Abstract
Previous studies have suggested that tumor hypoxia could be exploited for cancer gene therapy. Using hypoxia-responsive elements derived from the human vascular endothelial growth factor gene, we have generated vectors expressing a bacterial nitroreductase (NTR) gene that can activate the anticancer prodrug CB1954. Stable transfectants of human HT1080 tumor cells with hypoxia-inducible vectors were established with G418 selection. Hypoxic induction of NTR protein correlated with increased sensitivity to in vitro exposure of HT 1080 cells to the prodrug. Growth delay assays were performed with established tumor xenografts derived from the same cells to detect the in vivo efficacy of CB1954 conversion to its cytotoxic form. Significant antitumor effects were achieved with intraperitoneal injections of CB1954 both in tumors that express NTR constitutively or with a hypoxia-inducible promoter. In addition, respiration of 10% O2 increased tumor hypoxia in vivo and enhanced the antitumor effects. Taken together, these results demonstrate that hypoxia-inducible vectors may be useful for tumor-selective gene therapy, although the problem of delivery of the vector to the tumors, particularly to the hypoxic cells in the tumors, is not addressed by these studies.
Keywords: tumor hypoxia, nitroreductase, CB 1954, gene therapy, vascular endothelial growth factor
Introduction
Hypoxia, a unique feature of human solid tumors, is considered to be a major problem in cancer treatment [1]. Clinical studies of patients with cervical, head, and neck cancers, and soft tissue sarcomas treated with radiotherapy or surgery have shown that local tumor control and overall survival are significantly reduced in patients with hypoxic tumors compared to those with better-oxygenated tumors [2–6]. These results have been supported by the findings that hypoxia in the tumor microenvironment can accelerate malignant progression and metastasis [7]. Tumor hypoxia has been proposed to stimulate angiogenesis and tumor development through the induction of proangiogenic proteins, such as vascular endothelial growth factor (VEGF). A number of genes, including erythropoietin (Epo) and various glycolytic enzymes, are also induced under hypoxic conditions [8–10]. A hypoxia-responsive element (HRE) was originally reported in the 3′ flanking region of the human and mouse Epo genes, and a hypoxia-inducible factor 1 (HIF-1) activates transcription by binding to the Epo HRE [11,12]. Similar HIF-1 binding sites have been found in regulatory regions of other hypoxia-induciblegenes [13–15].TheHIF-1 transcription factor is composed of an oxygen-sensitive HIF-1α subunit and a constitutively expressed HIF-1β subunit. Recently, a murine hepatoma cell line lacking a HIF-1β subunit showed decreased expression of hypoxia-inducible genes in situ, reduced vascularity, and slower growth as tumor xenografts [16], suggesting that HIF-1-mediated gene regulation is essential for angiogenesis and tumor development in vivo. Such regulatory mechanisms for hypoxia-responsive gene expression could provide a potential target for tumor-specific therapeutic approaches.
A number of fundamental problems must be overcome for cancer gene therapy in vivo to be realized. One requirement for tumor-specific gene therapy is to regulate expression of the therapeutic gene at a specific site or in a particular cell type in a tumor. Regulated gene expression can be achieved through transcriptional targeting by the use of inducible enhancer/promoter units. Several strategies for transcriptional targeting have been proposed and used for gene therapy, including a tyrosinase gene promoter for melanomas [17], a prostate-specific antigen promoter for prostate cancers [18], and an α-fetoprotein promoter for hepatomas [19]. We and others have proposed that hypoxia may represent a means to target gene expression selectively to tumors [1,20–22]. Solid tumors have abnormal vasculature with sluggish, irregular blood flow, and the resulting hypoxia provides a clear distinction between tumor and normal tissues, except for certain pathological conditions. However, to date, there have been no reports of successful in vivo implementation of gene therapy exploiting tumor hypoxia. Possible reasons include inadequate specificity or potency of the vectors expressing therapeutic genes. An earlier study of Dachs et al. [21], using mouse phosphoglycerate kinase-1, thymidine kinase, and 9–27 promoters with HRE driving a cytosine deaminase (CD) gene in stably transfected cells, reported a 5.4-fold increase in sensitivity to 5-fluorocytosine (5-FC) as judged by an IC50 assay after 16 hours of hypoxia. Also, studies by Ruan et al. [23], who transfected a reporter plasmid with multiple copies of HRE into U-87 MG and U-251 MG-NCI human brain tumor cells, showed that increased expression levels of 4-to 12-fold could be achieved, as well as increased apoptosis in hypoxia when the hypoxia-activated promoter was used to drive the BAX gene. We have previously shown that a vector with HRE derived from the human VEGF gene exhibited a significant increase in gene expression under hypoxia when transiently transfected into human tumor cells [22]. In our recent study to develop hypoxia-inducible vectors with higher levels of gene expression [24], we found that a construct composed of 5HRE ligated to a human CMV minimal promoter (5HRE/hCMVmp) increased gene expression over 500-fold in response to hypoxia for 18 hours, reaching levels comparable to that obtained by the CMV/IE promoter in transient transfection assays. In addition, we also examined whether the 3′ UTR of the human VEGF gene would confer increased posttranscriptional mRNA stability under hypoxic conditions. However, despite increases in the hypoxic/aerobic ratio of luciferase activity, gene expression with 3′ UTR was lower due to mRNA destabilization by AU-rich elements, so that we found no benefit from the inclusion of the 3′ UTR in our vectors. Thus, a 5HRE/hCMVmp construct may be superior in terms of robust hypoxia responsiveness and quantitative production of a desired gene to obtain tumor-specific gene expression through the exploitation of tumor hypoxia.
5-Aziridinyl 2,4-dinitrobenzamide (CB 1954), originally synthesized over 30 years ago, exhibits a dramatic and highly specific antitumor activity against rat Walker 256 carcinoma cells [25]. This antitumor effect is due to efficient drug activation by rat DT-diaphorase [26]. CB 1954 entered into clinical trials in 1970s, but little antitumor activity was observed as human DT-diaphorase is much less active in reduction of CB 1954 than the rat enzyme. Recent studies revealed that amino acid difference at residue 104 between human and rat enzymes is responsible for the catalytic differences to CB 1954 [27]. A nitroreductase (NTR) gene isolated from Escherichia coli has been demonstrated to activate the prodrug CB 1954 to its toxic form approximately 90-fold more rapidly than rat DT-diaphorase, suggesting the possibility of using CB 1954 with NTR in antibody-directed enzyme prodrug therapy (ADEPT) [28,29]. Also, several authors have suggested using NTR with CB 1954 for gene directed enzyme prodrug therapy (GDEPT) [30–34].
The purpose of this study is to show proof-of-principle for performance of our hypoxia-inducible system with plasmids expressing an E. coli NTR as a prodrug-activating enzyme. We examined whether selective expression of this enzyme could be detected under hypoxic conditions in stably transfected human tumor cells in vitro. We also investigated the efficacy of the prodrug CB 1954 in inducible sensitization in response to hypoxia and tested the antitumor effects of CB 1954 using tumor xenografts in SCID mice in which we manipulated the level of O2 respired. Our results suggest that hypoxia-inducible promoters with GDEPT may provide a means of selectively producing cytotoxicity in solid tumors.
Materials and Methods
Culture and Hypoxic Treatment
HT1080 human fibrosarcoma cells were obtained from the American Type Culture Collection (Rockville, MD) and cultured in α-MEM with 10% FCS in a well-humidified incubator with 5% CO2 at 37°C. For transfection experiments, 5x105 exponentially growing cells were plated on the culture dish overnight as described previously [22]. Hypoxic conditions were achieved using prewarmed aluminum hypoxic chambers [35] by evacuation and gassing with 95% N2/5% CO2, and then the tightly sealed chambers were incubated at 37°C. Some experiments were also performed in an anaerobic tissue culture hood (Sheldon Corp., Cornelius, OR). These two techniques produced similar levels of hypoxia, and no difference was noted between similar experiments performed using the two techniques.
Plasmid Construction and Transfection
The methods for construction of a 5HRE/hCMVmp as a luciferase reporter vector were described previously [22]. In this study, we replaced the luciferase gene with an E. coli NTR gene as a prodrug-activating enzyme for CB 1954. The NTR cDNA as a 651-bp fragment was obtained by polymerase chain reaction (PCR) using Elongase enzyme mixture (Gibco BRL, Gaithersburg, MD) with the following paired primers: 5′-GAGACCATGGATATCATTTCTGTCGCCT-3′ and 5′-AGAAGCGGCCGCCACTTCGGTTAAGGTGATGTTTTGCGG-3′. The PCR conditions were 94°C, 1 minute; 56°C, 1 minute; 68°C, 2 minutes for 35 cycles. The amplified NTR gene fragment was digested and subcloned at NcoI-NotI sites of pEF/myc/cyto vector (Invitrogen, Carlsbad, CA) to make a C-terminal fusion with a c-myc epitope and checked by sequencing. To generate a hypoxia-inducible expression vector for the NTR gene, the enhancer/promoter sequence digested by KpnI and NcoI from a 5HRE/hCMVmp vector was inserted into a pEF/myc/cyto vector with NTR. Schematic diagrams of the plasmids are shown in Figures 1A and 4A.
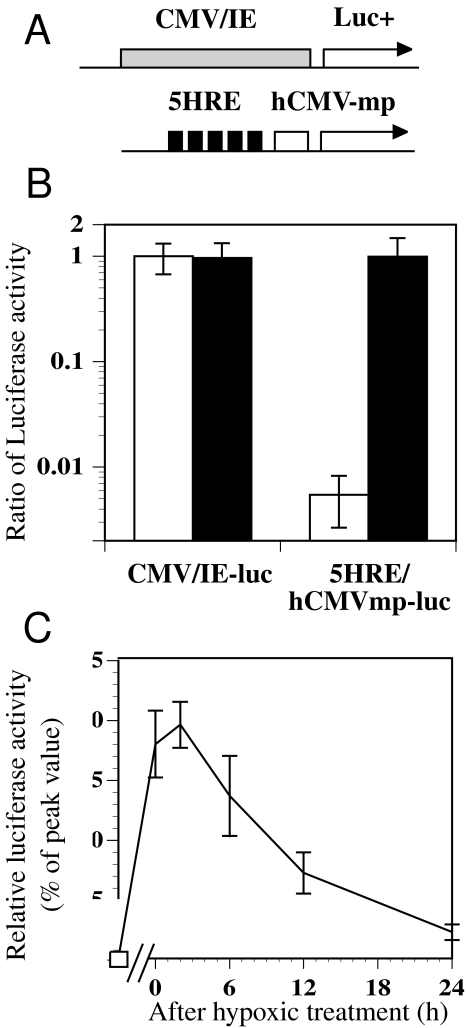
Figure 1.
The luciferase reporter assay using stably transfected cells under aerobic and hypoxic conditions. (A) Schematic diagram of the luciferase reporter plasmids with CMV/IE and 5HRE/hCMVmp expression units. (B) Comparison of the luciferase activity under aerobic (clear columns) and hypoxic (black columns) conditions. Stably transfected clones with the plasmids shown above were exposed to aerobic and hypoxic conditions (0.02% O2) for 6 hours and assayed for luciferase activity. The luciferase activities were normalized to that of a CMV/IE-luc clone under aerobic conditions. The error bars show the standard deviations (SD) of four independent samples. (C) The time course of luciferase activity after reoxygenation following hypoxic treatment of a 5HRE/hCMVmp-luc clone. The cells were treated under hypoxic conditions for 24 hours and then returned to aerobic conditions. The relative luciferase activity to its peak value was shown. Bars, SD of four independent experiments.
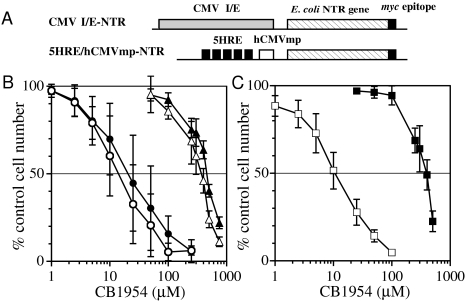
Figure 4.
Sensitivity to CB 1954 of wild-type HT1080 cells and stable transfectants of NTR expression vectors. (A) Schematic diagram of the plasmids with an E. coli NTR gene combined with CMV/IE and 5HRE/hCMVmp expression units. (B) Comparison of the sensitivity of wild-type HT1080 cells to CB 1954 under aerobic (▵) and hypoxic (▴) conditions, of the CMV/IE-NTR clone under aerobic (○) and hypoxic (●) conditions. (C) The sensitivity of the 5HRE/hCMVmp-NTR clone to CB 1954 under aerobic (□) and hypoxic (■) conditions. The error bars show SD of four to six independent experiments.
To obtain stable transfectants of the luciferase plasmid, 5 µg of the plasmids was transfected into tumor cells with the Superfect reagent (Qiagen, Valencia, CA) followed by incubation for 3 hours. The culture dishes were washed twice with PBS and fresh growth media added. At 48 hours after transfection, the cells were trypsinized and plated for clonal selection of stable transfectants in 400 µg/ml G-418. Several clones with good expression of the genes were selected out of 48 individual clones in each group. Stable transfectants of the CMV I/E-NTR and the 5HRE-NTR plasmids were obtained in a similar manner.
To measure gene expression under hypoxic conditions, stable transfectants were incubated for 6 to 18 hours under aerobic or hypoxic conditions. After 2 hours of reoxygenation, cell lysates were prepared with 400 µl of passive lysis buffer using a Dual Luciferase Assay kit (Promega Co., Madison, WI) and the luciferase activities were measured with a luminometer, as described previously [22].
In Vitro Assay for Antitumor Effects
The prodrug CB 1954, kindly provided by Dr. William R, Wilson (University of Auckland School of Medicine, New Zealand), was dissolved in dimethylsulfoxide and stored at -20°C. Cells were seeded at 5x103 cells/well in a 96-well plate and incubated overnight. The growth media were equilibrated under anaerobic conditions for at least 24 hours prior to use. The cells were treated under hypoxic conditions for 24 hours with CB 1954, then incubated for an additional 24 hours in air as increased levels of hypoxia-induced expression persist after reoxygenation for 12 to 24 hours (Figure 1C). The cells were washed in PBS, allowed to recover for 2 days, and measured for growth inhibition effects by an MTS assay using CellTiter96 AQ kit (Promega Co.). The percent control cell number was calculated as the absorbance (proportional to cell numbers) of the treated cells divided by that of the controls. The IC50 values were calculated as the concentration that inhibits growth of tumor cells by 50% compared to untreated cells.
Detection of Hypoxia-Inducible NTR Gene Expression
Stable transfectants were seeded on Lab-Tek Chamber Slides (Nalge Nunc International, Naperville, IL). The cells were treated under hypoxic conditions at 0.02% O2 or under aerobic conditions for 24 hours, then fixed in 4% paraformaldehyde for 10 minutes, rinsed in PBS, and treated with 0.1% Triton X-100 for 5 minutes. After incubation with 1% bovine serum albumin, the cells were treated with a 1:100 dilution of primary anti-myc antibody (Invitrogen) for 1 hour, followed by a 1:200 dilution of secondary antimouse IgG antibody conjugated with fluorescein isothiocyanate (FITC; Santa Cruz Biotechnology, Santa Cruz, CA). The specimens were examined by a fluorescence microscope.
Western blots were also performed for quantitative comparisons of protein levels. Wild-type HT1080 cells and each transfected clone were plated on glass dishes, treated under hypoxic or aerobic conditions for 6 to 18 hours, and then were lysed in the sample buffer (4% SDS, 15% sucrose, 100 mM Tris-HCl, and 5 mM EDTA) followed by boiling for 5 minutes. Protein concentrations were measured by Protein DC assay kit (Bio-Rad, Hercules, CA). Twenty-five micrograms of total proteins was separated on a 10% SDS-PAGE polyacrylamide gel, and transferred to nitrocellulose membrane (Schleicher&Schuell, Keene, NH). Membranes were blocked by 5% nonfat dry milk in Tris buffered saline (TBS) for 30 minutes, incubated for 1 hour with a 1:5000 dilution of anti-myc antibody for NTR-myc fusion protein, washed in TBS three times for 5 minutes, and then incubated with goat antimouse IgG conjugated with alkaline phosphatase (Santa Cruz Biotecnology). To confirm equal loading between samples, duplicate membranes were examined with a control antibody for monoclonal anti-β-actin (Sigma, St. Louis, MO) in parallel. Chemifluorescence signals on membranes were developed with ECF Western blotting kit (Amersham, Arlington Heights, IL) and scanned by Molecular Dynamics Storm System according to the manufacturer's instructions (Molecular Dynamics, Sunnyvale, CA).
Animal Studies
Human HT1080 cells and stably transfected clones were injected intradermally in the midline of the back of SCID mice and allowed to grow until the tumors reached a mean size of 100 mm3. Mice with a similar range of tumor sizes were chosen and divided among different treatment groups, such that the mean tumor sizes of four to six tumors per group were similar. CB 1954 dissolved in 10% DMSO, 40% polyethylene glycol 400, and 50% water at a concentration of 5 mg/ml was injected intraperitoneally at the dose of 50 mg/kg body weight. For hypoxic treatment, mice were placed in a plastic box and allowed to breathe 10% oxygen for 4 hours and then normal air for 2 hours prior to drug injections. Drug injections were performed twice, on day 0 (when the tumors were approximately 100 mm3) and 7 days later. For the growth delay assay, the average tumor volume was determined by measuring three orthogonal diameters with calculation of the volume by the formula: (d1·d2·d3)π/6. The growth delay effect was determined as the time required for the tumors to reach three times the initial volume at day 0. The difference of the growth delay effects between the control and each treatment group was examined by Student's t-test.
The oxygenation status of tumor xenografts was measured using a commercially available polarographic needle electrode (pO2 histograph; Eppendorf, Hamburg, Germany) using methods reported previously [36]. Briefly, tumor-bearing mice were anesthesized by an intraperitoneal injection of 0.5 mg/g pentobarbital (Abbott Laboratories, North Chicago, IL), placed in a specially designed jig to reduce mobilization to a minimum, and the electrode was inserted into the tumors followed by automatic measurements moving through the tissue in steps of 0.2 mm. The Mann-Whitney rank sum test was used to compare the median pO2 values of tumors in air breathing with 10% oxygen breathing animals as reported previously [36].
Results
Hypoxia Responsiveness of a 5HRE/hCMVmp Construct in Stable Transfectants
In our previous study, a 5HRE/hCMVmp construct showed potent hypoxia-inducible gene expression using a luciferase assay in transient transfected human tumor cells [24]. To confirm the hypoxia responsiveness of this construct in stably transfected cells, we generated a luciferase reporter vector driven by a 5HRE/hCMVmp expression unit along with a neomycin-resistant selectable marker. After establishing stably transfected clones using G418 selection, we assessed luciferase expression under hypoxic conditions. Stable transfectants with a 5HRE/hCMVmp-luc vector showed a 182-fold increase with a 6-hour hypoxic treatment, whereas a clone with CMV/IE-luc vector showed no significant change in luciferase activity following hypoxic exposure. The hypoxia/aerobic ratio of luciferase activity was comparable to previously published data using transient transfection. Importantly, the luciferase activity of a 5HRE/hCMVmp-luc clone under hypoxia reached levels similar to that of a CMV/IE-luc clone (Figure 1B), indicating that our construct could act as a strong enhancer/promoter in response to hypoxia in these stable transfected clones, although the expression levels might be affected by the sites of integration.
We also examined the time course of luciferase activity after reoxygenation following hypoxic exposure in a 5HRE/hCMVmp-luc clone. Activities reached a maximum at the end of the 24-hour period at approximately 280 times control at 2 hours after reoxygenation, and then gradually decreased over the next 24 hours (Figure 1C). As the half-life of luciferase in the cells is about 3 hours [37], the kinetics of luciferase activity decay was more prolonged than expected. A plausible explanation is that translation is still occurring from mRNA induced under hypoxia, even after reoxygenation inhibits HIF-1 activation.
Hypoxia-Induced Expression of the E. coli NTR gene
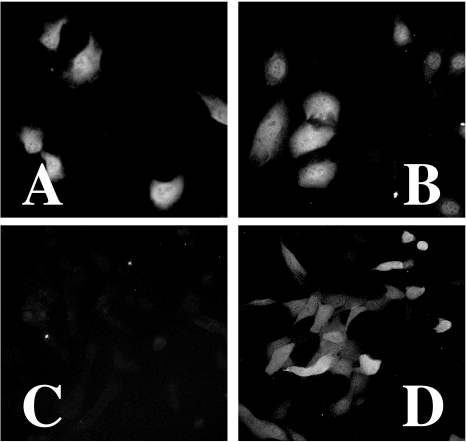
After establishing stably transfected clones with desired plasmid constructs, we examined expression of the prodrug-activating enzyme under hypoxic and aerobic conditions. Each clone was seeded on a glass slide and was incubated under hypoxic or aerobic conditions for 24 hours. Using an anti-myc primary antibody and an FITC-conjugated antimouse IgG secondary antibody, we assessed the protein expression in the cells with fluorescent microscopy (Figure 2). For a CMV/IE-NTR clone, significant FITC signal was detected under both hypoxic and aerobic conditions. In contrast, with the 5HRE/hCMVmp-NTR clone, only a background level was observed under aerobic conditions, while a strong FITC signal occurred after a 24-hour hypoxic treatment.
Figure 2.
Detection of E. coli NTR by immunofluorescence. Stable transfectants of HT1080 cells were seeded on glass slides and were incubated under aerobic or hypoxic conditions (0.02% O2) for 24 hours. The specimens were treated with a 1:100 dilution of primary anti-myc antibody, followed by a 1:200 dilution of secondary antimouse IgG antibody conjugated with FITC as described in Materials and Methods section. CMV/IE-NTR cells under aerobic (A) and hypoxic (B) conditions. 5HRE/hCMVmp-NTR cells under aerobic (C) and hypoxic (D) conditions.
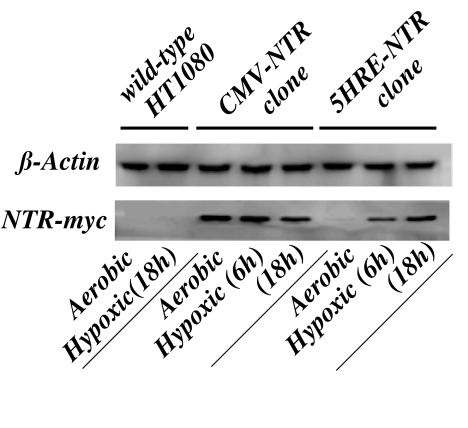
We quantitated NTR protein expression on aerobic and hypoxic extracts of these clones by Western blots. As shown in the Figure 3, NTR-myc tagged proteins have a molecular mass of 26 kDa. In the left two lanes, the negative controls of wildtype HT1080 cells showed only the β-actin bands. For the CMV-NTR clone, NTR-myc bands were readily seen under both aerobic and hypoxic conditions. In contrast, only a trace amount of NTR protein was present under aerobic conditions for 5HRE-NTR, whereas the expression levels were markedly increased after 6-and 18-hour hypoxic treatment in a time-dependent manner, demonstrating a marked induction of NTR protein under hypoxic conditions in the stably transfected cells with the 5HRE/hCMV-NTR construct.
Figure 3.
Western blot analysis of NTR protein expression under aerobic and hypoxic conditions. Protein lysates from wild-type HT1080, CMV/IE-NTR, and 5HRE/hCMVmp-NTR cells after treatment under aerobic or hypoxic conditions (0.02% O2) for 6 to 18 hours were analyzed by SDS-PAGE and immunoblotting with a monoclonal antimyc antibody as described in Materials and Methods section. A duplicate membrane was also treated for an anti-β-actin antibody as a loading control.
Hypoxia-Induced Sensitization to CB 1954
To examine whether the expression of E. coli NTR could confer increased sensitivity of human tumor cells to CB 1954, we assessed cytotoxicity/growth inhibition by a colorimetric assay. The cells were treated with CB1954 under hypoxia for 24 hours and under aerobic conditions for an additional 24 hours, the prodrug was then removed, and growth inhibition was measured after a further 2 days. For controls, the cells were treated with drug for 48 hours under aerobic conditions. For positive controls, we chose a stable transfectant with a CMV-NTR vector, which exhibited sensitivity to CB 1954 with an IC50 value of around 10 to 20 µM (Figure 4B). Under aerobic conditions, the 5HRE/hCMVmp-NTR clone showed similar sensitivity to the wildtype HT1080 cells, indicating that this clone did not express sufficient levels of NTR to affect prodrug activation. In contrast, the IC50 value was reduced by 40-to 50-fold by the 24-hour hypoxic treatment (Figure 4C). Thus, the hypoxia-inducible sensitization of the transfectants in vitro correlated with their increased NTR expression.
Tumor Xenografts in SCID Mice
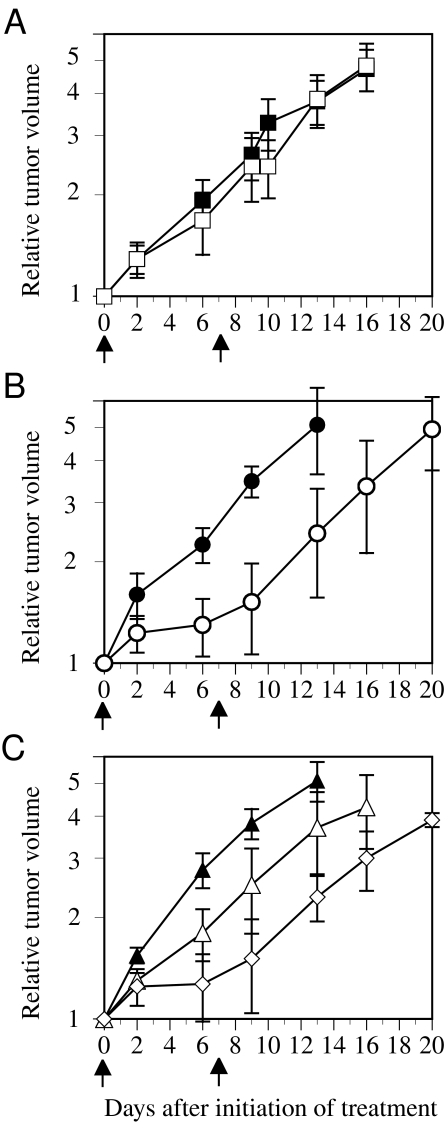
To examine whether hypoxia-induced gene expression could be activated in response to in vivo tumor hypoxia, we transplanted wildtype and stably transfected clones of HT1080 cells into SCID mice. We also treated tumorbearing mice by exposure to low oxygen to determine whether increased tumor hypoxia in vivo increased antitumor effect. The tumor take rates of each clone were similar, and there were no significant differences in growth rates between clones. For wildtype HT1080-derived tumors, no growth delay was observed with two injections of CB 1954 at 50 mg/kg. For CMV-NTR-derived tumors, a substantial growth delay was evident with two injections of CB 1954 when compared with the control (P=.032) (Figure 5B). Tumors derived from 5HRE/hCMVmp-NTR cells exhibited a small growth delay that did not quite reach statistical significance (P=.052) at this drug dose. However, when the mice breathed 10% oxygen for 4 hours prior to each of the two CB 1954 injections, there was a significant tumor growth delay when compared with the control (P=.018), suggesting that hypoxic breathing enhanced the expression of NTR gene in tumor tissues (Figure 5C).
Figure 5.
Antitumor effects in xenografts in SCID mice determined by the growth delay assay. Drug injections (50 mg/kg) were performed twice, on day 0 (when the tumors were approximately 100 mm3) and 7 days later. (A) Tumors derived from wild-type HT1080 cells with no drug (■) or CB 1954 (□) treatment. (B) Tumors derived from the CMV/IE-NTR cells with no drug (●) or CB 1954 (○) treatment. (C) Tumors derived from the 5HRE/hCMVmp-NTR cells with no drug (▴), CB 1954 (▵), or CB 1954 with hypoxic breathing of 10% O2 for 4 hours prior to injection (◊). The error bars show SE of four to six tumors. The time to reach 3x treatment volume for the tumors in the mice breathing 10% oxygen was significantly greater (P<.02) than those from controls.
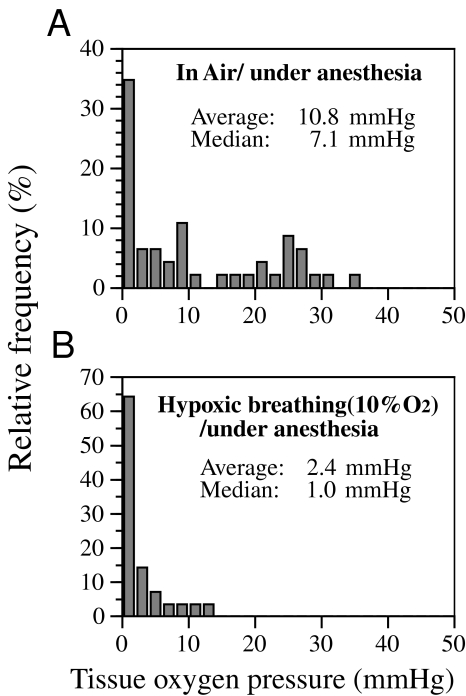
To confirm the oxygenation status of HT1080 tumor xenografts before and after 10% oxygen breathing, we performed pO2 measurements using the Eppendorf electrode. Tumors in anesthesized mice had a median pO2 of 7.1 mm Hg, with no significant differences between different clones. During hypoxic breathing, tumors showed a marked reduction of pO2 values with a median value of 1.0 mm Hg (Figure 6). These changes of median pO2 values between air breathing and 10% oxygen breathing animals were significant (P=.008), as assessed by the Mann-Whitney rank sum test.
Figure 6.
Tissue oxygen pressure measurements for transplanted tumors by the Eppendorf microelectrode. Tumor-bearing SCID mice were anesthesized and immobilized on a heating pad. Sequential measurements were done for each tumor before (A) and after (B) 10% O2 breathing. The data are shown as pooled histograms. The two groups were significantly different (P<.01) (Mann-Whitney rank sum test).
Discussion
Hypoxia is a tumor-specific microenvironmental stress that may provide an opportunity for tumor-specific therapy. In recent reports, the average median pO2 for carcinomas of the breast, prostate, head and neck, and cervix are in the range of 10 to 15 mm Hg (1.3% to 2%), with most tumors having areas with pO2 values <5 mm Hg [2–5,38,39]. In contrast, normal tissues have a median pO2 of 40 to 60 mm Hg with no values less than 10 mm Hg [36,38,40]. These differences in oxygenation status provide the specificity for hypoxia-responsive gene expression. In a study for HIF-1 DNA-binding activity in HeLa cells, a maximum response was obtained at 0.5% oxygen with a half-maximum at about 1.5% oxygen (10 to 12 mm Hg) [41]. Our previous results suggest that the 5HRE/hCMVmp construct gives maximum gene expression in human tumor cells at an oxygen concentration of 0.2% (1.5 mm Hg) and half-maximum activity at 1% oxygen (5 to 7 mm Hg) [24]. Thus, the activation of our hypoxia-inducible construct using the human VEGF HRE is in the correct range for obtaining selective gene expression in human tumors.
In the present studies, we used the E. coli NTR gene driven by the hypoxia-inducible promoter with the drug CB1954. This has been reported to be one of the most promising enzyme/prodrug systems [34]. NTR efficiently catalyses the reduction of CB 1954 to its 4-hydroxylamino derivative in the presence of NADH or NADPH as a cofactor, and is further converted by thioesters, such as acetyl coenzyme A, into a bifunctional alkylating agent [26]. In the studies of ADEPT by Anlezark et al. [28] and Knox et al. [29], this activated species is reported to be some 104 times more cytotoxic to V79 cells in vitro when the cells were incubated with CB 1954 plus NTR in the presence of NADH. In addition, the cytotoxicity of CB 1954 is not proliferation dependent, as it is for ganciclovir and 5-fluorouracil, and CB 1954 can kill noncycling cells by forming DNA crosslinks. With regard to the bystander effect, studies have suggested that a few percentages of activator cells produced a substantial bystander cell killing effect using human tumor cell lines in vitro [42]. To our knowledge, there have been no data on bystander effect in in vivo tumor models with CB 1954.
The extent of antitumor effect in vivo depends on a variety of factors, including the sensitivity of the target cells to activated prodrug and the percentage of cells expressing the enzyme. Stable transfectants expressing NTR by CMV or hypoxia-inducible promoters in this study had an IC50 of 10 to 20 µM with 48 hours of exposure and were 40-to 50-fold more sensitive to CB 1954 than wildtype HT1080 cells. However, these values are not as large as those reported in other studies using similar treatment conditions. McNeish et al. [33] reported that the IC50 of a transfected clone of a human ovarian cancer line SKOV3 was 0.4 µM compared to an IC50 of 305 µM for parental cells after a 72-hour exposure. In a study by Friedlos et al. [42], stable clones of NTR expressing lines of WiDr, SKOV3, and V79 cells were 82-, 670-, and 2600-fold more sensitive than the parent lines following a 96-hour exposure, respectively. Clearly, considerable differences in sensitivity to the activated form of CB 1954 are found among different cell lines. It has been suggested that above a threshold level of enzyme activity in stably transfected cells, the dose modification factor is not simply related to levels of expression of NTR: where NTR is in excess, the rate-limiting step may be the availability of acetyl CoA required for the formation of the cytotoxic species [42]. The availability of co-factors could also be the rate limiting step in this prodrug/enzyme system. To test another prodrug-activating enzyme with CB 1954, we have also established HT1080 clones transfected with mutated human DT-diaphorase (Shibata et al., unpublished observations) at amino acid residue 104, which has been shown to have similar catalytic activity for CB 1954 to the rat enzyme [27]. Interestingly, the IC50 for these clones was similar to that for the stable clones in this study, suggesting that the HT1080 cell line may be intrinsically resistant to CB 1954.
Although breathing 10% oxygen greatly increased hypoxia of the transplanted tumors and augmented the antitumor effect of CB 1954, this procedure can also affect the oxygenation status of the normal tissue. Experimental data obtained in our laboratory showed that breathing of 10% oxygen resulted in a dramatic fall of a median pO2 to 15 mm Hg in mouse subcutaneous tissue compared to a median in air breathing of anesthesized mice of 40 mm Hg [36]. However, because other tissues might have lower oxygen levels caused by breathing 10% oxygen, it is unclear that this strategy will increase the therapeutic ratio, and may not be a realistic approach to cancer therapy. However, induction of tumor hypoxia can be achieved by a variety of agents, including flavone acetic acid [43], tumor necrosis factor α [44], vinblastine, and combretastatins [45].
Finally, HIF-1 expression has been reported to be upregulated specifically during malignant progression of cancers. Previous studies demonstrated that loss of p53 function [46], or activation of the Src and Ras oncogenes, could increase HIF-1-mediated transcription by hypoxia [47,48]. In addition, a recent analysis by immunohistochemistry has demonstrated that HIF-1α was overexpressed in a number of specimens of primary cancers and metastasis compared with the normal tissues, and that HIF-1α expression levels correlated with aberrant p53 accumulation, bcl2 expression, and Ki67 index [49]. These findings indicate that HIF-1α expression may be a biomarker of malignant progression and metastatic potential of human cancers. Thus, HIF-1α dysregulation in human cancers, as well as synergistic effects of abnormalities of tumor suppressor genes and oncogenes on HIF-1-mediated transcription, may enhance this approach for tumor-specific gene therapy.
The present study suggests that hypoxia-inducible vectors have the potential of providing selective expression of therapeutic genes in the poorly oxygenated regions of solid tumors. In vivo experiments using a variety of enzyme/prodrug combinations and human tumor xenografts with different hypoxic fractions are currently underway. However, it has to be emphasized that the data we have reported are from model studies and do not address the problem of delivery of the hypoxia-activated promoters to the tumors. Recent studies, however, with macrophages transduced with a hypoxia-inducible promoter [50] clearly illustrate the potential of hypoxia-targeted gene therapy, and the possibility of combining it with a treatment such as radiotherapy, which preferentially kills aerobic cells, is attractive.
Acknowledgements
We thank Douglas Menke, Mary Jo Dorie, and Mark S. Gilbert for their excellent technical assistance.
Abbreviations
- CB 1954
5-aziridinyl 2,4-dinitrobenzamide
- 5-FC
5-fluorocytosine
- CD
cytosine deaminase
- Epo
erythropoietin
- FITC
fluorescein isothiocyanate
- GDEPT
gene-directed enzyme prodrug therapy
- HIF-1
hypoxia-inducible factor-1
- HRE
hypoxia responsive element
- NTR
nitroreductase
- VEGF
vascular endothelial growth factor
Footnotes
This work was supported by US National Cancer Institute grant PO1 CA-67166.
References
- 1.Brown JM, Giaccia AJ. The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer Res. 1998;58:1408–1416. [PubMed] [Google Scholar]
- 2.Hockel M, Knoop C, Schlenger K, Vorndran B, Baussmann E, Mitze M, Knapstein PG, Vaupel P. Intratumoral pO2 predicts survival in advanced cancer of the uterine cervix. Radiother Oncol. 1993;26:45–50. doi: 10.1016/0167-8140(93)90025-4. [DOI] [PubMed] [Google Scholar]
- 3.Sundfor K, Lyng H, Rofstad EK. Tumour hypoxia and vascular density as predictors of metastasis in squamous cell carcinoma of the uterine cervix. Br J Cancer. 1998;78:822–827. doi: 10.1038/bjc.1998.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nordsmark M, Overgaard M, Overgaard J. Pretreatment oxygenation predicts radiation response in advanced squamous cell carcinoma of the head and neck. Radiother Oncol. 1996;41:31–39. doi: 10.1016/s0167-8140(96)91811-3. [DOI] [PubMed] [Google Scholar]
- 5.Brizel DM, Sibley GS, Prosnitz LR, Scher RL, Dewhirst MW. Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 1997;38:285–289. doi: 10.1016/s0360-3016(97)00101-6. [DOI] [PubMed] [Google Scholar]
- 6.Brizel DM, Scully SP, Harrelson JM, Layfield LJ, Bean JM, Prosnitz LR, Dewhirst MW. Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer Res. 1996;56:941–943. [PubMed] [Google Scholar]
- 7.Graeber TG, Osmanian C, Jacks T, Housman DE, Koch CJ, Lowe SW, Giaccia AJ. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours [see comments] Nature. 1996;379:88–91. doi: 10.1038/379088a0. [DOI] [PubMed] [Google Scholar]
- 8.Goldberg MA, Glass GA, Cunningham JM, Bunn HF. The regulated expression of erythropoietin by two human hepatoma cell lines. Proc Natl Acad Sci USA. 1987;84:7972–7976. doi: 10.1073/pnas.84.22.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12:5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 11.Semenza GL, Nejfelt MK, Chi SM, Antonarakis SE. Hypoxia-inducible nuclear factors bind to an enhancer element located 3′ to the human erythropoietin gene. Proc Natl Acad Sci USA. 1991;88:5680–5684. doi: 10.1073/pnas.88.13.5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madan A, Curtin PT. A 24-base pair sequence 3′ to the human erythropoietin gene contains a hypoxia-responsive transcriptional enhancer. Proc Natl Acad Sci USA. 1993;90:3928–3932. doi: 10.1073/pnas.90.9.3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia inducible factor 1. J Biol Chem. 1994;269:23757–23763. [PubMed] [Google Scholar]
- 14.Semenza GL, Jiang BH, Leung SW, Passantino R, Concordet JP, Maire P, Giallongo A. Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J Biol Chem. 1996;271:32529–32537. doi: 10.1074/jbc.271.51.32529. [DOI] [PubMed] [Google Scholar]
- 15.Goldberg MA, Schneider TJ. Similarities between the oxygen-sensing mechanisms regulating the expression of vascular endothelial growth factor and erythropoietin. J Biol Chem. 1994;269:4355–4359. [PubMed] [Google Scholar]
- 16.Maxwell PH, Dachs GU, Gleadle JM, Nicholls LG, Harris AL, Stratford IJ, Hankinson O, Pugh CW, Ratcliffe PJ. Hypoxia inducible factor-1 modulates gene expression in solid tumors and influences both angiogenesis and tumor growth. Proc Natl Acad Sci USA. 1997;94:8104–8109. doi: 10.1073/pnas.94.15.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vile RG, Hart IR. In vitro and in vivo targeting of gene expression to melanoma cells. Cancer Res. 1993;53:962–967. [PubMed] [Google Scholar]
- 18.Pang S, Taneja S, Dardashti K, Cohan P, Kaboo R, Sokoloff M, Tso CL, Dekernion JB, Belldegrun AS. Prostate tissue specificity of the prostate-specific antigen promoter isolated from a patient with prostate cancer. Hum Gene Ther. 1995;6:1417–1426. doi: 10.1089/hum.1995.6.11-1417. [DOI] [PubMed] [Google Scholar]
- 19.Huber BE, Richards CA, Krenitsky TA. Retroviral mediated gene therapy for the treatment of hepatocellular carcinoma: an innovative approach for cancer therapy. Proc Natl Acad Sci USA. 1991;88:8039–8043. doi: 10.1073/pnas.88.18.8039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dachs GU, Stratford IJ. The molecular response of mammalian cells to hypoxia and the potential for exploitation in cancer therapy. Br J Cancer. 1996;74:S126–S132. [PMC free article] [PubMed] [Google Scholar]
- 21.Dachs GU, Patterson AV, Firth JD, Ratcliffe PJ, Townsend KM, Stratford IJ, Harris AL. Targeting gene expression to hypoxic tumor cells. Nat Med. 1997;3:515–520. doi: 10.1038/nm0597-515. [DOI] [PubMed] [Google Scholar]
- 22.Shibata T, Akiyama N, Noda M, Sasai K, Hiraoka M. Enhancement of gene expression under hypoxic conditions using fragments of the human vascular endothelial growth factor and the erythropoietin genes. Int J Radiat Oncol Biol Phys. 1998;42:913–916. doi: 10.1016/s0360-3016(98)00298-3. [DOI] [PubMed] [Google Scholar]
- 23.Ruan H, Hang J, Hu L, Lin CS, Lamborn KR, Deen DF. Killing of brain tumor cells by hypoxia-responsive element-mediated expression of BAX. Neoplasia. 1999;1:431–437. doi: 10.1038/sj.neo.7900059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shibata T, Giaccia AJ, Brown JM. Development of a hypoxia-responsive vector for tumor-specific gene therapy. Gene Ther. 2000;7:493–498. doi: 10.1038/sj.gt.3301124. [DOI] [PubMed] [Google Scholar]
- 25.Cobb LM, Connors TA, Elson LA, Khan AH, Mitchley BC, Ross WC, Whisson ME. 2,4-Dinitro-5-ethyleneiminobenzamide (CB 1954): a potent and selective inhibitor of the growth of the Walker carcinoma 256. Biochem Pharmacol. 1969;18:1519–1527. doi: 10.1016/0006-2952(69)90267-6. [DOI] [PubMed] [Google Scholar]
- 26.Knox RJ, Boland MP, Friedlos F, Coles B, Southan C, Roberts JJ. The nitroreductase enzyme in Walker cells that activates 5 (aziridin-1-yl)-2,4-dinitrobenzamide (CB 1954) to 5-(aziridin-1-yl)-4-hydroxylamino-2-nitrobenzamide is a form of NAD(P)H dehydrogenase (quinone) (EC 1..99.2) Biochem Pharmacol. 1988;37:4671–4677. doi: 10.1016/0006-2952(88)90336-x. [DOI] [PubMed] [Google Scholar]
- 27.Chen S, Knox R, Wu K, Deng P, Zhou D, Bianchet MA, Amzel LM. Molecular basis of the catalytic differences among DTdiaphorase of human, rat, and mouse. J Biol Chem. 1997;272:1437–1439. doi: 10.1074/jbc.272.3.1437. [DOI] [PubMed] [Google Scholar]
- 28.Anlezark GM, Melton RG, Sherwood RF, Coles B, Friedlos F, Knox RJ. The bioactivation of 5-(aziridin-1-yl)-2,4-dinitrobenzamide (CB1954): I. Purification and properties of a nitroreductase enzyme from Escherichia coli — a potential enzyme for antibody directed enzyme prodrug therapy (ADEPT) Biochem Pharmacol. 1992;44:2289–2295. doi: 10.1016/0006-2952(92)90671-5. [DOI] [PubMed] [Google Scholar]
- 29.Knox RJ, Friedlos F, Sherwood RF, Melton RG, Anlezark GM. The bioactivation of 5-(aziridin-1-yl)-2,4-dinitrobenzamide (CB1954): II. A comparison of an Escherichia coli nitroreductase and Walker DT-diaphorase. Biochem Pharmacol. 1992;44:2297–2301. doi: 10.1016/0006-2952(92)90672-6. [DOI] [PubMed] [Google Scholar]
- 30.Bridgewater JA, Springer CJ, Knox RJ, Minton NP, Michael NP, Collins MK. Expression of the bacterial nitroreductase enzyme in mammalian cells renders them selectively sensitive to killing by the prodrug CB1954 [published erratum appears in Eur J Cancer 1996;32A(1):186] Eur J Cancer. 1995;31(A(13–14)):2362–2370. doi: 10.1016/0959-8049(95)00436-x. [DOI] [PubMed] [Google Scholar]
- 31.Clark AJ, Iwobi M, Cui W, Crompton M, Harold G, Hobbs S, Kamalati T, Knox R, Neil C, Yull F, Gusterson B. Selective cell ablation in transgenic mice expression E. coli nitroreductase [see comments] Gene Ther. 1997;4:101–110. doi: 10.1038/sj.gt.3300367. [DOI] [PubMed] [Google Scholar]
- 32.Drabek D, Guy J, Craig R, Grosveld F. The expression of bacterial nitroreductase in transgenic mice results in specific cell killing by the prodrug CB1954 [see comments] Gene Ther. 1997;4:93–100. doi: 10.1038/sj.gt.3300366. [DOI] [PubMed] [Google Scholar]
- 33.McNeish IA, Green NK, Gilligan MG, Ford MJ, Mautner V, Young LS, Kerr DJ, Searle PF. Virus-directed enzyme prodrug therapy for ovarian and pancreatic cancer using retrovirally delivered E. coli nitroreductase and CB1954. Gene Ther. 1998;5:1061–1069. doi: 10.1038/sj.gt.3300744. [DOI] [PubMed] [Google Scholar]
- 34.Connors TA. The choice of prodrugs for gene-directed enzyme prodrug therapy of cancer. Gene Ther. 1995;2:702–709. [PubMed] [Google Scholar]
- 35.Brown JM. SR 4233 (tirapazamine): a new anticancer drug exploiting hypoxia in solid tumours. Br J Cancer. 1993;67:1163–1170. doi: 10.1038/bjc.1993.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adam MF, Dorie MJ, Brown JM. Oxygen tension measurements of tumors growing in mice. Int J Radiat Oncol Biol Phys. 1999;45:171–180. doi: 10.1016/s0360-3016(99)00157-1. [DOI] [PubMed] [Google Scholar]
- 37.Thompson JF, Hayes LS, Lloyd DB. Modulation of firefly luciferase stability and impact on studies of gene regulation. Gene. 1991;103:171–177. doi: 10.1016/0378-1119(91)90270-l. [DOI] [PubMed] [Google Scholar]
- 38.Vaupel P, Schlenger K, Knoop C, Hockel M. Oxygenation of human tumors: evaluation of tissue oxygen distribution in breast cancers by computerized O2 tension measurements. Cancer Res. 1991;51:3316–3322. [PubMed] [Google Scholar]
- 39.Movsas B, Chapman JD, Horwitz EM, Pinover WH, Greenberg RE, Hanlon AL, Iyer R, Hanks GE. Hypoxic regions exist in human prostate carcinoma. Urology. 1999;53:11–18. doi: 10.1016/s0090-4295(98)00500-7. [DOI] [PubMed] [Google Scholar]
- 40.Becker A, Hansgen G, Bloching M, Weigel C, Lautenschlager C, Dunst J. Oxygenation of squamous cell carcinoma of the head and neck: comparison of primary tumors, neck node metastases, and normal tissue. Int J Radiat Oncol Biol Phys. 1998;42:35–41. doi: 10.1016/s0360-3016(98)00182-5. [DOI] [PubMed] [Google Scholar]
- 41.Jiang BH, Semenza GL, Bauer C, Marti HH. Hypoxia inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am J Physiol. 1996;271:C1172–C1180. doi: 10.1152/ajpcell.1996.271.4.C1172. [DOI] [PubMed] [Google Scholar]
- 42.Friedlos F, Court S, Ford M, Denny WA, Springer C. Gene-directed enzyme prodrug therapy: quantitative bystander cytotoxicity and DNA damage induced by CB1954 in cells expressing bacterial nitroreductase. Gene Ther. 1998;5:105–112. doi: 10.1038/sj.gt.3300569. [DOI] [PubMed] [Google Scholar]
- 43.Bibby MC, Double JA, Loadman PM, Duke CV. Reduction of tumor blood flow by flavone acetic acid: a possible component of therapy. J Natl Cancer Inst. 1989;81:216–220. doi: 10.1093/jnci/81.3.216. [DOI] [PubMed] [Google Scholar]
- 44.Mahadevan V, Malik ST, Meager A, Fiers W, Lewis GP, Hart IR. Role of tumor necrosis factor in flavone acetic acid-induced tumor vasculature shutdown. Cancer Res. 1990;50:5537–5542. [PubMed] [Google Scholar]
- 45.Chaplin DJ, Pettit GR, Parkins CS, Hill SA. Antivascular approaches to solid tumour therapy: evaluation of tubulin binding agents. Br J Cancer Suppl. 1996:S86–S88. [PMC free article] [PubMed] [Google Scholar]
- 46.Kieser A, Weich HA, Brandner G, Marme D, Kolch W. Mutant p53 potentiates protein kinase C induction of vascular endothelial growth factor expression. Oncogene. 1994;9:963–969. [PubMed] [Google Scholar]
- 47.Grugel S, Finkenzeller G, Weindel K, Barleon B, Marme D. Both vHa-Ras and v-Raf stimulate expression of the vascular endothelial growth factor in NIH 3T3 cells. J Biol Chem. 1995;270:25915–25919. doi: 10.1074/jbc.270.43.25915. [DOI] [PubMed] [Google Scholar]
- 48.Mazure NM, Chen EY, Yeh P, Laderoute KR, Giaccia AJ. Oncogenic transformation and hypoxia synergistically act to modulate vascular endothelial growth factor expression. Cancer Res. 1996;56:3436–3440. [PubMed] [Google Scholar]
- 49.Zhong H, De MA, Laughner E, Lim M, Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL, Simons JW. Overexpression of hypoxia-inducible factor 1 alpha in common human cancers and their metastases. Cancer Res. 1999;59:5830–5835. [PubMed] [Google Scholar]
- 50.Griffiths L, Binley K, Iqball S, Kan O, Maxwell P, Ratcliffe P, Lewis C, Harris A, Kingsman S, Naylor S. The macrophage — a novel system to deliver gene therapy to pathological hypoxia. Gene Ther. 2000;7:255–262. doi: 10.1038/sj.gt.3301058. [DOI] [PubMed] [Google Scholar]