruginosa.
Characteristics of patients colonized with P. aeruginosa.
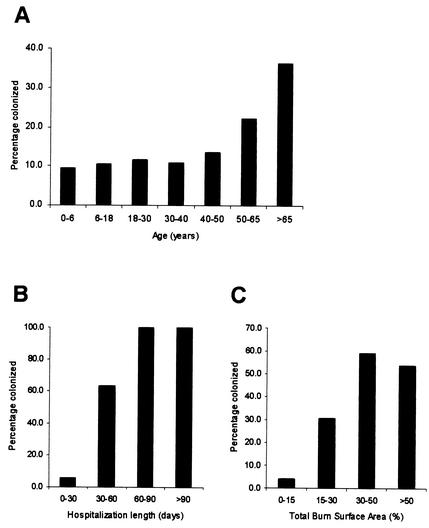
During the study period, 441 patients (mean age, 35.6 years; age range, 2.5 months to 95 years; mean total burn surface area [TBSA], 14.1%; TBSA range, 0 to 95%; mean duration of hospitalization, 14.2 days; range of duration of hospitalization, 1 to 270 days) were treated in the BWC. P. aeruginosa was isolated from 70 patients (16%; mean age, 46 years; age range, 1 to 92 years; mean TBSA, 25.0%; TBSA range, 0 to 75%; mean duration of hospitalization, 52.9 days; range of duration of hospitalization, 1 to 270 days). Twelve patients (17%) were colonized on admission, while nosocomially acquired colonization was demonstrated in 58 patients (83%). As could be expected, the incidence of P. aeruginosa colonization was proportional to the extent of the burn wound, the age of the patient, and the duration of the stay in the BWC (Fig. 1). Eight patients colonized with P. aeruginosa died. For three of them death was directly attributed to P. aeruginosa infection. A 73-year-old man with a TBSA of 50% and a 75-year-old woman with a TBSA of 36% died as a consequence of a clinically confirmed P. aeruginosa burn wound sepsis. A 43-year-old man with a TBSA of 75% died of a polymicrobial (P. aeruginosa and Aspergillus) burn wound sepsis. A 65-year-old man with a TBSA of 50% and inhalation injury survived severe inhalative pneumonia caused by P. aeruginosa. In addition, P. aeruginosa infection represented a contributing factor in the death of an 86-year-old woman with a TBSA of 12% and a history of heart disease. Three patients survived clinically confirmed burn wound sepsis caused by P. aeruginosa.
FIG. 1.
Percentages of burn wound patients colonized with P. aeruginosa by age (A), duration of hospitalization (B), and TBSA (C).
AFLP analysis.
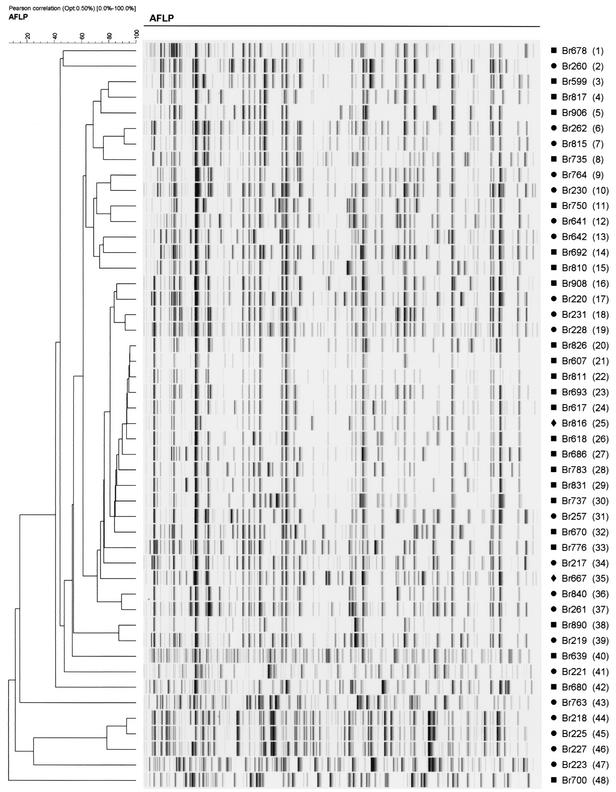
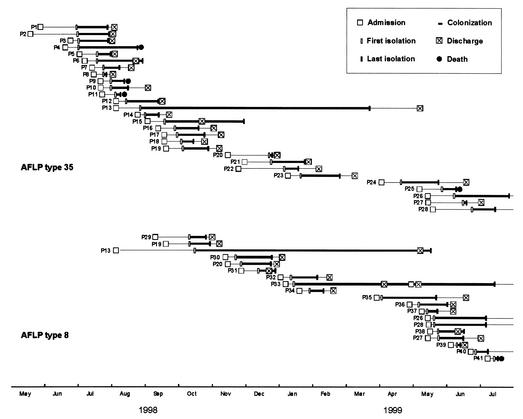
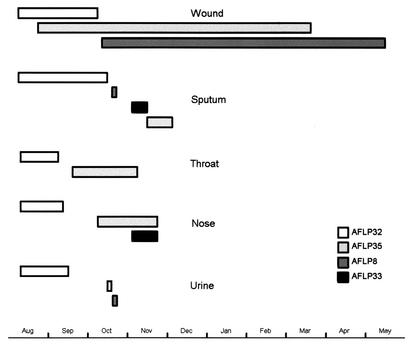
AFLP analysis of the 366 selected P. aeruginosa isolates revealed 48 AFLP genotypes (Fig. 2 and Table 1). These have been arbitrarily named AFLP genotype 1 (AFLP1) to AFLP48. The AFLP patterns of the strains were consistent over time; occasionally, differences of one or two bands were observed between the AFLP patterns of multiple isolates of the same strain. Fifteen individual AFLP genotypes were isolated from individual patients, 10 genotypes were isolated from 2 or more patients, 21 genotypes were isolated exclusively from the hospital environment, and 2 genotypes were isolated from the environment and patients. The most frequently identified clones were AFLP35 (131 isolates) and AFLP8 (76 isolates). Twenty-nine patients were colonized with AFLP35, 19 were colonized with AFLP8, and 6 were colonized with isolates of both AFLP types. AFLP35 was responsible for several outbreaks during the entire study period, while AFLP8 was first introduced in the BWC in October 1998 (Fig. 3). The AFLP35 strain was isolated from the hospital environment on six occasions. It was recovered from a 5% chlorhexidine solution (Hibidil) and repeatedly from a “cleaned” stretcher of a hydrotherapy facility, respiratory therapy equipment, and the sink of a patient room. Fifty-seven events of cross-acquisition were observed. Nineteen patients (27%) were coinfected with P. aeruginosa strains belonging to two to four different genotypes. Patient P13 (age, 25 years; TBSA, 75%) was simultaneously colonized with isolates belonging to four distinct genotypes (AFLP8, AFLP32, AFLP33, and AFLP35) (Fig. 4). During his 10-month hospitalization period, this patient was continuously colonized with epidemic strains AFLP8 and/or AFLP35. The AFLP8 strain was still isolated from his burn wounds 1 week after his discharge from the BWC (Fig. 3). MDR strain AFLP35 was more frequently isolated from sputum than AFLP8, which was associated with burn wounds (Table 1). Colonization with AFLP35 appeared on average 17.4 days (range, 6 to 43 days) after admission, with a median colonization length of 33.2 days (range, 5 to 207 days). The mean interval between admission and first isolation of AFLP8 isolates was 10.7 days (range, 2 to 38 days), and the median period of colonization was 47.1 days (range, 9 to 215 days).
FIG. 2.
Normalized AFLP patterns and dendrogram for the type isolates of the 48 genotypes from patients and/or the environment of the BWC during a 1-year period according to the differentiation criteria proposed by Speijer et al. (53). AFLP types are indicated in parentheses. Cluster analysis was performed with BioNumerics software by using the Pearson correlation and the unweighted pair group method with arithmetic averages. Percent similarities are shown above the dendrogram. ▪, isolated exclusively from clinical samples; •, isolated exclusively from the hospital environment; ⧫, isolated from clinical samples as well as from the hospital environment (secondary to patient carriage).
TABLE 1.
Origin and characteristics of the P. aeruginosa isolates
| AFLP type | Clonal complexa | Type isolate code | O serotypeb | No. of isolates | No. of patients | No. of isolates with the following origin:
|
Antibiotic resistancec
|
Resistance to topicals (zone size [mm])d
|
|||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wound | Sputum | Throat | Nose | Urine | Blood | Other | Catheter | Probe | Environment | AMK | ATM | FEP | CAZ | CRO | CIP | IPM | MEM | PIP | TZP | TOB | FlamZ | FlamC | Sulfa | Isobet | Fura | ||||||
| 1 | G | Br678 | 11 | 11 | 3 | 5 | 4 | 2 | I | R | R | R | R | R | R | R | R* | R* | R | 20 | 16 | >30 | 19 | 9 | |||||||
| 2 | Br260 | 10 | 3 | 3 | 23 | 23 | >30 | 24 | 10 | ||||||||||||||||||||||
| 3 | Br599 | 10 | 5 | 3 | 1 | 1 | 3 | 17 | 15 | >30 | 17 | 12 | |||||||||||||||||||
| 4 | Br817 | 11 | 6 | 1 | 5 | 1 | 18 | 20 | >30 | 18 | 10 | ||||||||||||||||||||
| 5 | Br906 | 6 | 4 | 2 | 2 | 1 | 1 | 20 | 19 | >30 | 15 | 12 | |||||||||||||||||||
| 6 | Br262 | 11 | 1 | 1 | 20 | 25 | >30 | 20 | 8 | ||||||||||||||||||||||
| 7 | Br815 | NA | 1 | 1 | R | 21 | 22 | >30 | 24 | 14 | |||||||||||||||||||||
| 8 | Br735 | E | 76 | 19 | 47 | 2 | 5 | 6 | 5 | 1 | 1 | 7 | 2 | R* | 13 | 14 | >30 | 18 | 10 | ||||||||||||
| 9 | Br764 | 11 | 4 | 4 | 20 | 17 | >30 | 17 | 9 | ||||||||||||||||||||||
| 10 | Br230 | 11 | 1 | 1 | R | 19 | 21 | >30 | 20 | 10 | |||||||||||||||||||||
| 11 | Br750 | 11 | 1 | 1 | 1 | R | I | 18 | 13 | >30 | 22 | 20 | |||||||||||||||||||
| 12 | Br641 | 12 | 1 | 1 | R | 18 | 17 | >30 | 21 | 10 | |||||||||||||||||||||
| 13 | E | Br642 | 1 | 3 | 3 | R* | 19 | 18 | >30 | 18 | 10 | ||||||||||||||||||||
| 14 | G | Br692 | 11 | 4 | 1 | 4 | R | R | 20 | 17 | >30 | 20 | 9 | ||||||||||||||||||
| 15 | Br810 | 8 | 8 | 1 | 5 | 1 | 2 | I | 20 | 20 | >30 | 16 | 11 | ||||||||||||||||||
| 16 | Br908 | 8 | 1 | 1 | 1 | 20 | 20 | >30 | 15 | 10 | |||||||||||||||||||||
| 17 | Br220 | 1/13 | 1 | 1 | 21 | 25 | >30 | 16 | 22 | ||||||||||||||||||||||
| 18 | Br231 | 11 | 4 | 4 | R | 16 | 20 | >30 | 22 | 10 | |||||||||||||||||||||
| 19 | Br228 | NA | 1 | 1 | I | 15 | 20 | >30 | 22 | 8 | |||||||||||||||||||||
| 20 | Br826 | 6 | 1 | 1 | 1 | 22 | 22 | >30 | 18 | 9 | |||||||||||||||||||||
| 21 | Br607 | 8 | 2 | 1 | 2 | 21 | 21 | >30 | 17 | 10 | |||||||||||||||||||||
| 22 | Br811 | 9 | 9 | 1 | 4 | 1 | 3 | 1 | 18 | 21 | >30 | 14 | 8 | ||||||||||||||||||
| 23 | Br693 | 11 | 8 | 3 | 4 | 1 | 1 | 1 | 1 | I | R | 20 | 20 | >30 | 17 | 11 | |||||||||||||||
| 24 | Br617 | 9 | 1 | 1 | 1 | I | 20 | 22 | >30 | 16 | 10 | ||||||||||||||||||||
| 25 | Br816 | 6 | 2 | 1 | 1 | 1 | R | 20 | 20 | >30 | 18 | 11 | |||||||||||||||||||
| 26 | Br618 | 1 | 2 | 2 | 1 | 1 | 20 | 19 | >30 | 13 | 9 | ||||||||||||||||||||
| 27 | Br686 | 5 | 2 | 1 | 2 | 18 | 16 | >30 | 17 | 9 | |||||||||||||||||||||
| 28 | Br783 | 1 | 1 | 1 | 1 | 24 | 24 | >30 | 18 | 10 | |||||||||||||||||||||
| 29 | Br831 | 6 | 4 | 2 | 3 | 1 | 20 | 20 | >30 | 16 | 8 | ||||||||||||||||||||
| 30 | Br737 | 1 | 1 | 1 | 1 | 21 | 20 | >30 | 18 | 10 | |||||||||||||||||||||
| 31 | A | Br257 | 6 | 5 | 5 | 20 | 21 | >30 | 22 | 11 | |||||||||||||||||||||
| 32 | A | Br670 | 6 | 28 | 2 | 3 | 9 | 6 | 3 | 1 | 3 | 3 | R* | R | R* | R | R | R* | R* | 19 | 18 | >30 | 18 | 11 | |||||||
| 33 | A | Br776 | 6 | 15 | 2 | 1 | 3 | 4 | 2 | 3 | 2 | R* | R | R | R | 20 | 19 | >30 | 18 | 11 | |||||||||||
| 34 | Br217 | 6 | 1 | 1 | R | 17 | 23 | >30 | 20 | 10 | |||||||||||||||||||||
| 35 | C | Br667 | 12 | 131 | 29 | 59 | 18 | 9 | 9 | 10 | 3 | 1 | 11 | 3 | 6 | R* | I* | R | R* | R | R | R* | R* | R | R | R | 18 | 16 | >30 | 18 | 10 |
| 36 | Br840 | NA | 1 | 1 | 19 | 22 | >30 | 17 | 12 | ||||||||||||||||||||||
| 37 | Br261 | 6 | 1 | 1 | 22 | 23 | >30 | 22 | 8 | ||||||||||||||||||||||
| 38 | Br890 | 3 | 4 | 2 | 3 | 1 | I | 21 | 20 | >30 | 14 | 10 | |||||||||||||||||||
| 39 | Br219 | 1/13 | 1 | 1 | R | 20 | 24 | >30 | 20 | 15 | |||||||||||||||||||||
| 40 | Br639 | NA | 1 | 1 | 1 | I | 20 | 20 | >30 | 20 | 14 | ||||||||||||||||||||
| 41 | Br221 | NA | 1 | 1 | R | 20 | 25 | >30 | 22 | 16 | |||||||||||||||||||||
| 42 | Br680 | 12 | 1 | 1 | 1 | 21 | 24 | >30 | 15 | 10 | |||||||||||||||||||||
| 43 | Br763 | 11 | 3 | 3 | 19 | 23 | >30 | 16 | 10 | ||||||||||||||||||||||
| 44 | Br218 | 6 | 1 | 1 | I | 23 | 27 | >30 | 20 | 10 | |||||||||||||||||||||
| 45 | Br225 | 11/PA | 2 | 2 | R | 21 | 22 | >30 | 20 | 20 | |||||||||||||||||||||
| 46 | Br227 | 11 | 1 | 1 | R | 16 | 24 | >30 | 20 | 8 | |||||||||||||||||||||
| 47 | Br223 | NA | 1 | 1 | R | R | >30 | >30 | >30 | >30 | 26 | ||||||||||||||||||||
| 48 | Br700 | NA | 1 | 1 | 1 | 18 | 17 | >30 | 21 | 13 | |||||||||||||||||||||
According to Pirnay et al. (42).
PA, polyagglutination; NA, no agglutination.
R, resistant; I, intermediate; blank cell, susceptible; *, acquired during this study; AMK, amikacin; ATM, aztreonam; FEP, cefepime; CAZ, ceftazidime; CRO, ceftriaxone; CIP, ciprofloxacin; IPM, imipenem; MEM, meropenem; PIP, piperacillin; TZP, piperacillin-tazobactam; TOB, tobramycin.
Mean zone of inhibition. FlamZ, Flammazine (1% SSD); FlamC, Flammacerium (1% SSD plus 2.2% cerium nitrate); Sulfa, Sulfamylon (8.5% mafenide acetate); Isobet, Isobetadine cream (10% povidone-iodine); Fura, Furacine (0.2% nitrofurazone).
FIG. 3.
Time course of P. aeruginosa AFLP35 and AFLP8 colonization in the BWC. P, patient.
FIG. 4.
Colonization of different sites of patient P13 with four P. aeruginosa genotypes. The horizontal bars represent the length of time that patient P13 was colonized.
Retrospective analysis of cryopreserved serotype O12 P. aeruginosa isolates showed that AFLP35 had been isolated from 12 patients in 1997.
Further analysis of the predominant clones observed in this study, which was done by combining the AFLP pattern, the nucleotide sequences of three (lipo)protein genes (oprI, oprL, and oprD), serotype, and pyoverdine type, revealed that all strains except the AFLP8 strain belonged to recently identified clonal complexes (42).
Isolates of the AFLP18 strain were recovered from the water of a “clean” trolley, the sinks of a patient room and the cafeteria, and the floor of a bathroom. Isolates of the AFLP2 strain were recovered from the unfiltered water of the main water tap of one ICU and two MCU bathrooms. Closely related genotypes AFLP36 and AFLP37 were recovered from the water from a faucet in an operating theater and an ICU patient room. Genetically related strains AFLP44, AFLP45, and AFLP46 were isolated from the sinks of three rooms in the MCU.
Serotyping.
Of the 48 AFLP genotypes, 28 (58.3%) belonged to serotypes 1 (n = 6), 6 (n = 10), and 11 (n = 12) (Table 1). All isolates of each AFLP type exhibited the same serotype. Isolates belonging to the predominant genotypes exhibited serotypes O12 (AFLP35), O6 (AFLP32 and AFLP33), and O11 (AFLP1). AFLP8 isolates agglutinated with antiserum mixture E (O2, O5, O15, and O16) but not with monovalent antisera.
Drug susceptibility testing.
Table 1 shows the average drug sensitivities of the 48 genotypes investigated. Most strains were found to be susceptible to most of the antibiotics tested. Isolates of four AFLP types (AFLP1, AFLP32, AFLP33, and AFLP35) were multiply antibiotic resistant. The AFLP1 strain was already MDR when it was first introduced into the BWC by admission of a colonized patient who had been transferred from Turkey. This MDR strain acquired further resistance to piperacillin to become resistant to all commercially available antibiotics. The AFLP35 strain, which was initially resistant to cefepime, ceftriaxone, ciprofloxacin, piperacillin, and tazobactam, acquired, step by step, resistance to amikacin, aztreonam, ceftazidime, imipenem, and meropenem to become an MDR strain. Strain AFLP32 was originally resistant to ceftriaxone, imipenem, and meropenem, but it soon acquired additional resistance to aztreonam, ciprofloxacin, and piperacillin. The statistics based on the results of all drug sensitivity tests performed from 1996 to 1998 show a disturbing amount of resistance in P. aeruginosa strains (Table 2), mainly due to the very high frequency of isolation of the epidemic MDR AFLP35 strain. The rates of resistance in the P. aeruginosa were higher than those observed in Staphylococcus aureus isolates.
TABLE 2.
Antibiotic susceptibilities of P. aeruginosa and S. aureus isolates recovered in the BWC from 1996 to 1998a
| Organism and antibiotic | Percentb
|
No. of isolates tested | ||
|---|---|---|---|---|
| R | S | I | ||
| P. aeruginosa | ||||
| Imipenem-cilastin | 7.5 | 85 | 7.5 | 146 |
| Imipenem | 16.7 | 74.5 | 8.8 | 317 |
| Amikacin | 21.7 | 45.5 | 32.8 | 686 |
| Ceftazidime | 21.9 | 75.9 | 2.2 | 686 |
| Meropenem | 28.4 | 63.4 | 8.2 | 134 |
| Aztreonam | 29.2 | 51.5 | 19.3 | 685 |
| Cefepime | 48 | 38.6 | 13.4 | 171 |
| Piperacillin | 67.3 | 32.7 | 0 | 688 |
| Cefsulodin | 69.3 | 29.2 | 1.5 | 685 |
| Norfloxacin | 71.2 | 27 | 1.8 | 685 |
| Ticarcillin | 71.3 | 28.6 | 0.1 | 688 |
| Ciprofloxacin | 95.9 | 2.7 | 1.4 | 73 |
| S. aureus | ||||
| Vancomycin | 0 | 100 | 0 | 12 |
| Netilmicin | 0.6 | 98.9 | 0.6 | 544 |
| Minocycline | 1.6 | 98.2 | 0.2 | 546 |
| Trimethoprim-sulfamethoxazole | 4.7 | 94.7 | 0.5 | 551 |
| Oxacillin | 17.5 | 82.5 | 0 | 553 |
| Amoxicillin-clavulanic acid | 17.9 | 81.7 | 0.4 | 553 |
| Erythromycin | 38.6 | 60.3 | 1.1 | 544 |
| Penicillin V | 91.3 | 8.5 | 0.2 | 554 |
Determined by the Bauer-Kirby agar disk diffusion method.
R, resistant; S, sensitive; I, intermediate level of resistance.
As shown in Table 1, 8.5% mafenide acetate (Sulfamylon) was by far the most effective topical antipseudomonal agent in vitro, with a mean inhibition zone (MIZ) of >30 mm. SSD (1%) and 2.2% cerium nitrate (Flammacerium) (MIZ = 21.0 mm), 1% SSD (Flammazine) (MIZ = 20.2 mm), and 10% povidone-iodine (Isobetadine cream) (MIZ = 19.1 mm) showed comparable in vitro activities against P. aeruginosa. Nitrofurazone (0.2%) was less effective, with an MIZ of 11.6 mm. The antibiotic-sensitive epidemic AFLP8 strain was less sensitive to 1% SSD and 1% SSD-2.2% cerium nitrate, the most widely used SSD-based antibacterial creams, than isolates of the other genotypes. Some AFLP8 isolates showed virtually no inhibition zones with either of those agents in the AWD test. Decreased in vitro sensitivities caused by prolonged use were not observed for either of the creams.
DISCUSSION
Acquisition routes.
The simultaneous isolation of antibiotic-resistant serotype O12 P. aeruginosa isolates in five burn wound patients was recognized as unusual. As a result, an epidemiological study was initiated in order to investigate the routes of P. aeruginosa acquisition in our BWC. P. aeruginosa colonization may originate from endogenous sources such as the intestinal tract (38) or from exogenous sources such as contaminated equipment or other patients colonized with P. aeruginosa (20). Understanding the relative importance of the routes of colonization is crucial to the development of effective preventive measures against infection. Even if the overall rate of P. aeruginosa colonization is not significantly reduced, it is important to recognize cross-infecting strains, especially if they exhibit resistance to a variety of antibiotics and give rise to severe infections.
As demonstrated in other studies (6), the inanimate hospital environment was not an important source of the P. aeruginosa isolates recovered from patients, even though 21 strains of P. aeruginosa were found in the burn unit environment. Isolates of strain AFLP2 and two groups of closely related environmental genotypes, AFLP36 and 37 and AFLP44, 45, and 46, were isolated from remote, tap water-related sites in the BWC. We suspect a common origin for these strains somewhere upstream from the water faucets, possibly the water reservoir of the hospital.
Colonized patients represent a continuous reservoir of (epidemic) strains from which other patients can be colonized via cross-acquisition (33). A limitation of this study is that no rectal swab or stool samples were collected on admission. As a result, we have no direct information concerning the colonization of the intestinal tracts of the patients on admission. The large number of unique genotypes observed in the patients (n = 25), however, suggests that a large number of patients were colonized from an endogenous source. On the other hand, 29 patients were colonized with the AFLP35 strain, 19 patients were colonized with the AFLP8 strain, and 6 patients were colonized with both genotypes. Although a common-source outbreak was initially suspected, a thorough survey of the inanimate hospital environment failed to identify an ongoing reservoir of AFLP35 and AFLP8 strains. Six AFLP35 isolates were recovered from the hospital environment, but all seemed to originate from colonized patients.
The observation that the antibiotic resistance of environmental AFLP35 isolates increased in parallel with that of the clinical isolates strengthened our conclusion that AFLP35 contamination of the environment was secondary to patient carriage. Since AFLP8 isolates were isolated from patients only 2 months before the last environmental survey, detection of this strain in the hospital environment was less likely than detection of AFLP35, which persisted in the BWC for years. The absence of an ongoing reservoir for P. aeruginosa AFLP35 and AFLP8 in the hospital environment and the presence of at least one colonized patient in the burn unit during the outbreak period (Fig. 3) suggest that the spread of both epidemic strains was related to cross-contamination between patients. The primary source of the two epidemic P. aeruginosa strains, the inanimate environment or a patient who was colonized on admission (intestinal tract or respiratory tract), remains unknown. The observation of 57 cases of cross-colonization indicates that cross-colonization was the main route of P. aeruginosa acquisition in our BWC. Several studies have demonstrated that cross-acquisition can play an important part in the epidemiology of nosocomial colonization and infection with P. aeruginosa (3, 5, 44).
In our BWC, horizontal transmission from colonized patients presumably occurred via transient contamination of staff or equipment involved in patient care. This conclusion is supported by the following three observations. (i) Several patients who were hospitalized during overlapping periods were colonized with the same P. aeruginosa strain. Patient P13, who was continuously colonized with isolates of both genotypes, especially in his wounds (Fig. 4), during his 10-month hospitalization was probably the reservoir from which both genotypes disseminated through horizontal transmission during patient nursing. (ii) P. aeruginosa isolates of the AFLP35 strain were detected in the patient's surroundings, such that cross-acquisition is more than likely. AFLP35 was isolated from materials which were directly contaminated by the patient, such as the stretcher of a hydrotherapy facility, respiratory therapy equipment, and a 5% chlorhexidine solution (Hibidil), which was used during hydrotherapy. The isolation of the MDR AFLP35 strain from a chlorhexidine solution is not surprising. Recently, Koljalg et al. (28) observed a relation between antibiotic resistance and increased chlorhexidine resistance. There are indications that triclosan, another widely used synthetic antimicrobial agent, selects for antibiotic resistance (51). Triclosan is used in a multitude of health care and consumer products including hand soaps, surgical scrubs, health care personnel hand washes, toothpastes, and mouthwashes (26). An environment in which antimicrobial agents like chlorhexidine and triclosan are routinely used could select for bacteria that are (inherently) resistant to these agents, like P. aeruginosa. Hydrotherapy equipment has been strongly linked to a P. aeruginosa epidemic in burn wound patients (57). A possible route of transmission for the SSD-resistant (SSDr) AFLP8 strain is the contamination of primary sterile supplies of 1% SSD or 1% SSD-2.2% cerium nitrate. An investigation of 19 brands of topical creams demonstrated high counts of bacteria and fungi, including P. aeruginosa, in 6 of them (40). On the other hand, the AFLP18 strain was also recovered from crucial points in the patients' surroundings, but none of the patients were colonized with this strain. (iii) During the multiple outbreaks, the length of exposure before known colonization dropped dramatically, sometimes to 1 or 2 days, for both epidemic clones (Fig. 3). This phenomenon is observed when the repetitive early inoculation of wounds with P. aeruginosa (during hydrotherapy, for example) overcomes the prophylactic effects of the topical agents (36).
Breaks in barrier nursing techniques, like touching the patient without sterile gloves, inefficient hand washing between patient contacts, inefficient cleaning of hydrotherapy equipment facilities, the nontimely replacement of used antiseptics and antibacterial creams, and inefficient grouping of infected and noninfected patients, were occasionally observed or admitted to by the medical staff and provided the opportunity for patient-to-patient transmission. Circumstances such as a shortage of personnel and the outdated architecture of the BWC contributed to unnecessary patient movement and breaks in barrier nursing techniques.
We also observed the frequent occurrence of polyclonal P. aeruginosa colonization. Nineteen burn wound patients were simultaneously colonized with more than one strain of P. aeruginosa. Patient P13 harbored at least four distinct P. aeruginosa strains (Fig. 4). The simultaneous cocolonization of individual patients with different P. aeruginosa strains, exhibiting different antibiotic resistance patterns, demands reasoned sampling of isolates used for antibiotic susceptibility testing.
Serotyping.
The usefulness of serotyping is limited by the high frequency of nonserotypeable and polyagglutinable strains and the high prevalence of a few serotypes. Serotyping was performed to support the similarities found on the molecular level and to compare our results with those of earlier epidemiological studies. The predominant serotypes were O1, O6, and O11, which is in concordance with the results of earlier studies (4, 42). The MDR AFLP35 strain belonged to serogroup O12. Epidemic strains are frequently of serotypes O11 and O12 (13, 14, 15, 19, 43, 47, 55). Detection of the simultaneous presence of three distinct P. aeruginosa O12 strains, one of which was an environmental strain from the BWC, by AFLP analysis illustrates the advantage of genotyping over serotyping.
Drug susceptibility.
Several strains, including the epidemic MDR AFLP35 strain, acquired resistance to amikacin, aztreonam, ceftazidime, imipenem, meropenem, and piperacillin, which are, not accidentally, the first-line antibiotics used in our BWC. This illustrates the importance of the selective pressure of antibiotics in the emergence and selection of MDR epidemic strains (47). Retrospective analysis demonstrated that the AFLP35 strain was already omnipresent in the BWC in 1997 and can be qualified as endemic. Nowadays, outbreaks with MDR P. aeruginosa strains have become rather frequent (12, 41), and the persistence of an MDR P. aeruginosa clone in a burn unit has been reported (25). However, in this study we have reported on the persistence of a moderately antibiotic-resistant strain of a widespread (serotype O12) clone which gradually became MDR.
SSD is the most widely used silver compound in antimicrobial creams (27). Because of the considerable differences in the diffusion rates of the different topical agents, it has been suggested that the inhibition zone sizes measured in the AWD test should not be interpreted as an indicator of the superiority of one topical agent over another (56). However, one can argue that the diffusion capacity of a topical agent in agar can be an indicator for its penetration strength in wound beds, which is a very important aspect of the use of topical agents for burns. Furthermore, Thomson et al. (56) found that the clinical efficacy data for SSD and mafenide acetate were in good agreement with the results of the AWD test. The 8.5% mafenide acetate cream was by far the most active topical agent in vitro against the P. aeruginosa isolates analyzed in this study. This is partly due to the high diffusion capacity of this agent in the AWD test. Since systemic toxicity (metabolic acidosis) attends the use of mafenide acetate, this topical agent should be used as briefly as possible for the treatment of established microbial burn wound infections, especially those caused by P. aeruginosa (36). Despite its high degree of penetration (24), 0.2% nitrofurazone had comparatively poor activity against P. aeruginosa in the AWD test. This parallels the findings of earlier studies, which showed that nitrofurazone is more effective against gram-positive cocci than against gram-negative rods (24, 56).
SSD (1%), 1% SSD-2.2% cerium nitrate, and 10% povidone-iodine showed comparable in vitro activities against the P. aeruginosa strains studied. Unfortunately, povidone-iodine is readily absorbed in the blood and has been associated with lethal metabolic acidosis (36). Since no significant toxicity attributable to SSD or cerium has been observed, 1% SSD and 1% SSD-2.2% cerium nitrate can be used prophylactically and may delay colonization of extensive burn wounds with gram-negative bacilli for from 10 to 14 days (36). Although cerium nitrate was shown to have a potent antiseptic effect in burn wounds, especially against gram-negative bacteria and fungi (37), in this study, 1% SSD was found to be almost as effective against P. aeruginosa as 1% SSD-2.2% cerium nitrate. Marone et al. (34) also found no difference in the in vitro activities of SSD alone and SSD in combination with cerium nitrate against staphylococci and gram-negative bacteria. The benefit of cerium in reducing infection in contaminated wounds remains controversial (27). The emergence of SSDr strains is rare, even when the agent is routinely used (36). Plasmid-mediated silver resistance has been observed in Acinetobacter baumannii (9), Escherichia coli (54), Salmonella enterica serovar Typhimurium (35), and Pseudomonas stutzeri (22). Little is known, however, about the molecular genetic basis of bacterial silver resistance. Plasmid-mediated resistance to silver was studied in detail in Salmonella (21). The resistance determinant encoded a periplasmic silver-specific binding protein and two parallel efflux pumps. Silver-resistant mutants of E. coli displayed active efflux of silver ions and were deficient in porins (29). The silver resistance in a P. stutzeri strain was assumed to be due to the formation of silver-sulfide complexes (52). Resistance of clinical P. aeruginosa isolates to SSD was also detected (23) and was assumed to be plasmid related. From 1977 to 1998 some gentamicin-resistant P. aeruginosa strains were found to be resistant to silver nitrate, but this resistance was revealed to be unstable and disappeared upon repeated subculture (8). The resistance of the AFLP8 strain to SSD also diminished upon subculture and subsequent cryopreservation. The mechanisms of silver resistance in P. aeruginosa have not been thoroughly investigated. The fact that the AFLP8 strain persisted in our BWC, colonizing various body sites of multiple patients for long periods of time, with subsequent severe infections, shows that SSDr P. aeruginosa strains can pose problems comparable to those caused by antibiotic-resistant strains.
Although it is a somewhat controversial technique, the AWD test can eliminate antibacterial creams inappropriate for use against particular bacterial strains. In this specific case, 1% SSD and 1% SSD-2.2% cerium nitrate could have been excluded for use on burn wounds colonized with the AFLP8 strain.
The implementation of a strict infection control strategy with a more rational use of antibiotics and the topical use of antibiotics could probably have prevented the dissemination of the MDR and SSDr epidemic strains in our BWC.
Disease habitat selection.
The rate of detection of the MDR strain in sputum (14%) was significantly higher than that of the SSDr strain (3%), which, on the other hand, showed a higher prevalence in burn wound samples (62%) compared to that of the MDR strain (45%). The mean interval between admission and recovery of the first isolate was significantly shorter for the SSDr AFLP8 strain (10.7 days) than for the MDR AFLP35 strain (17.4 days). The mean colonization length was longer for the SSDr AFLP8 strain (47.1 days) than for the MDR AFLP35 strain (33.2 days). The SSDr AFLP8 strain colonized the burn wounds of patient P13 for more than 7 months but was only sporadically isolated from sputum and urine (Fig. 4). These results indicate an advantage of the SSDr strain in colonizing burn wounds, probably due to the very low concentrations of systemically administered antibiotics in burn wounds caused by wound ischemia (36) and its insensitivity to the topical creams 1% SSD and 1% SSD-2.2% cerium nitrate. In the lungs, the SSDr strain, being multiantibiotic sensitive, is efficiently eradicated by antibiotics, in contrast to the MDR strain. Several studies have demonstrated that there is no strong association of particular P. aeruginosa clones with a particular pathovar (1, 17, 42, 46, 48, 49). In contrast, Lomholt et al. (32) recently found evidence for an epidemic clone that is pathogenic for the eye. In this study we demonstrate that particular (acquired) properties of strains, like drug resistance, can elicit selection for a particular (human disease-related) habitat. Hence, which came first: the drug resistance or clinical habitat selection? We hypothesize that differences in drug susceptibility (MDR versus SSDr) were the basis of the niche selection observed and were accentuated through the systemic administration of antibiotics and topical burn wound treatment. The AFLP35 strain appeared to be more invasive than the AFLP8 strain. The AFLP35 strain caused severe, occasionally lethal, burn wound sepsis in five patients (patients P11, P13, P19, P23, and P25) and inhalative pneumonia in one patient (patient P4), whereas the AFLP8 strain caused lethal burn wound sepsis in only one patient (patient P41) (Fig. 3).
Conclusion.
We observed cycles of ongoing transmission of two persistent P. aeruginosa strains with distinctive features. The first strain, an MDR AFLP35 serotype O12 strain which was endemic in our BWC, belonged to a previously described successful and widespread clonal complex (42), and the second strain was the supposedly rare SSDr AFLP8 serotype E strain. Both strains apparently persisted in the BWC through insufficient compliance with infection control measures and selective pressure from antibiotics and SSD-based antibacterial creams. The background for these recurrent outbreaks was one of high rates of endogenous colonization and very low rates of acquisition from the inanimate hospital environment, which was characterized by polyclonality and which strongly resembled the state of polyclonal endemicity described by Bonten et al. (7) in ICUs. Fingerprinting by AFLP analysis provides discriminatory power comparable to that of pulsed-field gel electrophoresis for the epidemiological study of P. aeruginosa (53). The present study demonstrates the usefulness of genotyping in the fight against nosocomial infections. Acquisition times, rates of colonization, and phenotypic characteristics like serotype and antibiotic susceptibility patterns can provide alerts to an emerging outbreak. Additional genotyping of surveillance isolates by RAPD-PCR, which is less reproducible but which is also less expensive and time-consuming than AFLP or pulsed-field gel electrophoresis analysis, enables clinicians to confirm an outbreak. A detailed prospective epidemiological study by a multiparametric approach, like the one demonstrated in this work, can provide useful information on the routes of colonization. AFLP20 to AFLP26 strains exhibited similar genotypes but distinct serotypes. Sequencing of the oprL gene and pyoverdine typing revealed that these strains were not epidemiologically related. This illustrates the advantages of a polyphasic approach. Routine fingerprinting of P. aeruginosa isolates could have prevented the two P. aeruginosa outbreaks. The medical staff of the BWC concluded on 10 August that an outbreak was occurring, while retrospective epidemiological analysis demonstrated that on 3 July four patients were already colonized with the AFLP35 epidemic strain (Fig. 3). Nowadays, several genotyping methods are in use, although they are mostly in use in large university laboratories or research-oriented medical microbiology units. Public health authorities should support the provision of genotyping techniques like AFLP analysis or molecular epidemiology in general in the routine microbiology laboratory.
Acknowledgments
This work was supported by grant WB14/2002 of the Belgian Department of Defense.
We thank Y. Denis, M. Pauwels, C. Taquet, M. Arnolds, J. Jacobs, and A. Van De Sompel for contributions to this research.
REFERENCES
- 1.Alonso, A., F. Rojo, and J. L. Martinez. 1999. Environmental and clinical isolates of Pseudomonas aeruginosa show pathogenic and biodegradative properties irrespective of their origin. Environ. Microbiol. 1:421-430. [DOI] [PubMed] [Google Scholar]
- 2.Bauer, A. N., W. M. M. Kirby, J. C. Sherris, and M. Turk. 1966. Antibiotic susceptibility testing and standardised single disc method. Am. J. Clin. Pathol. 45:493-496. [PubMed] [Google Scholar]
- 3.Bergmans, D. C. J. J., M. J. M. Bonten, F. H. van Tiel, C. A. Gaillard, S. van der Geest, R. M. Wilting, P. W. de Leeuw, and E. E. Stobberingh. 1998. Cross-colonisation with Pseudomonas aeruginosa of patients in an intensive care unit. Thorax 53:1053-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bert, F., and N. Lambert-Zechovsky. 1996. Comparative distribution of resistance patterns and serotypes in Pseudomonas aeruginosa isolates from intensive care units and other wards. J. Antimicrob. Chemother. 37:809-813. [DOI] [PubMed] [Google Scholar]
- 5.Bertrand, X., M. Thouverez, D. Talon, A. Boillot, G. Capellier, C. Floriot, and J. P. Helias. 2001. Endemicity, molecular diversity and colonisation routes of Pseudomonas aeruginosa in intensive care units. Intensive Care Med. 27:1263-1268. [DOI] [PubMed] [Google Scholar]
- 6.Blanc, D. S., C. Petignat, B. Janin, J. Bille, and P. Francioli. 1998. Frequency and molecular diversity of Pseudomonas aeruginosa upon admission and during hospitalization: a prospective epidemiologic study. Clin. Microbiol. Infect. Dis. 4:242-247. [DOI] [PubMed] [Google Scholar]
- 7.Bonten, M. J. M., D. C. J. J. Bergmans, H. Speijer, and E. E. Stobberingh. 1999. Characteristics of polyclonal endemicity of Pseudomonas aeruginosa colonization in intensive care units. Am. J. Respir. Crit. Care Med. 160:1212-1219. [DOI] [PubMed] [Google Scholar]
- 8.Bridges, K., A. Kidson, E. J. Lowbury, and M. D. Wilkins. 1979. Gentamycin- and silver-resistant Pseudomonas in a burns unit. Br. Med. J. 1:446-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deshpande, L. M., and B. A. Chopade. 1994. Plasmid-mediated silver resistance in Acinetobacter baumannii. Biometals 7:49-56. [DOI] [PubMed] [Google Scholar]
- 10.De Vos, D., A. Lim Jr., J.-P. Pirnay, L. Duinslaeger, H. Revets, A. Vanderkelen, R. Hamers, and P. Cornelis. 1997. Analysis of epidemic Pseudomonas aeruginosa isolates by isoelectric focusing of pyoverdine and RAPD-PCR: modern tools for an integrated anti-nosocomial infection strategy in burn wound centres. Burns 23:379-386. [DOI] [PubMed] [Google Scholar]
- 11.De Vos, D., A. Lim, Jr., J.-P. Pirnay, M. Struelens, C. Vandenvelde, L. Duinslaeger, A. Vanderkelen, and P. Cornelis. 1997. Direct detection and identification of Pseudomonas aeruginosa in clinical samples such as skin biopsy specimens and expectorations by multiplex PCR based on two outer membrane lipoprotein genes, oprI and oprL. J. Clin. Microbiol. 35:1295-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Douglas, M. W., K. Mulholland, V. Denyer, and T. Gottlieb. 2001. Multi-drug resistant Pseudomonas aeruginosa outbreak in a burns unit—an infection control study. Burns 27:131-135. [DOI] [PubMed] [Google Scholar]
- 13.Dubois, V., C. Arpin, M. Melon, B. Melon, C. Andre, C. Frigo, and C. Quentin. 2001. Nosocomial outbreak due to a multiresistant strain of Pseudomonas aeruginosa P12: efficacy of cefepime-amikacin therapy and analysis of β-lactam resistance. J. Clin. Microbiol. 39:2072-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elaichouni, A., G. Verschraegen, G. Claeys, M. Devleeschouwer, C. Godard, and M. Vaneechoutte. 1994. Pseudomonas aeruginosa serotype O12 outbreak studied by arbitrary primer PCR. J. Clin. Microbiol. 32:666-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farmer, J. J., III, R. A. Weinstein, C. H. Zierdt, and C. D. Brokopp. 1982. Hospital outbreaks caused by Pseudomonas aeruginosa: importance of serogroup O11. J. Clin. Microbiol. 16:266-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fluit, A. C., J. Verhoef, F. J. Schmitz, and The European SENTRY Participants. 2001. Frequency of isolation and antimicrobial resistance of gram-negative and gram-positive bacteria from patients in intensive care units of 25 European university hospitals participating in the European arm of the SENTRY Antimicrobial Surveillance Program 1997-1998. Eur. J. Clin. Microbiol. Infect. Dis. 20:617-625. [DOI] [PubMed] [Google Scholar]
- 17.Foght, J. M., D. W. S. Westlake, W. M. Johnson, and H. F. Ridgway. 1996. Environmental gasoline-utilizing isolates of Pseudomonas aeruginosa are taxonomically indistinguishable by chemotaxonomic and molecular techniques. Microbiology 142:2333-2340. [DOI] [PubMed] [Google Scholar]
- 18.Gilligan, P. H. 1995. Pseudomonas and Burkholderia, p. 509-519. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 6th ed. American Society for Microbiology, Washington, D.C.
- 19.Grattard, F., O. G. Gaudin, B. Pozzetto, A. Ros, and A. D. Mbida. 1993. Genotypic homogeneity of nosocomial Pseudomonas aeruginosa O12 strains demonstrated by analysis of protein profiles, DNA fingerprints and rRNA gene restriction patterns. Eur. J. Clin. Microbiol. Infect. Dis. 12:57-61. [DOI] [PubMed] [Google Scholar]
- 20.Grundmann, H., A. Kropec, D. Hartung, R. Berner, and F. Daschner. 1993. Pseudomonas aeruginosa in a neonatal intensive care unit: reservoirs and ecology of the nosocomial pathogen. J. Infect. Dis. 168:943-947. [DOI] [PubMed] [Google Scholar]
- 21.Gupta, A., K. Matsui, J. F. Lo, and S. Silver. 1999. Molecular basis for resistance to silver cations in Salmonella. Nat. Med. 5:183-188. [DOI] [PubMed] [Google Scholar]
- 22.Haefeli, C., C. Franklin, and K. Hardy. 1984. Plasmid-determined silver resistance in Pseudomonas stutzeri isolated from a silver mine. J. Bacteriol. 158:389-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heggers, J. P., and M. C. Robson. 1978. The emergence of silver sulphadiazine resistant Pseudomonas aeruginosa. Burns 5:184-187. [Google Scholar]
- 24.Herruzo-Cabrera, R., V. Garcia-Torres, J. Rey-Calero, and M. J. Vizcaino-Alcaide. 1992. Evaluation of the penetration strength, bactericidal efficacy and spectrum of action of several antimicrobial creams against isolated microorganisms in a burn centre. Burns 18:39-44. [DOI] [PubMed] [Google Scholar]
- 25.Hsueh, P. R., L. J. Teng, P. C. Yang, Y. C. Chen, S. W. Ho, and K. T. Luh. 1998. Persistence of a multidrug-resistant Pseudomonas aeruginosa clone in an intensive care burn unit. J. Clin. Microbiol. 36:1347-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones, R. D., H. B. Jampani, J. L. Newman, and A. S. Lee. 2000. Triclosan: a review of effectiveness and safety in health care settings. Am. J. Infect. Control 28:184-196. [PubMed] [Google Scholar]
- 27.Klasen, H. J. 2000. A historical review of the use of silver in the treatment of burns. II. Renewed interest for silver. Burns 26:131-138. [DOI] [PubMed] [Google Scholar]
- 28.Koljalg, S., P. Naaber, and M. Mikelsaar. 2002. Antibiotic resistance as an indicator of bacterial chlorhexidine susceptibility. J. Hosp. Infect. 51:106-113. [DOI] [PubMed] [Google Scholar]
- 29.Li, X. Z., H. Nikaido, and K. E. Williams. 1997. Silver-resistant mutants of Escherichia coli display active efflux of Ag+ and are deficient in porins. J. Bacteriol. 179:6127-6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu, P. V., H. Matsumoto, H. Kusama, and T. Bergan. 1983. Survey of heat-stable, major somatic antigens of Pseudomonas aeruginosa. Int. J. Syst. Bacteriol. 33:256-264. [Google Scholar]
- 31.Livermore, D. M. 2002. Multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: our worst nightmare? Clin. Infect. Dis. 34:634-640. [DOI] [PubMed] [Google Scholar]
- 32.Lomholt, J. A., K. Poulsen, and M. Kilian. 2001. Epidemic population structure of Pseudomonas aeruginosa: evidence for a clone that is pathogenic to the eye and that has a distinct combination of virulence factors. Infect. Immun. 69:6284-6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maki, D. G. 1978. Control of colonization and transmission of pathogenic bacteria in the hospital. Ann. Intern. Med. 89:777-780. [DOI] [PubMed] [Google Scholar]
- 34.Marone, P., V. Monzillo, L. Perversi, and E. Carretto. 1998. Comparative in vitro activity of silver sulfadiazine, alone and in combination with cerium nitrate, against staphylococci and gram-negative bacteria. J. Chemother, 10:17-21. [DOI] [PubMed] [Google Scholar]
- 35.McHugh, S. L., R. C. Moellering, C. C. Hopkins, and M. N. Swartz. 1975. Salmonella typhimurium resistant to silver nitrate, chloramphenicol, and ampicillin. Lancet i:235-240. [DOI] [PubMed]
- 36.Monafo, W. W., and V. H. Ayvazian. 1978. Topical therapy. Surg. Clin. N. Am. 58:1157-1171. [DOI] [PubMed] [Google Scholar]
- 37.Monafo, W. W., S. N. Tandon, V. H. Ayvazian, J. Tuchschmidt, A. M. Skinner, and F. Deitz. 1976. Cerium nitrate: a new topical antiseptic for extensive burns. Surgery 80:465-473. [PubMed] [Google Scholar]
- 38.Murthy, S. K., A. L. Baltch, R. P. Smith, E. K. Desjardin, M. C. Hammer, J. V. Conroy, and P. B. Michelsen. 1989. Oropharyngeal and fecal carriage of Pseudomonas aeruginosa in hospital patients. J. Clin. Microbiol. 27:35-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.National Committee for Clinical Laboratory Standards. 2000. Performance standards for antimicrobial disk susceptibility testing, 7th ed. M2A7. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 40.Na'was, T., and A. Alkofahi. 1994. Microbial contamination and preservative efficacy of topical creams. J. Clin. Pharmacol. Ther. 19:41-46. [DOI] [PubMed] [Google Scholar]
- 41.Pellegrino, F. L., L. M. Teixeira, M. G. S. Carvalho, S. Aranha Nouér, M. Pinto de Oliveira, J. L. Mello Sampaio, A. D'Avila Freitas, A. L. P. Ferreira, E. L. T. Amorim, L. W. Riley, and B. M. Moreira. 2002. Occurrence of a multidrug-resistant Pseudomonas aeruginosa clone in different hospitals in Rio de Janeiro, Brazil. J. Clin. Microbiol. 40:2420-2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pirnay J.-P., D. De Vos, C. Cochez, F. Bilocq, A. Vanderkelen, M. Zizi, B. Ghysels, and P. Cornelis. 2002.. Pseudomonas aeruginosa displays an epidemic population structure. Environ. Microbiol. 4:898-911. [DOI] [PubMed]
- 43.Pitt, T. L., D. M. Livermore, D. Pitcher, A. C. Vatopoulos, and N. J. Legakis. 1989. Multiresistant serotype O12 Pseudomonas aeruginosa: evidence for a common strain in Europe. Epidemiol. Infect. 103:565-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pradella, S., M. Pletschette, F. Mantey-Stiers, and W. Bautsch. 1994. Macrorestriction analysis of Pseudomonas aeruginosa in colonized burn patients. Eur. J. Clin. Microbiol. Infect. Dis. 13:122-128. [DOI] [PubMed] [Google Scholar]
- 45.Pruitt, B. A., Jr., A. T. McManus, S. H. Kim, and C. W. Goodwin. 1998. Burn wound infections: current status. World J. Surg. 22:135-145. [DOI] [PubMed] [Google Scholar]
- 46.Rahme, L. G., E. J. Stevens, S. F. Wolfort, J. Shao, R. G. Tompkins, and F. M. Ausubel. 1995. Common virulence factors for bacterial pathogenicity in plants and animals. Science 268:1899-1902. [DOI] [PubMed] [Google Scholar]
- 47.Richard, P., R. Le Floch, C. Chamoux, M. Pannier, E. Espaze, and H. Richet. 1994. Pseudomonas aeruginosa outbreak in a burn unit: role of antimicrobials in the emergence of multiply resistant strains. J. Infect. Dis. 170:377-383. [DOI] [PubMed] [Google Scholar]
- 48.Römling, U., J. Wingender, H. Müller, and B. Tümmler. 1994. A major Pseudomonas aeruginosa clone common to patients and aquatic habitats. Appl. Environ. Microbiol. 60:1734-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ruimy, R., E. Genauzeau, C. Barnabe, A. Beaulieu, M. Tibayrenc, and A. Andremont for the Pseudomonas aeruginosa Study Group. 2001. Genetic diversity of Pseudomonas aeruginosa strains isolated from ventilated patients with nosocomial pneumonia, cancer patients with bacteremia, and environmental water. Infect. Immun. 69:584-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Savelkoul, P. H. M., H. J. M. Aarts, J. de Haas, L. Dijkshoorn, B. Duim, M. Otsen, J. L. W. Rademaker, L. Schouls, and J. A. Lenstra. 1999. Amplified-fragment length polymorphism analysis: the state of an art. J. Clin. Microbiol. 37:3083-3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schweizer, H. P. 2001. Triclosan: a widely used biocide and its link to antibiotics. FEMS Microbiol. Lett. 202:1-7. [DOI] [PubMed] [Google Scholar]
- 52.Slawson, R. M., E. M. Lohmeier-Vogel, H. Lee, and J. T. Trevors. 1994. Silver resistance in Pseudomonas stutzeri. Biometals 7:30-40. [DOI] [PubMed] [Google Scholar]
- 53.Speijer, H., P. H. M. Savelkoul, M. J. Bonten, E. E. Stobberingh, and J. H. T. Tjhie. 1999. Application of different genotyping methods for Pseudomonas aeruginosa in a setting of endemicity in an intensive care unit. J. Clin. Microbiol. 37:3654-3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Starodub, M. E., and J. T. Trevors. 1990. Mobilization of Escherichia coli R1 silver-resistance plasmid pjT1 by Tn5-Mob into Escherichia coli C600. Biol. Metals 3:24-27. [DOI] [PubMed] [Google Scholar]
- 55.Tassios, P. T., V. Gennimata, A. N. Maniatis, C. Fock, and N. J. Legakis. 1998. Emergence of multidrug resistance in ubiquitous and dominant Pseudomonas aeruginosa serogroup O:11. J. Clin. Microbiol. 36:897-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thomson, P. D., T. E. Taddonio, M. J. Tait, and J. K. Prasad. 1989. Susceptibility of pseudomonas and staphylococcus wound isolates to topical antimicrobial agents: a 10-year review and clinical evaluation. Burns 15:190-192. [DOI] [PubMed] [Google Scholar]
- 57.Tredget, E. E., H. A. Shankowsky, A. M. Joffe, T. I. Inkson, K. Volpet, W. Paranchych, P. C. Kibsey, J. D. Alton, and J. F. Burke. 1992. Epidemiology of infections with Pseudomonas aeruginosa in burn patients: the role of hydrotherapy. Clin. Infect. Dis. 15:941-949. [DOI] [PubMed] [Google Scholar]
- 58.Vos, P., R. Hogers, M. Bleeker, M. Reijans, T. van der Lee, M. Hornes, A. Frijters, J. Pot, J. Peleman, M. Kuiper, and M. Zabeau. 1995. AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res. 23:4407-4414. [DOI] [PMC free article] [PubMed] [Google Scholar]