Abstract
Blastocystis has a widespread distribution in a variety of animals, which is a potential source of infection for humans. However, the contribution of zoonotic transmission remains unclear due to the absence of molecular proof of these organisms being identical to those found in humans. We report herein the similar subgroup of Blastocystis isolates from humans, pigs, and a horse using a restriction fragment length polymorphism (RFLP) analysis of partial small-subunit ribosomal DNA (ssu rDNA). Additionally, sequence and phylogenic analysis of partial ssu rDNA of Blastocystis from a human, a pig, and a horse sharing a common subgroup shows that Blastocystis isolates from a pig and a horse were monophyletic and closely related to B. hominis, with 92 to 94% identity. These results suggest the possibility of zoonotic potential of Blastocystis.
Blastocystis hominis is one of the most common intestinal protozoa found in humans. This organism has been recognized as a causative agent of diarrhea both in immunocompromised and immunocompetent hosts in several studies (6, 8). However, its role in human disease is still intensely debated since most cases are asymptomatic (2, 18, 21, 23). It has been reported from both developed and developing countries, with a high prevalence in tropical areas, ranging between 30 and 50% (2, 15, 21). Our knowledge of this organism in several aspects, including its transmission, is still unclear. Zoonotic transmission of B. hominis has been speculated since epidemiological studies suggested a linkage between close contact with animals and blastocystosis in humans (8, 17). In addition, Blastocystis-like organisms were detected in a wide range of animals (1, 4, 9, 16). However, there was no conclusive evidence demonstrating animal-to-human transmission and zoonotic potential of B. hominis. One of the difficulties is the identification of these organisms since infection with Blastocystis has been diagnosed on the basis of morphology, host of origin, and in vitro culture characteristics (4). Although the morphology of Blastocystis detected in some animals was similar to those found in humans, only few animal isolates of Blastocystis were genetically proven to be identical to human isolates (7, 25). Using a restriction fragment length polymorphism (RFLP) analysis of small-subunit ribosomal DNA (ssu rDNA), Clark identified a ribodeme of Blastocystis isolated from guinea pigs similar to that found in B. hominis (7). Yoshikawa et al. also showed that the pattern of random amplified polymorphic DNA in Blastocystis isolated from a chicken was similar to that displayed by B. hominis isolate HE87-1 (25). These observations, however, were limited and lack epidemiological data to support the association. Therefore, to determine the zoonotic potential of B. hominis, we conducted a study of genotypic characterization using RFLP analysis of partial ssu rDNAs of Blastocystis isolated from humans compared to those isolated from animals. ssu rDNAs of Blastocystis isolated from a human, a pig, and a horse were also partially sequenced for phylogenic analysis.
MATERIALS AND METHODS
Collection of fecal samples.
Stool specimens of the army personnel who lived on an army base in Chonburi Province, Thailand, were collected and examined for Blastocystis by wet preparation under light microscopy. Every specimen was then cultivated for Blastocystis using Jones' medium supplemented with 10% horse serum and incubated at 37°C for 2 to 3 days. Stool specimens of pigs and horses raised on this army base were also collected and examined by the same method. Culture-positive specimens were centrifuged at 300 × g for 5 min at 4°C, and the pellets were washed three times in phosphate-buffered saline (pH 7.4) and stored at −80°C until processed for DNA.
DNA extraction.
Extraction of genomic DNA was performed by using the QIAamp DNA stool mini kit (Qiagen, Hilden, Germany), following the manufacturer's instructions. Genomic DNA of each sample was kept at −20°C until used.
PCR-RFLP analysis.
Genotypic characterization of Blastocystis from humans and animals was determined by an RFLP analysis of partial ssu rDNA (4). Although a few different primer pairs were used for the PCR-RFLP analysis of this gene in recent reports (3, 7, 12, 19), we chose a primer pair specific to partial ssu rDNA of B. hominis (3) since mixed infection with intestinal protozoa might be found in some specimens. Genomic DNA and a primer pair (forward, 5′-GGAGGTAGTGACAATAAATC-3′; reverse, 5′-CGTTCATGATGAACAATT-3′) were used in PCR with conditions as described by Böhm-Gloning et al. (3). PCR amplification was performed using a Perkin-Elmer 480 thermal cycler. After an initial 4-min denaturing step at 94°C, 35 PCR cycles were carried out, each consisting of a 30-s annealing at 54°C, 30-s extension at 72°C, 30-s denaturing at 94°C, and an additional cycle with a 5-min chain elongation at 72°C. The PCR products and molecular markers were electrophoresed in 2% agarose gel (FMC Bioproducts, Philadelphia, Pa.) with 1.5% Tris-borate-EDTA (TBE) buffer using a Mupid gel electrophoresis device (Cosmo Bio, Tokyo, Japan). Bands were visualized under UV light after being stained with ethidium bromide and documented on Polaroid film using a FoTo Prep (Fotodyne). Digestions of the PCR products were performed using three restriction enzymes, HinfI, RsaI, and AluI (Gibco-BRL, Gaithersburg, Md.), and separated by 2% agarose gel electrophoresis.
Amplification of ssu rDNA and sequencing.
A partial sequence of ssu rDNA of Blastocystis isolated from a horse had been amplified by a pair of primers described by Böhm-Gloning et al. (3), but the complete ssu rDNA region of Blastocystis isolated from a human and a pig was amplified by a pair of primers described by Sogin (20). DNA sequencing was performed by an automated ABI PRISM 377 DNA sequencer at the Armed Forces Research Institute of Medical Sciences, Bangkok, Thailand. Nucleotide sequences were determined with the Sequencer program (Gene Codes Corporation, Inc. [1995 release]).
Sequence and phylogenic analysis.
There were two sets of partial ssu rDNA sequence alignments since total DNA of Blastocystis isolated from horse feces was not enough to reamplify for the complete region of ssu rDNA. The first set of partial ssu rDNA sequence alignment consists of 1,054 bp from nine taxa, including four taxa of B. hominis, four taxa of Blastocystis, and Caecitellus parvulus. The second set of ssu rDNA sequence alignment was 1,738 bp from four taxa of B. hominis, three taxa of Blastocystis, and C. parvulus. The details of these taxa are shown in Table 1.
TABLE 1.
Selected Thai Blastocystis taxa for the ssu rDNA region sequenced and additional taxa from GenBank
| Taxon | Strain no. | Host or source | Geographic origin | GenBank accession no. | No. of bp |
|---|---|---|---|---|---|
| Blastocystis hominis, subgroup III | Human | Thailand | AF439782 | 1,700 | |
| Blastocystis sp., subgroup III | Pig | Thailand | AF538348 | 1,707 | |
| Blastocystis sp., subgroup III | Horse | Thailand | AF538349 | 1,025 | |
| B. hominis | HE87 | Human | Japan | AB023499 | 1,740 |
| B. hominis | HE87 | Human | Japan | AB023578 | 1,740 |
| B. hominis | Nand II (ATCC 50177) | Human | United States | U51151 | 1,770 |
| Blastocystis sp. | NIH 1295:1 | Unidentified guinea pig | U51152 | 1,778 | |
| Blastocystis sp., isolate U26177 | Unidentified guinea pig | U26177 | 1,776 | ||
| Caecitellus parvulus | NBH4 | Deep-sea hydrothermal vents | Eastern Pacific Ocean | AF174368 | 1,772 |
For simple phylogenic analysis, two sets of partial ssu rDNA sequence alignments were aligned separately using the multiple alignment program CLUSTAL W (22), and this was followed by manual alignment. The total number of identical base pairs was reported as percentage of base identity. Gap positions were introduced into the alignment to account for nucleotide insertion-deletion event.
The data were analyzed using the parsimony heuristic search, neighbor joining, and bootstrapping parsimony heuristic search of the Phylogeny Analysis Using Parsimony program (version 4.0b8). The stepwise addition option was used to find the most-parsimonious bootstrap trees. Bootstrapping by using the full heuristic search option of the Phylogeny Analysis Using Parsimony program was performed to calculate the robustness of each branch. The sequence of C. parvulus was considered to be an out-group. The analysis was set with the following parameters: 1,000 bootstrap replicates (10, 11), with gaps treated as missing data, tree bisection-reconnection branch swapping, and random sequence addition. All sites were weighted equally. The percentage of bootstrap replicates that confirmed each clade is indicated in Fig. 3 and 6. Hosts and geographical locations were also mapped onto a bootstrap single-most-parsimonious tree inferred from partial ssu rDNA.
FIG. 3.
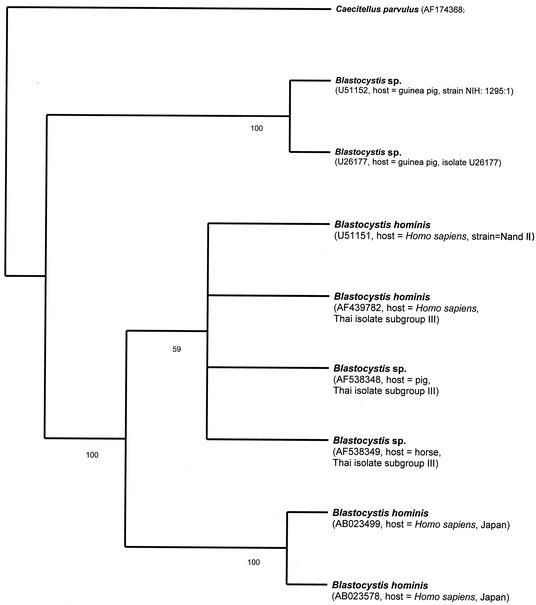
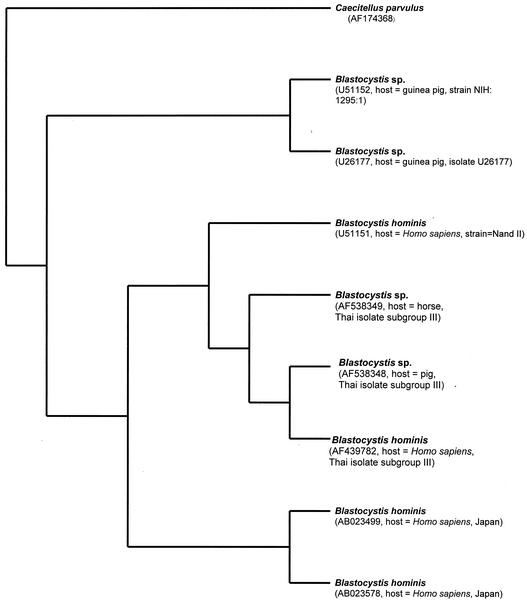
Phylogenetic relationships of selected Blastocystis spp. inferred from partial ssu rDNA sequence (1,054 bp). A single most-parsimonious tree with tree length of 355, consistency index of 0.9352, and retention index of 0.8663 was found. Bootstrap replicates, 1,000. Bootstrap frequencies of more than 50 are indicated on each branch. GenBank accession numbers of sequences are in parentheses.
FIG. 6.
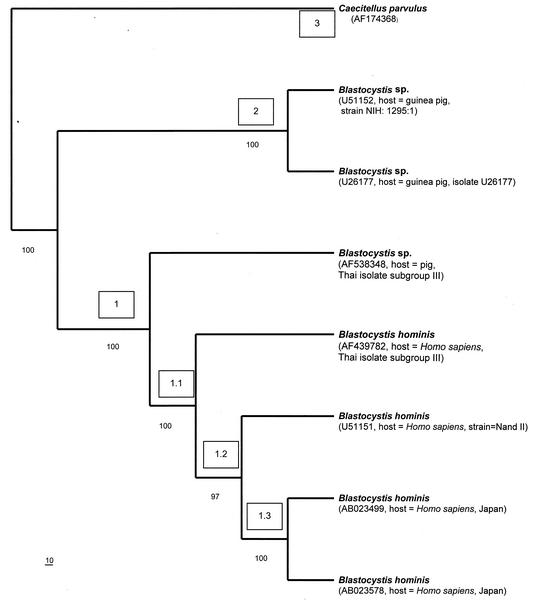
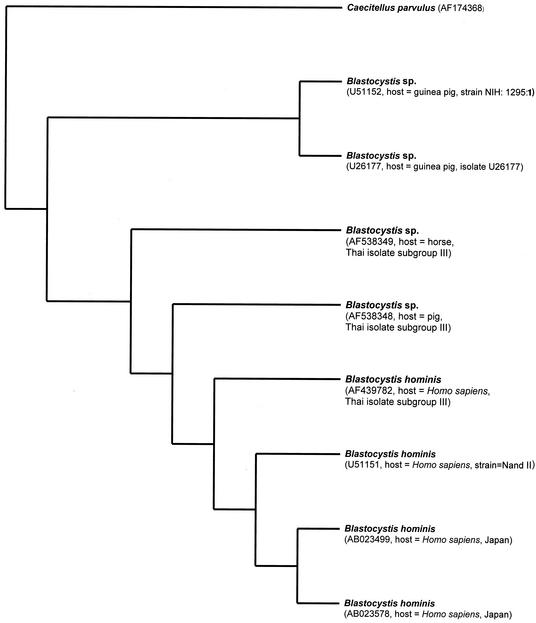
Phylogenetic relationships of selected Blastocystis spp. inferred from partial ssu rDNA sequence (1,738 bp). A single most-parsimonious tree with tree length of 520, consistency index of 0.9577, and retention index of 0. 9185 is shown. Bootstrap replicates, 1,000. Bootstrap frequencies of more than 50 are indicated on each branch. Nodes and clades are numbered in parentheses in the order in which they are described in the text. Bar, 10 substitutions. GenBank accession numbers of sequences are in parentheses.
RESULTS
PCR-RFLP analysis of partial ssu rDNA of Blastocystis isolated from humans and animals.
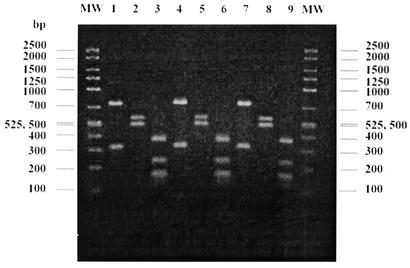
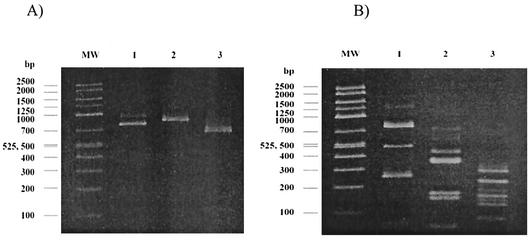
Stool specimens were collected from the army personnel (n = 924), pigs (n = 26), and horses (n = 8). Of these specimens, 334 (36.9%), 25 (96.1%), and 1 (12.5%), respectively, were positive for Blastocystis by in vitro cultivation. Compared to in vitro cultivation, we found that wet preparation was rather insensitive for the detection of Blastocystis. The comparison of these methods has been reported elsewhere (14). All culture-positive specimens were then processed for DNA extraction and PCR amplification; however, only 153, 20, and 1 specimen from humans, pigs, and a horse, respectively, were positive for PCR product. Since we used stool-containing specimens, it is not surprising that approximately half of the specimens did not produce PCR products. PCR amplification using stool specimens is rather insensitive because of PCR inhibitors (24). Many researchers have used different methods to overcome this problem, including purification and concentration. However, we did not perform these processes of purification or concentration since Blastocystis might have been easily ruptured by these steps, so reducing the number of organisms (14). All isolates from pigs and a horse were positive for the expected ssu rDNA amplicon at 1,100 bp, while amplification from 146 and 13 human isolates revealed a band at 1,100 and 850 bp, respectively (data not shown). These PCR products were used for the genotypic determination by PCR-RFLP. Genotypic characterization of Blastocystis isolated from humans and animals is shown in Table 2. The most-common banding patterns in the specimens from humans were identical to group III of B. hominis reported by Böhm-Gloning et al. (3). This subgroup was also found in all specimens from pigs and a horse. Figure 1 shows the representative Blastocystis partial ssu rDNA RFLP banding patterns produced by HinfI, RsaI, and AluI in the isolate from a human, a pig, and a horse. Another seven human isolates that presented the amplicon at 850 bp showed different banding patterns after being digested by the same enzymes. These banding patterns, shown in Fig. 2A, were identified as group I of B. hominis. Mixed infection with group I and group III of B. hominis was found in six specimens. Banding patterns that have not been identified by Böhm-Gloning et al. (3) were found in two specimens from humans and described as group VI in this study (Fig. 2B).
TABLE 2.
Distribution of Blastocystis subgroups among humans, pigs, and a horse using PCR-RFLP analysis of partial ssu rDNA (3)
| Source of specimen | No. (%) with indicated genotype
|
Total no. | |||
|---|---|---|---|---|---|
| I | III | I + III | VI | ||
| Human | 7 (4.6) | 138 (90.2) | 6 (3.9) | 2 (1.3) | 153 |
| Pig | 20 (100) | 20 | |||
| Horse | 1 (100) | 1 | |||
FIG. 1.
Comparison of the representative PCR-RFLP analysis of partial ssu rDNA of Blastocystis isolates from a human (lanes 1 to 3), a pig (lanes 4 to 6), and a horse (lanes 7 to 9). These banding patterns were identified as subgroup III as previously described (3). The PCR product in lanes 1, 4, and 7 was digested by HinfI; the PCR product in lanes 2, 5, and 8 was digested by RsaI; and the PCR product in lane 3, 6, and 9 was digested with AluI. MW, molecular weight marker.
FIG. 2.
Banding patterns of subgroup I (A) and subgroup VI (B) using PCR-RFLP analysis of partial ssu rDNA of Blastocystis isolated from humans. The PCR product in lane 1 was digested by HinfI, the PCR product in lane 2 was digested by RsaI, and the PCR product in lane 3 was digested by AluI. MW, molecular weight marker.
Sequence and phylogenic analysis.
In the first set of ssu rDNA sequence alignment, there are 1,054 positions, comprising 774 constant sites from four taxa of B. hominis, four taxa of Blastocystis isolated from animals, and C. parvulus. In 1,054 alignable sites, the 160 variable sites are parsimony uninformative, whereas the number of parsimony-informative sites equals 120. In the preliminary analysis, 1,054 alignable sites of ssu rDNA from nine taxa were used for bootstrap analysis, heuristic search, and neighbor-joining analysis (Fig. 3 to 5). A single most-parsimonious tree of 355 steps, with a consistency index of 0.9352 and a retention index of 0.8663 was found (Fig. 3). The dendrograms derived from the bootstrap analysis, heuristic search, and neighbor-joining analysis of 1,054 alignable sites of ssu rDNA sequence alignment from eight taxa of Blastocystis showed that the six taxa of Blastocystis, isolated from a pig, a horse, and humans, form a monophyletic group sharing the same common ancestor. This topology was strongly supported by 100% bootstrap values (Fig. 3). Comparison of the 1,054 bp of the partial ssu rDNA region showed divergence to be 5.87% between the genus Blastocystis isolate from a horse and the B. hominis isolates from humans (GenBank accession number U51151 and Thai isolate subgroup III). Only 61 positions were found to be variable, over a total of 1,054 bp among these three taxa. Examining 61 positions more closely, there were 13 insertion-deletion positions (1.23%), 14 transition positions (1.33% [seven A/G and seven C/T]) and 34 transversion positions (3.32% [6 A/C, 11 A/T, 6 G/C, and 11 G/T]).
For sequence analysis, the second data set consists of 1,738 alignable sites from seven taxa of Blastocystis mentioned previously, except Blastocystis sp. isolated from a horse (GenBank accession number AF538349, Thai isolate subgroup III; source, horse, Thailand). In 1,738 alignable sites, the 234 variable sites are parsimony uninformative, whereas the number of parsimony-informative sites equals 180. One thousand three hundred twenty-four base pairs are constant. Phylogenetic analysis of 1,738 bp of ssu rDNA resulted in parsimonious trees of 520 steps, with a consistency index of 0.9577 and a retention index of 0.9185. Comparison of the 1,738 bp of ssu rDNA showed 96% identity between two B. hominis isolates from humans (Thai isolate subgroup III AB023578 and GenBank accession number U51151) and a Blastocystis sp. isolated from a pig. In 4% (70 of 1,738) of base pair differences in ssu rDNA between B. hominis isolates from humans and a Blastocystis sp. isolate from a pig, transversion was about 2% (36 of 1,738 bp [9 A/C, 13 A/T, 3 G/C, and 11 G/T]) and transition was about 1% (17 of 1,738 bp [12 A/G and 5 C/T]), with 1% insertion-deletion events (17 of 1,738 bp). To facilitate discussion, we have defined these clades as groups 1 to 3 (Fig. 6). Group 1 was also divided into subgroups 1.1 to 1.3 (Fig. 6).
DISCUSSION
In the present study, PCR-RFLP analysis of partial ssu rDNA allowed us to study genetic variation in human isolates of B. hominis. Heterogeneity in B. hominis has been described in previous reports (3, 5, 7, 12, 13, 25). To date, there is no standardized RFLP analysis of ssu rDNA to evaluate genetic variation in B. hominis. We chose the method described by Böhm-Gloning et al. because of its specificity (3). This method is also cheaper than others to perform because only three restriction enzymes are used. Compared to the study of Böhm-Gloning et al. (3), our study found less variation, which could be explained by the origin of B. hominis isolates. Böhm-Gloning et al. (3) used B. hominis isolates from various groups of patients, including travelers and immigrants who must have gotten the infection in different parts of the world. In contrast to our study, B. hominis was isolated from army personnel who lived in the same area and also shared the same risks.
We also used this method to preliminarily compare Blastocystis isolates from humans and animals that lived in the same area. Banding patterns identical to subgroup III could be found in humans, pigs, and a horse, while other banding patterns were only found in human isolates. This suggests the existence of different host specificity among many different genotypes of Blastocystis. Snowden et al. found a common genotype in different animal hosts (19), which indicates that some genotypes of Blastocystis are not host specific. Moreover, cross infection of Blastocystis between humans and animals has been suggested by the observation of similar patterns of RFLP (7) and random amplified polymorphic DNA (25) analysis. Our data confirm these findings.
We then analyzed the partial ssu rDNA sequences of Blastocystis isolates from a human, a pig, and a horse that exhibited similar banding patterns of RFLP. There are two lines of evidence suggesting that the Blastocystis isolates from a pig and a horse are very closely related to B. hominis isolates from humans. First, phylogenetic analysis based on ssu rDNA sequences depicted that the Blastocystis isolates from pigs and horses always form a sister clade to B. hominis isolates from humans. Second, the 92 to 94% (∼6% divergence) identity of partial ssu rDNA sequences is found among B. hominis isolates from humans and Blastocystis isolates from a pig and a horse. Furthermore, this result is consistent with reports done by Kukoschke and Müller (13) and Boreham et al. (5). The results of sequence analysis of ssu rDNA here perhaps reveal host nonspecificity of Blastocystis species.
The results of comparison of 1,054 bp of ssu rDNA sequences of Blastocystis species also imply that the level of similarity of partial ssu rDNA is quite correlated with hosts from which Blastocystis species were isolated. High similarity (97 to 99%) of partial ssu rDNA sequences is found among B. hominis strains isolated from the same host, while low identity (79 to 89%) of partial ssu rDNA sequences is noticeable among Blastocystis species isolated from different hosts, except that 92% homology was found between partial ssu rDNA sequences from a pig and a horse. For example, a comparison of ssu rDNA sequences of B. hominis (Thai isolate subgroup III) and B. hominis (GenBank accession number U51151; strain Nand) showed 99% homology (Fig. 3). There was 97% homology of the ssu rDNA region among B. hominis (Thai isolate subgroup III), B. hominis (GenBank accession number AB023578; strain HE87, Japan), and B. hominis (GenBank accession number AB023499; strain HE87, Japan), while there was 89% homology of the ssu rDNA region among B. hominis (Thai isolate subgroup III), Blastocystis sp. (GenBank accession number U26177; source, unidentified guinea pig; isolate U26177) and Blastocystis sp. (GenBank accession number U51152; source, unidentified guinea pig; strain NIH:1295:1) (Fig. 3). There was 80% identity of partial ssu rDNA between B. hominis and C. parvulus.
However, the dendrogram from the bootstrapping analysis (Fig. 3) suggested that 1,054 alignable sites are not divergent enough to distinguish between four taxa of B. hominis (GenBank accession number AF439782; Thai isolate subgroup III), Blastocystis sp. (GenBank accession number AF538348, Thai isolate subgroup III; source, pig, Thailand), Blastocystis sp. (GenBank accession number AF538349; Thai isolate subgroup III; source, horse, Thailand), and B. hominis (GenBank accession number U51151; strain Nand). This clade was only supported by 59% bootstrap values and unresolved topologies of four taxa of Blastocystis derived from the bootstrap analysis. Thus, the 1,738 bp of ssu rDNA sequence analysis of the second data set from seven taxa of Blastocystis was constructed.
Phylogenetic analysis based on ssu rDNA sequences from the second data set indicated that B. hominis isolated from humans and Blastocystis isolated from a pig form a monophyletic clade separately from Blastocystis isolates from guinea pigs. This result was strongly supported by 100% bootstrap values and identical topologies of all dendrograms derived from the bootstrap analysis, neighbor joining analysis, and heuristic search (data not shown). The other evidence is the 98 to 99% similarity of ssu rDNA sequences between B. hominis isolates from Japan, Thailand, and the United States and the 93 to 95% identity between B. hominis and Blastocystis isolates from a pig. In contrast, there is 88 to 89% nucleotide similarity between B. hominis isolates from humans, Blastocystis isolates from a pig, and Blastocystis isolates from guinea pigs. In the meantime, we regard the overall topology in Fig. 6 as a working hypothesis of evolutionary relationships that requires further testing. The remaining discussion focuses on the taxonomically evolutionary relationships of groups 1 to 3.
Group 1 represents taxa including B. hominis GenBank accession number AB023578; B. hominis GenBank accession number AB023499; B. hominis Thai isolate, subgroup III, GenBank accession number AF439782; B. hominis GenBank accession number U51151; and Blastocystis sp. subgroup III, isolated from a pig. This branch was supported with a 100% confident bootstrap value (Fig. 6). Four different B. hominis isolates from humans in this clade have shown 1 to 2% variation of ssu rDNA sequence alignment. Group 1 was divided into three subgroups, which are 1.1, 1.2, and 1.3. Subgroup 1.1 is composed of B. hominis Thai isolate subgroup III, B. hominis GenBank accession number AB023578 and AB023499, and B. hominis GenBank accession number U51151, with 100% bootstrapping and 99% identity of partial ssu rDNA sequences. Subgroup 1.2 includes B. hominis GenBank accession number U51151 and B. hominis GenBank accession number AB023578 and AB023499 (97% bootstrapping). B. hominis GenBank accession numbers AB023578 and AB023499 in group 1.3 were strains isolated from Japan with 100% bootstrapping and 99% identity of partial ssu rDNA sequences. The phylogenic tree of ssu rDNA sequences showed that Blastocystis sp. subgroup III, isolated from a pig, is part of a sister group with B. hominis isolates from humans, with a 100% confident bootstrap value, and that the sequences of B. hominis, isolated from humans, have been diverged recently. This result is consistent with 93 to 96% similarity of ssu rDNA between B. hominis isolates from humans and Blastocystis sp. isolates from a pig. In 1,738 bp, the homology of ssu rDNA between B. hominis isolates from humans in Japan and Blastocystis sp. isolates from a pig are 93 to 94%. There is about 96% identity of 1,738 bp of ssu rDNA between two B. hominis isolates from humans (Thai isolate subgroup III AB023578 and GenBank accession number U51151) and a Blastocystis sp. isolated from pig.
In group 2, both Blastocystis species were isolated from guinea pigs (GenBank accession numbers U51152 and U26177), with the strong robustness of a 100% bootstrap value. The homologies of ssu rDNA sequences of Blastocystis sp. isolates from guinea pigs and those from humans and a pig are 88 and 89%, respectively.
In conclusion, phylogeny analyses of partial ssu rDNA sequences of selected isolates of Blastocystis with the same pattern of restriction site analysis of amplified ssu rDNA suggests that B. hominis isolates are monophyletic, forming a sister group to Blastocystis species isolated from a pig and a horse. These results perhaps imply that B. hominis has evolved from Blastocystis sp. isolates from domestic animals closely linked with humans, such as pigs and horses. There is no correlation between host and geographical origin for clades of the inferred trees. The high values of the consistency index and retention index suggest that there is no homoplasy in these tested sequences. Using PCR-RFLP and phylogeny analyses of partial ssu rDNA, our data might be evidence of the zoonotic potential of Blastocystis. Nonetheless, interpretation of the phylogenetic results is restricted to selected species in Blastocystis; more samples from pigs, horses, and humans from different geographic locations (examined by RFLP analysis of ssu rDNA) and complete sequences of ssu rDNA should be examined for further biodiversity studies of Blastocystis. Since one molecular phylogeny of one gene cannot explain all evolutionary relationships among organisms, other genes, such as complete internal transcribed spacers 1 and 2 and 5.8S rDNA, from many species of Blastocystis as well as epidemiological data may imply more evolutionary relationships between Blastocystis isolates from different hosts, the origin of the pathogenic Blastocystis, and the correlation between species of Blastocystis and geographic locations.
FIG. 4.
Maximum analysis of ssu rDNA sequences of 1,054 bp for selected genera in Blastocystis and Caecitellus parvulus as a reference taxon. GenBank accession numbers and hosts of each taxon are in parentheses.
FIG. 5.
Neighbor-joining based on ssu rDNA analysis containing 9 taxa of selected genera in Blastocystis. The designated outgroup is Caecitellus parvulus. GenBank accession numbers and hosts of each taxon are in parentheses.
Acknowledgments
We thank Tawee Naaglor for his technical assistance.
This work was financially supported by Thailand-Tropical Diseases Research Programme (T2) (ID 00-1-HEL-24-011).
REFERENCES
- 1.Abe, N., M. Nagoshi, K. Takami, Y. Sawano, and H. Yoshikawa. 2002. A survey of Blastocystis sp. in livestock, pets, and zoo animals in Japan. Vet. Parasitol. 106:203-212. [DOI] [PubMed] [Google Scholar]
- 2.Ashford, R. W., and E. A. Atkinson. 1992. Epidemiology of Blastocystis hominis infection in Papua New Guinea: age-prevalence and associations with other parasites. Ann. Trop. Med. Parasitol. 86:129-136. [DOI] [PubMed] [Google Scholar]
- 3.Böhm-Gloning, B., J. Knobloch, and B. Walderich. 1997. Five subgroups of Blastocystis hominis isolates from symptomatic and asymptomatic patients revealed by restriction site analysis of PCR-amplified 16S-like rDNA. Trop. Med. Int. Health 2:771-778. [DOI] [PubMed] [Google Scholar]
- 4.Boreham, P. F. L., and D. J. Stenzel. 1993. Blastocystis in humans and animals: morphology, biology, and epizootiology. Adv. Parasitol. 32:1-70. [DOI] [PubMed] [Google Scholar]
- 5.Boreham, P. F. L., J. A. Upcroft, and J. A. Dunn. 1992. Protein and DNA evidence for two demes of Blastocystis hominis for humans. Int. J. Parasitol. 22:49-53. [DOI] [PubMed] [Google Scholar]
- 6.Cirioni, O., A. Giacometti, D. Drenaggi, F. Ancarani, and G. Scalise. 1999. Prevalence and clinical relevance of Blastocystis hominis in diverse patient cohorts. Eur. J. Epidemiol. 15:989-993. [DOI] [PubMed] [Google Scholar]
- 7.Clark, C. G. 1997. Extensive genetic diversity in Blastocystis hominis. Mol. Biochem. Parasitol. 87:79-83. [DOI] [PubMed] [Google Scholar]
- 8.Doyle, P. W., M. M. Helgason, R. G. Mathias, and E. M. Proctor. 1990. Epidemiology and pathogenicity of Blastocystis hominis. J. Clin. Microbiol. 28:116-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duda, A., D. J. Stenzel, and P. F. L. Boreham. 1998. Detection of Blastocystis sp. in domestic dogs and cats. Vet. Parasitol. 76:9-17. [DOI] [PubMed] [Google Scholar]
- 10.Efron, B. 1979. Bootstrapping methods: another look at the jackknife. Ann. Stat. 7:1-26. [Google Scholar]
- 11.Felsenstein, J. 1985. Confidence intervals on phylogenies: an approach using the bootstrap. Evolution 39:783-791. [DOI] [PubMed] [Google Scholar]
- 12.Hoevers, J., P. Holman, K. Logan, M. Hommel, R. Ashford, and K. Snowden. 2000. Restriction-fragment-length polymorphism analysis of small-subunit rRNA genes of Blastocystis hominis isolates from geographically diverse human hosts. Parasitol. Res. 86:57-61. [DOI] [PubMed] [Google Scholar]
- 13.Kukoschke, K. G., and H. E. Müller. 1991. SDS-PAGE and immunological analysis of different axenic Blastocystis hominis strains. J. Med. Microbiol. 35:35-39. [DOI] [PubMed] [Google Scholar]
- 14.Leelayoova, S., P. Taamasri, R. Rangsin, T. Naaglor, U. Thathaisong, and M. Mungthin. In-vitro cultivation: a sensitive method for detecting Blastocystis hominis. Ann. Trop. Med. Parasitol., in press. [DOI] [PubMed]
- 15.Nimri, L. F. 1993. Evidence of an epidemic of Blastocystis hominis infections in preschool children in northern Jordan. J. Clin. Microbiol. 31:2706-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pakandl, M. 1991. Occurrence of Blastocystis sp. in pigs. Folia Parasitol. 38:297-301. [PubMed] [Google Scholar]
- 17.Rajah-Salim, H., G. Suresh-Kumar, S. Vellayan, J. W. Mak, A. Khairul-Anuar, I. Init, G. D. Vennila, R. Saminathan, and K. Ramakrishnan. 1999. Blastocystis in animal handlers. Parasitol. Res. 85:1032-1033. [DOI] [PubMed] [Google Scholar]
- 18.Senay, H., and D. MacPherson. 1990. Blastocystis hominis: epidemiology and natural history. J. Infect. Dis. 162:987-990. [DOI] [PubMed] [Google Scholar]
- 19.Snowden, K., K. Logan, C. Blozinski, J. Hoevers, and P. Holman. 2000. Restriction-fragment-length polymorphism analysis of small-subunit rRNA genes of Blastocystis isolates from animal hosts. Parasitol. Res. 86:62-66. [DOI] [PubMed] [Google Scholar]
- 20.Sogin, M. L. 1990. Amplification of ribosomal RNA genes for molecular evolution studies, p. 307-314. In M. A. Innis, D. H. Gelfand, J. J. Sninsky, and T. J. White (ed.), PCR protocol: a guide to methods and applications. Academic Press, New York, N.Y.
- 21.Taamasri, P., S. Leelayoova, R. Rangsin, T. Naaglor, A. Ketupanya, and M. Mungthin. 2002. Prevalence of Blastocystis hominis carriage in Thai army personnel based in Chonburi, Thailand. Mil. Med. 167:643-646. [PubMed] [Google Scholar]
- 22.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Udkow, M. P., and E. K. Markell. 1993. Blastocystis hominis: prevalence in asymptomatic versus symptomatic hosts. J. Infect. Dis. 168:242-244. [DOI] [PubMed] [Google Scholar]
- 24.Wildmer, G. 1998. Genetic heterogeneity and PCR detection of Cryptosporidium parvum. Adv. Parasitol. 40:223-239. [DOI] [PubMed] [Google Scholar]
- 25.Yoshikawa, H., I. Nagano, E. H. Yap, M. Singh, and Y. Takahashi. 1996. DNA polymorphism revealed by arbitrary primers polymerase chain reaction among Blastocystis strains isolated from humans, a chicken, and a reptile. J. Eukaryot. Microbiol. 43:127-130. [DOI] [PubMed] [Google Scholar]