Abstract
MUC1 mucin is transcriptionally regulated by estrogen, progesterone, and glucocorticoids. Our objective was to determine whether androgen receptor (AR) activation regulates expression of MUC1. The following breast and prostatic cell lines were phenotyped and grouped according to AR and MUC1 protein expression: 1) AR+MUC1+ [DAR17+19 (AR transfectants of DU-145), ZR-75-1, MDA-MB-453, and T47D]; 2) AR-MUC1+ [DZeo1 (AR-vector control), DU-145, BT20,MDA-MB-231, and MCF7]; 3) AR+MUC1- (LNCaP and LNCaP-r). Cell proliferation was determined using the MTT assay in the presence of synthetic androgen R1881, 0.1 pM to 1 µM. Cell surface MUC1 expression was determined by flow cytometry in the presence or absence of oestradiol, medroxy progesterone acetate or R1881, with and without 4 hydroxy-flutamide (4-OH), a nonsteroidal AR antagonist. The functional significance of MUC1 expression was investigated with a cell-cell aggregation assay. Only AR+ MUC1+ cell lines showed a significant increase in MUC1 expression with AR activation (P (range) =.01 to.0001), reversed in the presence of 4-OHF. Cell proliferation was unaffected. Increased expression of MUC1 was associated with a significant (P (range) =.002 to.001) reduction in cell-cell adhesion. To our knowledge, this is the first description of androgen-dependent regulation of MUC1 mucin. This is also functionally associated with decreased cell-cell adhesion, a recognised feature of progressive malignancy. These findings have important implications for physiological and pathological processes.
Keywords: prostate cancer, breast cancer, androgen receptor, MUC1, hormone responsive element
Introduction
Mucins are major constituents of the glycocalyx — a predominantly negatively charged, highly glycosylated protein coat expressed at the luminal surface of glandular epithelia. Recently, the 14th mucin gene was described [1], although MUC1 mucin (syn: polymorphic epithelial mucin, sialomucin, episialin) remains the most extensively investigated [2,3]. A common feature of all mucin genes is a tandem repeat region, which in the case of MUC1 is a 20-amino acid sequence, repeated 30 to 120 times [2]. Mucins can be broadly grouped into those with (e.g., MUC1, MUC3, and MUC4) or without (e.g., MUC2, MUC5AC, MUC5B, MUC6) a transmembrane domain, resulting in the mucin being either membrane-bound or secreted. MUC1 mucin, however, is unique among mucins because of its ubiquitous expression by all glandular epithelia. Splice variants of the MUC1 gene have been identified, including secreted MUC1 (lacking the C-terminal membrane spanning subunit) and MUC1/Y, which lacks the tandem repeat region [4,5].
In vitro studies have demonstrated a number of different properties of MUC1, which have been correlated with physiological or pathological processes. These include 1) interference with immune surveillance by cancer cells through an immunosuppressive effect on T cells [6]; 2) inhibition of cell-cell and cell-matrix adhesion, which has been correlated to tumour progression [7], and also control of blastocyst implantation [8]; 3) signal transduction via the cytoplasmic tail, which has also been associated with inhibition of cell-cell adhesion [9]; 4) sequestration of β-catenin through interaction with the cytoplasmic tail [10]; 5) induction of glandular morphogenesis by epithelial cells [11]; and 6) heterotypic cell-cell adhesion through binding to specific ligands, such as the selectins [12] and ICAM-1 [13], which may facilitate intravascular cancer metastasis.
Hormonal regulation of MUC1 has been demonstrated in a number of organ systems. Cyclical change of endometrial MUC1 expression in animal models has been extensively documented, and may play an important role in determining the receptivity of endometrium to embryo implantation [8]. Recently, progesterone dependent regulation of MUC1 has also been demonstrated in human endometrium, although the effect was the opposite to that seen in animal models [14]. In human breast cancer cell lines, addition of estrogen and progesterone is associated with increased MUC1 expression [15] and glucocorticoid-dependent upregulation of MUC1 has been documented in DU-145, a prostatic cell line, and multiple myeloma lines [16]. There are, however, no data in the literature examining the role of androgens in the regulation of MUC1 expression.
Androgen receptor (AR) expression has been reported in up to 50% to 80% of breast [17,18], ovarian [19], and endometrial [18] carcinomas, and 80% or more of prostatic adenocarcinomas is initially androgen-sensitive [20]. A role for androgens in the regulation of MUC1 may have considerable clinical significance. Our objective was to investigate whether ligand-mediated activation of the AR modulates MUC1 protein expression.
Materials and Methods
Cell Culture
All cell lines were grown in phenol red free RPMI 1640 [Imperial Cancer Research Fund (ICRF), London, UK], containing penicillin-streptomycin-amphotericin B mixture at a final concentration of 100 U, 100 µg, and 0.25 µg/ml, respectively, supplemented with 10% fetal bovine serum (FBS) (Gibco Life Technologies, Paisley, Scotland).
LNCaP and DU-145 were purchased from American Type Cell Culture (ATCC, Manassas, VA). LNCaP-r was a generous gift from Dr. Van Steenbrugge (Department of Urology, Erasmus University, Rotterdam, Holland). MCF7, T47D, MDA-MB-231, MDA-MB-453, BT20, and ZR-75-1 were obtained from the ICRF (Lincoln's Inn Fields, London, UK). DAR17 and 19 are two transfectants of the DU-145 cell line, which constitutively express human AR under the control of the CMV promoter, and DZeo1, the vector control, is transfected with plasmid lacking AR. Details of transfection and characterisation are reported elsewhere [21]. Cells were maintained in standard cell culture incubation conditions in humidified air with 10% CO2 supplementation at 37°C. Cells were split at 80% confluence using trypsin/versene as previously reported [22]. Cells were routinely subcultured at 1:6, and medium changed every 72 hours.
Sex Steroid Receptor Agonists and Antagonist
Sex steroid receptor agonists: 17β-hydroxy-5α-androstan-3-one (dihydro-testosterone: DHT), 17β-hydroxy-17α-methyl-estra-4,9,11-trien-3-one (R1881 — a nonmetabolised AR agonist), 17β-estradiol (E2), and 6α-methyl 17α-hydroxy-progesterone acetate (medroxy progesterone acetate: MPA) were diluted to stock solutions of 1000x final concentration desired in 100% ethanol, and stored at -20°C. Sex steroids were obtained from Sigma (Poole, Dorset, UK), except for R1881, which was obtained from NEN Life Science Products (Zaventem, Belgium). For negative controls, an equal volume of ethanol vehicle alone was added at a final concentration of 0.1% v/v. Sex steroids were tested for effect on MUC1 expression between 0.1 and 10 nM. Following this, DHT, R1881, and MPA were used at 1 nM, and oestradiol (E2) at 10 nM, which are comparable to concentrations established in previous reports [15,23,24] and physiological ranges [25]. The time course of MUC1 response was tested with the addition of sex steroids for 12, 24, 36, 48, 72, and 96 hours.
4-Hydroxy flutamide (4-OHF) was a generous gift from AstraZeneca pharmaceuticals, and was also stored as a stock solution of 1000x in ethanol; the final concentration used in all experiments was 1 µM as described previously [26]. In experiments in which both 4-OHF and steroid agonist were added, 4-OHF was added 6 hours prior to the addition of sex steroid.
Antibodies
The antibodies used, their sources, and dilutions are detailed in Table 1.
Table 1.
Source and Dilutions for Antibodies Used in this Study.
| Source | Minimum Epitope | Dilution Factors | |
| ICC | FC | ||
| Primary antibodies | |||
| HMFG1 [47] | DTRP [48,49] | 10–50 | 10 |
| HMFG2 [47] | DTR [48,49] | 1–10 | 1 |
| SM3 [50] | PDTRP [49,51] | 1–10 | 1 |
| C595* | RPAP [49] | 100 | 200 |
| AR† | human AR | 100 | |
| LE61 [52] | CK8/18 dimer (shared epitope) [52] | 10 | |
| Secondary antibody | |||
| FITC‡ | rabbit anti mouse | 500 | 50 |
Abbreviations: ICC — immunocytochemistry; FC — flow cytometry; FITC — fluorescein isothyanate; HRP — horseradish peroxidase.
Serotec (Kidlington, Oxford, UK).
Biogenex, distributed by Menarini Diagnostics (Finchampstead, UK).
Dako (High Wycombe, Bucks, UK).
Immunocytochemistry (ICC)
All reagents were of molecular grade and were obtained from BDH/Merck (Poole, Dorset, UK), unless otherwise stated. Cells were cultured on four well slides (Hendley, Loughton, Essex, UK) in medium containing steroids, as described above, for 48 hours, washed in phosphatebuffered saline (PBS) (pH 7.4±0.2), and fixed in methanol at 4°C for 15 minutes. Slides were incubated in washing medium (final concentrations of 3% horse serum, 1% bovine serum albumin, 0.05% Tween 20, 0.5 M NaCl in PBS) for 2 hours at room temperature (RT) [27], after which 75 µl of antibody (see Table 1) was added and incubated overnight at 4µC. CK8/18 are constitutively expressed by glandular epithelial cells [22] and served as positive controls. Slides were washed three times, then incubated with fluoresceinlabeled secondary antibody at RT for 2 hours in the dark. Slides were washed and dried in the dark at RT and counterstained with propidium iodide-containing Vectashield (Vector Laboratories, Burlingame, CA, USA). UV light photography (Olympus BX60) was performed within a week of preparation. ICC was used to screen cell lines for the presence of AR, and determine expression of MUC1 prior to flow cytometry.
Cell Proliferation Assay
For cell proliferation studies, cells were plated at a density of 3000 cells/well in 96-well plates (Corning, New York, USA) and were allowed to recover for 4 hours prior to the addition of the appropriate concentration of R1881. The final concentration of ethanol was 0.1 µl/well. Ethanol vehicle was added in the same concentration to control wells. Following incubation was 10 µl. 3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) stock solution (5 mg/ml diluted in phenol red-free RPMI) was added to each well, and incubated at 37°C, 5% CO2 for 4 hours. The medium was removed, and insoluble formazan crystals were solubilized with 100 µl of acidic isopropanol (0.1 N HCl in absolute isopropanol) per well according manufacturer's protocol (Sigma). Plates were shaken for 5 minutes and absorbance measured at 570 nm on a BioRad 550 microplate reader (BioRad, Hemel Hempstead, UK).
Flow Cytometry
Cells were plated in T75 culture flasks and allowed to attach overnight, prior to addition of steroid/ethanol vehicle, and the flasks incubated for 12 to 72 hours. Cells were detached using versene only (ICRF) plus gentle agitation, and washed twice at 4°C using washing buffer (PBSA containing 1% FBS, 1 mM CaCl2, and 1 mM MgCl2). Samples were then resuspended in 75 µl of primary antibody (Table 1) per sample, and incubated at 4°C for 1 hour. One additional sample per cell line was incubated with PBS instead of antibody, which served as a negative control. Following washing, samples were resuspended in 300 µl of secondary antibody, and incubated at 4°C for 45 minutes. Following further washing, samples were resuspended in 500 µl of washing buffer. Flow cytometric analysis was performed on a Coulter Epics XL (Coulter Electronics, Luton, UK). Gating parameters were set to exclude clumped or dead cells, and 20,000 events within the gated area were counted per individual experiment.
Cell Aggregation Assay
Cell-cell adhesion was determined using a previously described protocol [28] with minor modifications. Cells were cultured in the presence of steroids for 72 hours, detached from plates with versene only, passed through a 24-gauge needle, and resuspended in Ca2+/Mg2+ free PBS. Cell density was determined by automated cell counting (Multisizer II; Coulter Electronics). 106 cells were then resuspended in 1.5 ml of RPMI/FBS 1% with appropriate steroids and incubated on a shaking platform at 150 rpm at 37°C. At appropriate time points, the percentage of clumped and single cells was determined by cell counting using a haemocytometer (Neubauer Improved; Weber Scientific International, Sussex, UK). Dead cells were excluded by trypan blue staining. Counts were performed in quadruplicate for each time point.
Statistical Analysis
Flow cytometric data were analysed as follows. Clumped or apoptotic cells were excluded by gating. Sample size for each experiment was constant (20,000 cells). The plot of fluorescent intensity versus frequency was integrated to obtain the area under the curve, corresponding to overall MUC1 expression in each sample. Absolute levels of MUC1 expression varied with different cell lines and MUC1 antibodies. To allow comparison, MUC1 expression with each of the different steroid protocols (4OHF, R1881, 4OHF/R1881, MPA, 4OHF/MPA, E2) was expressed as a percentage of MUC1 expression in control medium (which was set at 100%). These were analysed using a one-way ANOVA on log10 transformed data. If the ANOVA indicated significant difference (P<.05) between steroid protocols, the estimated marginal means for each steroid, expressed as a ratio of control medium, and 95% CI were examined to indicate where the significant differences lay with respect to control medium.
Cell aggregation data were analysed by regression analysis using ANOVA with time as the covariate, assessing steroid, and time x steroid interaction terms to indicate differences in intercept and slope for different steroids. All analyses were performed on SPSS version 9 (Chicago, USA). The significance level was set at P=.05.
Results
AR/MUC1 Expression Status
All cell lines were phenotyped for expression of AR and MUC1 proteins in control medium using ICC, and results are summarised in Table 2. DU-145 and its derivatives all expressed heterogeneous membranous and diffuse cytoplasmic MUC1 recognised by HMFG1. A comparable pattern of expression was detected using C595. MUC1 expression was not detected in any of the prostatic cell lines using HMFG2 or SM3 at 1:10 dilution, with minimal expression seen at 1:1 dilution. LNCaP and LNCaP-r were both negative for MUC1 with all antibodies consistent with our previous report [22]. MUC1 expression was observed in all breast cell lines with HMFG1, HMFG2, SM3, and C595. The flow cytometry data were consistent with ICC for all cell lines with the exception of MDA-MB-231 and-453, both of which expressed extremely low levels of membrane-associated MUC1.
Table 2.
Summary of Basal Antigen Expression in the Absence of Added Exogenous Steroid.
| (A) Prostatic Cell Lines | ||||||
| Antibody | DU145 | DAR17 | DAR19 | DZeo1 | LNCaP | LNCaP-r |
| HMFG1 | ++ (het) | ++ (het) | ++ (het) | ++ (het) | - | - |
| HMFG2 | -* | -* | -* | -* | - | - |
| C595 | ++ (het) | ++ (het) | ++ (het) | ++ (het) | - | - |
| SM3 | -* | -* | -* | -* | - | - |
| AR | - | ++ | ++ | - | ++ | + |
| LE61 | ++ | ++ | ++ | ++ | ++ | ++ |
| (B) Breast Cell Lines | ||||||
| Antibody | MDA-MB-231 | BT20 | T47D | MCF7 | ZR-75-1 | MDA-MB-453 |
| HMFG1 | ++ (het) | ++ | ++ | ++ | ++ | + |
| HMFG2 | ± | + | + | + | + | ± |
| C595 | ++ (het) | ++ | ++ | ++ | ++ | + |
| SM3 | + (het) | ++ | + | + | ++ | ± |
| AR | - | - | + | - | ++ | ++ |
| LE61 | ++ | ++ | ++ | ++ | ++ | ++ |
(A) Cell lines derived from PC. (B) Cell lines derived from breast cancer. (++/-) Give a guide as to comparative intensity of immunostaining. (het): heterogeneous expression.
Heterogeneous weak immunofluorescence seen with HMFG2 and SM3 at 10 times higher concentration (1:1).
On the basis of their expression profile, cell lines were grouped as follows:
AR+MUC1+): DAR17, DAR19, ZR-75-1, T47D, MDA-MB-453;
(AR-MUC1+): DU-145, DZeo1, MCF-7, BT20, MDA-MB-231; and
(AR+MUC1-): LNCaP, LNCaP-r.
Effects of Exogenous Androgens on Proliferation
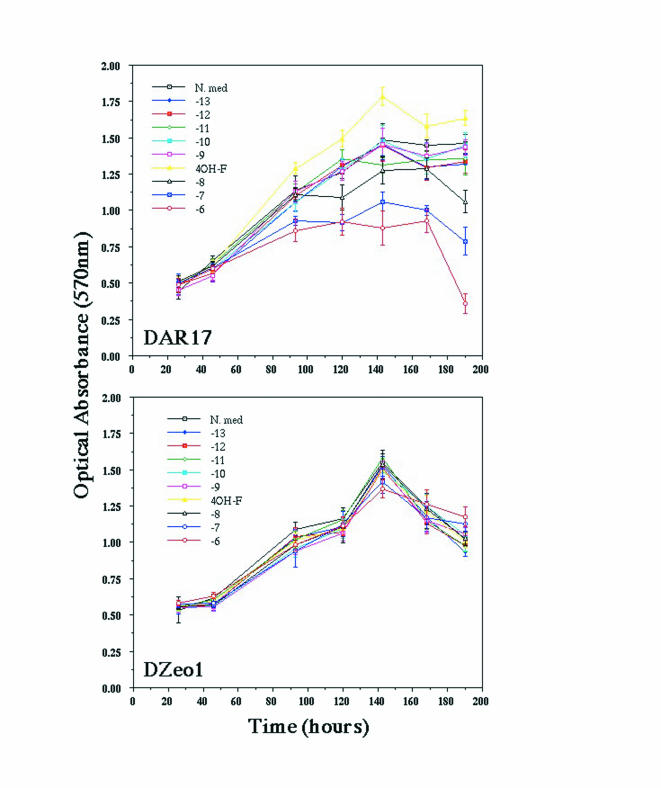
Before investigating the effect of androgens on MUC1 expression, we assessed whether they were mitogenic or inhibitory over the concentration range 10-13 to 10-6 M. It was found that R1881 exerted no effect on cell proliferation until 10 nM and above. Representative results are shown in Figure 1. For all subsequent experiments, DHT, R1881, or MPA were added at a final concentration of 1 nM.
Figure 1.
Effect of exogenous androgen (R1881) on proliferation of AR+ (DAR17) and AR- (DZeo1) cells. Proliferation in control medium (N.med) was compared with proliferation in the presence of R1881 over a concentration range of 10-13 to 10-6 M, and also 4-hydroxyflutamide (4-OHF). None of the exogenous steroids has any effect on the proliferation of DZeo1. In contrast, there is an increasing inhibitory effect of R1881 on the growth of DAR19 first apparent at 10 nM. The decline in cell number seen in both cell lines from 150 hours onwards is a result of serum starvation.
Effects of Exogenous Steroids on MUC1 Expression
Flow cytometric analysis of cells incubated with sex steroids revealed increased MUC1 expression after 12 hours and maximal expression by 72 hours (data not shown). On the basis of this, we incubated all cell lines with sex steroids for 72 hours. Initial experiments performed with DHT and R1881 were similar, and R1881 was subsequently used in all experiments.
Group 1 (AR + MUC1+):
DAR17; DAR19; ZR-75-1; MDA-MB-453; T47D)
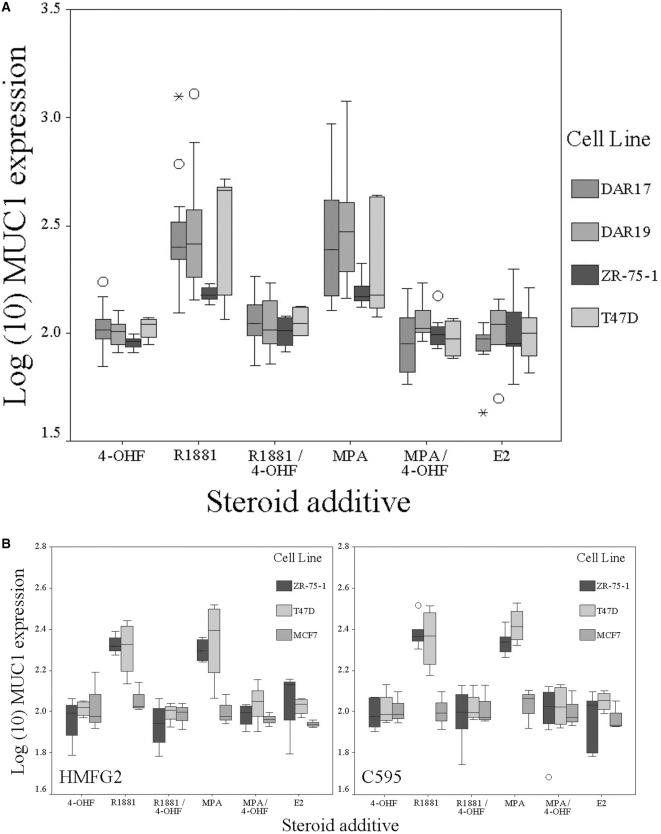
Flow cytometric analysis of membranous MUC1 expression using four different antibodies (see below) showed that all cell lines except MDA-MB-453 expressed membrane associated MUC1, and that MUC1 expressed by DAR17 and -19 was not recognised by HMFG2 or SM3. Addition of R1881 or MPA to group 1 (AR+MUC1+) cell lines was associated with a significant increase (<.0001) (Figure 2A, Table 3) in membranous expression of MUC1 with the exception of MDA-MB-453, in which membrane-associated MUC1 could not be detected. Oestradiol had no effect on membranous MUC1 expression (Figure 2A). To ensure reproducibility and specificity, MUC1 expression was analysed with four different antibodies (each recognises a different epitope in the tandem repeat region; Table 1). Although there was some variation in the magnitude, a consistent response to exogenous androgens was seen (Table 3). None of the cell lines demonstrated any change in MUC1 expression with the addition of ethanol vehicle alone.
Figure 2.
Effect of exogenous steroids on membrane expression of MUC1 mucin. In each graph, log10 (change in MUC1 expression) is shown compared to control medium. (A) AR+ cell lines demonstrate a significant increase in membranous expression of MUC1 in the presence of R1881 or MPA, which is blocked in the presence of AR antagonist 4-OHF. (B) Representative results (HMFG2 and C595) obtained in AR+ (T47D and ZR-75-1) and AR- (MCF-7) breast cell lines using different antibodies to MUC1. A comparable increase of membrane MUC1 expression is seen in the presence of R1881 and MPA with different the antibodies. Expression in MCF7 is not affected by AR agonist or antagonist. In each graph, boxes represent interquartile range and error bars show confidence interval. Circles represent outlier values (1.5 to 3 boxlengths from interquartile range) and asterisks represent extreme values (greater than three boxlengths from interquartile range). For statistical analysis, see Table 3.
Table 3.
Summary of Statistical Significance Obtained from Pooled Flow Cytometry Data.
| Cell Line | ANOVA | Pr | Steroid | Mean Increase | CI | Pr |
| HMFG1 | ||||||
| DAR17 | F(5,59)=17.229 | <.0001 | R1881 | 0.443 | (0.348, 0.537) | <.0001 |
| MPA | 0.440 | (0.314, 0.566) | <.0001 | |||
| DAR19 | F(5,79)=25.510 | <.0001 | R1881 | 0.455 | (0.381, 0.530) | <.0001 |
| MPA | 0.495 | (0.393, 0.596) | <.0001 | |||
| ZR-75-1 | F(5,45)=11.738 | <.0001 | R1881 | 0.180 | (0.122, 0.238) | <.0001 |
| MPA | 0.191 | (0.133, 0.249) | <.0001 | |||
| T47D | F(5,27)=7.843 | <.0001 | R1881 | 0.506 | (0.362, 0.649) | <.0001 |
| MPA | 0.330 | (0.160, 0.500) | .008 | |||
| DU-145 | F(5,37)=1.660 | .169 | ||||
| DZeo1 | F(5,38)=2.158 | .079 | MPA | 0.048 | (0.011, 0.085) | .005 |
| MCF7 | F(5,16)=0.613 | .699 | ||||
| BT20 | F(5,24)=0.050 | .998 | ||||
| C595 | ||||||
| DAR17 | F(5,18)=46.913 | <.0001 | R1881 | 0.301 | (0.249, 0.353) | <.0001 |
| MPA | 0.311 | (0.260, 0.363) | <.0001 | |||
| DAR19 | F(5,29)=13.751 | <.0001 | R1881 | 0.437 | (0.320, 0.554) | <.0001 |
| MPA | 0.348 | 0.221, 0.474) | <.0001 | |||
| ZR-75-1 | F(5,24)=13.060 | <.0001 | R1881 | 0.388 | (0.279, 0.497) | <.0001 |
| MPA | 0.337 | (0.228, 0.447) | <.0001 | |||
| T47D | F(5,12)=10.706 | <.0001 | R1881 | 0.377 | (0.248, 0.507) | .001 |
| MPA | 0.450 | (0.320, 0.579) | <.0001 | |||
| MCF7 | F(5,12)=0.148 | .977 | ||||
| HMFG2 | ||||||
| ZR-75-1 | F(5,18)=11.094 | <.0001 | R1881 | 0.325 | (0.215, 0.436) | .002 |
| MPA | 0.297 | (0.186, 0.407) | .004 | |||
| T47D | F(5,17)=7.330 | .001 | R1881 | 0.307 | (0.183, 0.430) | .003 |
| MPA | 0.343 | (0.219, 0.466) | .001 | |||
| MCF7 | F(5,11)=0.847 | .545 | ||||
| BT20 | F(5,6)=0.067 | .995 | ||||
| SM3 | ||||||
| ZR-75-1 | F(5,45)=12.989 | <.0001 | R1881 | 0.390 | (0.279, 0.500) | .007 |
| MPA | 0.450 | (0.333, 0.567) | .001 | |||
| E2 | 0.171 | (0.061, 0.282) | .003 | |||
| T47D | F(5,27)=4.866 | .003 | R1881 | 0.255 | (0.142, 0.367) | .001 |
| MPA | 0.272 | (0.149, 0.395) | .001 | |||
| MCF7 | F(5,16)=2.542 | .071 | R1881 | 0.090 | (0.040, 0.156) | .048 |
| BT20 | F(5,12)=0.584 | .712 | ||||
The significance of added steroids was assessed with ANOVA. Where this showed a significant outcome (P<.05), the estimated marginal means with their 95% CI were examined. Mean increase = log10 (increase in MUC1 expression as ratio of control).
Group 2 (AR-MUC1+):
DU-145; DZeol; MCF-7; BT20; MDA-MB-231)
All cell lines except for MDA-MB-231 expressed membrane-associated MUC1. Addition of sex steroid receptor agonists was not associated with a significant increase in MUC1 (Table 3 and Figure 2B).
Group 3 (AR+MUC1-): LNCap; LNCap-r
No MUC1 was detectable in these cell lines, and addition of R1881, MPA, and E2 did not induce MUC1 expression.
Effects of AR Blockade
Addition of 4-OHF abolished R1881-and MPA-mediated increase in MUC1 in the group 1 (AR+MUC1+) cell lines (Figure 2A). Expression of MUC1 in group 2 (AR-MUC1+) was unaffected by the addition of 4OHF (Figure 2B). 4-OHF was without effect when added to control medium alone, or in the presence of E2.
Cellular Aggregation
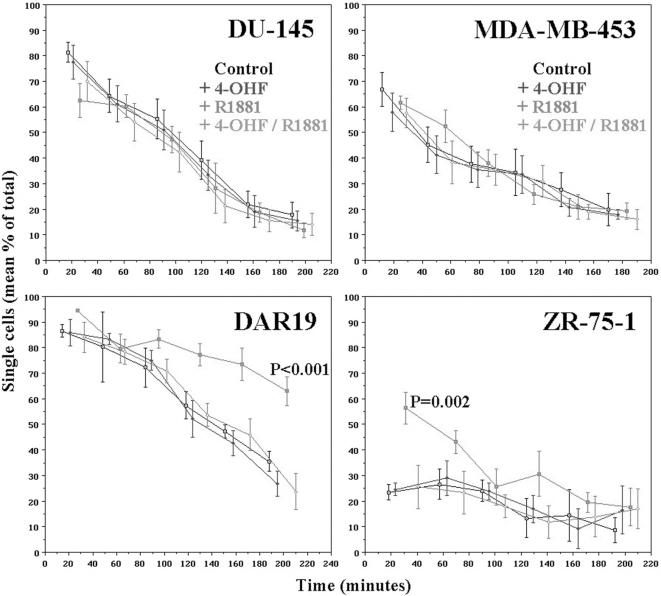
To investigate the functional significance of increased MUC1 expression in the group 1 cell lines, we analysed cellular aggregation in the presence of R1881 and/or 4-OHF. Aggregation was significantly inhibited in group 1 (AR+MUC1+) cell lines DAR17, DAR19 (P<.001), and ZR-75-1 (P<.002) when cells were incubated in the presence of R1881 (Figure 3). The anti-adhesive effect was abrogated when 4-OHF was added together with R1881. 4-OHF did not have any effect when added to control medium. MDA-MB-453 (AR+MUC1+), which expresses minimal cell surface MUC1, did not demonstrate any change in cell adhesion in the presence of R1881, 4-OHF, or both. Cellular aggregation in the groups 2 (AR-MUC1+) and 3 (AR+MUC1-) cell lines was unaffected by any of the steroid protocols.
Figure 3.
Representative results of cell-cell aggregation assay. Aggregation of prostatic (DAR19 and DU-145) and breast (MDA-MB-453 and ZR-75-1) cell lines with time in the presence of 4-OHF, R1881, or 4-OHF+ R1881. AR+ cell lines (DAR19 and ZR-75-1) demonstrate reduced cell aggregation in the presence of R1881, which is blocked in the presence of 4-OHF. AR-MUC1+ (e.g., DU-145) cell lines do not demonstrate any change in cell aggregation in the presence of R1881 and/or 4-OHF. MDA-MB-453, which is AR+MUC1+ but does not express cell surface MUC1, also does not show any demonstrable change in cellular aggregation in the presence of androgens, consistent with this effect being mediated by cell surface MUC1. Results with each steroid protocol were compared with regression analysis. Statistically significant results are indicated. The differences between DAR19 and ZR-75-1 are attributed to different kinetics of cellular aggregation in each cell line.
Discussion
In the early 1990s, several groups identified consensus sequences for estrogen, progesterone, and glucocorticoid steroid response elements on the human MUC1 promoter. However, in the intervening years, only a few studies have further explored the effect of steroids on MUC1 expression. Treon et al. [16] demonstrated dexamethasone-mediated increase in surface MUC1 in a variety of multiple myeloma and adenocarcinoma cell lines [16]. A regulatory role for ovarian steroids on MUC1 in endometrium has also been reported in a variety of animals including human, rabbit, and mouse [8,14,29].
In this report, we have demonstrated that ligand activation of the AR causes a significant increase in membrane-associated MUC1 (P<.0001) in AR+MUC1+ breast and prostatic cell lines but not in AR-MUC1+ or AR+MUC1-cell lines. This observed increase in MUC1 was abrogated when cells were incubated in the presence of 4OHF, an AR antagonist. Our data demonstrate that ligand activation of the AR upregulates membrane-associated MUC1. A search of the human MUC1 gene promoter sequence [30] for previously reported androgen responsive elements (ARE) [31] identified several partial consensus sequences, two of which are shown below:
| (+20) | GCCTGAATCTGTTCT (+40) |
| Consensus ARE | GGTACANNNTGTTCT |
| (-2546) | AGTCCTCCCAGACCC (-2532) |
| Probasin ARE-2 [32] | AGTACTCCAAGAACC |
There are several mechanisms by which MUC1 is thought to influence cell adhesion. The extracellular domain of MUC1 can extend 500 nm or more from the cell surface. Its length, rigidity, and negative charge result in steric hindrance and electrostatic repulsion, masking the interactions of much smaller membrane-associated molecules [32,33]. The cytoplasmic tail of MUC1 is able to sequester β- and γ-catenin, which may influence the cadherin/catenin system [10]. In addition, the cytoplasmic tail of MUC1 also interacts with the EGFR pathway [9] and its overexpression was associated with increased activity of MAPK — a downstream effector of EGFR ligand binding. One known consequence of increased MAPK activity is negative regulation of tight junction formation [9]. In breast cancer [7] and melanoma [33] cell lines, cell-cell adhesion may be virtually absent as a result of high MUC1 expression, even with high levels of E-cadherin expression. Abrogation of MUC1 expression restores cellular aggregation without any change in expression level of E-cadherin. Our data reveal a significant decrease in aggregation in the presence of R1881 (P<.001), consistent with an antiadhesive role mediated by MUC1. It is conceivable that the observed reduction in cellular aggregation could be mediated by other molecules. However, our data on MDA-MB-453 (AR+ MUC1+), which expresses cytoplasmic MUC1 but virtually no membranous MUC1, and LNCaP-r (AR+MUC1-) suggest that the likely reason is an alteration in the expression of cell surface MUC1. The exact mechanism governing the antiadhesive effect requires further investigation.
Overexpression of MUC1 has been described in most human adenocarcinomas, including lung [34], stomach [35], and most extensively in breast cancer [36]. It has been associated with prognostic indicators of poor clinical outcome, including lymph node metastasis, blood vessel invasion [37], early recurrence following surgical resection [34], and overall disease-specific survival [35] reflecting its multifunctional nature. Increased serum androgens [38] or urinary androgen metabolites [39] have been correlated to increased risk of postmenopausal breast cancer, disease recurrence [40], or rate of disease progression [41]. Menopausal androgen replacement therapy (MART) in symptomatic women following natural or surgical menopause is in current clinical practice [42]. In light of our data, it is conceivable that MART administration may create a steroid environment in which occult glandular malignancy may progress through the actions of MUC1. The majority of ovarian, endometrial, and breast adenocarcinomas are AR+ [17–19], and approximately 80% of prostatic adenocarcinomas are sensitive to androgen deprivation therapy on initial presentation [20]. MUC1 expression has been correlated to pathological stage and Gleason grade in prostate cancer [43]. The androgen-dependent regulation of MUC1 in androgen-sensitive carcinomas is a potentially important area of investigation.
In animal models investigated thus far, endometrial MUC1 is expressed in the prereceptive phase and its downregulation by progesterone coincides with the receptive phase [8,29]. Whereas in the human endometrium the converse is observed, MUC1 is expressed throughout the menstrual cycle and its mRNA levels increase several fold in the post ovulatory phase, consistent with transcriptional regulation by progesterone [14]. Okon et al. compared the plasma androgen concentrations in women with recurrent miscarriage to fertile controls and correlated this with the endometrial protein PP14. They found that levels of both testosterone and androstenedione were higher in the recurrent miscarriage group [44]. Current understanding of embryo implantation suggests that MUC1 is important in this process and alterations of this glycoprotein could significantly impact successful implantation of the embryo [14,45,46].
In summary, our study provides evidence for receptor-mediated upregulation of MUC1 protein expression by androgen in an in vitro model of hormone-responsive (prostate and breast) cancer. This is the first report exploring androgen-dependent regulation of MUC1 in any organ system. The majority of breast, ovarian, endometrial, and prostate cancers are AR+ and (with the exception of prostate) the function of AR is not clear. Given that overexpression of MUC1 has been correlated with poor prognostic features in many adenocarcinomas, androgen-dependent regulation of MUC1 may be important in AR+ tumours as well as normal physiological processes and merits further study.
Abbreviations
- 4-OHF
4-hydroxyflutamide
- AR
androgen receptor
- CK
cytokeratin
- DHT
dihydrotestosterone
- E2
estradiol
- FBS
fetal bovine serum
- FITC
fluorescein isothyanate
- ICC
immunocytochemistry
- ICRF
Imperial Cancer Research Fund
- MPA
medroxy progesterone acetate
- MTT
(3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide
- PBS
phosphate-buffered saline
- PC
prostate cancer
References
- 1.Yin BW, Lloyd KO. Molecular cloning of the CA125 ovarian cancer antigen: Identification as a new mucin (MUC16) J Biol Chem. 2001;276:27371–27375. doi: 10.1074/jbc.M103554200. [DOI] [PubMed] [Google Scholar]
- 2.Seregni E, Botti C, Massaron S, Lombardo C, Capobianco A, Bogni A, Bombardieri E. Structure, function and gene expression of epithelial mucins. Tumori. 1997;83:625–632. doi: 10.1177/030089169708300301. [DOI] [PubMed] [Google Scholar]
- 3.Lagow E, DeSouza MM, Carson DD. Mammalian reproductive tract mucins. Hum Reprod Update. 1999;5:280–292. doi: 10.1093/humupd/5.4.280. [DOI] [PubMed] [Google Scholar]
- 4.Baruch A, Hartmann M, Yoeli M, Adereth Y, Greenstein S, Stadler Y, Skornik Y, Zaretsky J, Smorodinsky NI, Keydar I, Wreschner DH. The breast cancer-associated MUC1 gene generates both a receptor and its cognate binding protein. Cancer Res. 1999;59:1552–1561. [PubMed] [Google Scholar]
- 5.Hanisch FG, Muller S. MUC1: the polymorphic appearance of a human mucin. Glycobiology. 2000;10:439–449. doi: 10.1093/glycob/10.5.439. [DOI] [PubMed] [Google Scholar]
- 6.Gimmi CD, Morrison BW, Mainprice BA, Gribben JG, Boussiotis VA, Freeman GJ, Park SY, Watanabe M, Gong J, Hayes DF, Kufe DW, Nadler LM. Breast cancer-associated antigen, DF3/MUC1, induces apoptosis of activated human T cells. Nat Med. 1996;2:1367–1370. doi: 10.1038/nm1296-1367. [DOI] [PubMed] [Google Scholar]
- 7.Kondo K, Kohno N, Yokoyama A, Hiwada K. Decreased MUC1 expression induces E-cadherin-mediated cell adhesion of breast cancer cell lines. Cancer Res. 1998;58:2014–2019. [PubMed] [Google Scholar]
- 8.Hewetson A, Chilton BS. Molecular cloning and hormone-dependent expression of rabbit Muc1 in the cervix and uterus. Biol Reprod. 1997;57:468–477. doi: 10.1095/biolreprod57.2.468. [DOI] [PubMed] [Google Scholar]
- 9.Schroeder JA, Thompson MC, Gardner MM, Gendler SJ. Transgenic MUC1 interacts with epidermal growth factor receptor and correlates with mitogen-activated protein kinase activation in the mouse mammary gland. J Biol Chem. 2001;276:13057–13064. doi: 10.1074/jbc.M011248200. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto M, Bharti A, Li Y, Kufe D. Interaction of the DF3/MUC1 breast carcinoma-associated antigen and beta-catenin in cell adhesion. J Biol Chem. 1997;272:12492–12494. doi: 10.1074/jbc.272.19.12492. [DOI] [PubMed] [Google Scholar]
- 11.Hudson MJ, Stamp GWH, Chaudhary KS, Hewitt R, Stubbs AP, Abel PD, Lalani E-N. Human MUC1 mucin: a potent glandular morphogen. J Pathol. 2001;194:373–383. doi: 10.1002/1096-9896(200107)194:3<373::AID-PATH898>3.0.CO;2-6. (DOI: 10.1002/path.898) [DOI] [PubMed] [Google Scholar]
- 12.Mannori G, Crottet P, Cecconi O, Hanasaki K, Aruffo A, Nelson RM, Varki A, Bevilacqua MP. Differential colon cancer cell adhesion to E-, P-, and L-selectin: role of mucin-type glycoproteins. Cancer Res. 1995;55:4425–4431. [PubMed] [Google Scholar]
- 13.Kam JL, Regimbald LH, Hilgers JH, Hoffman P, Krantz MJ, Longenecker BM, Hugh JC. MUC1 synthetic peptide inhibition of intercellular adhesion molecule-1 and MUC1 binding requires six tandem repeats. Cancer Res. 1998;58:5577–5581. [PubMed] [Google Scholar]
- 14.Meseguer M, Aplin JD, Caballero-Campo P, O'Connor JE, Martin JC, Remohi J, Pellicer A, Simon C. Human endometrial mucin MUC1 is up-regulated by progesterone and down-regulated in vitro by the human blastocyst. Biol Reprod. 2001;64:590–601. doi: 10.1095/biolreprod64.2.590. [DOI] [PubMed] [Google Scholar]
- 15.McGuckin MA, Quin RJ, Ward BG. Progesterone stimulates production and secretion of MUC1 epithelial mucin in steroid-responsive breast cancer cell lines. Int J Oncol. 1998;12:939–945. doi: 10.3892/ijo.12.4.939. [DOI] [PubMed] [Google Scholar]
- 16.Treon SP, Mollick JA, Urashima M, Teoh G, Chauhan D, Ogata A, Raje N, Hilgers JHM, Nadler L, Belch AR, Pilarski LM, Anderson KC. Muc-1 core protein is expressed on multiple myeloma cells and is induced by dexamethasone. Blood. 1999;93:1287–1298. [PubMed] [Google Scholar]
- 17.Hall REA, Horsfall DJ, Birrell SN, JM B, Sutherland RL, Tilley WD. Expression of the androgen receptor and androgen-responsive protein, apolipoprotein D, in human breast cancer. Br J Cancer. 1996;74:1175–1180. doi: 10.1038/bjc.1996.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hackenberg R, Schulz KD. Androgen receptor-mediated growth control of breast cancer and endometrial cancer modulated by antiandrogen- and androgen-like steroids. J Steroid Biochem Mol Biol. 1996;56:113–117. doi: 10.1016/0960-0760(95)00228-6. [DOI] [PubMed] [Google Scholar]
- 19.Ilekis JV, Connor JP, Prins GS, Ferrer K, Niederberger C, Scoccia B. Expression of epidermal growth factor and androgen receptors in ovarian cancer. Gynecol Oncol. 1997;66:250–254. doi: 10.1006/gyno.1997.4764. [DOI] [PubMed] [Google Scholar]
- 20.Stenner J, Crawford DE. Combined Androgen Blockade. In: Kaisary AV, Murphy GP, Denis L, Griffiths K, editors. Prostate Cancer. Pathology, Diagnosis and Treatment. London: Martin Duntz; 1999. pp. 303–31. [Google Scholar]
- 21.Chaudhary KS, Nightingale J, Stubbs AP, Stamp GWH, Abel PD, Lalani E-N. Ligand mediated inhibition of cell proliferation, and apoptotic cell death in DU145 prostatic adenocarcinoma cell line expressing full length androgen receptor complementary deoxyribonucleic acid. Eur Urol. 1998;34:251. [Google Scholar]
- 22.Mitchell S, Abel P, Ware M, Stamp G, Lalani E. Phenotypic and genotypic characterization of commonly used human prostatic cell lines. BJU Int. 2000;85:932–944. doi: 10.1046/j.1464-410x.2000.00606.x. [DOI] [PubMed] [Google Scholar]
- 23.Birrell SN, Bentel JM, Hickey TE, Ricciardelli C, Weger MA, Horsfall DJ, Tilley WD. Androgens induce divergent proliferative responses in human breast cancer cell lines. J Steroid Biochem Mol Biol. 1995;52:459–467. doi: 10.1016/0960-0760(95)00005-k. [DOI] [PubMed] [Google Scholar]
- 24.Hall RE, Birrell SN, Tilley WD, Sutherland RL. MDA-MB-453, an androgen-responsive human breast carcinoma cell line with high level androgen receptor expression. Eur J Cancer. 1994;4:484–490. doi: 10.1016/0959-8049(94)90424-3. [DOI] [PubMed] [Google Scholar]
- 25.Giles AM, Holloway P. Reference Intervals for Biochemical Data. In: Weatherall DJ, Ledingham JGG, Warrell DA, editors. Oxford Textbook of Medicine. Oxford: Oxford Univ. Press; 1996. 3rd ed., vol 3. [Google Scholar]
- 26.Terouanne B, Tahiri B, Georget V, Belon C, Poujol N, Avances C, Orio FJ, Balaguer P, Sultan C. A stable prostatic bioluminescent cell line to investigate androgen and antiandrogen effects. Mol Cell Endocrinol. 2000;160:39–49. doi: 10.1016/s0303-7207(99)00251-8. [DOI] [PubMed] [Google Scholar]
- 27.Nightingale J. Effect on cellular growth, apoptosis and adhesion. London: PhD Thesis. London University; 2000. Investigation of androgen receptor gene transfection into human prostate cancer cells. 322 pp. [Google Scholar]
- 28.Hamaguchi M, Matsuyoshi N, Ohnishi Y, Gotoh B, Takeichi M, Nagai Y. p60v-src causes tyrosine phosphorylation and inactivation of the N-cadherin-catenin cell adhesion system. EMBO J. 1993;12:307–314. doi: 10.1002/j.1460-2075.1993.tb05658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Surveyor GA, Gendler SJ, Pemberton L, Das SK, Chakraborty I, Julian J, Pimental RA, Wegner CC, Dey SK, Carson DD. Expression and steroid hormonal control of Muc-1 in the mouse uterus. Endocrinology. 1995;136:3639–3647. doi: 10.1210/endo.136.8.7628404. [DOI] [PubMed] [Google Scholar]
- 30.Kovarik A, Lu PJ, Peat NMJ, Taylor-Papadimitriou J. Two GC boxes (Sp1 sites) are involved in regulation of the activity of the epithelium-specific MUC1 promoter. J Biol Chem. 1996;271:18140–18147. doi: 10.1074/jbc.271.30.18140. [DOI] [PubMed] [Google Scholar]
- 31.Funder JW. Importance of Steroidogenesis in Specific Hormone Action. In: Parker MG, editor. Steroid Hormone Action. Oxford: Oxford Univ. Press; 1993. pp. 26–44. [Google Scholar]
- 32.Wesseling J, van der Valk SW, Vos HL, Sonnenberg A, Hilkens J. Episialin (MUC1) overexpression inhibits integrin-mediated cell adhesion to extracellular matrix components. J Cell Biol. 1995;129:255–265. doi: 10.1083/jcb.129.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wesseling J, van der Valk SW, Hilkens J. A mechanism for inhibition of E-cadherin-mediated cell-cell adhesion by the membrane-associated mucin episialin/MUC1. Mol Biol Cell. 1996;7:565–577. doi: 10.1091/mbc.7.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohgami A, Tsuda T, Osaki T, Mitsudomi T, Morimoto Y, Higashi T, Yasumoto K. MUC1 mucin mRNA expression in stage I lung adenocarcinoma and its association with early recurrence. Ann Thorac Surg. 1999;67:810–814. doi: 10.1016/s0003-4975(99)00041-7. [DOI] [PubMed] [Google Scholar]
- 35.Utsunomiya T, Yonezawa S, Sakamoto H, Kitamura H, Hokita S, Aiko T, Tanaka S, Irimura T, Kim YS, Sato E. Expression of MUC1 and MUC2 mucins in gastric carcinomas: its relationship with the prognosis of the patients. Clin Cancer Res. 1998;4:2605–2614. [PubMed] [Google Scholar]
- 36.McGuckin MA, Walsh MD, Hohn BG, Ward BG, Wright RG. Prognostic significance of MUC1 epithelial mucin expression in breast cancer. Hum Pathol. 1995;26:432–439. doi: 10.1016/0046-8177(95)90146-9. [DOI] [PubMed] [Google Scholar]
- 37.Nakagawa K, Akagi J, Takai E, Tamori Y, Okino T, Kako H, Egami H, Ogawa M. Prognostic values of MUC-1 molecule expressing cytokine receptor-like epitope and DF3 in patients with gastric carcinoma. Int J Oncol. 1999;14:425–435. doi: 10.3892/ijo.14.3.425. [DOI] [PubMed] [Google Scholar]
- 38.Dorgan J, Boudou P, Stanczyk FZ, Longcope CTA, Falk RT, Schusler N, Stephenson HE. Sources of elevated serum androgens in postmenopausal women who develop breast cancer (Abstract) Proc AACR 92nd Annu Meet (March 2001, #4086) 2001;42:761. [PubMed] [Google Scholar]
- 39.Wang DY, Allen DS, De Stavola BL, Fentiman IS, Brussen J, Bulbrook RD, Thomas BS, Hayward JL, Reed MJ. Urinary androgens and breast cancer risk: results from a long-term prospective study based in Guernsey. Br J Cancer. 2000;82:1577–1584. doi: 10.1054/bjoc.1999.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ballerini P, Oriana S, Duca P, Martinetti A, Venturelli E, Ferrari L, Dolci S, Secreto G. Urinary testosterone as a marker of risk of recurrence in operable breast cancer. Breast Cancer Res Treat. 1993;26:1–6. doi: 10.1007/BF00682694. [DOI] [PubMed] [Google Scholar]
- 41.Bulbrook RD, Thomas BS. Hormones are ambiguous risk factors for breast cancer. Acta Oncol. 1989;28:841–847. doi: 10.3109/02841868909092319. [DOI] [PubMed] [Google Scholar]
- 42.Slayden S. Risks of menopausal androgen supplementation. Semin Reprod Endocrinol. 1998;16:145–152. doi: 10.1055/s-2007-1016265. [DOI] [PubMed] [Google Scholar]
- 43.Kirschenbaum A, Itzkowitz SH, Wang JP, Yao S, Eliashvili M, Levine AC. MUC1 expression in prostate carcinoma: correlation with grade and stage. Mol Urol. 1999;3:163–168. [PubMed] [Google Scholar]
- 44.Okon MA, Laird SM, Tuckerman EM, Li TC. Serum androgen levels in women who have recurrent miscarriages and their correlation with markers of endometrial function. Fertil Steril. 1998;69:682–690. doi: 10.1016/s0015-0282(98)00007-7. [DOI] [PubMed] [Google Scholar]
- 45.Horne AW, White JO, Margara RA, Williams R, Winston RM, Lalani E. MUC1: a genetic susceptibility to infertility? Lancet. 2001;357:1336–1337. doi: 10.1016/s0140-6736(00)04502-5. [DOI] [PubMed] [Google Scholar]
- 46.Horne AW, White JO, Lalani EN. The endometrium and embryo implantation. A receptive endometrium depends on more than hormonal influences. BMJ. 2000;321:1301–1302. doi: 10.1136/bmj.321.7272.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taylor Papadimitriou J, Peterson JA, Arklie J, Burchell J, Ceriani RL, Bodmer WF. Monoclonal antibodies to epithelium-specific components of the human milk fat globule membrane: production and reaction with cells in culture. Int J Cancer. 1981;28:17–21. doi: 10.1002/ijc.2910280104. [DOI] [PubMed] [Google Scholar]
- 48.Briggs S, Price MR, Tendler SJ. Immune recognition of linear epitopes in peptide fragments of epithelial mucins. Immunology. 1991;73:505–507. [PMC free article] [PubMed] [Google Scholar]
- 49.Petrakou E, Murray A, Rosamund C, Graves L, Price MR. Evaluation of Pepscan analyses for epitope mapping of anti-MUC1 monoclonal antibodies — a comparative study and review of five antibodies. Anticancer Res. 1998;18:4419–4421. [PubMed] [Google Scholar]
- 50.Burchell J, Gendler S, Taylor Papadimitriou J, Girling A, Lewis A, Millis R, Lamport D. Development and characterization of breast cancer reactive monoclonal antibodies directed to the core protein of the human milk mucin. Cancer Res. 1987;47:5476–5482. [PubMed] [Google Scholar]
- 51.Dokurno P, Bates PA, Band HA, Stewart LMD, Lally JM, Burchell JM, Taylor Papadimitriou J, Snary D, Sternberg MJE, Freemont PS. Crystal structure at 1. 5 A resolution of the breast tumour-specific antibody SM3 complexed with its peptide epitope reveals novel hypervariable loop recognition. J Mol Biol. 1998;284:713–728. doi: 10.1006/jmbi.1998.2209. [DOI] [PubMed] [Google Scholar]
- 52.Waseem A, Lane EB, Harrison D, Waseem N. A keratin antibody recognizing a heterotypic complex: epitope mapping to complementary locations on both components of the complex. Exp Cell Res. 1996;223:203–214. doi: 10.1006/excr.1996.0074. [DOI] [PubMed] [Google Scholar]
- 53.Rennie PS, Bruchovsky N, Leco KJ, Sheppard PC, McQueen SA, Cheng H, Snoek R, Hamel A, Bock ME, MacDonald BS, et al. Characterization of two cis-acting DNA elements involved in the androgen regulation of the probasin gene. Mol Endocrinol. 1993;7(1):23–36. doi: 10.1210/mend.7.1.8446105. [DOI] [PubMed] [Google Scholar]