Abstract
A decision analysis was conducted to evaluate the cost-effectiveness of programs in which the Amplified Mycobacterium Tuberculosis Direct test (MTD) (Gen-Probe) is used to rapidly exclude Mycobacterium tuberculosis complex as a cause of disease in smear-positive respiratory specimens. MTD sensitivity, specificity, and probability of inhibition for smear-positive specimens were estimated from literature reports. Costs and laboratory performance characteristics were determined from review of records and practices at an urban hospital in the mid-Atlantic United States. In the base case, 31.4% of smear-positive specimens were assumed to be culture positive for M. tuberculosis. Under these conditions, the marginal cost of the MTD testing program was estimated as $338 per smear-positive patient, or $494 per early exclusion of tuberculosis based on negative MTD results. By comparison, the cost of respiratory isolation ($27.77/day) and drugs ($5.66/day) averted by MTD testing was estimated at $201 per early tuberculosis exclusion. MTD testing was therefore not cost-effective in this scenario. Sensitivity analysis revealed that cost-effectiveness estimates are sensitive to the number of smear-positive specimens processed annually, the relative prevalence of M. tuberculosis in smear-positive specimens, and the marginal daily cost of respiratory isolation. A decision tool is therefore presented for assessing the cost-effectiveness of MTD under various combinations of those three variables. While routine MTD testing of smear-positive specimens is not expected to be cost-saving for most individual hospitals, centralized reference laboratories may be able to implement MTD in a cost-effective manner across a wide range of situations.
Tuberculosis (TB) is a leading infectious killer worldwide and is far from elimination as a public health threat in the United States and other developed countries (14, 22). Nevertheless, in countries such as France (23), the United Kingdom (12), and the United States (9), incidence rates of TB (caused by Mycobacterium tuberculosis) are beginning to fall relative to rates of disease from mycobacteria other than TB (MOTT), particularly M. avium complex. Rapid diagnosis of TB currently relies on the examination of smears stained for acid-fast bacilli (AFB). However, AFB smears cannot distinguish TB from MOTT. Mycobacterial culture readily differentiates between TB and MOTT, but culture results are often not available for 2 to 3 weeks after specimen collection. Since the sputum AFB smear identifies the individuals who are potentially most infectious, current clinical guidelines are to isolate patients who are suspected to have TB on the basis of sputum smear-positive results in negative-pressure rooms (4) and to place such individuals on presumptive therapy for TB, pending either results from culture or response to therapy. However, MOTT are not typically transmitted from person to person by the respiratory route and do not necessarily respond to antibiotics directed against M. tuberculosis.
In recent years, rapid diagnostic tests for TB based on nucleic acid amplification techniques have been developed. Two such tests are currently approved in the United States for the detection of M. tuberculosis complex organisms in smear-positive respiratory specimens: the AMPLICOR Mycobacterium tuberculosis test (Roche Diagnostics, Inc., Indianapolis, Ind.) and the Amplified Mycobacterium Tuberculosis Direct test (MTD) (Gen-Probe, Inc., San Diego, Calif.). The MTD is currently marketed as an “enhanced” version that, unlike the AMPLICOR test, is also approved for use with smear-negative specimens (5). For smear-positive respiratory specimens, the sensitivity of the enhanced MTD has been reported between 91.7% (17) and 100% (2, 6, 11, 20), with specificity between 99.6% (6) and 100% (2, 11, 19). Despite such demonstrated high validity, the routine implementation of nucleic acid amplification tests in clinical settings has been slow, due in large part to concerns of their high cost, previously estimated at $50 to $100 per test in most laboratories (10). To determine whether this cost may be offset by savings in the form of isolation room and medication expense, we conducted a cost-effectiveness analysis of the routine use of MTD during the evaluation of patients suspected of having pulmonary TB at a large urban hospital in the mid-Atlantic United States.
MATERIALS AND METHODS
Cost-effectiveness analysis.
A decision-analytic model was constructed to measure the cost-effectiveness of implementing MTD for the routine detection of M. tuberculosis in smear-positive respiratory specimens. Cost-effectiveness was measured in terms of cost per early TB exclusion. “Early exclusion of TB” was defined as the proper exclusion of M. tuberculosis as the etiologic agent of smear-positive respiratory disease on the basis of MTD results. This analysis was carried out from the perspective of the hospital or health care system. The base case was assumed to be a hospitalized person with symptoms consistent with active pulmonary TB and positive AFB smear results on at least one respiratory specimen.
MTD diagnostic strategy.
In order to measure the cost-effectiveness of MTD relative to the standard of care (no MTD), it was necessary to outline a diagnostic strategy for the use of MTD. We assumed an MTD strategy in which a single MTD test was run on the first smear-positive respiratory specimen submitted from any patient in whom a diagnosis of mycobacterial disease had not been made in the previous 30 days. Tests would be performed according to the manufacturer's instructions. Positive and negative amplification controls would be run on each day that MTD testing was performed. Results of >30,000 relative light units (RLU) would be considered positive or insufficient for the exclusion of M. tuberculosis, whereas those of ≤30,000 RLU would be considered negative or sufficient to exclude M. tuberculosis. All MTD-negative specimens would be tested for inhibitors by spiking the sample with an aliquot of M. tuberculosis, either in a separate reaction run simultaneously with the original test (a strategy in which MTD-positive specimens would also be tested for inhibitors) or immediately following the initial MTD result. Samples found to contain MTD-inhibitory substances would be considered to provide insufficient evidence for the exclusion of M. tuberculosis and would not be retested. Repeat runs in our institution were rarely necessary outside the context of training or equipment fault; costs of failed training runs were included in our estimates, but we assumed that additional failures on patient samples would not occur. All MTD results were assumed to be uniformly available 24 h after the initial positive AFB smear result. The results of both MTD and smear were assumed to be available 6 days per week.
Smear-positive patients were assumed to be placed immediately in respiratory isolation and started on presumptive therapy for TB, consisting of isoniazid, rifampin, pyrazinamide, and ethambutol. Respiratory isolation would be continued until the earliest of the following: a negative MTD result, isolation of MOTT from culture, or successful completion of 2 weeks of therapy.
Estimation of analysis variables.
The sensitivity and specificity of the MTD test, as well as the likelihood of sample inhibition, were estimated from the literature. For this purpose, we conducted a literature search using Medline to identify studies that reported data sufficient to determine the sensitivity and specificity of the enhanced MTD in smear-positive respiratory specimens. Eight such reports (2, 6, 8, 11, 16, 17, 19, 20) were identified. A total of 852 smear-positive samples were evaluated in these reports, 517 of which were from a single laboratory (6). To determine the validity of the MTD test, we used culture (or culture plus clinical criteria, when reported) as the “gold standard.” The likelihood of MTD inhibition was estimated from the subset of reports that provided this information.
The relative prevalence of TB among individuals with smear-positive respiratory specimens, as well as the average length of time between smear and culture result, was ascertained directly using data from our hospital's clinical microbiology laboratory. This facility processed 4,607 specimens for mycobacterial culture in 2001. We evaluated laboratory records of all smear- or culture-positive specimens submitted from patients who provided at least one smear-positive sample to our hospital between the years of 1996 and 2001, inclusive. A total of 82 specimens met these criteria and were used for this analysis. Data abstracted on each specimen included anatomic source, date of submission, date of smear and/or culture positivity, date of M. tuberculosis-specific DNA probe result, date of final identification to species level, and species identity. The presence or absence of comorbid conditions, including human immunodeficiency virus and AIDS, was not ascertained. Identification of cultured mycobacteria to the species level was determined by DNA probe or by other standard methods (15).
Estimation of costs.
For purposes of determining the incremental cost of MTD testing, the annual costs associated with the MTD testing program at our hospital's clinical microbiology laboratory, implemented in January 2000, were enumerated and estimated. The cost per smear-positive patient was then obtained by dividing the annual program cost by the number of MTD candidates per year. The MTD detection reagents are sold in kits of 50 tests at an estimated cost of $1,200/kit; these reagents have a maximum shelf life of 6 months, after reconstitution. Analysis of records between January 2000 and December 2001 indicated that three new technicians were trained to use MTD per year, the most recent training sessions requiring 12 MTD tests over 3 days per technician trained. During the 2-year period, 28 additional MTD tests were run on six different days for research or quality control purposes. We assumed that an MTD test series requires 2.5 h of technician time (3 h, minus 30 min deemed to be free during a 60-minute incubation) at the midpoint 2001 wage of $25.47 per h (includes 22% benefit rate) for medical technologists at our hospital. Three-day training sessions for new employees were assumed to involve 3 h for an experienced technician at the wage listed above and 10 hours for a starting technician at a wage (including benefits) of $19.58 per h. The cost of supplies (such as gloves and microcentrifuge tubes) needed in addition to the detection reagents was estimated at $5.85 per daily series of MTD tests. In addition, positive and negative control samples were maintained in the laboratory, at an estimated cost of $13.17 per 6 months. Additional costs for proficiency testing were not included.
The marginal daily cost of respiratory isolation was estimated by consultation with the senior operations engineer at our hospital. The refitting of an existing room to accommodate negative-pressure isolation was assumed to cost $120,000 per room. Such rooms have a minimum operational life expectancy of 20 years; we therefore amortized costs for a 30-year operating life, with an annual discount rate of 5% per year, giving an annuity rate of 15.37%. Estimated annual costs included 26 h per year for pressure testing and 16 h for heating, ventilation, and air conditioning maintenance, at a billed rate of $50/h. Furthermore, each two-room unit was assumed to undergo two annual HEPA filter and prefilter changes, with a cost of $600 per filter for parts and installation. Using these estimates, we calculated the mean daily cost of maintaining an available negative-pressure room and took this figure to equal the incremental cost per day of placing a patient in respiratory isolation. The marginal costs of personal protective equipment (masks) were not included in this analysis. Drug costs were estimated from the average wholesale price list in 2001; listed prices were found to be similar to those charged by a nationwide commercial pharmacy.
Sensitivity analysis.
One-way sensitivity analyses were performed on all variables in the decision analysis, with the exception of drug costs. Ranges for different analysis variables were determined from literature reports unless such reports were unavailable or identified reports did not appear to cover the range of reasonable possibilities. If one-way variation of a variable throughout its entire range did not change the estimated MTD cost-effectiveness by more than 10%, the analysis was reported as insensitive to that variable. Three-way sensitivity analysis was carried out on the annual number of specimens processed, the relative prevalence of TB, and the marginal cost of respiratory isolation, as the analysis was found to be particularly sensitive to these variables. Decision tree construction and sensitivity analysis were performed using DATA software (version 4.0; TreeAge Software, Inc., Williamstown, Mass.). All cost estimates from the literature were adjusted to 2001 dollars using the medical-care portion of the Consumer Price Index (U.S. Bureau of Labor Statistics [http://data.bls.gov/cgi-bin/surveymost]).
Ethical review.
The institutional review board for our hospital approved this study.
RESULTS
Estimates of probabilities and costs.
Table 1 shows the sensitivity, specificity, and probability of indeterminate results for the MTD test. When considering only smear-positive specimens, our analysis of compiled studies from the literature indicated an overall sensitivity of 99.6% and specificity of 99.7% for MTD, with 2.3% of specimens from TB-infected individuals containing MTD-inhibitory substances. Table 2 shows the variables used in the decision analysis and their base case values, along with the ranges over which each variable was tested in one-way sensitivity analysis. The estimated parameters, particularly for smear-positive, M. tuberculosis-negative specimens, are dominated by a single study (6) from a reference laboratory that annually processes 25,000 clinical specimens for isolation of mycobacteria. The results from other identified studies, however, are generally consistent with results from that laboratory.
TABLE 1.
Estimation of MTD validity measuresf
| Study | MTD resultsa of smear-positive samples
|
Sensitivityc (%) | Specificityc (%) | |||||
|---|---|---|---|---|---|---|---|---|
| TB positiveb
|
TB negativeb
|
|||||||
| + | − | I | + | − | I | |||
| Chedore and Jamieson (6) | 194 | 0 | —d | 1 | 318 | —d | 100 | 99.7 |
| Gamboa et al. (11) | 48 | 0 | 0 | 0 | 19 | 0 | 100 | 100 |
| Bergmann et al. (2)e | 13 | 0 | 0 | 0 | 9 | 0 | 100 | 100 |
| Smith et al. (19)e | 15 | 1 | 0 | 0 | 7 | 0 | 93.8 | 100 |
| Scarparo et al. (17) | 89 | 1 | 7 | 0 | 0 | 0 | 99.0 | NA |
| Wang and Tay (20) | 66 | 0 | 0 | 0 | 0 | 0 | 100 | NA |
| Piersimoni et al. (16) | 36 | 0 | 0 | 0 | 0 | 0 | 100 | NA |
| Della-Latta and Whittier (8) | 38 | 0 | 0 | 0 | 0 | 0 | 100 | NA |
| Total | 499 | 2 | 7 | 1 | 353 | |||
| Mean | 99.6 | 99.7 | ||||||
+, positive; −, negative with no inhibitors detectable; I, negative with inhibitors detectable.
TB status was determined by culture result, combined with clinical criteria when reported.
Sensitivity and specificity are reported here after removal of specimens with MTD inhibitors. NA, not applicable.
—, authors did not report rates of inhibition.
Study used a different diagnostic algorithm, whereby MTDs giving results between 30,000 and 300,000 RLU or 30,000 and 500,000 RLU were retested. The sensitivity of this alternative algorithm is lower and its specificity is higher than those of the diagnostic approach we have taken.
The estimated total probability of sample inhibition was 2.3% (7 of 305) specimens if culture positive [the specimens tested by Chedore and Jamieson {6} were not included in this calculation because the presence or absence of inhibitory substances was not reported] and was 0% (0 of 35 specimens) if culture negative.
TABLE 2.
Variables used in cost-effectiveness and sensitivity analysesa
| Variable | Base value | Lower bound | Upper bound |
|---|---|---|---|
| Sensitivity (%) of MTD in smear-positive specimensb | 99.6 | 91.8 (17) | 100 (2, 6, 8, 11, 16, 20) |
| Specificity (%) of MTD in smear-positive specimensb | 99.7 | 95 | 100 (2, 11, 19) |
| Proportion (%) of smear-positive specimens containing detectable MTD inhibitorsb | 0, 2.3c | 0 (2, 11, 19) | 7.2d (17) |
| Proportion (%) of first smear-positive respiratory specimens that are culture-positive for M. tuberculosis | 31.4e | 25 | 98.4 (7) |
| Annual no. of MTD-candidate patients submitting smear-positive respiratory samples | 14e | 1 | 500 |
| Hourly cost of labor ($) | 25.47e | 15 | 35 |
| Marginal cost of reagents per test ($) | 24e | 0 | 100f |
| Marginal daily cost of respiratory isolationg ($) | 27.77e | ||
| Daily cost of four-drug presumptive TB therapyg ($) | 5.66h | ||
| Median length of time from MTD result to M. tuberculosis speciation resultg (days) | 6e |
References from the literature are listed in parentheses. Lower and upper bounds refer to the range over which each given variable was tested in a one-way sensitivity analysis.
See Table 1 for a description of how the variable was estimated.
0% in individuals without TB; 2.3% in individuals with TB.
Sensitivity of the MTD without a test for inhibitors. (A test for inhibitors was assumed in the present analysis.)
Estimate obtained directly from our hospital.
The marginal reagent cost per test of a program in which reagents are purchased at list price, and only 15 of 50 tests are performed before the reagent expiration date.
Variable not used in the decision analysis to determine the cost of MTD testing per early TB exclusion. Rather, the variable factors into the value that individual hospitals will assign to an early TB exclusion. See Table 3 and Fig. 1.
Average wholesale price in 2001.
Cost-effectiveness of MTD.
The cost-effectiveness of our diagnostic strategy implementing routine MTD testing of patients' first smear-positive respiratory specimens is shown in Table 3. In the base case, a routine MTD testing program is expected to cost $494 per early exclusion of TB. By contrast, the expected financial savings from an early TB exclusion, in terms of averted isolation and medication, was $201. Therefore, MTD testing was not cost-effective in the base case scenario. However, as seen in Table 3, the estimates of MTD cost-effectiveness were acutely sensitive to changes in the relative prevalence of TB among smear-positive patients, annual number of specimens processed by the laboratory, and the marginal cost of reagents. Furthermore, the counterbalancing estimates of savings depended heavily on the marginal daily cost of respiratory isolation, as well as the speed with which MTD and culture results would become available. The model was not sensitive to changes in MTD sensitivity, specificity, or probability of inhibition (less than 10% change in cost-effectiveness across the variable's range).
TABLE 3.
MTD cost-effectiveness and one-way sensitivity analysis of variables
| Scenarioc | Marginal MTD cost/patienta | No. of early TB exclusions/100 patientsa | Cost ($)/early exclusion of TB |
|---|---|---|---|
| Base caseb | 338 | 68 | 494 |
| Relative prevalence (%) of TB in smear-positive patients | |||
| 25 | 338 | 75 | 452 |
| 50 | 338 | 50 | 677 |
| 65 | 338 | 35 | 966 |
| 75 | 338 | 25 | 1,355 |
| 90 | 331 | 10 | 3,321 |
| 98.4 | 320 | 2 | 20,087 |
| No. of smear-positive specimens processed by MTD/yr | |||
| 1 | 2,564 | 68 | 3,750 |
| 10 | 407 | 68 | 595 |
| 25 | 260 | 68 | 381 |
| 50 | 208 | 68 | 304 |
| 100 | 176 | 68 | 258 |
| 250 | 141 | 68 | 206 |
| 500 | 114 | 68 | 168 |
| Cost of labor ($/h) | |||
| 15 | 306 | 68 | 448 |
| 35 | 366 | 68 | 535 |
| Marginal cost of reagents ($/test) | |||
| 0 | 144 | 68 | 210 |
| 25 | 346 | 68 | 506 |
| 50 | 548 | 68 | 801 |
| 100 | 952 | 68 | 1,392 |
Eligible patients are those with symptoms consistent with TB, no mycobacterial diagnosis or history of treatment in the past 30 days, and at least one AFB-positive smear.
Using values from our hospital and from the literature, as described in Table 2.
Additionally, we calculated the cost of isolation plus presumptive therapy for a base care ($201), for the case in which the cost of respiratory isolation is $50/day ($334), and for the case in which the time between MTD and culture results is 10 days ($334).
Three-way sensitivity analysis.
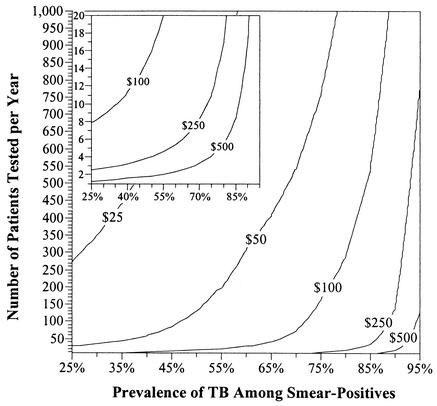
As shown in Table 3, the cost-effectiveness of MTD testing is highly sensitive to three variables that are likely to differ substantially from one site to another: the relative prevalence of TB in smear-positive specimens, the number of specimens processed per year, and the marginal daily cost of respiratory isolation. Figure 1 presents the results of a three-way sensitivity analysis on these variables. At sites where the marginal daily cost of placing a patient in respiratory isolation is $25 or less, MTD testing is unlikely to be cost-saving except in areas of extremely low relative TB prevalence with access to a reference laboratory capable of processing multiple specimens per day. On the other hand, if the marginal daily cost of respiratory isolation is $250 or more, MTD testing may be cost-effective even if performed by individual hospitals in areas of moderate-to-high rates of MOTT infection.
FIG. 1.
MTD cost-effectiveness, by TB prevalence, patient load, and isolation cost. Each isocontour represents the daily marginal cost of respiratory isolation needed to offset the costs of a hypothetical MTD testing program at a laboratory with a given number of patients tested per year (y axis) and relative prevalence of pulmonary TB among patients with AFB smear-positive respiratory specimens (x axis). Areas above a given isocontour represent those situations in which the implementation of MTD is expected to be cost-saving relative to the standard of care. The inset at the upper left details MTD cost-effectiveness in laboratories processing small numbers of smear-positive specimens on an annual basis.
DISCUSSION
Our analysis suggests that institutions considering the cost-effectiveness of implementing routine MTD testing of first smear-positive respiratory specimens should take four factors into account: the relative prevalence of TB among the candidate population to be tested, the number of smear-positive respiratory specimens processed per year, the marginal cost of placing a patient in respiratory isolation, and the marginal cost of test reagents. In general, if at least two of these factors are favorable for MTD testing, MTD is likely to be a cost-effective option as a component of the standard diagnostic evaluation for TB. More specifically, Fig. 1 may serve as a decision tool for hospital laboratory managers interested in determining whether routine MTD testing of smear-positive respiratory specimens is likely to be cost-effective in any particular setting. In addition to high rates of infection with MOTT, specific circumstances likely to increase the cost-effectiveness of MTD testing include the existence of regional reference laboratories capable of processing specimens submitted by multiple laboratories, the presence of excess MTD reagents (from an existing research program, for example) that may drive down the marginal cost of using those reagents, or a low supply (and therefore high cost) of respiratory isolation rooms.
In the base case analysis presented here, the costs of implementing an MTD program ($494 per early exclusion of TB) were not recoverable in terms of averted isolation and treatment ($201 per presumptive TB patient). However, a number of considerations indicate that this analysis may underestimate the cost-effectiveness of MTD testing in other situations. First of all, the marginal cost of respiratory isolation at our hospital is low, due to our hospital's relatively large number of respiratory isolation rooms. In addition, the mean time of 7 days between positive smear result and definitive identification to species level from culture at our hospital is substantially lower than the turnaround time reported from other institutions (21). Finally, this analysis did not consider factors such as decreased length of hospital stay among patients infected with MOTT able to start appropriate therapy sooner or averted toxicity from drugs used for presumptive treatment of TB in individuals with MOTT infections. Although these outcomes are likely to be rare, they are also associated with great costs to the health care system and may therefore have great impact on the cost-effectiveness of MTD testing programs.
In contrast to the above considerations, other components of our analysis may have led us to overestimate the cost-effectiveness of MTD. The relative prevalence of TB (31.4%) among smear-positive respiratory specimens at our hospital is relatively low. Furthermore, this analysis did not include the costs of purchasing equipment such as a luminometer or sonicator or the costs of shipping samples to a hypothetical reference laboratory. Finally, our estimate of a uniform 24 h between smear and culture results may be low, particularly in laboratories incapable of MTD testing 6 days per week. In settings where MTD testing is performed fewer than 6 days per week the amount of respiratory isolation time, and therefore costs, saved by MTD testing may be lower than those in our analysis.
One key limitation of the present analysis is that we were unable to assess the potential costs and outcomes due to false-negative MTD results. As can be seen in Table 1, previous reports have shown the sensitivity of MTD to be extremely high when applied to smear-positive respiratory specimens. Nevertheless, concern justifiably exists that sensitivity in the clinical setting may be lower than that reported in the literature, and the potential consequences of releasing an individual with smear-positive, active TB onto the general hospital ward are serious.
Observations from our analysis indicate that, in sites where the implementation of MTD is expected to be cost-effective, fears of false-negative MTD results may not be sufficient to outweigh cost-effectiveness concerns. First of all, two of the six reports listed in Table 1 (2, 6) identified a total of nine specimens that were positive by MTD but were, based on clinical criteria, false negative by culture. Were MTD not performed on these individuals in the clinical setting, they may have been released with active TB. Secondly, most reports of false-negative MTD results (as in reference 13, for example) involve specimens with initial results in the range of 30,000 to 500,000 RLU. The manufacturer recommends retesting samples in this equivocal range, but the diagnostic strategy promulgated in this analysis—that of considering all results above 30,000 RLU to be positive—not only minimizes cost due to retesting but also maximizes sensitivity. Thirdly, our analysis indicates that MTD is likely to be cost-effective mainly in those laboratories processing large numbers of specimens. As demonstrated by Chedore and Jamieson (6), whose laboratory performed 517 MTD tests on smear-positive respiratory specimens from 1997 to 1998 without a single false-negative result, such large laboratories are the ideal setting for maximizing sensitivity through standardization of procedures. A similar reference laboratory in Florida (S. Rungruanghiranya, D. F. Romero-Fischmann, M. Narita, E. S. Hollender, D. Nolan, Y. M. Hale, and D. Ashkin, Abstr. 4th World Congress Tuberc., abstr. 139, 2002) found no significant difference between the sensitivity of MTD and that of culture in smear-positive respiratory specimens, although five false-negative MTD results were reported out of 257 smear-positive TB cases tested (98% sensitivity). Finally, if concerns regarding imperfect sensitivity persist, a diagnostic strategy based on the current Centers for Disease Control and Prevention guidelines (5), which require two negative MTD results in order to exclude TB, may be adopted. This strategy involves greater expense but could still be cost-effective, for example, at a reference laboratory (500 samples/year) with a relative TB prevalence of 70% among smear-positive samples and a marginal daily cost of $100 for respiratory isolation.
In addition to its inability to assess the impact of false-negative MTD results, this analysis is further limited by the fact that it is restricted to the inpatient setting. For outpatients, the measures implemented to prevent potential M. tuberculosis transmission are likely to vary from one site to another. Furthermore, outpatients are sometimes required to purchase drugs for presumptive treatment in bulk and may not be able to start therapy immediately after a positive smear result is reported by the laboratory. As a result, the estimates of MTD cost and effectiveness are likely to differ substantially in the outpatient setting from those described here.
Although the analysis presented here demonstrates that routine MTD testing can be cost-effective in certain circumstances for routine testing of smear-positive respiratory samples, the greatest promise of rapid molecular TB diagnostics is for use in TB suspects whose specimens are smear negative (1, 3). With evidence emerging (18) that smear-negative TB patients can be infectious, routine MTD testing could play a vital role in disease-prevention efforts by identifying such individuals days or weeks before culture results become available. Catanzaro and colleagues (3) have presented evidence that MTD testing can be carried out on smear-negative specimens with high positive and negative predictive value, if combined with clinical judgment in identifying those patients at high risk for TB. Nevertheless, cost-effectiveness remains a critical obstacle to the routine use of MTD for smear-negative patients; the need for development and analysis of a cost-effective diagnostic strategy is critical, if nucleic acid amplification tests are to reach their full potential in aiding the diagnosis and improving the clinical outcomes of TB patients.
Acknowledgments
This work was supported by NIH grants U19-AI45432 and 1K24AI01637, both to Richard E. Chaisson, as well as Master's support funding from the Johns Hopkins Department of Epidemiology.
We thank George Comstock, Michael Sweat, and David Bishai for critical review of the manuscript. We also thank Gregory Bova for assistance in estimating costs associated with respiratory isolation at our hospital and Richard Chaisson for many helpful discussions in the design and analysis of this project.
REFERENCES
- 1.American Thoracic Society Workshop. 1997. Rapid diagnostic tests for tuberculosis: what is the appropriate use? Am. J. Respir. Crit. Care Med. 155:1804-1814. [DOI] [PubMed] [Google Scholar]
- 2.Bergmann, J. S., G. Yuoh, G. Fish, and G. L. Woods. 1999. Clinical evaluation of the enhanced Gen-Probe Amplified Mycobacterium Tuberculosis Direct Test for rapid diagnosis of tuberculosis in prison inmates. J. Clin. Microbiol. 37:1419-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Catanzaro, A., S. Perry, J. E. Clarridge, S. Dunbar, S. Goodnight-White, P. A. LoBue, C. Peter, G. E. Pfyffer, M. F. Sierra, R. Weber, G. Woods, G. Mathews, V. Jonas, K. Smith, and P. Della-Latta. 2000. The role of clinical suspicion in evaluating a new diagnostic test for active tuberculosis: results of a multicenter prospective trial. JAMA 283:639-645. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 1994. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care facilities, 1994. Morb. Mortal. Wkly. Rep. 43(RR-13):1-132. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2000. Update: nucleic acid amplification tests for tuberculosis. Morb. Mortal. Wkly. Rep. 49:593-594. [PubMed] [Google Scholar]
- 6.Chedore, P., and F. B. Jamieson. 1999. Routine use of the Gen-Probe MTD2 amplification test for detection of Mycobacterium tuberculosis in clinical specimens in a large public health mycobacteriology laboratory. Diagn. Microbiol. Infect. Dis. 35:185-191. [DOI] [PubMed] [Google Scholar]
- 7.Conde, M. B., C. M. Figueira, R. Moraes, L. S. Fonseca, K. Deriemer, and A. L. Kritski. 1999. Predictive value of the acid-fast smear for detection of Mycobacterium tuberculosis in respiratory specimens in a reference center of HIV/AIDS in Rio de Janeiro, Brazil. Mem. Inst. Oswaldo Cruz 94:787-790. [DOI] [PubMed] [Google Scholar]
- 8.Della-Latta, P., and S. Whittier. 1998. Comprehensive evaluation of performance, laboratory application, and clinical usefulness of two direct amplification technologies for the detection of Mycobacterium tuberculosis complex. J. Clin. Pathol. 110:301-310. [DOI] [PubMed] [Google Scholar]
- 9.Detels, R., P. Tarwater, J. P. Phair, J. Margolick, S. A. Riddler, and A. Muñoz. 2001. Effectiveness of potent antiretroviral therapies on the incidence of opportunistic infections before and after AIDS diagnosis. AIDS 15:347-355. [DOI] [PubMed] [Google Scholar]
- 10.Drobniewski, F. A., B. Watt, E. G. Smith, J. G. Magee, R. Williams, J. Holder, and J. Ostrowski. 1999. A national audit of the laboratory diagnosis of tuberculosis and other mycobacterial diseases within the United Kingdom. J. Clin. Pathol. 52:334-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gamboa, F., G. Fernandez, E. Padilla, J. M. Manterola, J. Lonca, P. J. Cardona, L. Matas, and V. Ausina. 1998. Comparative evaluation of initial and new versions of the Gen-Probe amplified Mycobacterium tuberculosis direct test for direct detection of Mycobacterium tuberculosis in respiratory and nonrespiratory specimens. J. Clin. Microbiol. 36:684-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ives, N. J., B. G. Gazzard, and P. J. Easterbrook. 2001. The changing pattern of AIDS-defining illnesses with the introduction of highly active antiretroviral therapy (HAART)in a London clinic. J. Infect. 42:134-139. [DOI] [PubMed] [Google Scholar]
- 13.Jorgensen, J. H., J. R. Salinas, R. Paxson, K. Magnon, J. E. Patterson, and T. F. Patterson. 1999. False-positive Gen-Probe direct Mycobacterium tuberculosis amplification test results for patients with pulmonary M. kansasii and M. avium infections. J. Clin. Microbiol. 37:175-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minino, A. M., and B. L. Smith. 2001. Deaths: preliminary data for 2000. National vital statistics reports 2001, vol. 49, no. 12. National Center for Health Statistics, Hyattsville, Md. [PubMed]
- 15.Nolte, F., and Metchock, B. 1995. Mycobacterium, p. 400-437. In P. Murray, E. Baron, M. Pfaller, F. Tenover, and R. Yolken (ed.), Manual of clinical microbiology. American Society for Microbiology, Washington, D.C.
- 16.Piersimoni, C., A. Callegaro, C. Scarparo, V. Penati, D. Nista, S. Bornigia, C. Lacchini, M. Scagnelli, G. Santini, and G. de Sio. 1998. Comparative evaluation of the new Gen-Probe Mycobacterium tuberculosis Amplified Direct Test and the semiautomated Abbott LCx Mycobacterium tuberculosis assay for direct detection of Mycobacterium tuberculosis complex in respiratory and extrapulmonary specimens. J. Clin. Microbiol. 36:3601-3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scarparo, C., P. Piccoli, A. Rigon, G. Ruggiero, M. Scagnelli, and C. Piersimoni. 2000. Comparison of enhanced Mycobacterium tuberculosis amplified direct test with COBAS AMPLICOR Mycobacterium tuberculosis assay for direct detection of Mycobacterium tuberculosis complex in respiratory and extrapulmonary specimens. J. Clin. Microbiol. 38:1559-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sepkowitz, K. A. 1996. How contagious is tuberculosis? Clin. Infect. Dis. 23:954-962. [DOI] [PubMed] [Google Scholar]
- 19.Smith, M. B., J. S. Bergmann, M. Onoroto, G. Matthews, and G. L. Woods. 1999. Evaluation of the enhanced amplified Mycobacterium tuberculosis direct test for direct detection of Mycobacterium tuberculosis complex in respiratory specimens. Arch. Pathol. Lab. Med. 123:1101-1103. [DOI] [PubMed] [Google Scholar]
- 20.Wang, S. X., and L. Tay. 1999. Evaluation of three nucleic acid amplification methods for direct detection of Mycobacterium tuberculosis complex in respiratory specimens. J. Clin. Microbiol. 37:1932-1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watterson, S. A., and F. A. Drobniewski. 2000. Modern laboratory diagnosis of mycobacterial infections. J. Clin. Pathol. 53:727-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization. 2001. World health report 2001: mental health, new understanding, new hope. World Health Organization, Geneva, Switzerland.
- 23.Yazdanpanah, Y., G. Chene, E. Losina, S. J. Goldie, L. D. Merchadou, S. Alfandari, G. R. Seage III, L. Sullivan, C. Marimoutou, A. D. Paltiel, R. Salamon, Y. Mouton, and K. A. Freedberg. 2001. Incidence of primary opportunistic infections in two human immunodeficiency virus-infected French clinical cohorts. Int. J. Epidemiol. 30:864-871. [DOI] [PubMed] [Google Scholar]