Abstract
The gene encoding a truncated merozoite antigen-2 (EMA-2t) of Babesia equi was cloned and highly expressed in Escherichia coli as a glutathione S-transferase fusion protein (G-rEMA-2t). Both G-rEMA-2t and rEMA-2t (after the removal of glutathione S-transferase) had good antigenicity. Either Western blot analysis with rEMA-2t or enzyme-linked immunosorbent assay (ELISA) with G-rEMA-2t clearly discriminated the sera of horses experimentally infected with B. equi from sera of horses infected with Babesia caballi and healthy horses, although rEMA-2t was not suitable for ELISA, probably owing to its poor absorbability to the plates. The specific antibodies in B. equi-infected horses were detectable during both acute and latent infection (6 to 244 days postinfection). Horse sera from Jilin Province, China, were examined by the two tests. The seroprevalence of B. equi was 49.2% (31 of 63 sera) by Western blot analysis with rEMA-2t and 47.6% (30 of 63 sera) by ELISA with G-rEMA-2t. The correspondence was 98.4% (62 of 63 sera) between the two tests. The results indicate that G-rEMA-2t and rEMA-2t proteins should be suitable antigens for the development of an effective immunodiagnostic assay due to their high sensitivity, specificity, and great yield.
Babesia equi is a tick-borne hemoprotozoan parasite which causes an economically important disease, equine piroplasmosis (11). This disease is characterized clinically by fever, anemia, and icterus (6). The mortality rate is high during the initial infection of horses introduced into regions of endemicity (6). Horses that survive the initial infection are lifelong carriers of the parasites (6), which are very difficult to demonstrate by microscopic examination. Currently, no drug or vaccine is available to clear the parasites completely or prevent horses from the parasite infection. Due to the widespread occurrence of both B. equi and various tick vectors, diagnosis and prevention of these diseases are important in both areas of endemicity and areas of nonendemicity. Therefore, it is necessary to develop a reliable, sensitive, specific, and inexpensive immunodiagnosis kit to detect both acute and latent infections with the parasite.
For immunodiagnosis, sensitivity, specificity, and cost mainly depend on the antigen. Native crude antigens can nonspecifically react to test sera, and preparation on a large scale is very complicated and laborious. These results are partially responsible for the limitation of the complement fixation test (CFT) and have hindered the development of the enzyme-linked immunosorbent assay (ELISA) (3, 15, 16). Hence, it is quite plausible to use recombinant antigens in detection of B. equi infection (14, 17, 18).
Merozoite surface antigens play important roles in parasite recognition of, attachment to, and penetration of host erythrocytes (8). They are, hence, logical targets of host immune responses. B. equi merozoite antigen-2 (EMA-2) is a major surface antigen; therefore, it is a good candidate for a diagnostic regent for the detection of antibody against the parasite. In the present study, the gene encoding the entire EMA-2 (10) was initially expressed in Escherichia coli by using a recombinant pGEX-4T vector. However, expression of whole EMA-2 was low level and incorrect. Therefore, a truncated EMA-2 (EMA-2t) gene without sequences encoding hydrophobic signal peptide and C terminus was then amplified and expressed in E. coli to improve the expression and hydrophilicity of the protein. The recombinant EMA-2t fusion protein and the recombinant EMA-2t after removal of glutathione S-transferase (GST) were evaluated for the potential use in immunodiagnosis by Western blot analysis and ELISA.
MATERIALS AND METHODS
Parasite.
A U.S. Department of Agriculture strain of B. equi was cultured in equine erythrocytes as described previously (1, 2). When the level of B. equi parasitemia reached 10 to 20%, cultured erythrocytes were washed three times with phosphate-buffered saline (PBS) by centrifugation, and the pellets were then stored at −80°C.
Cloning of the EMA-2 and EMA-2t genes.
B. equi-infected erythrocytes were washed with PBS and lysed in 0.1 M Tris-HCl (pH 8.0) containing 1% sodium dodecyl sulfate, 0.1 M NaCl, and 10 mM EDTA. They were then digested with protease K (100 μg/ml) for 2 h at 55°C. The DNA was extracted with phenol-chloroform and precipitated with ethanol. The pellets were resuspended in 10 mM Tris-HCl (pH 8.0) containing 1 mM EDTA and used as a template DNA for PCR. The EMA-2 gene was amplified by PCR using the oligonucleotide primers 5′-ACGAATTCTAAAATGTTGAGCAAG-3′ and 5′-ACGAATTCGCGCTTAGTAGAACAA-3′ (10). The EMA-2t gene was amplified by using a sense primer, 5′-ACGAATTCCGATGAGGCACCAAAG-3′, which was located at the 23rd codon (the first codon behind the cleavage site of signal sequence), and the antisense primer, 5′-ACGAATTCTTATTGGGTCTTGTAG-3′, which was located at the 251st codon (the last codon before the hydropheotic C-terminal sequence) (Fig. 1) (10). The amplified DNA was inserted into the EcoRI site of pGEX-4T and transformed into the DH5α strain of E. coli. The resulting recombinant plasmids were cloned and designated pGEX-4T/EMA-2 and pGEX-4T/EMA-2t, respectively.
FIG. 1.
Hydrophilicity plot of EMA-2 antigen sequence and location of EMA-2t. The plot shown was derived from the amino acid sequence of the open reading frame of the EMA-2 gene by using a computer analysis programs (window = 7) developed by Hopp and Woods (7). nt, nucleotide.
Expression of the recombinant EMA-2 and EMA-2t proteins in E. coli.
E. coli colonies transformed with the recombinant plasmids pGEX-4T/EMA-2 and pGEX-4T/EMA-2t were cultured, respectively, in LB medium (1% Bacto Tryptone, 0.5% yeast extract, 1% NaCl, and 0.1% 5 N NaOH) with ampicillin sodium (50 μg/ml) at 37°C. When the optical density at 600 nm reached 0.30, E. coli was induced to express the recombinant EMA-2 and EMA-2t proteins by the addition of 0.5 mM IPTG (isopropyl-β-d-thiogalactopyranoside) and incubation for another 4 h.
Extraction of the recombinant fusion proteins.
The recombinant EMA-2 and EMA-2t fusion proteins with GST were extracted with TNE (50 mM Tris-HCl at pH 7.5, 100 mM NaCl, and 2 mM EDTA) containing lysozyme (100 μg/ml) and 1% Triton X-100 combined with sonication. After centrifugation at 12,000 × g for 10 min, both the soluble and insoluble fractions were harvested and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) to compare the levels of expression.
Purification with glutathione-Sepharose 4B and thrombin protease cleavage.
The recombinant EMA-2t fusion protein (G-rEMA-2t) was purified from the soluble fraction with glutathione-Sepharose 4B (Amersham Pharmacia Biotech, Uppsala, Sweden). To remove the GST affinity tail from the fusion protein, thrombin protease cleavage was used to combine with glutathione-Sepharose 4B according to the manufacturer's instructions. After the removal of GST, the recombinant EMA-2t was designated rEMA-2t.
SDS-PAGE and Western blot analysis.
The samples were boiled for 5 min in a sample buffer (62.5 mM Tris-HCl at pH 6.8, 2% SDS, 5% β-mercaptoethanol, 10% glycerol, and 0.02% bromophenol blue) and subjected to SDS-PAGE using 12% acrylamide gels as described previously (12). Sequentially, proteins were transferred electrophoretically onto polyvinylidene difluoride membranes (Millipore). The blots were incubated with diluted serum samples (1:100) for 1 h and then washed with PBS three times. The membranes were incubated with horseradish peroxidase-conjugated goat anti-horse immunoglobulin G antibody (1:2,000; ICN Biochemicals, Aurora, Ohio) for 1 h. After three washes with PBS, the membranes were exposed to a substrate solution containing diaminobenzidine (0.5 mg/ml) and 0.03% H2O2 to develop the color and visualize the specific antigen bands.
ELISA.
ELISA was performed in 96-well microplates (Nunc, Roskilde, Denmark). The plates were coated with the diluted antigen (5 μg/ml) at 4°C overnight. After the unabsorbed antigen was discarded, the wells were blocked with 3% skim milk in PBS (blocking solution) at 37°C for 1 h. Then, the blocking solution was discarded, and 50 μl of serum sample diluted in blocking solution was added to each well. After 1 h of incubation at 37°C, the wells were washed for six cycles with a wash solution (PBS containing 0.05% Tween 20) and then incubated with horseradish peroxidase-conjugated goat anti-horse immunoglobulin G (ICN Biochemicals) diluted in the blocking solution at 37°C for 1 h (50 μl per well). After six cycles of wash, substrate [0.1 M citric acid, 0.2 M sodium phosphate, 0.003% H2O2, 0.3 mg of 2, 2′-azino-di-(3-ethylbenzthiazoline sulfonate) per ml] was added (100 μl per well). The absorbance at 415 nm was read after 1 h by means of an MTP-120 ELISA reader (Corona Electric, Ibaraki, Japan). GST was used as a control antigen for G-rEMA-2t. The ELISA result was determined for each sample by taking the mean optical density value of two readings with the G-rEMA-2t and subtracting the mean value of two readings with GST protein. A sample was considered positive if the calculated absorbance value was equal to or greater than 0.1.
Sera.
Sixty sequential serum samples from four horses experimentally infected with B. equi or Babesia caballi, sera from 11 B. equi-infected horses bled from 30 to 244 days postinfection (d.p.i.), 10 B. caballi-infected horses bled from 28 to 395 d.p.i., and 20 healthy horses were obtained from the Equine Research Institute, the Japan Racing Association, and the Onderstepoort Veterinary Institute. Sixty-three field serum samples were from horses in Jilin Province, in the northeast of the People's Republic of China.
RESULTS
Cloning and expression of EMA-2 and EMA-2t in E. coli.
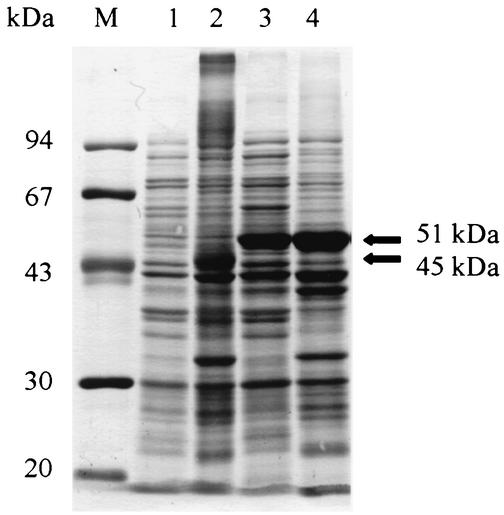
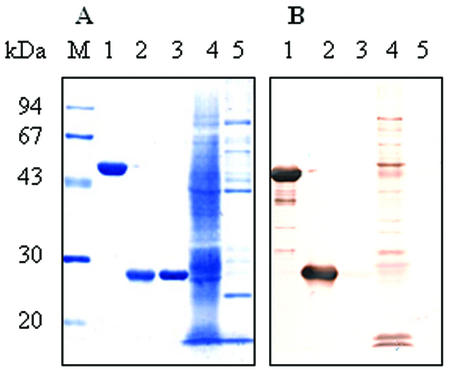
The DNA encoding EMA-2 and a predicted 684-bp DNA encoding the EMA-2t (Fig. 1) were amplified from the parasites by PCR and inserted into the EcoRI site of pGEX-4T-3. The recombinant plasmids were then transformed into E. coli (DH5α) and cloned. The cloned genes were expressed in E. coli as fusion proteins with GST under induction of IPTG. Soluble and insoluble fractions were extracted and subjected to SDS-PAGE to compare the expression in different fractions. As shown in Fig. 2, there was almost no recombinant EMA-2 fusion protein expressed in soluble form (lane 1) and only some expressed in the insoluble form, with an incorrect molecular mass (45 kDa) (lane 2). However, G-rEMA-2t at 51 kDa, as expected, was expressed at a high level, with approximately 40% of the protein in soluble form (lane 3), which indicates that removal of the hydrophobic signal peptide and C terminus had dramatically improved its expression and hydrophilicity. G-rEMA-2t in the soluble fraction was purified with glutathione-Sepharose 4B (Fig. 3A, lane 1). To eliminate the probable presence of antibody directed to GST, thrombin protease was used to cleave GST from the fusion protein, and, as expected, rEMA-2t at 25 kDa was sequentially obtained (Fig. 3A, lane 2). About 5 to 6 mg of highly purified G-rEMA-2t or rEMA-2t was recovered from l liter of culture. The antigenicity of G-rEMA-2t and rEMA-2t was examined using Western blot analysis, which showed that both antigens were recognized by sera from horses experimentally infected with B. equi (Fig. 3B).
FIG. 2.
Expression of the recombinant fusion protein of entire (lanes 1 and 2) and truncated (lanes 3 and 4) EMA-2 in E. coli. Lane M, molecular mass standard; lanes 1 and 3, proteins in soluble fractions; lanes 2 and 4, proteins in insoluble fractions. Proteins were stained with amido black 10B.
FIG. 3.
Purified G-rEMA-2t and rEMA-2t and their antigenicity. (A) Antigens stained with amido black 10B; (B) reactivity of G-rEMA-2t and rEMA-2t to serum from B. equi-infected horse by Western blot analysis. Lane M, molecular mass standard; lane 1, G-rEMA-2t (51 kDa); lane 2, rEMA-2t (25 kDa); lane 3, GST; lane 4, B. equi-infected erythrocyte lysate; lane 5, normal erythrocyte lysate.
Detection of specific antibody in sera from the known infected and noninfected horses, and in field sera from horses in Jilin Province, China.
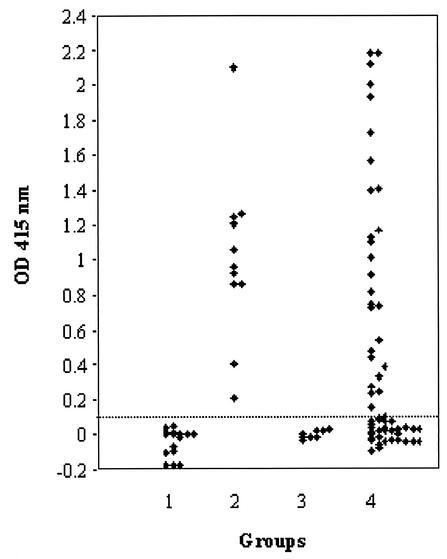
Sera from 11 and 10 horses infected experimentally with B. equi and B. caballi, respectively, and sera from 20 healthy horses were examined by ELISA with G-rEMA-2t and ELISA with rEMA-2t. The specific antibody response to G-rEMA-2t was detected only in sera from B. equi-infected horses but not in sera from B. caballi-infected and normal horses (Fig. 4, groups 1 to 3). However, the antibody responses to rEMA-2t in the ELISA were very weak and could not differentiate B. equi-infected sera from other sera (data not shown). Since rEMA-2t was not suitable for use as an antigen in ELISA, rEMA-2t was evaluated by Western blot analysis with the same serum samples as mentioned above to further confirm the antigenicity of rEMA-2t. All sera from B. equi-infected horses had strong reactions to rEMA-2t, but no sera from healthy horses and B. caballi-infected horses did. The fact that rEMA-2t had poor reactivity in ELISA but had good reactivity in Western blot analysis suggests that the removal of GST from the recombinant protein impaired the absorbability of the protein to ELISA plates but did not affect its antigenicity and its absorbability to polyvinylidene difluoride membranes.
FIG. 4.
ELISA with G-rEMA-2t. Group 1, sera from normal horses; groups 2 and 3, sera from horses infected experimentally with B. equi and B. caballi, respectively; group 4, sera from horses in Jilin Province, China.
ELISA with G-rEMA-2t and Western blot analysis with rEMA-2t were used to examine B. equi infection in 63 horses in Jilin Province, China. As shown in Fig. 4 (group 4) and Table 1, the seroprevalence of B. equi was 47.6% (30 of 63 horses) and 49.2% (31 of 63 horses), respectively. The correspondence between ELISA and Western blot analysis was 98.4% (62 of 63 horses).
TABLE 1.
Comparison between results obtained by ELISA with G-rEMA-2t and by Western blot analysis with rEMA-2t in the detection of antibody to B. equi in serum from horses in Jilin Province, China
| Western blot result | No. (%) with ELISA result
|
Total no. (%) | |
|---|---|---|---|
| +a | − | ||
| + | 30 (47.6) | 1 (1.6) | 31 (49.2) |
| − | 0 (0) | 32 (50.8) | 32 (50.8) |
| Total | 30 (47.6) | 33 (52.4) | 63 (100) |
ELISA was considered positive when the calculated absorbance value, the mean optical density value of two readings at 415 nm with the G-rEMA-2t minus the mean value of two readings with GST protein, was equal to or greater than 0.1.
Kinetics of antibody responses to B. equi in experimentally infected horses measured by ELISA with G-rEMA-2t and Western blot analysis with rEMA-2t.
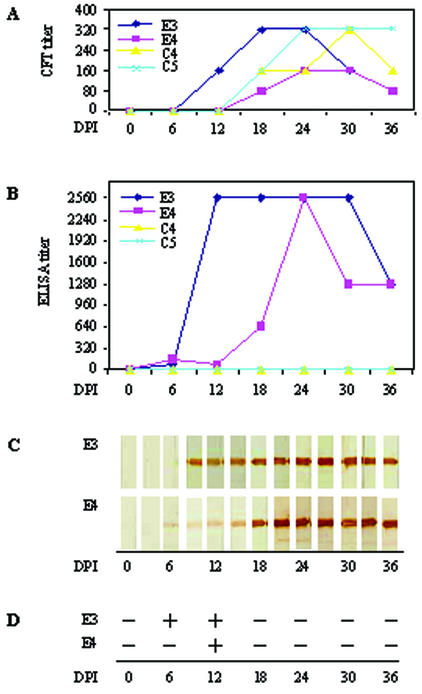
Sixty sequential sera from four horses infected experimentally with B. equi and B. caballi, respectively, were examined by ELISA with G-rEMA-2t and by Western blot analysis with rEMA-2t. The results were compared with that by the CFT (Fig. 5A) (17). The specific antibody response to G-rEMA-2t in sera from B. equi-infected horses was detectable from 6 d.p.i. (Fig. 5B), which was earlier than that by the CFT. The specific antibody response to rEMA-2t in the Western blot analysis was detectable from 6 d.p.i. (E4) or 7 d.p.i. (E3) (Fig. 5C). Although parasitemia was only detectable during 6 to 12 d.p.i. (Fig. 5D), strong antibody responses to G-rEMA-2t and rEMA-2t remained until the end of the experiment (36 d.p.i.) (Fig. 5). No reactions were found in sera from B. caballi-infected horses or sera from normal horses (data not shown).
FIG. 5.
Comparison of results obtained by CFT (A), ELISA (B), Western blot analysis (C), and microscopic examination (D) in the diagnosis of B. equi infection. E3 and E4 were two horses experimentally infected with B. equi. C4 and C5 were two horses experimentally infected with B. caballi.
DISCUSSION
The “gold standard” for the diagnosis of equine piroplasmosis should be a test that is (i) sensitive enough for both the early detection of acute infection and the detection of latent infections, (ii) specific for the differentiation between the two parasite species, and (iii) economical with regards to materials and time (3). So far, no immunological assays with native antigens can meet the standards due to the nonspecific reaction and complicated preparation of the antigen and the limitations of tests such as CFT and IFAT (3, 9, 13). Recently, recombinant EMA-1 and EMA-2 expressed in a baculovirus-insect cell system have improved the ELISAs in both sensitivity and specificity (14, 17). However, they were still contaminated with some proteins excreted by insect cells or produced by baculovirus, which are not suitable for some other immunodiagnostic assays. Purification is very difficult if the recombinant proteins expressed in insect cells do not carry a His tag, as mentioned above. Moreover, the expression in insect cells takes more time. Therefore, with regard to cost performance, the procedures for expression and purification are still too complicated and expensive.
A bacterial expression system has an advantage over others because the procedures of expression and purification are much easier. This is more relevant when producing a protein on a large scale if its antigenicity will not be impaired by incorrect folding, which may sometimes happen (4, 5). The present study takes advantage of the bacterial expression system. Since the entire EMA-2 was expressed improperly in E. coli for unknown reasons, a truncated EMA-2 gene without fragments encoding a hydrophobic signal peptide and C terminus was cloned and expressed in E. coli. The result showed that the recombinant protein was expressed at a high level, with about 40% in soluble form. The fusion protein could be purified under nonreducing conditions by affinity chromatography using glutathione-Sepharose 4B beads, and GST could be removed by thrombin protease cleavage. A large amount of highly purified G-rEMA-2t or rEMA-2t could be easily recovered, which may warrant an economical test. The rEMA-2t will facilitate some tests other than ELISA because the removal of GST has eliminated the probable presence of antibodies against GST so that the tests will be more specific and no control antigen is required. Moreover, the smaller molecular mass of rEMA-2t might be an outstanding advantage in development of rapid immunochromatographic assay for diagnosis of B. equi infection.
Both G-rEMA-2t and rEMA-2t could react strongly only with sera from B. equi-infected horses, as judged by ELISA and/or Western blot analysis, which indicated that the truncation of EMA-2 did not affect the antigenicity of the proteins. The approach may be suitable for improving the expression and hydrophilicity of some other antigens, which could not be expressed in E. coli properly or expressed as only insoluble proteins. G-rEMA-2t had strong reactions to B. equi-infected sera in ELISA and Western blot analysis; however, rEMA-2t was not a suitable antigen in ELISA but had a strong reactivity with B. equi-infected sera in Western blot analysis. This indicates that the removal of GST did not affect the protein's antigenicity or the absorbance to polyvinylidene difluoride membranes but impaired its absorbance to the ELISA plates.
ELISA with G-rEMA-2t and Western blot analysis with the rEMA-2t could clearly differentiate B. equi-infected horse sera from B. caballi-infected or normal horse sera. Both could detect the antibody response as early as 6 or 7 d.p.i., which was 6 to 12 days earlier than detection by the ELISA with EMA-1 expressed in insect cells or by the CFT (17). Although the parasites were only detectable from 6 to 12 d.p.i., the specific antibody responses to G-rEMA-2t and rEMA-2t remained at a high level until the end of the experiment (36 d.p.i.) in sequential sera and 244 d.p.i. in individual sera from B. equi-infected horses. The result indicates that both tests could detect the early acute and latent infection of B. equi and that they are specific enough for the differentiation between the infection of two parasite species, B. equi and B. caballi. Both tests were also concordant with each other, as confirmed further in the examination of serum samples from field horses in Jilin Province, China, which indicates that the results are both reliable to a certain extent. The high seroprevalence of B. equi in this area serves as a reminder to pay attention to the issue.
In conclusion, rEMA-2t and G-rEMA-2t have improved the Western blot analysis and the ELISA, respectively, to reach the first two items on the gold standard list and half of the third item, which makes it economical with regard to materials. Moreover, rEMA-2t has a low molecular weight, which will facilitate some rapid immunodiagnostic tests. Further work is required to develop a rapid, reliable, sensitive, and specific immunodiagnostic test with the antigens.
Acknowledgments
This study was supported by a grant from The 21st Century COE Program (A-1), Ministry of Education, Culture, Sports, Science, and Technology, Japan, and a grant-in-aid for scientific research (A) from the Japan Society for the Promotion of Science (no. 13356007).
REFERENCES
- 1.Avarazed, A., D. T. de Waal, I. Igarashi, A. Saito, T. Oyamada, Y. Toyoda, and N. Suzuki. 1997. Prevalence of equine piroplasmosis in central Mongolia. Onderstepoort J. Vet. Res. 64:141-145. [PubMed] [Google Scholar]
- 2.Avarazed, A., I. Igarashi, D. T. DeWaal, S. Kawai, Y. Oomori, N. Inoue, Y. Maki, Y. Omata, A. Saito, H. Nagasawa, Y. Toyoda, and N. Suzuki. 1998. Monoclonal antibody against Babesia equi: characterization and potential application of antigen for serodiagnosis. J. Clin. Microbiol. 36:1835-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruning, A. 1996. Equine piroplasmosis: an update on diagnosis, treatment, and prevention. Br. Vet. J. 152:139-151. [DOI] [PubMed] [Google Scholar]
- 4.Burg, J. D., D. Perelmam, L. H. Kasper, P. L. Ware, and J. C. Boothroyd. 1988. Molecular analysis of the gene encoding the major surface antigen of Toxoplasma gondii. J. Immunol. 41:3584-3591. [PubMed] [Google Scholar]
- 5.Chen, X., Y. Gong, H. Li, Z. Lun, and M. Fung. 2001. High-level expression and purification of immunogenic recombinant SAG1 (P30) of Toxoplasma gondii in Escherichia coli. Protein Express. Purif. 23:33-37. [DOI] [PubMed] [Google Scholar]
- 6.Holbrook, A. A. 1969. Biology of equine piroplasmosis. Am. J. Vet. Med. Assoc. 155:453-454. [PubMed] [Google Scholar]
- 7.Hopp, T. P., and K. R. Woods. 1981. Predication of protein antigenic determinants from amino acid sequences. Proc. Natl. Acad. Sci. USA 78:3824-3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jack, R. M., and P. A. Ward. 1981. Mechanisms of entry of Plasmodium and Babesia into red cells, p. 445-458. In M. Ristic and J. P. Kreier (ed.), Babesiosis. Academic Press, Inc., New York, N.Y.
- 9.Knowles, D. P., L. E. Perryman, L. S. Kappmeyer, and S. G. Hennager. 1991. Detection of equine antibody to Babesia equi merozoite proteins by a monoclonal antibody-based competitive inhibition enzyme-linked immunosorbent assay. J. Clin. Microbiol. 29:2056-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knowles, D. P., L. S. Kappmeyer, and L. E. Perryman. 1997. Genetic and biochemical analysis of erythrocyte-stage surface antigens belonging to a family of highly conserved proteins of Babesia equi and Theileria species. Mol. Biochem. Parasitol. 90:69-79. [DOI] [PubMed] [Google Scholar]
- 11.Knowles, R. C. 1988. Equine babesiosis: epidemiology, control, and chemotherapy. Equine Vet. Sci. 8:61-64. [Google Scholar]
- 12.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 13.McGuire, T. C., G. L. Van Hoosier, Jr., and J. B. Henson. 1971. The complement-fixation reaction in equine infectious anemia: demonstration of inhibition by IgG (T). J. Immunol. 107:1738-1744. [PubMed] [Google Scholar]
- 14.Tanaka, T., X. Xuan, H. Ikadai, I. Igarashi, H. Nagasawa, K. Fujisaki, T. Mikami, and N. Suzuki. 1999. Expression of Babesia equi merozoite antigen-2 by recombinant baculovirus and its use in the ELISA. Int. J. Parasitol. 29:1803-1808. [DOI] [PubMed] [Google Scholar]
- 15.Tenter, A. M., and K. T. Friedhoff. 1986. Serodiagnosis of experimental and natural Babesia equi and B. caballi infections. Vet. Parasitol. 20:49-61. [DOI] [PubMed] [Google Scholar]
- 16.Weiland, G. 1986. Species-specific serodiagnosis of equine piroplasma infections by means of complement fixation test, immunofluorescence, and enzyme-linked immunosorbent assay. Vet. Parasitol. 20:43-48. [DOI] [PubMed] [Google Scholar]
- 17.Xuan, X., A. Larsen, H. Ikadai, T. Tanaka, I. Igarashi, H. Nagasawa, K. Fujisaki, Y. Toyoda, N. Suzuki, and T. Mikami. 2001. Expression of Babesia equi merozoite antigen 1 in insect cells by recombinant baculovirus and evaluation of its diagnostic potential in an enzyme-linked immunosorbent assay. J. Clin. Microbiol. 39:705-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xuan, X., A. Nagai, B. Battsetseg, S. Fukumoto, L. H. Makala, N. Inoue, I. Igarashi, T. Mikami, and K. Fujisaki. 2001. Diagnosis of equine piroplasmosis in Brazil by serodiagnostic methods with recombinant antigens. J. Vet. Med. Sci. 63:1159-1160. [DOI] [PubMed] [Google Scholar]