Abstract
The heterotetrameric adaptor protein complex AP-3 has been shown to function in the sorting of proteins to the endosomal/lysosomal system. However, the mechanism of AP-3 recruitment onto membranes is poorly understood, and it is still uncertain whether AP-3 nucleates clathrin-coated vesicles. Using purified components, we show that AP-3 and clathrin are recruited onto protein-free liposomes and Golgi-enriched membranes by a process that requires ADP-ribosylation factor (ARF) and GTP but no other proteins or nucleotides. The efficiency of recruitment onto the two sources of membranes is comparable and independent of the composition of the liposomes. Clathrin binding occurred in a cooperative manner as a function of the membrane concentration of AP-3. Thin-section electron microscopy of liposomes and Golgi-enriched membranes that had been incubated with AP-3, clathrin, and ARF·GTP showed the presence of clathrin-coated buds and vesicles. These results establish that AP-3–containing clathrin-coated vesicles form in vitro and are consistent with AP-3–dependent protein transport being mediated by clathrin-coated vesicles.
INTRODUCTION
The directed movement of proteins along the exocytic or endocytic pathways is largely mediated by coated vesicles that are formed by the recruitment of coat proteins from a cytosolic pool to their proper target membrane (reviewed by Rothman and Wieland, 1996). Once on the membrane, the coat proteins assemble into a higher-order structure resulting in physical deformation of the underlying membrane and ultimate budding of the vesicle. To date, three distinct classes of coated vesicles have been described: coat protein I (COPI-), COPII-, and clathrin-coated vesicles (CCVs) (reviewed by Hirst and Robinson, 1998). The coat of CCVs is composed of two principal protein complexes: clathrin and adaptor proteins. Clathrin exists as a triskelion and acts as a molecular scaffold, forming the outer coat (reviewed by Ungewickell, 1999). Clathrin is linked to a portion of the underlying membrane that is ultimately captured into a coated vesicle via the adaptor protein complexes. At present, four distinct heterotetrameric adaptor complexes have been identified, designated AP-1 to AP-4. Each is composed of a related ∼100-kDa β subunit, a unique subunit of ∼100–160 kDa (designated γ for AP-1, α for AP-2, δ for AP-3, and ε for AP-4), a related μ subunit of ∼50 kDa, and a related ς subunit of ∼20 kDa.
Within the endoplasmic reticulum and Golgi apparatus, coat recruitment is coordinated by small GTP-binding proteins (reviewed by Robinson, 1997). Sar1p appears specific for COPII binding, whereas members of the ADP-ribosylation factor (ARF) family initiate recruitment of both the COPI and AP-1 complexes onto Golgi membranes and AP-3 onto endosomes. ARF recruitment is dependent on the action of a guanine nucleotide exchange factor (GEF) whose activity is blocked by the fungal metabolite brefeldin A. ARF1 serves as the prototype among ARF family members and is capable of promoting recruitment of the COPI (Donaldson et al., 1992), AP-1 (Stamnes and Rothman, 1993; Traub et al., 1993), and AP-3 (Ooi et al., 1998) complexes onto membranes.
COPI- and COPII-coated vesicles mediate trafficking events between the endoplasmic reticulum and Golgi apparatus, and COPI-coated vesicles also function in intra-Golgi transport. At the trans-Golgi network (TGN), AP-1–containing CCVs mediate the transport of lysosomal hydrolases and lysosomal membrane proteins to an endosomal compartment, whereas at the plasma membrane, AP-2–containing CCVs function in receptor-mediated endocytosis for delivery to early endosomes. The AP-3 coat protein complex is believed to mediate protein sorting and delivery from the TGN or endosomes to specialized organelles such as lysosomes and related structures, including melanosomes and platelet-dense granules. Evidence to support this concept comes from the multiple reports of naturally occurring mutations in subunits of the AP-3 complex in Drosophila (Ooi et al., 1997; Simpson et al., 1997; Mullins et al., 1999), mouse (Kantheti et al., 1998; Feng et al., 1999; Zhen et al., 1999), and human (Dell'Angelica et al., 1999b) that result in hypopigmentation and storage pool deficiency phenotypes. These sorting events are likely to involve interaction between the AP-3 complex and both dileucine-based (Darsaw et al., 1998; Höning et al., 1998; Blagoveshchenskaya et al., 1999) and tyrosine-based (Dell'Angelica et al., 1997b; Le Borgne et al., 1998) motifs in the cytoplasmic domains of the membrane proteins present in these organelles.
Whereas AP-1 and AP-2 initiate coated bud formation in mammalian cells by linking clathrin to the TGN and plasma membrane, respectively, conflicting reports have brought into question whether AP-3 associates with clathrin inside the cell to generate AP-3–containing CCVs. In in vitro binding (Dell'Angelica et al., 1998) and crystallographic studies (ter Haar et al., 2000), AP-3 interacts with clathrin via a “clathrin box” sequence within the β3 subunit and has been shown to colocalize with clathrin on intracellular membranes by both immunofluorescence and electron microscopy (Dell'Angelica et al., 1998). Isolated CCV preparations, however, contain little AP-3 (Simpson et al., 1996; Dell'Angelica et al., 1997b), and in vitro, AP-3–containing vesicles appear capable of budding from endosomes in the absence of clathrin (Faundez et al., 1998). Furthermore, no consensus clathrin-binding motif is present in yeast AP-3, which appears to function independently of clathrin (Cowles et al., 1997; Panek et al., 1997; Stepp et al., 1997; Vowels and Payne, 1998; Yeung et al., 1999) in mediating cargo-selective transport to the yeast vacuole. Interestingly, the very recently described AP-4 complex does not contain a consensus clathrin-binding motif within its β subunit (Dell'Angelica et al., 1999a; Hirst et al., 1999) and failed to bind clathrin during in vitro experiments (Dell'Angelica et al., 1999a), consistent with the suggestion that adaptor protein complex–mediated trafficking events may occur without the formation of an overlying clathrin coat.
Ooi et al. (1998) have demonstrated in in vitro assays that AP-3 is recruited from cytosol onto endosome-enriched membranes in an ARF1·GTP-dependent manner. This recruitment was abolished by pretreating the membranes with trypsin, implying the need for a protein component in AP-3 recruitment. Because the trypsin treatment also prevented ARF1 binding to the membranes, it was not possible to determine whether there was a specific membrane protein required for AP-3 binding. To address the issue of whether AP-3 can nucleate CCV formation and to better understand the factors that regulate its association with membranes, we have studied the recruitment of the AP-3 adaptor protein complex onto Golgi-enriched membranes and protein-free liposomes. We find that AP-3 is efficiently recruited onto both types of membranes in a reaction that is strictly dependent on both ARF and GTP. In these assays, ARF5 is as effective as ARF1. Once bound to the membrane, AP-3 is able to recruit clathrin to form CCVs. The clathrin binding is dependent on the membrane surface concentration of AP-3.
MATERIALS AND METHODS
Materials
l-α-Phosphatidylcholine (PC) from soybeans containing 20% PC, phosphatidylinositol (PI) 4-phosphate (PI4P), PI 4,5-bisphosphate (PIP2), phosphatidic acid (PA), the dioleoyl forms of pure PC (DOPC) and phosphatidylethanolamine (DOPE), DTT, trypsin, soybean trypsin inhibitor, BSA, ATP, creatine kinase, creatine phosphate, and other common reagents were from Sigma Chemical (St. Louis, MO). PI 3,4,5-triphosphate (PIP3) was from Matreya (Pleasant Gap, PA). Phosphatidylserine (PS) and PI were from Avanti Polar Lipids (Alabaster, AL). GTP and GTPγS were purchased from Boehringer Mannheim (Indianapolis, IN). Nitrocellulose membranes were from Schleicher & Schuell (Keene, NH). Siliconized microfuge tubes were from Midwest Scientific (St. Louis, MO). CNBr-activated Sepharose 4B, molecular weight standards used for electrophoresis, and reagents for ECL detection were obtained from Amersham Pharmacia Biotech (Piscataway, NJ).
Antibodies
The anti-clathrin heavy chain mAb TD.1 (Nathke et al., 1992) was generously provided by Dr. Frances Brodsky (University of California, San Francisco, CA). Affinity-purified antibodies AE/1 and RY/1, which recognize the γ and μ subunits of the AP-1 complex, respectively, were kindly provided by Dr. Linton Traub (Washington University, St. Louis). Affinity-purified antibodies against the ς and μ subunits of AP-3 were generous gifts of Dr. Juan Bonifacino (National Institutes of Health, Bethesda, MD). The anti-ARF mAb 1D9 was a kind gift of Dr. Richard Kahn (Emory University, Atlanta, GA). mAb M3A5 specific for β-COP, mAb 100/2 directed against the α subunit of AP-2, and mAb 100/3 specific for the γ subunit of AP-1 were purchased from Sigma. mAb 100/3 was subsequently used for immunodepletion of AP-1 from bovine adrenal cytosol. The neuron-specific anti-clathrin light chain mAb clone 57.4 was purchased from Synaptic Systems (Göttingen, Germany). Antiserum KQ/1 was raised in rabbits against a 16-residue synthetic peptide (KQEQANNPFYIKSSPS) derived from the sequence of the human AP-3 δ subunit coupled to keyhole limpet hemocyanin (Pierce, Rockford, IL). Peptide conjugation, immunization, and screening were as described previously (Traub and Sagi-Eisenberg, 1991). KQ/1 was further affinity purified from immune serum on a column on which the immunizing peptide was coupled to CNBr-activated Sepharose 4B. Peptide-specific antibodies were eluted with 100 mM glycine-HCl, pH 2.5, followed by immediate neutralization. Affinity-purified HRP-conjugated anti-rabbit and anti-mouse immunoglobulin antibodies were from Amersham Pharmacia Biotech.
Preparation of Cytosols, Membranes, Recombinant ARF Proteins, and Clathrin-coated Vesicles
Bovine adrenal cytosol was prepared from fresh adrenal glands obtained at a local slaughterhouse. After removal, glands were placed on ice and residual fat was removed and transferred to homogenization buffer (25 mM HEPES-KOH, pH 7.4, 250 mM sucrose, 1 mM EDTA supplemented with 0.1 trypsin inhibitory unit/ml aprotinin, 1 mM PMSF, 5 μg/ml leupeptin) on ice. Homogenization was performed with the use of a Potter-Elvehjem homogenizer with a 2:1 ratio (wt/wt) of buffer to tissue. This and all subsequent tissue preparation was at 4°C. The crude homogenate was centrifuged sequentially at 3000 × g for 10 min, 10,000 × g for 20 min, and 100,000 × g for 60 min. The final supernatant served as the cytosolic fraction and was frozen on dry ice in aliquots before storage at −80°C. Bovine brain cytosol was prepared from fresh brains essentially as described above after the initial removal of surrounding blood vessels and excess white matter. Before use, cytosols were rapidly thawed and desalted over PD-10 columns (Amersham Pharmacia Biotech) previously equilibrated with 1× assay buffer (25 mM HEPES-KOH, pH 7.2, 125 mM potassium acetate, 2.5 mM magnesium acetate, 1 mM DTT) and subsequently centrifuged at 245,000 × g (70,000 rpm) for 20 min at 4°C in a Beckman (Palo Alto, CA) TLA 100.3 rotor to remove insoluble material.
Bovine adrenal cytosol devoid of AP-1 was prepared by immunodepletion with the use of mAb 100/3 coupled to CNBr-activated Sepharose 4B as described previously (Traub et al., 1995). Bovine adrenal cytosol lacking endogenous ARF proteins was prepared by gel filtration at 4°C over a Sephadex G-75 column equilibrated in 1× assay buffer. ARF-containing fractions were identified by immunoblotting after SDS-PAGE of column fraction aliquots. ARF-depleted fractions were pooled, rapidly frozen in aliquots on dry ice, and stored at −80°C. Clathrin-depleted bovine adrenal cytosol was prepared by gel filtration on Sepharose 4B as described previously (Traub et al., 1995). Purification of AP-3 from bovine brain was according to a protocol generously provided by the Tomas Kirchhausen laboratory (Harvard Medical School, Cambridge, MA). Before use, purified AP-3 was centrifuged at 245,000 × g (70,000 rpm) for 20 min at 4°C.
Golgi-enriched membranes were prepared from fresh rat liver as described (Zhu et al., 1998). To prepare membranes depleted of both endogenous AP-1 and ARF, purified Golgi-enriched membranes were diluted to a final concentration of 50 μg/ml in 1× assay buffer and incubated at 37°C for 20 min (Traub et al., 1993; Zhu et al., 1998). Tubes were then chilled on ice, and the membranes were recovered by centrifugation at 20,000 × g for 10 min at 4°C. The resultant AP-1– and ARF-depleted Golgi-enriched membranes were then resuspended in 10 mM HEPES-KOH, pH 7.0, 250 mM sucrose and used subsequently in recruitment assays. Soybean lipid-derived and chemically defined liposomes were prepared essentially as described (Zhu et al., 1999), with the exception that all liposomes were maintained at a final concentration of 2 mg/ml.
Recombinant myristoylated ARF1, ARF5, and ARF6 were prepared as described previously (Liang and Kornfeld, 1997). Before use, ARF proteins were diluted in 1× assay buffer and centrifuged at 245,000 × g (70,000 rpm) for 20 min at 4°C.
CCVs derived from both rat brain and liver were prepared according to described methods (Campbell et al., 1984) and further purified from contaminating vaults by discontinuous sucrose gradient centrifugation (Kedersha and Rome, 1986). Purified clathrin derived from the rat brain CCV pool was prepared by extraction of the coated vesicles in 1.0 M Tris-HCl, pH 7.0, for 1 h on ice. The extracted proteins were separated from the residual CCV membranes by centrifugation at 245,000 × g (70,000 rpm) for 20 min at 4°C as described above. Soluble clathrin was separated from other extracted coat proteins by gel filtration at 4°C over a Superose 6 HR 30 column (Amersham Pharmacia Biotech) previously equilibrated in 0.5 M Tris-HCl, pH 7.0. Clathrin-containing fractions were identified after SDS-PAGE of fraction aliquots by Coomassie blue staining. Clathrin-enriched fractions were pooled, and clathrin trimers were concentrated by the addition of ammonium sulfate to 50% saturation. Before use in recruitment assays, the purified clathrin was resuspended in 1.0 M Tris-HCl, pH 7.0, dialyzed at 4°C against 1× assay buffer containing 1 mM PMSF, and subsequently centrifuged at 245,000 × g (70,000 rpm) for 20 min at 4°C to remove insoluble material.
Coat Protein Recruitment Assays
Recruitment reactions were performed in presiliconized 1.5-ml microfuge tubes in a total volume of 200 μl. Typical recruitment reaction mixtures contained 1× assay buffer and a combination of gel-filtered cytosol at a final concentration of 5 mg/ml or purified AP-3 with or without purified clathrin (both at the concentrations noted in the figure legends), purified Golgi-enriched membranes at 50 μg/ml or liposomes at 200 μg/ml, GTP (1 mM) or GTPγS (100 μM), and/or recombinant ARF proteins at a final concentration of 50 μg/ml. In reactions containing purified AP-3 and/or purified clathrin, BSA was added to a final concentration of 2.5 mg/ml to reduce nonspecific protein binding. When used, the ATP regeneration system was composed of 1 mM ATP, 10 mM creatine phosphate, and 5 U/ml creatine kinase. All reactions were prepared on ice, and binding assays were begun by transferring the tubes to a 37°C water bath. Reactions were typically terminated (unless noted in the figure legends) after a 20-min incubation by returning the tubes to ice. Membranes were recovered by centrifugation at 20,000 × g for 15 min at 4°C. Membrane pellets were solubilized in 1× SDS sample buffer before fractionation on 12% polyacrylamide gels.
Controlled Tryptic Digestion
Tryptic digestions were performed with modifications to a method described previously (Traub et al., 1995). Briefly, AP-3 was recruited from clathrin-depleted cytosol onto Golgi-enriched membranes and liposomes as described above. The membranes were recovered by centrifugation at 20,000 × g for 10 min at 4°C and resuspended in 1× assay buffer to a final membrane concentration of 50 μg/ml for reactions containing Golgi-enriched membranes and 200 μg/ml for reactions containing liposomes. Tryptic reactions were prepared on ice with final trypsin concentrations as noted in the figure legends. The tubes were then incubated at 37°C for 10 min and returned to ice, and excess soybean trypsin inhibitor was added. Reactions in which purified AP-3 was digested with trypsin contained 50 μg/ml cytosolic protein. Samples were concentrated by methanol/chloroform precipitation (Wessel and Flugge, 1984) and subjected to SDS-PAGE for immunoblot analysis.
Gel Electrophoresis and Immunoblotting
Protein samples were subjected to discontinuous SDS-PAGE according to standard protocols after boiling for 5 min in 1× SDS sample buffer (2.3% SDS, 62.5 mM Tris-HCl, pH 6.8, 5% β-mercaptoethanol, 10% sucrose). The gels were prepared from an acrylamide/bisacrylamide stock solution of 30:0.4 rather than the usual 30:0.8, because we have found the reduced cross-linking to provide better resolution of the ς3A and ς3B subunits. After electrophoresis, proteins were transferred to nitrocellulose membranes in ice-cold buffer containing 15.6 mM Tris, 120 mM glycine, pH 8.3, at 110 V for 75 min. Blots were blocked overnight in 5% (wt/vol) skim milk prepared in 10 mM Tris-HCl, pH 7.5, 150 mM NaCl, and 0.1% (vol/vol) Tween 20 (TBS-T buffer), and subsequently portions of each blot were probed with primary antibodies diluted in 1% (wt/vol) skim milk in TBS-T buffer as indicated in the figure legends. Both before and after incubation with HRP-conjugated anti-mouse or anti-rabbit immunoglobulin G diluted as described above, blots were washed three times with TBS-T buffer for 5 min per wash. ECL detection was used for visualization of immunoreactive protein bands. For quantitative analysis, autoradiographs were analyzed with a Personal Densitometer with the use of Image-Quant software (Molecular Dynamics, Sunnyvale, CA).
Electron Microscopy
Electron microscopy of Golgi-enriched membranes and liposome preparations was essentially as described (Zhu et al., 1999). Briefly, Golgi-enriched membranes and liposomes were incubated at 37°C for 20 min in 1× assay buffer supplemented with AP-3, clathrin, recombinant myristoylated ARF, and GTPγS. After recruitment assays, membranes were returned to ice and recovered by centrifugation at 20,000 × g. Membrane pellets were fixed with 1% glutaraldehyde in 0.1 M sodium cacodylate buffer, pH 7.0, for 1 h on ice, postfixed with 1% osmium tetroxide, and impregnated with tannic acid to enhance visualization of coat-containing membranes (Orci et al., 1986). Membranes were then embedded in Epon for thin sectioning. Thin sections were further contrasted with uranyl acetate and lead citrate and analyzed with the use of a Zeiss (Thornwood, NY) 902 electron microscope.
RESULTS
Binding of AP-3 to Membranes Is Nucleotide, ARF, and Temperature Dependent and Sensitive to Brefeldin A
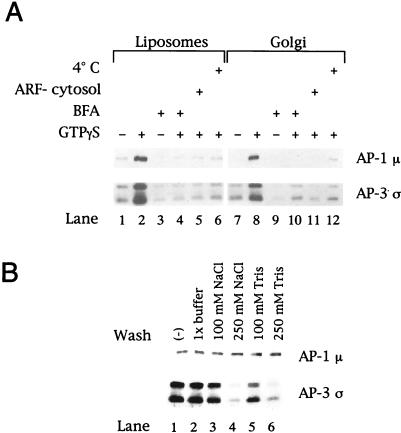
We initially determined the ability of liposomal membranes prepared from soybean lipids containing 20% PC to support recruitment of the AP-3 complex. Such liposomes have been shown to bind AP-1 at levels comparable to those obtained with isolated Golgi-enriched membranes (Zhu et al., 1999). As seen in Figure 1A (lower blot), AP-3 was recruited from bovine adrenal cytosol onto the liposomes in a GTPγS-dependent manner (lane 2 versus lane 1), and the extent of recruitment was comparable to the binding observed when identical reactions were performed with Golgi-enriched membranes (lane 8). AP-3 translocation onto either liposomes or Golgi-enriched membranes did not occur in the presence of brefeldin A (lanes 4 and 10), a fungal metabolite shown to inhibit both the Golgi-associated ARF GEF and some cytosolic ARF GEFs (Randazzo et al., 1993; Sata et al., 1998). Furthermore, AP-3 binding did not occur in reactions containing ARF-depleted cytosol (lanes 5 and 11) or at 4°C (lanes 6 and 12). Concomitant assessment of AP-1 membrane association (Figure 1A, upper blot) demonstrated identical requirements to those described for the AP-3 complex, consistent with previous studies of AP-1 recruitment onto both liposomes (Zhu et al., 1999) and Golgi-enriched membrane fractions (Traub et al., 1993). Additional experiments to study the association of the AP-3 complex with membranes (Figure 1B) revealed that whereas neither AP-1 nor AP-3 recruited onto either liposomes or Golgi-enriched membranes in the presence of GTPγS could be extracted by incubation in 1× assay buffer, the AP-3 complex was quantitatively dissociated from both membrane sources when incubated with either 250 mM NaCl or 250 mM Tris-HCl, conditions under which the AP-1 complex remained quantitatively membrane associated. Thus, although the nucleotide, ARF, and temperature requirements of AP-3 complex membrane association resemble those described for membrane binding of the AP-1 adaptor protein coat, differences clearly exist in the membrane-binding properties of these related adaptor protein complexes.
Figure 1.
AP-3 membrane recruitment is nucleotide, ARF, and temperature dependent and sensitive to brefeldin A. (A) Liposomes (200 μg/ml) prepared from soybean 20% PC material (lanes 1–6) or AP-1– and ARF-depleted Golgi-enriched membranes (50 μg/ml; lanes 7–12) were incubated in the presence or absence of 100 μM GTPγS in 200-μl reactions containing either 5 mg/ml gel-filtered bovine adrenal cytosol or 5 mg/ml bovine adrenal cytosol that had been depleted of ARF proteins by gel filtration over a Sephadex G-75 column (lanes 5 and 11). In reactions containing brefeldin A (BFA; lanes 3, 4, 9, and 10), a final concentration of 100 μg/ml BFA was used. After incubation at either 37 or 4°C (lanes 6 and 12) for 20 min, membranes were collected by centrifugation at 20,000 × g for 15 min at 4°C, fractionated on 12% SDS-polyacrylamide gels, and transferred to nitrocellulose membranes. Portions of the blot were probed with affinity-purified antibodies directed against the μ subunit of AP-1 (antibody RY-1) or specific for the ς3A and ς3B isoforms of the AP-3 complex (Dell'Angelica et al., 1997a). (B) After recruitment of AP-1 and AP-3, the liposomal membranes were resuspended in 1× assay buffer or assay buffer containing 100 or 250 mM NaCl or 100 or 250 mM Tris-HCl, pH 7.0. After incubation on ice for 10 min, the membranes were recovered by centrifugation and subjected to SDS-PAGE and immunoblotting as described in A. Shown are results with the liposomes. Similar findings were noted with the Golgi-enriched membranes.
Lipid Requirements for AP-3 Recruitment
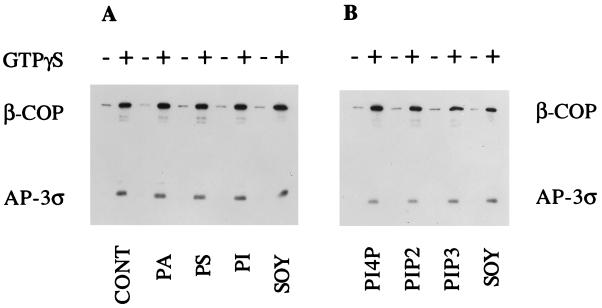
To determine the individual lipids necessary for binding of AP-3 to membranes, we prepared liposomes from purified phospholipids. Liposomes composed of DOPC/DOPE/cholesterol (50:40:10, wt/wt) served as the control liposomes. The DOPE fraction was adjusted accordingly for the addition of the others lipids assayed. In preliminary experiments, we found that the control liposomes bound AP-3 as well as liposomes prepared from the 20% PC soybean lipid mixture. As shown in Figure 2, the addition of PA, PS, or various phosphoinositides did not alter the amount of AP-3 recruited onto the membranes. COPI was recruited efficiently from cytosol onto all of the liposome preparations, in agreement with previous reports (Spang et al., 1998; Zhu et al., 1999). These results differ from those obtained with AP-1 recruitment, in which acidic lipids, especially PS, stimulated AP-1 binding (Zhu et al., 1999), and from studies of COPII binding, in which acidic phospholipids, particularly PI4P and PIP2, were required, although higher concentrations of PA also worked (Matsuoka et al., 1998). Thus, AP-3 appears to more closely resemble COPI in its lipid requirements than either the clathrin-associated AP-1 or the clathrin-independent COPII coat protein complex.
Figure 2.
Lipid requirements for AP-3 binding to chemically defined liposomes. Liposomes (200 μg/ml) prepared from various combinations of purified phospholipids as described below were assayed for the binding of AP-3 and COPI. Liposomes were incubated with 5 mg/ml gel-filtered bovine adrenal cytosol in the presence or absence of 100 μM GTPγS as described in Figure 1. Liposomes prepared from DOPC/DOPE/cholesterol (50:40:10, wt/wt) were designated as control liposomes (CONT). In liposomes containing additional phospholipids, the DOPE fraction was adjusted accordingly. Liposomes designated as PA, PS, and PI contained 10% of each phospholipid added to control liposomes. Liposomes denoted as PI4P and PIP2 contained 5% of each phospholipid added to control liposomes, and liposomes identified as PIP3 contained 2.5% PIP3 added to control liposomes. Liposomes prepared from soybean lipids containing 20% PC are denoted as SOY. Liposome-bound proteins were separated by SDS-PAGE, and binding of β-COP (detected with mAb M3A5) and ς3 was determined by immunoblotting and densitometry. Coat protein binding in the absence of GTPγS was considered to be nonspecific.
Effect of ARF Class on AP-3 Membrane Recruitment
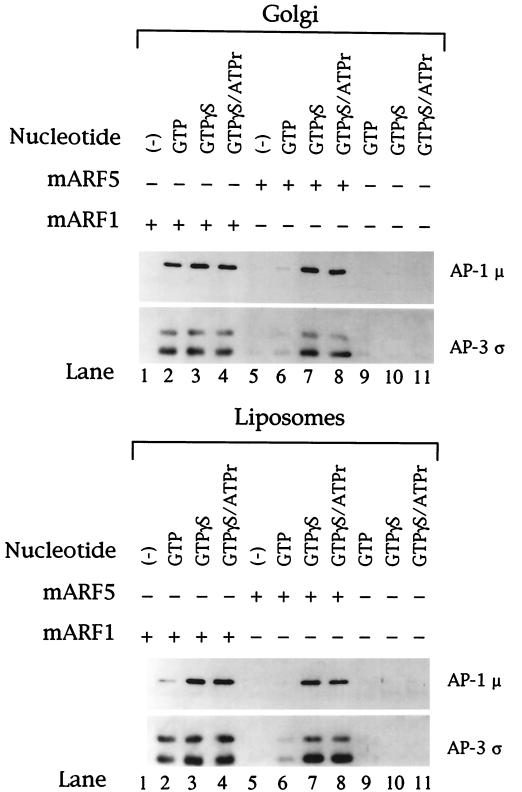
The mammalian ARF family has been classified into three distinct groups based on primary structure. Class I ARFs consist of ARF1 to ARF3, class II consists of ARF4 and ARF5, and class III contains only ARF6. ARF1 serves as the prototype among ARF family members, and previous studies have shown a role of ARF1 in AP-3 recruitment onto synaptic vesicle and endosome-enriched membranes in vitro (Faundez et al., 1998; Ooi et al., 1998) as well as in in vivo recruitment assays with the use of permeabilized normal rat kidney cells (Ooi et al., 1998). To extend these previous studies, we assessed the ability of a number of purified recombinant myristoylated ARF protein isoforms to promote the in vitro binding of cytosolic AP-3 to both liposomal and Golgi-enriched membranes. Bovine adrenal cytosol that had been depleted of endogenous ARF by gel filtration was used in standard binding assays with or without supplemental ARF proteins added at a final concentration of 50 μg/ml. As seen in Figure 3, no AP-3 binding was observed in the absence of ARF addition (lanes 9–11), in confirmation of the previous reports demonstrating a requirement for ARF in AP-3 recruitment onto membranes (Simpson et al., 1996; Faundez et al., 1998; Ooi et al., 1998). When the ARF-depleted cytosol was supplemented with either mammalian ARF1 or ARF5, AP-3 binding to both Golgi-enriched and liposome membranes occurred in the presence of GTPγS (lanes 3 and 7) and GTP (lanes 2 and 6), although recruitment was not as robust with ARF5 and GTP (lane 6). Neither yeast ARF2 nor mammalian ARF6 stimulated AP-3 membrane translocation, showing that AP-3 recruitment is selective for ARF class I and II members (our unpublished results). AP-3 binding was not enhanced when an ATP regeneration system was included in addition to GTPγS (compare lanes 4 and 8 with lanes 3 and 7), in contrast to previous reports that have described a requirement for ATP in AP-3 membrane association (Simpson et al., 1996; Faundez et al., 1998). The recruitment of AP-1 onto the membranes occurred in a similar manner, being equally stimulated by ARF1 and ARF5, as reported previously (Liang and Kornfeld, 1997; Zhu et al., 1998).
Figure 3.
ARF1 and ARF5 mediate recruitment of AP-3 onto liposomes and Golgi-enriched membranes. Liposomes (200 μg/ml) prepared from soybean 20% PC material (lower panel) or AP-1– and ARF-depleted Golgi-enriched membranes (50 μg/ml; upper panel) were incubated in a reaction mixture containing ARF-depleted bovine adrenal cytosol at 5 mg/ml supplemented with recombinant myristoylated ARF1 or ARF5 at a final concentration of 50 μg/ml. Nucleotides were added as indicated above each lane. Final guanine nucleotide concentrations were 1 mM GTP and 100 μM GTPγS. The ATP regeneration system consisted of 1 mM ATP, 10 mM creatine phosphate, and 5 U/ml creatine kinase. After recruitment reactions, membranes were recovered by centrifugation and subjected to SDS-PAGE as described in Figure 1. Portions of each blot were probed with antibodies specific for the AP-1 μ and AP-3 ς coat proteins.
Two isoforms of the AP-3 ς subunit exist: a ς3A isoform of 23 kDa and a ς3B isoform of 20 kDa (Dell'Angelica et al., 1997a). The bovine adrenal cytosol used in the experiment shown in Figure 3 contains twice as much ς3B subunit as ς3A subunit, as determined by densitometric analysis of Western blots. Interestingly, whereas ARF5 stimulates recruitment of the two forms of AP-3 in the same ratio as they occur in the adrenal cytosol, ARF1 shows a preference for the AP-3 with the ς3A isoform (compare lanes 3 and 4 with lanes 7 and 8). This phenomenon was most striking on liposomes. The significance of this observation is unclear at this time.
Recruitment of Clathrin onto Membranes by AP-3
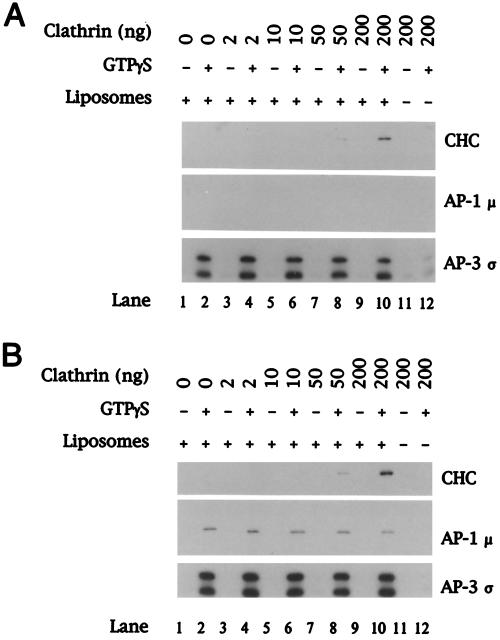
We next examined whether clathrin is recruited onto liposomes in conjunction with AP-3. In our initial experiments, we used bovine adrenal cytosol from which AP-1 had been removed by immunodepletion. This was necessary because the AP-1 complex is known to recruit clathrin from cytosol (reviewed by Scales et al., 2000). Furthermore, because we had found previously that the concentration of clathrin in the diluted cytosol used in these experiments is rate limiting for clathrin recruitment by AP-1 (Zhu et al., 1999), the adrenal cytosol was supplemented with clathrin. As seen in Figure 4A, clathrin recruitment from the AP-1–depleted cytosol that was not supplemented with clathrin could not be detected in spite of good AP-3 recruitment (lane 2). However, the addition of increasing amounts of clathrin to the assays resulted in the GTPγS-dependent translocation of both AP-3 and clathrin. Parallel experiments with the use of complete cytosol (Figure 4B) demonstrate increased clathrin binding, presumably from the concomitant recruitment of AP-1. No adaptor or clathrin binding was seen in control reactions containing cytosol and clathrin but lacking liposomes (lanes 11 and 12). Although this result suggests that clathrin is able to form a stable association with the AP-3 complex on membranes, the potential existence of another protein in the AP-1–depleted cytosol that can bind to the membranes and recruit clathrin cannot be excluded.
Figure 4.
GTPγS-dependent recruitment of AP-3 and clathrin onto liposomes. Liposomes (200 μg/ml) prepared from soybean 20% PC material were incubated in a reaction mixture containing either AP-1–depleted (A) or complete (B) bovine adrenal cytosol at 5 mg/ml in the absence or presence of 100 μM GTPγS. Cytosol devoid of AP-1 was prepared by immunodepletion with the use of mAb 100/3 specific for the γ subunit of AP-1. Reactions were supplemented with soluble unassembled clathrin at the final concentration shown above each lane in nanograms. After recruitment reactions, membranes were recovered by centrifugation and bound proteins were fractionated by 12% SDS-PAGE. Portions of each blot were probed with mAb TD.1 specific for the clathrin heavy chain (CHC) or affinity-purified antibodies against the AP-1 μ and AP-3 ς coat proteins.
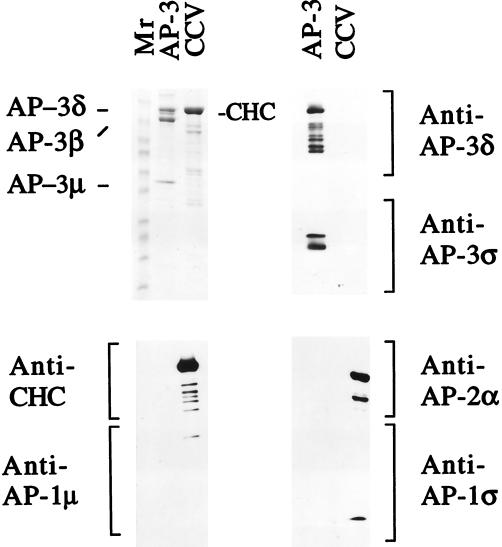
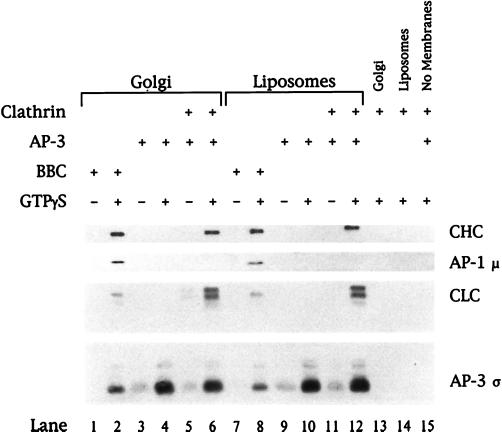
To show definitively that AP-3 is capable of translocating clathrin onto membranes, a recruitment reaction containing only purified components was developed. To accomplish this, AP-3 was purified to apparent homogeneity from bovine brain cytosol (Figure 5). Immunoblot analysis of the AP-3–containing fraction revealed no contamination with either AP-1 or AP-2. The AP-4 adaptor protein complex was also undetectable in this material (our unpublished results). As seen in Figure 6, the addition of purified clathrin trimers to in vitro recruitment reactions containing purified AP-3 resulted in the GTPγS-dependent association of clathrin with both liposomes and Golgi-enriched membranes. The Golgi-enriched membranes used in this experiment had been preincubated to remove the small amount of AP-1 that is present when the membranes are isolated. Importantly, clathrin recruitment did not occur in reactions lacking purified AP-3 (lanes 13 and 14), nor was clathrin or AP-3 binding seen in the absence of membranes (lane 15). These results demonstrate that, in vitro, the AP-3 complex is able to associate with clathrin on membranes.
Figure 5.
Characterization of the purified AP-3. Ten micrograms of the purified AP-3 material and 35 μg of purified rat brain CCVs were boiled in 50 μl of 1× SDS sample buffer. Thirty-five microliters of each sample was loaded onto a 6–15% gradient gel for Coomassie blue staining, and 5 μl of each sample was loaded in triplicate on the same gel for subsequent immunoblotting with different antibodies. The Coomassie blue staining indicates that the purified AP-3 fraction has only three major bands, labeled AP-3δ, AP-3β, and AP-3μ (upper left panel). Because AP-3δ and the clathrin heavy chain (CHC) comigrate in this gel system, they were identified by immunoblotting. The anti-AP-3δ antibody detected only AP-3δ in the AP-3 fraction (upper right panel, top), whereas the anti-CHC antibody detected only the CHC in the CCV fraction (lower left panel, top). The AP-3ς subunit was not visible by Coomassie blue staining but was clearly detected by immunoblotting with anti-AP-3ς antibody (upper right panel, bottom). Immunoblotting with anti-AP-1 and anti-AP-2 antibodies demonstrated that these adaptors were undetectable in the purified AP-3 fraction (lower left and right panels). The lower molecular weight bands seen in the CHC and AP-3δ blots most likely represent degradation products of these proteins.
Figure 6.
Recruitment of clathrin onto membranes requires AP-3. Golgi-enriched membranes depleted of AP-1 and ARF (50 μg/ml; lanes 1–6 and 13) or liposomes (200 μg/ml) prepared from soybean 20% PC material (lanes 7–12 and 14) were incubated in reaction mixtures containing either bovine brain cytosol at 5 mg/ml (lanes 1, 2, 7, and 8) or purified AP-3 at 8 μg/ml (lanes 3–6 and 9–12) in the absence or presence of 100 μM GTPγS. Clathrin trimers purified from rat brain CCVs were added to a final concentration of 5 μg/ml (lanes 5, 6, and 11–15). All reactions containing AP-3 and/or clathrin also contained recombinant myristoylated ARF1 (50 μg/ml) and BSA (2.5 mg/ml) to reduce nonspecific protein binding. After recruitment reactions, membranes were recovered and recruited proteins were separated by 12% SDS-PAGE followed by transfer to nitrocellulose. Bound proteins were detected by immunoblotting with a neuron-specific anti-clathrin light chain (CLC) mAb (clone 57.4) or antibodies against the clathrin heavy chain (CHC), AP-1 μ, or AP-3 ς. Species differences between the bovine (lanes 1, 2, 7, and 8) and rat (lanes 5, 6, and 11–15) sources of clathrin are reflected in the migration of the CLCs and the blotting intensity.
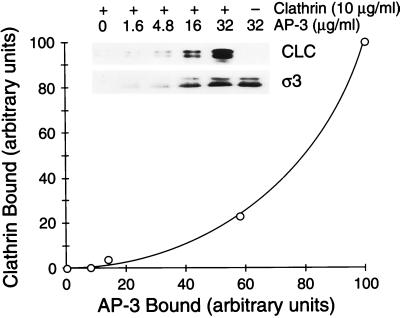
To further characterize the association of clathrin with the AP-3 complex on membranes, we assessed the effect of the concentration of the adaptor complex on the membrane on clathrin recruitment. In this experiment, various amounts of purified AP-3 were added to liposomes in reactions containing a constant concentration (10 μg/ml) of purified unassembled clathrin. As seen in Figure 7 (inset, lower panel), the addition of increasing amounts of AP-3 resulted in a concomitant and nearly linear increase in the amount of the AP-3 complex bound to the liposomal membranes, as monitored by immunoblotting of the AP-3 ς subunit. As anticipated, the binding of AP-3 was independent of clathrin, as demonstrated by the fact that addition of the same amount of AP-3 to reactions performed in the presence (lane 5) or absence (lane 6) of added clathrin resulted in equal amounts of the AP-3 ς subunit being associated with the recovered membranes.
Figure 7.
Clathrin is recruited onto AP-3–containing membranes in a cooperative manner. Liposomes (200 μg/ml) prepared from soybean 20% PC material were incubated in 200-μl reactions containing increasing concentrations of AP-3 as noted and 10 μg/ml purified rat brain clathrin in the presence of 100 μM GTPγS. All reactions contained recombinant myristoylated ARF1 (50 μg/ml) and BSA (2.5 mg/ml). After recruitment reactions, membranes were recovered, separated by 12% SDS-PAGE, and transferred to nitrocellulose, and bound proteins were analyzed by immunoblotting with antibodies directed against the clathrin light chain (CLC) or AP-3 ς followed by densitometric quantitation. Clathrin binding in the absence of added AP-3 (inset, lane 1) was considered to be nonspecific and was subtracted from the other values. The amount of clathrin bound (in arbitrary units) to the liposomal membranes is plotted as a function of the amount of AP-3 bound (in arbitrary units). This experiment was done three times with similar results.
Strikingly, the increase in membrane-bound AP-3 resulted in a nonlinear increase in the amount of clathrin associated with the membranes (Figure 7, inset, upper panel). When the amounts of bound clathrin and AP-3 were quantitated by densitometry and these values were plotted on a linear scale, it became apparent that clathrin binding occurred in a cooperative manner as a function of the membrane concentration of AP-3 (Figure 7). This is consistent with the idea that engagement of clathrin by adaptor proteins on the plane of the membrane likely involves multiple sites of contact between the adaptor protein complexes and a clathrin triskelion to establish an interaction of sufficiently high affinity. Essentially identical results were obtained in parallel experiments in which clathrin binding as a function of AP-3 recruitment onto AP-1– and ARF-depleted Golgi-enriched membranes was determined (our unpublished results).
AP-3–containing CCVs Assemble on Liposomes and Golgi-enriched Membranes
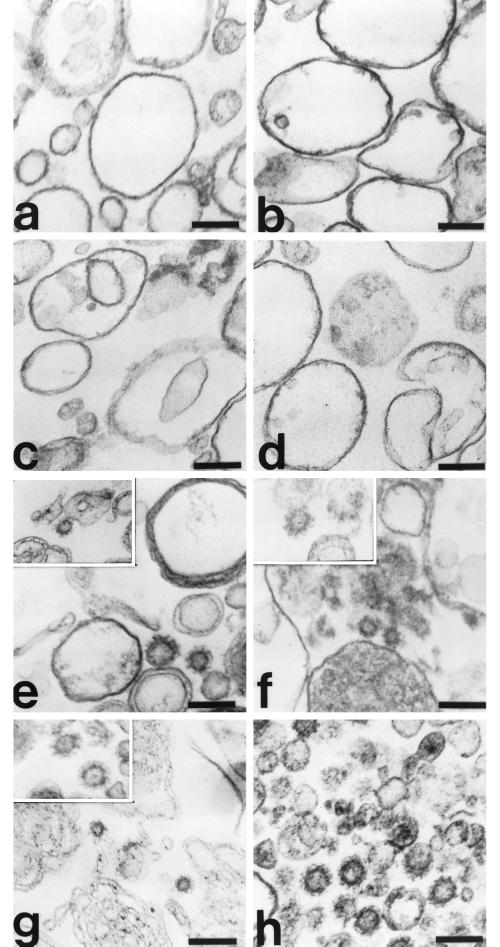
To further confirm our results demonstrating a cooperative association between clathrin and AP-3 on membranes in vitro, we undertook thin-section electron microscopic analyses of both liposomal and AP-1– and ARF-depleted Golgi-enriched membranes after recruitment reactions containing optimal concentrations of purified coat proteins. As seen in Figure 8, when liposomes prepared from the 20% PC soybean source were incubated with 30 μg/ml AP-3, 10 μg/ml clathrin, and 50 μg/ml ARF1 in the presence of GTPγS, clathrin-coated buds and vesicles were identified readily (panels e and g). Importantly, assembly of CCVs on liposomes did not occur in the absence of GTPγS (panel a) or in the absence of clathrin (panel c). Parallel studies in which AP-1– and ARF-depleted Golgi-enriched membranes served as a membrane source for the recruitment of purified AP-3 and clathrin (panels b, d, and f) showed identical requirements for CCV formation. Endogenous CCVs isolated from rat liver are shown for comparative purposes (panel h).
Figure 8.
Morphology of AP-3–containing CCVs assembled on liposomes and Golgi-enriched membranes. Liposomes (200 μg/ml) prepared from soybean 20% PC material (a, c, e, and g) or AP-1– and ARF-depleted Golgi-enriched membranes (50 μg/ml; b, d, and f) were incubated in reactions containing AP-3 (30 μg/ml; a–g), purified clathrin (10 μg/ml; a, b, and e–g), and recombinant myristoylated ARF1 (50 μg/ml; a–g) in the absence (a and b) or presence (c–g) of 100 μM GTPγS at 37°C for 20 min. The membranes were recovered by centrifugation, fixed with 1% glutaraldehyde in 0.1 M sodium cacodylate buffer, pH 7.0, and processed for electron microscopy as detailed in MATERIALS AND METHODS. CCVs isolated from rat liver are shown for comparison (h). Assembly of CCVs in vitro did not occur in the absence of GTPγS (a and b) or in reactions performed in the absence of clathrin (c and d). Bar, 100 nm.
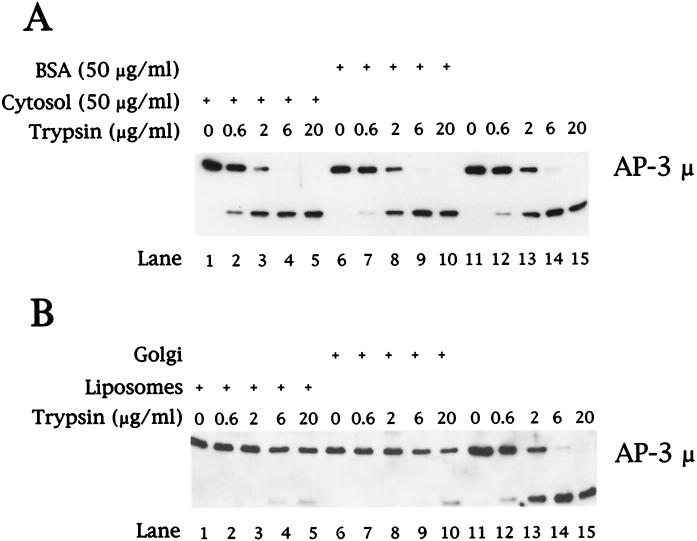
Controlled Proteolysis of the AP-3 Complex
To assess whether the AP-3 complex undergoes a conformational change upon membrane association, we used limited tryptic digestion conditions similar to those used for the controlled digestion of purified AP-1 (Schroder and Ungewickell, 1991) and AP-1 recruited onto Golgi-enriched membranes in vitro (Traub et al., 1995). As seen in Figure 9A, digestion of purified AP-3 in either the absence (lanes 11–15) or presence of 50 μg/ml cytosolic protein (lanes 1–5) or albumin (lanes 6–10) resulted in efficient cleavage of the AP-3 μ subunit. In contrast, the μ subunit of AP-3 that had been recruited onto either liposomal (Figure 9B, lanes 1–5) or Golgi-enriched membranes (lanes 6–10) exhibited markedly reduced sensitivity to trypsin digestion. Lanes 11–15 in Figure 9B represent tryptic digests of purified AP-3 resuspended in the presence of cytosolic protein.
Figure 9.
Controlled tryptic digestion of AP-3. (A) Purified AP-3 (300 ng) was digested with trypsin in the presence of 50 μg/ml bovine adrenal cytosol (lanes 1–5), 50 μg/ml BSA (lanes 6–10), or without exogenous protein (lanes 11–15). After 10 min at 37°C, the reactions were returned to ice and excess soybean trypsin inhibitor was added. Samples were concentrated by methanol/chloroform precipitation, separated by 12% SDS-PAGE followed by transfer to nitrocellulose, and analyzed by immunoblotting with antibodies specific for the μ subunit of the AP-3 complex. (B) Liposomes prepared from soybean 20% PC material (lanes 1–5) or AP-1– and ARF-depleted Golgi-enriched membranes (50 μg/ml; lanes 6–10) were incubated at 37°C for 20 min in reactions containing 5 mg/ml clathrin-depleted bovine adrenal cytosol in the presence of 100 μM GTPγS. Membranes were recovered by centrifugation and resuspended in 1× assay buffer, and equal aliquots were removed for incubation with trypsin as indicated in A. Reactions in which purified AP-3 (300 ng) was digested with trypsin (lanes 11–15) contained 50 μg/ml cytosolic protein.
DISCUSSION
In this study, we have used an in vitro biochemical approach to probe the molecular events regulating recruitment of the AP-3 complex onto membranes. Our data establish that purified AP-3 is recruited onto protein-free liposomes by a process that requires ARF·GTP but no other proteins. Previous studies by other researchers had established a role for ARF·GTP in this process, but it was unclear whether other proteins were required (Simpson et al., 1996; Faundez et al., 1998; Ooi et al., 1998). An important conclusion that follows from the liposome experiments is that AP-3 must interact directly with ARF·GTP on the lipid surface. This is similar to what has been found for COPI binding to ARF·GTP-containing liposomes (Spang et al., 1998) and by direct cross-linking experiments (Zhao et al., 1997). Although ARF·GTP and a lipid surface represent the minimal requirement for AP-3 binding in this experimental system, it is likely that other factors function to regulate AP-3 recruitment within cells. Otherwise, it is difficult to understand why AP-3 is recruited only onto selected membranes rather than associating with all membranes that contain ARF·GTP. In the case of COPI, the combination of ARF·GTP and the cytoplasmic tails of cargo molecules has been shown to be much more effective than ARF·GTP alone in recruiting this coat material (Bremser et al., 1999). Furthermore, the recruitment of AP-1 onto liposomes requires a cytosolic component in addition to ARF·GTP (Zhu et al., 1999). An important goal is to identify other factors that impart target membrane specificity to AP-3 binding.
One factor that could modulate AP-3 binding is the lipid composition of the target membrane. In our studies, AP-3 binding to liposomes was relatively independent of lipid composition, similar to the results obtained in studies of the lipid requirements for COPI membrane binding (Spang et al., 1998). This is in contrast to the finding that membrane lipid composition affects recruitment of COPII (Matsuoka et al., 1998) and AP-1 (Zhu et al., 1999). Although it is currently unknown whether organellar differences in lipid composition contribute to the membrane specificity of coat protein association, our results suggest that regional differences in intracellular lipids alone do not account for the markedly different subcellular distributions of the COPI and AP-3 coat protein complexes. Although AP-3 binding to liposomes was relatively independent of the lipid composition, it was influenced by the type of ARF present on the membrane. Thus, AP-3 recruitment was facilitated by myristoylated members of both class I and class II ARFs (ARF1 and ARF5, respectively) but not by the class III member, ARF6.
The finding that the μ3 subunit of membrane-associated AP-3 is more resistant to cleavage by trypsin than the μ3 subunit of soluble AP-3 may indicate that the AP-3 complex undergoes a conformational change upon membrane association. Another explanation is that this subunit becomes inaccessible to trypsin when AP-3 is bound to the membrane. In this regard, it has been shown that the μ2 subunit of AP-2 that is coassembled with clathrin into coats exhibits enhanced cleavage by trypsin compared with digestion of soluble AP-2 (Matsui and Kirchhausen, 1990). Interestingly, AP-2 in coats has a higher affinity for tyrosine-based motifs than cytosolic AP-2 (Rapoport et al., 1997), suggesting that a conformational change in μ2 may occur to expose the binding site. An attractive possibility is that the conformation of the μ3 subunit of AP-3 is altered upon membrane association so that binding to its cargo molecules is enhanced.
Previous studies have also reported conflicting results in terms of whether AP-3 associates with clathrin inside the cell to generate AP-3–containing CCVs. Confocal immunofluorescence studies of AP-3 and clathrin distribution in human HeLa cells have shown colocalization (Dell'Angelica et al., 1998), whereas similar studies in normal rat kidney cells found these two proteins on the same membranes but in different populations of coated buds (Simpson et al., 1997). Immunoelectron microscopy of human A431 and rat PC12 cells revealed colocalization of AP-3 and clathrin on buds of endosomes (Dell'Angelica et al., 1998). However, isolated CCVs contain little AP-3 (Simpson et al., 1996; Dell'Angelica et al., 1997b), and AP-3–containing vesicles have been shown to bud from endosomal membranes in vitro in the absence of clathrin (Faundez et al., 1998). Other in vitro studies have challenged these findings by providing evidence that AP-3 is able to associate with clathrin via a clathrin-binding motif within the β3 subunit (Dell'Angelica et al., 1998; Kirchhausen, 1999; ter Haar et al., 2000).
Our in vitro assays demonstrate that once AP-3 is recruited onto liposomes or Golgi-enriched membranes, it efficiently recruits clathrin and the assembly of CCVs ensues. Clathrin recruitment was highly dependent on the concentration of AP-3 on the membrane, indicative of a cooperative interaction between the soluble clathrin triskelions and the clathrin-binding motifs on multiple AP-3 molecules. Recently, ter Haar and colleagues have provided molecular insight into how such cooperative binding could occur (ter Haar et al., 2000). These investigators reported that an LLDLD sequence in the hinge of the AP-3 β subunit is capable of binding to the blade-1/blade-2 groove in the β-propeller module of the clathrin terminal domain. Because clathrin is a three-legged structure, it should be able to bind to the hinge of three β subunits simultaneously. This multivalent binding would be highly dependent on the concentration of AP-3 and would lead to a significant increase in binding affinity, as observed in our experiments. The importance of multivalent interactions between adaptor proteins and clathrin triskelions on the assembly of AP-1– and AP-2–containing CCVs has been stressed previously (Schroder and Ungewickell, 1991; Shih et al., 1995). These earlier studies were focused on the interaction of adaptors with clathrin assembled into cages or present in CCVs. Our experiments with AP-3 recruited onto biological membranes allow the extension of this concept to a more physiological setting.
Electron microscopic analysis of membranes recovered after recruitment reactions containing AP-3, ARF1, and clathrin revealed that the coat assembly process proceeded to the formation of clathrin-coated buds and deeply invaginated vesicles. Given this observation, it is curious that in purified CCV preparations, we and others (Simpson et al., 1996; Dell'Angelica et al., 1997b) have detected little AP-3. One possible explanation is that AP-3–containing CCVs are labile, as suggested by Stoorvogel et al. (1996) for endosome-derived CCVs. In this regard, it is of note that the bound AP-3 was more readily released from Golgi-enriched membranes and liposomes compared with AP-1, indicating that it binds to these membranes with a lower affinity. This would be consistent with AP-3 being lost from CCVs more readily than AP-1 and AP-2. It is of interest that non-clathrin– (COPI- and COPII-) coated vesicles have not been isolated by in vivo enrichment procedures but have been obtained from in vitro budding reactions in which guanine nucleotide hydrolysis was inhibited (Ostermann et al., 1993; Barlowe et al., 1994). It could be that the use of the poorly hydrolyzable GTP analogue GTPγS in our assays resulted in coat structure stabilization in the CCVs we have detected. In the absence of clathrin, coated vesicles were not detected by electron microscopic analysis. Non-clathrin–coated vesicles might have been seen if AP-3 served as a coat on its own. However, this possibility cannot be excluded because the fixation procedure may not have been adequate to reveal such a coat.
ACKNOWLEDGMENTS
We thank the many individuals who readily provided us with the antibodies used in this study. We also thank Rosalind Kornfeld and Linton Traub and members of the Kornfeld laboratory for helpful comments on the manuscript, and Lorrain LaRose for electron microscopic analysis. This research was supported by National Institutes of Health Grant CA 08759, Medical Scientist Training Grant T32 GM 07200, and the National Research Science Award Grant for Training in Molecular Hematology (T32 HL 07088).
Abbreviations used:
- AP
adaptor protein complex
- ARF
ADP-ribosylation factor
- CCV
clathrin-coated vesicle
- COP
coat protein complex
- DOPC
dioleoyl form of pure phosphatidylcholine
- DOPE
dioleoyl form of pure phosphatidylethanolamine
- GEF
guanine nucleotide exchange factor
- PA
phosphatidic acid
- PC
phosphatidylcholine
- PI
phosphatidylinositol
- PI4P
PI 4-phosphate, PIP2, PI 4,5-bisphosphate
- PIP3
PI 3,4,5-triphosphate
- PS
phosphatidylserine
- TGN
trans-Golgi network
REFERENCES
- Barlowe C, Orci L, Yeung T, Hosobuchi M, Hamamoto S, Salama N, Rexach MF, Ravazzola M, Amherdt M, Schekman R. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994;77:895–907. doi: 10.1016/0092-8674(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Blagoveshchenskaya AD, Hewitt EW, Cutler DF. Di-leucine signals mediate targeting of tyrosinase and synaptotagmin to synaptic-like microvesicles within PC12 cells. Mol Biol Cell. 1999;10:3979–3990. doi: 10.1091/mbc.10.11.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremser M, Nickel W, Schweikert M, Ravazzola M, Amherdt M, Hughes CA, Sollner TH, Rothman JE, Wieland FT. Coupling of coat assembly and vesicle budding to packaging of putative cargo receptors. Cell. 1999;96:495–506. doi: 10.1016/s0092-8674(00)80654-6. [DOI] [PubMed] [Google Scholar]
- Campbell C, Squicciarini J, Shia M, Pilch PF, Fine RE. Identification of a protein kinase as an intrinsic component of rat liver coated vesicles. Biochemistry. 1984;23:4420–4426. doi: 10.1021/bi00314a028. [DOI] [PubMed] [Google Scholar]
- Cowles CR, Odorizzi G, Payne GS, Emr SD. The AP-3 adaptor complex is essential for cargo-selective transport to the yeast vacuole. Cell. 1997;91:109–118. doi: 10.1016/s0092-8674(01)80013-1. [DOI] [PubMed] [Google Scholar]
- Darsaw T, Burd CG, Emr SD. Acidic di-leucine motif essential for AP-3-dependent sorting and restriction of the functional specificity of the Vam3p vacuolar t-SNARE. J Cell Biol. 1998;142:913–922. doi: 10.1083/jcb.142.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Angelica EC, Ohno H, Ooi CE, Rabinovich E, Roche KW, Bonifacino JS. AP-3: an adaptor-like protein complex with ubiquitous expression. EMBO J. 1997a;16:917–928. doi: 10.1093/emboj/16.5.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Angelica EC, Ooi CE, Bonifacino JS. Beta3A-adaptin, a subunit of the adaptor-like complex AP-3. J Biol Chem. 1997b;272:15078–15084. doi: 10.1074/jbc.272.24.15078. [DOI] [PubMed] [Google Scholar]
- Dell'Angelica EC, Klumperman J, Stoorvogel W, Bonifacino JS. Association of the AP-3 adaptor complex with clathrin. Science. 1998;280:431–434. doi: 10.1126/science.280.5362.431. [DOI] [PubMed] [Google Scholar]
- Dell'Angelica EC, Mullins C, Bonifacino JS. AP-4, a novel protein complex related to clathrin adaptors. J Biol Chem. 1999a;274:7278–7285. doi: 10.1074/jbc.274.11.7278. [DOI] [PubMed] [Google Scholar]
- Dell'Angelica EC, Shotelersuk V, Aguilar RC, Gahl WA, Bonifacino JS. Altered trafficking of lysosomal proteins in Hermansky-Pudlak syndrome due to mutations in the β3A subunit of the AP-3 adaptor. Mol Cell. 1999b;3:11–21. doi: 10.1016/s1097-2765(00)80170-7. [DOI] [PubMed] [Google Scholar]
- Donaldson JG, Finazzi D, Klausner RD. Brefeldin A inhibits Golgi membrane-catalyzed exchange of guanine nucleotide onto ARF protein. Nature. 1992;360:350–352. doi: 10.1038/360350a0. [DOI] [PubMed] [Google Scholar]
- Faundez V, Horng J-T, Kelly RB. A function for the AP3 coat complex in synaptic vesicle formation from endosomes. Cell. 1998;93:423–432. doi: 10.1016/s0092-8674(00)81170-8. [DOI] [PubMed] [Google Scholar]
- Feng L, et al. The β3A subunit gene (Ap3b1) of the AP-3 adaptor complex is altered in the mouse hypopigmentation mutant pearl, a model for Hermansky-Pudlak syndrome and night blindness. Hum Mol Genet. 1999;8:323–330. doi: 10.1093/hmg/8.2.323. [DOI] [PubMed] [Google Scholar]
- Hirst J, Bright NA, Rous B, Robinson MS. Characterization of a fourth adaptor-related protein complex. Mol Biol Cell. 1999;10:2787–2802. doi: 10.1091/mbc.10.8.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst J, Robinson MS. Clathrin and adaptors. Biochim Biophys Acta. 1998;1404:173–193. doi: 10.1016/s0167-4889(98)00056-1. [DOI] [PubMed] [Google Scholar]
- Höning S, Sandoval IV, von Figura K. A di-leucine-based motif in the cytoplasmic tail of LIMP-II and tyrosinase mediates selective binding of AP-3. EMBO J. 1998;17:1304–1314. doi: 10.1093/emboj/17.5.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantheti P, et al. Mutation in AP-3δ in the mocha mouse links endosomal transport to storage deficiency in platelets, melanosomes, and synaptic vesicles. Neuron. 1998;21:111–122. doi: 10.1016/s0896-6273(00)80519-x. [DOI] [PubMed] [Google Scholar]
- Kedersha NL, Rome LH. Isolation and characterization of a novel ribonucleoprotein particle: large structures contain a single species of small RNA. J Cell Biol. 1986;103:699–709. doi: 10.1083/jcb.103.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhausen T. Adaptors for clathrin-mediated traffic. Annu Rev Cell Dev Biol. 1999;15:705–732. doi: 10.1146/annurev.cellbio.15.1.705. [DOI] [PubMed] [Google Scholar]
- Le Borgne R, Alconda A, Bauer U, Hoflack B. The mammalian AP-3 adaptor-like complex mediates the intracellular transport of lysosomal membrane glycoproteins. J Biol Chem. 1998;273:29451–29461. doi: 10.1074/jbc.273.45.29451. [DOI] [PubMed] [Google Scholar]
- Le Borgne R, Hoflack B. Mechanisms of protein sorting and coat assembly: insights from the clathrin-coated vesicle pathway. Curr Opin Cell Biol. 1998;10:499–503. doi: 10.1016/s0955-0674(98)80065-3. [DOI] [PubMed] [Google Scholar]
- Liang JO, Kornfeld S. Comparative activity of ADP-ribosylation factor family members in the early steps of coated vesicle formation on rat liver Golgi membranes. J Biol Chem. 1997;272:4141–4148. doi: 10.1074/jbc.272.7.4141. [DOI] [PubMed] [Google Scholar]
- Matsui W, Kirchhausen T. Stabilization of clathrin coats by the core of the clathrin-associated protein complex AP-2. Biochemistry. 1990;29:10791–10798. doi: 10.1021/bi00500a011. [DOI] [PubMed] [Google Scholar]
- Matsuoka K, Orci L, Amherdt M, Bednarek SY, Hamamoto S, Schekman R, Yeung T. COPII-coated vesicle formation reconstituted with purified coat proteins and chemically defined liposomes. Cell. 1998;93:263–275. doi: 10.1016/s0092-8674(00)81577-9. [DOI] [PubMed] [Google Scholar]
- Mullins C, Hartnell LM, Wassarman DA, Bonifacino JS. Defective expression of the μ3 subunit of the AP-3 adaptor complex in the Drosophila pigmentation mutant carmine. Mol Gen Genet. 1999;262:401–412. doi: 10.1007/s004380051099. [DOI] [PubMed] [Google Scholar]
- Nathke IS, Heuser J, Lupas A, Stock J, Turek CW, Brodsky FM. Folding and trimerization of clathrin subunits at the triskelion hub. Cell. 1992;68:899–910. doi: 10.1016/0092-8674(92)90033-9. [DOI] [PubMed] [Google Scholar]
- Odorizzi G, Cowles CR, Emr SD. The AP-3 complex: a coat of many colors. Trends Cell Biol. 1998;8:282–288. doi: 10.1016/s0962-8924(98)01295-1. [DOI] [PubMed] [Google Scholar]
- Ooi CE, Dell'Angelica EC, Bonifacino JS. ADP-ribosylation factor 1 (ARF1) regulates recruitment of the AP-3 adaptor complex to membranes. J Cell Biol. 1998;142:391–402. doi: 10.1083/jcb.142.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi CE, Moreira JE, Dell'Angelica EC, Poy G, Wassarman DA, Bonifacino JS. Altered expression of a novel adaptin leads to defective pigment granule biogenesis in the Drosophila eye color mutant garnet. EMBO J. 1997;16:4508–4518. doi: 10.1093/emboj/16.15.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L, Glick BS, Rothman JE. A new type of coated vesicular carrier that appears not to contain clathrin: its possible role in protein transport within the Golgi stack. Cell. 1986;46:171–184. doi: 10.1016/0092-8674(86)90734-8. [DOI] [PubMed] [Google Scholar]
- Ostermann J, Orci L, Tani K, Amherdt M, Ravazzola M, Elazar Z, Rothman JE. Stepwise assembly of functionally active transport vesicles. Cell. 1993;75:1015–1025. doi: 10.1016/0092-8674(93)90545-2. [DOI] [PubMed] [Google Scholar]
- Panek HR, Stepp JD, Engle HM, Marks KM, Tan PK, Lemmon SK, Robinson LC. Suppressors of YCK-encoded yeast casein kinase 1 deficiency define the four subunits of a novel clathrin AP-like complex. EMBO J. 1997;16:4194–4204. doi: 10.1093/emboj/16.14.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo PA, Yang YC, Rulka C, Kahn RA. Activation of ADP-ribosylation factor by Golgi membranes: evidence for a brefeldin A- and protease-sensitive activating factor on Golgi membranes. J Biol Chem. 1993;268:9555–9563. [PubMed] [Google Scholar]
- Rapoport I, Miyazaki M, Boll W, Duckworth B, Cantley LC, Shoelson S, Kirchhausen T. Regulatory interactions in the recognition of endocytic sorting signals by AP-2 complexes. EMBO J. 1997;16:2240–2250. doi: 10.1093/emboj/16.9.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MS. Coats and vesicle budding. Trends Cell Biol. 1997;7:99–102. doi: 10.1016/S0962-8924(96)10048-9. [DOI] [PubMed] [Google Scholar]
- Rothman JE, Wieland FT. Protein sorting by transport vesicles. Science. 1996;272:227–234. doi: 10.1126/science.272.5259.227. [DOI] [PubMed] [Google Scholar]
- Sata M, Donaldson JG, Moss J, Vaughn M. Brefeldin-A inhibited guanine nucleotide-exchange activity of Sec7 domain from yeast Sec7 with yeast and mammalian ADP-ribosylation factors. Proc Natl Acad Sci USA. 1998;95:4204–4208. doi: 10.1073/pnas.95.8.4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scales SJ, Gomez M, Kreis TE. Coat proteins regulating membrane traffic. Int Rev Cytol. 2000;195:67–144. doi: 10.1016/s0074-7696(08)62704-7. [DOI] [PubMed] [Google Scholar]
- Schroder S, Ungewickell E. Subunit interaction and function of clathrin-coated vesicle adaptors from the Golgi and the plasma membrane. J Biol Chem. 1991;268:7910–7918. [PubMed] [Google Scholar]
- Shih W, Gallusser A, Kirchhausen T. A clathrin-binding site in the hinge of the beta 2 chain of mammalian AP-2 complexes. J Biol Chem. 1995;270:31083–31090. doi: 10.1074/jbc.270.52.31083. [DOI] [PubMed] [Google Scholar]
- Simpson F, Bright NA, West MA, Newman LS, Darnell RB, Robinson MS. A novel adaptor-related protein complex. J Cell Biol. 1996;133:749–760. doi: 10.1083/jcb.133.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson F, Peden AA, Christopoulou L, Robinson MS. Characterization of the adaptor-related protein complex, AP-3. J Cell Biol. 1997;137:835–845. doi: 10.1083/jcb.137.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spang A, Matsuoka K, Hamamoto S, Schekman R, Orci L. Coatomer, Arf1p, and nucleotide are required to bud coat protein complex I-coated vesicles from large synthetic liposomes. Proc Natl Acad Sci USA. 1998;95:11199–11204. doi: 10.1073/pnas.95.19.11199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamnes MA, Rothman JE. The binding of AP-1 clathrin adaptor particles to Golgi membranes requires ADP-ribosylation factor, a small GTP-binding protein. Cell. 1993;73:999–1005. doi: 10.1016/0092-8674(93)90277-w. [DOI] [PubMed] [Google Scholar]
- Stepp JD, Huang K, Lemmon SK. The yeast adaptor protein complex, AP-3, is essential for the efficient delivery of alkaline phosphatase by the alternate pathway to the vacuole. J Cell Biol. 1997;139:1761–1774. doi: 10.1083/jcb.139.7.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoorvogel W, Oorschot V, Geuze HJ. A novel class of clathrin-coated vesicles budding from endosomes. J Cell Biol. 1996;132:21–33. doi: 10.1083/jcb.132.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Haar E, Harrison SC, Kirchhausen T. Peptide-in-groove interactions link target proteins to the beta-propeller of clathrin. Proc Natl Acad Sci USA. 2000;97:1096–1100. doi: 10.1073/pnas.97.3.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub LM, Kornfeld S, Ungewickell E. Different domains of the AP-1 adaptor complex are required for Golgi membrane binding and clathrin recruitment. J Biol Chem. 1995;270:4933–4942. doi: 10.1074/jbc.270.9.4933. [DOI] [PubMed] [Google Scholar]
- Traub LM, Ostrom JA, Kornfeld S. Biochemical dissection of AP-1 recruitment onto Golgi membranes. J Cell Biol. 1993;123:561–573. doi: 10.1083/jcb.123.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub LM, Sagi-Eisenberg R. Purification of p100, a protein antigenically related to the signal transducing G proteins Gt and Gi: evidence for an adaptin-like protein. J Biol Chem. 1991;266:24642–24649. [PubMed] [Google Scholar]
- Ungewickell E. Clathrin: a good view of a shapely leg. Curr Biol. 1999;9:32–35. doi: 10.1016/s0960-9822(99)80040-2. [DOI] [PubMed] [Google Scholar]
- Vowels JJ, Payne GS. A dileucine-like sorting signal directs transport into an AP-3-dependent, clathrin-independent pathway to the yeast vacuole. EMBO J. 1998;17:2482–2493. doi: 10.1093/emboj/17.9.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel D, Flugge UI. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 1984;138:141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]
- Yeung BG, Phan HL, Payne GS. Adaptor complex-independent clathrin function in yeast. Mol Biol Cell. 1999;10:3643–3659. doi: 10.1091/mbc.10.11.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Helms JB, Brugger B, Harter C, Martoglio B, Graf R, Brunner J, Wieland FT. Direct and GTP-dependent interaction of ADP ribosylation factor 1 with coatomer subunit beta. Proc Natl Acad Sci USA. 1997;94:4418–4423. doi: 10.1073/pnas.94.9.4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen L, et al. Abnormal expression and subcellular distribution of subunit proteins of the AP-3 adaptor complex lead to platelet storage pool deficiency in the pearl mouse. Blood. 1999;94:146–155. [PubMed] [Google Scholar]
- Zhu Y, Drake MT, Kornfeld S. ADP-ribosylation factor 1 dependent clathrin-coat assembly on synthetic liposomes. Proc Natl Acad Sci USA. 1999;96:5013–5018. doi: 10.1073/pnas.96.9.5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Traub LM, Kornfeld S. ADP-ribosylation factor 1 transiently activates high-affinity adaptor protein complex AP-1 binding sites on Golgi membranes. Mol Biol Cell. 1998;9:1323–1337. doi: 10.1091/mbc.9.6.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]