Abstract
The MMP, matrilysin (MMP-7), has been shown to be overexpressed in prostate cancer cells and to increase prostate cancer cell invasion. Prostate stromal fibroblasts secrete factor(s), including fibroblast growth factor-1 (FGF-1) that induces promatrilysin expression in LNCaP cells. In the present study, we investigated the signal transduction pathway involved in the FGF-1-induced expression of promatrilysin. FGF-1 treatment significantly increased the activation of extracellular signal-regulated kinases 1 and 2 (ERK1 and ERK2). This induction was time-dependent and was sustained until 24 hours after treatment. Treating the cells with MEK1/2 inhibitor (PD98059) eliminated ERK activation completely and blocked FGF-1-mediated induction of promatrilysin expression. Transient transfection studies with human matrilysin promoter resulted in a four-to five-fold increase in reporter luciferase enzyme activity that was blocked by the MEK1/2 inhibitor (PD98059). Serine phosphorylation of signal transducer and activator of transcription 3 (STAT3) was observed after FGF-1 treatment and pretreatment with 20 µM PD98059-abolished STAT3 phosphorylation. Transient transfection with dominant negative STAT3 inhibited FGF-1-induced transactivation of the matrilysin promoter indicating that STAT3 plays an important role in FGF-1-induced matrilysin expression. We propose that the FGF-1-induced signaling pathway that leads to promatrilysin expression is ERK-dependent and leads to phosphorylation of Ser-727 on STAT3, phosphorylated STAT3, then binds and transactivates the matrilysin promoter. Our results demonstrate that ERK-MAP kinase and transcription factor STAT3 are important components of FGF-1—mediated signaling, which induce promatrilysin expression in LNCaP cells.
Keywords: matrilysin, prostate, STAT3, FGF-1, LNCaP
Introduction
Prostate cancer is the leading male visceral cancer diagnosed and the second leading cause of cancer death in North American men [1]. Even though extensive studies have been pursued concerning prostate cancer, the cellular and molecular mechanisms involved in the initiation and progression are not clear. Most prostate cancer-related deaths are due to metastasis of the cancer cells to other tissues or organs. Studies involving the mechanisms underlying the metastasis, and the interactions between the prostate cancer epithelial cells and their surrounding stromal cells, are important in understanding the natural history of prostate cancer. Prostate cancer has been associated with increased matrix metalloproteinase (MMP) expression, which contributes to tumor cell invasion and metastasis [2]. Proteolysis of the extracellular matrix proteins, which allows the migration of neoplastic cells through the basal lamina into the interstitial stroma, is an important event in the metastatic process of prostate cancer. Also, the degradation of extracellular matrix proteins helps in releasing the bound growth factors, such as fibroblast growth factors (FGFs), which are capable of influencing the function of neoplastic cells [3]. The MMPs, a family of metal-requiring enzymes, are capable of degrading ECM proteins [4] and are found to be elevated in many types of cancer including prostate cancer [5–7]. Matrilysin (PUMP-1, MMP-7) is capable of degrading many extracellular matrix proteins, including proteoglycans, fibronectin, entactin, laminin, gelatin, and elastin [8]. Matrilysin expression appears to predominate in epithelial cells of glandular tissue, whereas other MMPs, like stromelysin and the gelatinases, are more commonly expressed by cells in the stromal compartment [8]. Matrilysin is overexpressed in prostate cancer [7] and in inflamed ducts of the human prostate, but is not expressed in normal prostate glands [9]. This overexpression could be due to stimulation by paracrine factors from the surrounding stroma [10]. Previous reports from our laboratory showed that, in the prostate carcinoma cell line LNCaP, promatrilysin expression is enhanced by factors secreted from prostate-derived fibroblasts, and this enhancement is due to members of the FGF family [10]. FGFs have been implicated in the development of numerous malignancies including prostate cancer. There are at least 20 members of the FGF family, 10 of which have been characterized in humans [11]; and expression of FGF-1, FGF-2, FGF-7, FGF-9, and FGF-10 has been observed in prostate stromal fibroblasts [10]. Because FGFs are present in the prostate, a gain in sensitivity to FGF induction of promatrilysin expression could have important consequences with regard to prostate cancer progression. There are four different genes encoding high-affinity transmembrane receptors, FGF receptors 1 to 4. FGFR-1 to FGFR-4 are characterized by the presence of two or three immunoglobulin-like domains in the extracellular region and a tyrosine kinase domain in the intracellular region of the receptor [12]. These receptors are responsible for FGF-mediated signal transduction. Further diversity in the FGF receptor family is generated by alternative RNA splicing.
FGF receptor activation elicits phosphorylation of the receptor and intracellular proteins, including phospholipase Cγ [13], and extracellular signal-regulated kinases (ERKs) [14]. Mitogen-activated protein kinases (MAP kinase) are serine-threonine kinases that are activated in response to extracellular stimuli by a phosphorylation cascade involving multiple kinases. ERKs belong to the MAP kinase family and are one of the strong candidates in FGF-mediated signal transduction. ERK1/2 is predominantly stimulated by mitogens and hormones inducing proliferation, cell growth, or differentiation [15]. A number of transcription factors have been identified as targets for MAP kinase. These transcription factors enter the nucleus after phosphorylation by MAP/ERK kinase [16]. Recently, it was shown that signal transducer and activator of transcription 3 (STAT3) is a substrate for MAP kinase [17].
Previous reports from our laboratory showed that, in LNCaP cells, promatrilysin expression was mediated by FGF-1 [10]. However, the signaling pathway leading to the increase in promatrilysin expression is unknown. The goal of this present study was to investigate the possible mechanisms involved in FGF-1-mediated induction of promatrilysin expression in LNCaP cells. Our results show that the ERK1/2-STAT3 pathway is activated by FGF-1, and that blocking this pathway with a MAP kinase inhibitor abolishes the induction of promatrilysin by FGF-1. These results suggest that ERK1/2 and STAT3 may play roles as the downstream mediators for the FGF-1-induced increase in the promatrilysin expression in LNCaP cells.
Materials and Methods
Cell Culture and Reagents
LNCaP cells were obtained from the American Type Culture Collection (Rockville, MD). LNCaP cell cultures were maintained in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum, 100 µg/ml penicillin, and 100 µg/ml streptomycin, both from Gibco-BRL (Rockville, MD). All cells were maintained in a humidified incubator at 37°C and 5% CO2. Recombinant human FGFs were purchased from R&D Systems (Minneapolis, MN). MEK1/2 inhibitor PD98059, phospho-specific antibodies, and general antibodies against ERK and STAT3 were obtained from New England Biolaboratories (Beverly, MA). DMEM was obtained from Gibco-BRL (Gaithersburg, MD). Fetal bovine serum was obtained from JRH Biosciences (Lenexa, KS).
Plasmid Constructs
The heterologous human matrilysin promoter (HMAT luciferase) was generated using 1179 bp of the sequenced human matrilysin promoter (GenBank accession no. L22525) located directly upstream of the TATA box. This sequence was amplified by polymerase chain reaction (PCR) and subcloned into pTAL-luc (Promega, San Luis, CA). PCR amplification of the matrilysin promoter (kindly provided by the laboratory of Lynn Matrisian, Vanderbilt University) was carried out using the following heterologous primers, which contained either NheI (upstream primer) or XhoI (downstream primer) restriction site sequences linked to matrilysin promoter-specific sequences (upstream primer 5′-CGTCTTGTCATTGGCGAATTC-3′ and the downstream primer 5′-CCCCAGTGCAAGTGCAGGTGC-3′). The resultant 1217-bp amplification product was digested with NheI/Xho, gel purified, and directionally cloned into NheI/XhoI-digested pTAL-luc vector directly upstream of the thymidine kinase minimal promoter. The resultant plasmid construct was confirmed with DNA sequencing. The pTAL-luc construct was used as a control plasmid. The STAT3 dominant negative plasmid construct was provided by the laboratory of Ralph A. Bradshaw (University of California Medical School, Irvine, CA). The double mutant STAT3 contains both Tyr-to-Phe and Ser-to-Ala mutations preventing phosphorlylation at sites critical for STAT3 activity [18]. The parent vector (pCMV-1) into which the STAT3 double mutant was cloned was used as a control plasmid.
Transient Transfection and Reporter Gene Assays
LNCaP cells were grown to log phase by splitting 24 hours before being trypsinized, counted, and plated for the experiment at 4x104 cells/cm2 of a six-well plate. Cells, at approximately 60% confluence 24 hours later, were transfected with a DNA/DOTAP mixture made up of 2 µg/well DNA (in 50 µl/well of 20 mM HEPES, pH 7.3) and 4 µg/well sonicated DOTAP (1, 2-diacyl-3-trimethylammonium-propane) from Avanti Polar Lipids (Birmingham, AL) (in 50 µl/well of 20 mM HEPES, pH 7.3), preincubated together for 20 minutes before being mixed with 2 ml/well of serum-free medium. Cells were rinsed once in serum-free medium, incubated with the DNA/DOTAP medium for 12 hours, and allowed to grow for an additional 12 hours in complete medium. Then the cells were treated with FGF-1 (10 ng/ml). The cells were harvested between 2 and 24 hours after treatment with 100 µl of Luciferase Cell Culture Lysis Reagent from Promega (Madison, WI) (25 mM Tris phosphate, pH 7.8, 2 mM DTT, 2 mM 1,2-diaminocyclohexane-N,N,N′,N′-tetra acetic acid, 10% glycerol, 1% Triton X-100) and protein amounts were determined using a Bio-Rad (Hercules, CA) DC Protein Assay kit. Whole cell lysates (30 µg) were assayed in 50 µl of Promega's Luciferase Assay Reagent using 3-second delay and 15-second integration on a Turner Designs (Sunnyvale, CA) TD-20/20 Luminometer.
ELISA Assay for Promatrilysin
An antibody sandwich assay described previously [10] was used for detection and quantification of promatrilysin protein in culture medium. The capture antibody (10D2, a mouse monoclonal antibody produced in the laboratory of Dr. Raymond Nagle using purified matrilysin from Dr. Mark Navre; Syntex, Palo Alto, CA) is specific for human promatrilysin. The mouse monoclonal antibody, 10D2, was coated onto 96-well EIA plates (Costar, Cambridge, MA). The detection antibodies include antibody Rb2, a rabbit polyclonal antibody to human matrilysin [9], followed by a horseradish peroxidase-conjugated goat antirabbit antibody (Pierce, Rockford, IL) used to detect the bound Rb2. Horseradish peroxidase activity was quantitated using a hydrogen peroxide/o-phenyldiamine (Sigma, St. Louis, MO) colorimetric system. Purified promatrilysin (Syntex) was used to generate a standard curve for each assay. The assay was linear in the range of 0.2 to 12.5 ng/ml. Samples were diluted prior to analysis until the readings fell within the linear range of the assay and the results were multiplied by the dilution factor.
Western Blot Analysis to Determine the Change in Phosphorlylation Status of ERK1/2 and STAT3 Proteins
Detection of ERK1/2 and STAT3 proteins in the cells by Western analysis was carried out with total and phosphospecific anti-ERK1/2 and STAT3 antibody. The LNCaP cells were pretreated with 20 µM MAP kinase kinase inhibitor (PD98059; Alexis, San Diego, CA) followed by FGF-1 treatment. Cells were harvested and total cellular protein was extracted after 4, 12, 24, and 48 hours. Protein determination was performed on the cell extracts using DC protein assay kit (Bio-Rad) and 40 µg of total protein was loaded and separated on a 12% SDS polyacrylamide gel by electrophoresis. Proteins were then transferred to a polyvinylidene difluoride membrane (Millipore Immobilin P, Bedford, MA) by electroblotting at 50 V at 4°C for 12 to 16 hours using a transfer buffer containing 25 mM Tris, 190 mM glycine, and 20% methanol. The membrane was blocked in 5% nonfat milk in TBST (10 mM Tris, pH 8.0, 150 mM NaCl, and 0.05% Tween-20) at room temperature for 1 hour. Phospho-specific anti-ERK1/2 and STAT3 were used as the primary antibodies. They were diluted to 1:1000 in dry milk/TBST and the membranes were incubated for 1 hour at room temperature and then washed three times with TBST. Horseradish peroxidase-conjugated goat antirabbit IgG was used as the secondary antibody at a dilution 1:2000 in 5% dry milk/TBST. The membrane was incubated for 1 hour and then washed three times with TBST. Antigen-antibody complexes were visualized by treating the membranes with Western blotting ECL detection reagent (Amersham International, Little Chalfont, UK) and exposing the blot to Kodak autoradiographic film.
Results
FGF-1-Induced Promatrilysin Expression in LNCaP Cells is Time-Dependent
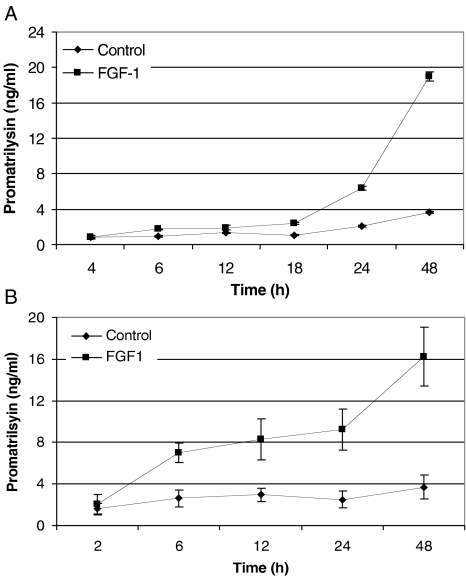
Previous studies from our laboratory showed that FGF-1 induces promatrilysin expression in LNCaP cells. It was also shown that nearly all of the matrilysin protein present in the culture was in the 28-kDa proenzyme form [10]. In the present study, we demonstrated that the promatrilysin induction in LNCaP cells was time-dependent. The cultured LNCaP cells were treated with FGF-1 (10 ng/ml) containing sodium heparin (2 µg/ml) under serum-free conditions. The culture medium and the cell lysates were collected at different time points. Following FGF-1 treatment, promatrilysin expression in the conditioned medium and the total cell lysate was measured by ELISA. FGF-1-treated LNCaP cells showed a time-dependent increase in promatrilysin expression in the conditioned media as well as the cell lysates. After FGF-1 addition, promatrilysin did not start to accumulate in the medium until 18 to 24 hours (Figure 1A). At 48 hours, there was a four-to five-fold increase in promatrilysin in the medium compared to the basal level at that time. In contrast, when intracellular promatrilysin was measured, there was a significant increase in promatrilysin as early as 6 hours after addition of FGF-1 (Figure 1B).
Figure 1.
FGF-1-induced promatrilysin expression in LNCaP cells. (A) LNCaP cells (4x104 cells/cm2) were treated with 10 ml of serum-free DMEM (control) or with FGF-1 (10 ng/ml), with 2 µg/ml sodium heparin for different time periods. The conditioned media were collected after 4, 6, 12, 18, 24, and 48 hours of incubation and ELISA was performed with antibodies specific to promatrilysin. (B) Total cell lysates were collected after 2, 6, 12, 24, and 48 hours of incubation and ELISA was performed with antibodies specific to matrilysin. Each bar represents the mean and standard deviation of three independent experiments run in triplicate.
Activation of ERK1/2 Induced by FGF-1 is Inhibited by MEK1/2 Inhibitor (PD98059)
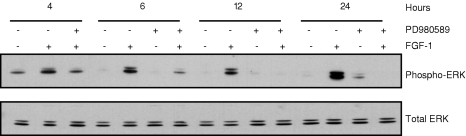
MAP kinases have established themselves as key regulators of gene expression through their ability to phosphorylate transcription factors and modulate their activity. Because the MEK/ERK pathway has been reported to be stimulated by growth factors [19,20], we investigated whether FGF-1 can activate the MEK/ERK pathway. To determine the effect of FGF-1 on ERK, cultures of LNCaP cells were stimulated with recombinant FGF-1 (10 ng/ml) in the presence of 2 µg/ml sodium heparin. After stimulation, the activation of ERKs was evaluated by monitoring changes in the phosphorylation status of ERK1/2. A time course of the immunoreactivity detected with the anti-phospho-ERK1/2 antibody by Western blot analyses of the whole cell extracts is shown in Figure 2. The antibody recognizes the kinases only when phosphorylated on Thr-202 and Tyr-204 residues in their regulatory sequence. FGF-1 stimulated the phosphorylation of ERK1/2 in a time-dependent manner. The activation of ERK1/2 was observed as early as 4 hours, increasing at 6 and 12 hours, and the maximum induction was observed at 24 hours. The phosphorylation of ERK1/2 in the presence of FGF-1 was sustained up to 24 hours and the increase in ERK activation decreased to undetectable levels by 48 hours (data not shown). There was no effect of FGF-1 on the total level of ERK proteins across the entire time course. To differentiate between the possible signaling pathways involved in FGF-1-induced promatrilysin expression, we used an inhibitor of MEK/ERK activation. The PD98059 has been described as a specific inhibitor of the ERK1/2-activating kinase, MEK1/2 [20,36]. A dose-response study was carried out for PD98059 [10, 20, and 50 µM (data not shown)] and 20 µM concentration did not alter the cell number and the basal expression of promatrilysin (data not shown), and this concentration of PD98059 was used in further experiments. LNCaP cells were pretreated for 1 hour with the MAP kinase kinase inhibitor, PD98059 (20 µM), prior to the addition of 10 ng/ml FGF-1 in the presence of 2 µg/ml sodium heparin. At various times after treatment, cellular protein was isolated from cells. The phosphorylated forms of ERK1/2 were analyzed by Western blotting with an antibody specific for the activated forms of ERK1/2. Pretreatment with the MEK1/2 inhibitor, PD98059 (20 µM), strongly inhibited the FGF-1-induced activation of ERK1/2 at all time points studied (Figure 2).
Figure 2.
FGF-1-induced activation of ERK1/2 was inhibited by MEK1/2 inhibitor PD98059 in LNCaP cells. MEK1/2 inhibitor PD98059 inhibited FGF-1-induced ERK activation. The LNCaP cells (4x104 cells/cm2) were pretreated with MEK1/2 inhibitor PD98059 (20 µM) for 1 hour in serum-free DMEM. After 1 hour, the cells were treated with 2 ml of serum-free DMEM (control) or with FGF-1 (10 ng/ml), with 2 µg/ml sodium heparin for different time periods. Western analyses were performed with phospho-specific ERK antibody for determining the inhibitory effect of PD98059 on FGF-1-induced ERK activation. Whole cell lysates (40 µg) from control and FGF-1-treated groups obtained from 4, 12, and 24 hours were analyzed. The results are representative of three independent experiments.
MEK1/2 Inhibitor (PD98059) Completely Abrogates Promatrilysin Expression Induced by FGF-1
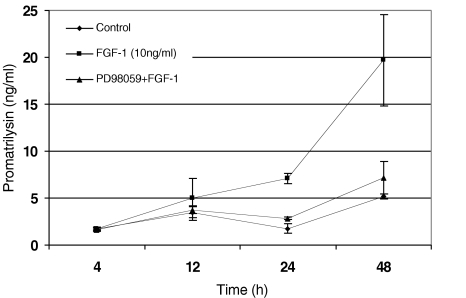
To further investigate the role of MAPKs in FGF-1-induced promatrilysin expression, and to determine whether the MEK1/2 inhibitor (PD98059) can inhibit the expression of promatrilysin induced by FGF-1, cultures of LNCaP cells were treated concurrently with PD98059 and recombinant FGF-1 (10 ng/ml) in the presence of 2 µg/ml sodium heparin. LNCaP cells were pretreated with PD98059 (20 µM) for 1 hour prior to FGF-1 treatment. The cells were then treated with FGF-1 for different time periods (4, 12, 24, and 48 hours). The media were collected and promatrilysin expression was measured by ELISA. Pretreatment with the MEK1/2 inhibitor completely abrogated the FGF-1-induced promatrilysin expression in LNCaP cells indicating that the FGF-1-induced promatrilysin expression requires the ERK1/2 signaling pathway (Figure 3).
Figure 3.
FGF-1-induced promatrilysin expression in LNCaP cells was inhibited by MEK1/2 inhibitor PD98059. MEK1/2 inhibitor PD98059 inhibited FGF-1-induced promatrilysin expression. LNCaP cells (4x104 cells/cm2) were pretreated with MEK1/2 inhibitor PD98059 (20 µM) for 1 hour in serum-free DMEM. After 1 hour, the cells were treated with 2 ml of serum-free DMEM (control) or with FGF-1 (10 ng/ml), with 2 µg/ml sodium heparin for different time periods (4, 12, 24, and 48 hours). The conditioned media were collected after 4, 12, 24, and 48 hours of incubation and ELISA was performed with antibodies specific to promatrilysin. Each bar represents mean and standard deviation of three independent experiments run in triplicate.
Transcription Enhancer Elements Present in the Matrilysin Promoter Region are Responsive to FGF-1 and the Induction was Blocked by PD98059
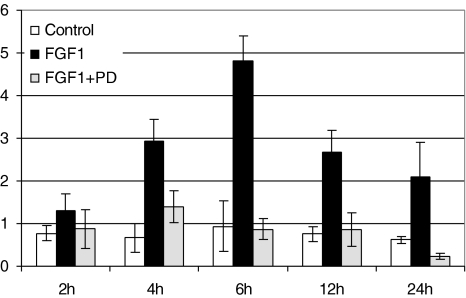
To determine if transcriptional activation of the matrilysin gene promoter is involved in the FGF-1-induced increase in steady state levels of matrilysin mRNA (data not shown), we cloned a plasmid construct encoding the 1.2-kb human matrilysin promoter driving a luciferase reporter gene. The 1.2-kb published human matrilysin promoter contains cis elements that include a TPA response element (TRE), cyclic AMP response element (CRE), serum response element (SRE), and an interferon stimulatory response element (ISRE). Apart from these cis elements, the human matrilysin promoter sequence has a number of NF IL-6 elements to which STAT3 may be capable of binding. We conducted transient transfection experiments in LNCaP cells with this 1.2-kb human matrilysin promoter-TK-luciferase reporter construct (HMAT-LUC). Treatment of HMAT-LUC-transfected cells with 10 ng/ml FGF-1 in the presence of 2 µg/ml sodium heparin for 6 hours resulted in a four-to five-fold increase in luciferase enzyme activity compared with untreated controls (Figure 4), whereas no induction was seen in cells transfected with the parental vector (pTAL-luc). The induction of 1.2-kb matrilysin promoter by FGF-1 was inhibited by the MEK1/2 inhibitor (PD98059) at all time periods studied. The four-to five-fold increase in the reporter gene was similar to the increase in promatrilysin protein levels measured by ELISA.
Figure 4.
PD98059 inhibited the FGF-1-induced transactivation of the 1.2-kb matrilysin promoter in LNCaP cells. LNCaP cells (4x104 cells/cm2) were transfected with a plasmid construct encoding 1.2 kb of the human matrilysin promoter driving a luciferase reporter gene. Following a 24-hour transfection, the cells were pretreated with PD98059 for 1 hour and then with FGF-1 (10 ng/ml). The cells were harvested at different time points indicated between 2 and 24 hours with 100 µl Luciferase Cell Culture Lysis Reagent from Promega. Proteins (30 µg) were assayed in 50 µl of Promega's Luciferase Assay Reagent on a luminometer. Each treatment group represents at least three wells per experiment, and at least three experiments were performed for each figure. Representative graphs are shown with error bars representing the replicates for a particular experiment.
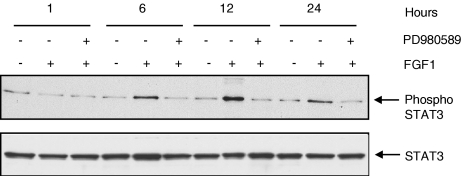
The MEK1/2 Inhibitor, PD98059, Completely Inhibits STAT3 Activation Induced by FGF-1
Recent reports have suggested that STAT3 is a substrate of ERK-MAP kinases that phosphorylate STAT3 at serine 727. Serine phosphorylation of STAT3 may affect both the nuclear translocation and DNA binding of the transcription factor [21]. Therefore, we examined the ability of FGF-1 to activate STAT3 in LNCaP cells in the presence of the MAP kinase kinase inhibitor, PD98059. Whole cell lysates were analyzed from LNCaP cells that were treated for different time periods with FGF-1 (10 ng/ml) to analyze the time-dependent activation of STAT3. LNCaP cells were harvested as described in the Materials and Methods section and the total cellular protein was examined for the presence of STAT3 activation by Western blot analyses. Serum-deprived LNCaP cells were pretreated with 20 µM PD98059 for 1 hour and then stimulated with FGF-1 (10 ng/ml) in the presence of 2 µg/ml sodium heparin. Western blot analyses revealed that STAT3 was phosphorylated on serine 727 to some extent in the basal conditions but was markedly enhanced with the addition of FGF-1. The phosphorylation of STAT3 was more potently stimulated between 6 and 12 hours after treatment with FGF-1, was decreased at 24 hours, and became undetectable at 48 hours (Figure 5). The ability of FGF-1 to induce the phosphorylation of STAT3 was inhibited completely by the MEK1/2 inhibitor PD98059 at all time periods studied.
Figure 5.
FGF-1-induced activation of STAT3 was inhibited by MEK1/2 inhibitor PD98059 in LNCaP cells. MEK-1/2 inhibitor PD98059 inhibited FGF-1-induced STAT3 activation. The LNCaP cells (4x104 cells/cm2) were pretreated with MEK1/2 inhibitor PD98059 (20 µM) for 1 hour in serum-free DMEM. After 1 hour, the cells were treated with 2ml of serum-free DMEM (control) or with FGF-1 (10 ng/ml), with 2 µg/ml sodium heparin for different time periods. Western analyses were performed with total and phospho-specific STAT3 antibodies for determining the inhibitory effect of PD98059 on FGF-1-induced STAT3 activation. Cell lysates (40 µg) from control and FGF-1-treated groups obtained from 2, 6, 12, and 24 hours were loaded analyzed. The results are representative of three independent experiments.
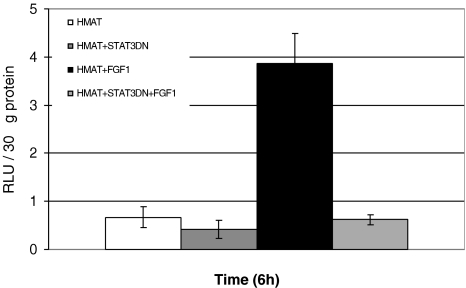
A Dominant Negative STAT3 Inhibits FGF-1-Induced Transactivation of the 1.2-kb Promoter
We then determined whether the FGF-1-induced activity of the 1.2-kb human matrilysin promoter could be inhibited by a STAT3 dominant negative construct. A dominant negative double mutant STAT3 construct with mutations in the Tyr and Ser phosphorylation sites, which prevent phosphorylation both at tyrosine and serine that are critical for STAT3 activation, has been used in this study. The parent vector (pCMV-1) into which the STAT3 double mutant was cloned was used as a control plasmid. After transient cotransfections with the 1.2-kb human matrilysin reporter and the STAT3 dominant negative or parent vector with equimolar amounts, FGF-1-induced transactivation of the 1.2-kb human matrilysin promoter was measured. Expression of dominant negative STAT3 inhibited the FGF-1-induced transactivation of the human matrilysin 1.2-kb promoter (Figure 6).
Figure 6.
A STAT3 dominant negative mutant construct inhibits FGF-1-induced transactivation from the 1.2-kb human matrilysin promoter in LNCaP cells. LNCaP cells (4x104 cells/cm2) were transiently cotransfected with HMAT-LUC and dominant negative STAT3 or HMAT-LUC and parent vector (pCMV-1), the parent vector into which the dominant negative STAT3 was cloned. Following a 24-hour transfection, the cells were treated with FGF-1 (10 ng/ml). The cells were harvested at different time points between 2 and 24 hours (here we show the 6-hour time point) with 100 µl of Luciferase Cell Culture Lysis Reagent from Promega. Total protein (30 µg) was assayed in 50 µl of Promega's Luciferase Assay Reagent on a luminometer. Each treatment group represents at least three wells per experiment, and at least three experiments were performed. Representative graphs are shown with error bars representing the replicates for a particular experiment.
Discussion
Matrilysin is known to play a role in prostate cancer progression and metastasis [8]. It was shown earlier that prostate cancer cells overexpress matrilysin [7,22]. Previously, we reported that stromally expressed FGF proteins induce promatrilysin expression in prostate cancer cell line; this finding may provide a mechanism for overexpression of matrilysin observed in prostate cancer [10]. FGF proteins have been shown to induce MMP expression in several mesenchymal [23–25] and cancer cell types [26–28] but not in normal prostate epithelial cells. Earlier, we have shown that normal prostate epithelial cells do not express FGFR-1 receptor and forced expression of FGFR-1 made them susceptible to FGF-mediated induction of promatrilysin expression [29]. In a recent study from our laboratory, we have shown that STAT3 plays a role downstream of IL-6 in IL-1β-induced promatrilysin expression in LNCaP cells [41].
In this study, we provided evidence for the mechanism involved in the signal transduction from FGF-1 to induced expression of promatrilysin in LNCaP cells. It was shown earlier from our laboratory that recombinant FGFs are capable of inducing promatrilysin expression in the prostate carcinoma cell line LNCaP [10] and FGF-1 was effective at a much lower dose level than other FGF proteins. FGF-1 was effective because of its high affinity towards all the four known FGF receptors [30]. The biological activities of the FGFs are initiated by binding to cell surface receptors. The FGF receptors are encoded by four genes, FGFR-1 to FGFR-4. The FGF receptors are receptor tyrosine kinases composed of three immunoglobulin-like extracellular domains, a transmembrane region, and a cytoplasmic domain that is autophosphorylated upon ligand binding [31]. Multiple alternative mRNA splicing events further contribute to the diversity in the FGF receptor family [32].
The data presented in this paper showed that FGF-1 induced promatrilysin expression in a time-dependent manner in LNCaP cells. We observed that the promatrilysin protein levels had accumulated by 24 and 48 hours with a four- to five-fold increase. In our experiments, it was found that the promatrilysin protein secreted into the medium is delayed compared to the intracellular levels, and the increase in the intracellular levels of promatrilysin protein was observed as early as 6 hours, indicating that the promatrilysin accumulates intracellularly before being secreted into the medium after treatment. Delayed secretion of proteins by LNCaP cells has been reported earlier [44]. It has been shown by densitometric analysis that laminin secretion was delayed in LNCaP cells compared to DU145 cells. Previously published data suggest that the lack of oligomerization has been found to be a cause of retention of many proteins in the rough endoplasmic reticulum [45,46], which would explain the present findings with LNCaP cells.
To determine the signaling pathways involved in the FGF-1-induced expression of promatrilysin, we investigated the role of MAP kinases in the FGF-1-induced promatrilysin expression. ERKs are coordinately activated in response to a wide range of mitogenic and nonmitogenic stimuli including the FGFs [14]. It was also shown that FGF receptor activation causes tyrosine phosphorylation of the receptor itself, and activation of extracellular regulated kinases (ERKs). In retinal-pigmented epithelial cells, it was observed that ERKs undergo rapid activation in response to FGF-1 and FGF-2 [33]. Our results demonstrate that ERK-MAP kinases play a major role in mediating the FGF-1-induced promatrilysin expression in LNCaP cells. Two pathways are known to transduce FGF-1 signals generated by FGFR-1 stimulation — one dependent on Ras and the other dependent on PLCγ [34,35]. Raf-1 integrates signals from these two pathways to activate the MAP kinase cascade and MAPKs are important upstream regulators of transcription factor activities.
Previously published reports suggest that transient activation of cell surface receptors and subsequent activation of MAP kinase cascade contribute to the transcriptional upregulation of MMPs. Activation of the ERK1/2 or the p38 MAP kinase pathway is sufficient to induce transcription from the MMP-1 promoter in human primary fibroblasts [47]. Wan et al. [48] have shown that in human keratinocytes, IL-1 β-induced expression of MMP-1 is mediated by activation of EGF receptor and through ERK-MAP kinase pathway. It was also demonstrated that P38 MAPK and ERK pathways contribute to the transcriptional regulation of MMP-9 in arterial smooth muscle cells [49]. Collectively, the literature suggests that growth factors and cytokines are capable of inducing MMPs through MAP kinase pathways, which corroborates with our present data.
Further, we determined whether the FGF-1-induced promatrilysin expression in LNCaP cells was mediated through transcription. In this study, we were able to show the transactivation of the matrilysin promoter by FGF-1 in prostate cancer cell line LNCaP. FGF-1-mediated transactivation was demonstrated using a 1.2-kb human matrilysin promoter that was cloned into a pTAL-luc expression vector. Transient transfections studies demonstrated that this matrilysin promoter was inducible by FGF-1 in LNCaP cells. We observed a four-to five-fold increase with this reporter gene, which was comparable to the FGF-1-induced matrilysin protein levels. The 1.2-kb region of the human matrilysin promoter, for which published sequence is available, contains a number of cis elements that are known to bind STAT3. These include multiple NF IL-6 binding elements and one IRF binding element. Further investigation showed that inhibiting the ERK pathway with a MEK1/2 inhibitor (PD98059) almost completely blocked FGF-1-induced transactivation of the 1.2-kb human matrilysin promoter. These data suggested that ERK pathway is required for the FGF-1-induced transactivation of the 1.2-kb human matrilysin promoter.
Biochemical studies have shown that ERKs can phosphorylate a large number of proteins including transcription factors, protein kinases, and cytosolic enzymes [37,38]. A potential substrate for MAP kinase, belonging to a distinct family of transcription factors, is signal transducers and activators of transcription 3 (STAT3) [39]. We provided evidence that ERK-MAP kinases are responsible for the phosphorylation of STAT3 serine 727 in vitro in LNCaP cells when induced with FGF-1. Treatment of the cells with the MEK1/2 inhibitor, PD98059, resulted in a decrease in the phosphorylation of STAT3 induced by FGF-1. Our data suggest that, the FGF-1 signal transduction pathway leading to induced promatrilysin expression involves ERK-dependent pathways to phosphorylate Ser-727 on STAT3. It has been shown early that basic FGF activates ERK1, ERK2, and STAT3 in myoblast cells [42]. The authors demonstrated that bFGF stimulation of myoblasts leads to a rapid tyrosine phosphorylation of STAT3. Recent studies show that ERKs, but not p38 or JNK, participate in STAT3 serine phosphorylation in growth factor-stimulated cells [22]. STAT1, STAT3, and STAT4 have conserved MAP kinase phosphorylation sites [39,40]. Though STAT1 has a conserved MAP kinase phosphorylation site, ERK-MAP kinases have been shown to poorly activate STAT1. The authors also reported that the difference in the activation of STATs is due the difference in the amino acid sequence surrounding the MAP kinase phosphorylation site of STAT1 and STAT3. The mechanism by which the serine phosphorylation influences the transcriptional activity is still unclear [43].
Chung et al. [21] have reported that both serine and tyrosine phosphorylation of STAT3 are induced in cytokine and growth factor signaling pathways. The predominant site of serine phosphorylation and activation of STAT3 is on serine 727. Using an antibody specific for STAT3 serine 727 phosphorylation, we showed that FGF-1 induces phosphorylation of this site in an ERK-dependent manner in LNCaP cells. In addition, we showed with a dominant negative double mutant STAT3 construct with mutations in Tyr (705) and Ser (727) that STAT3 activation is required for FGF-1-induced transactivation of the matrilysin promoter.
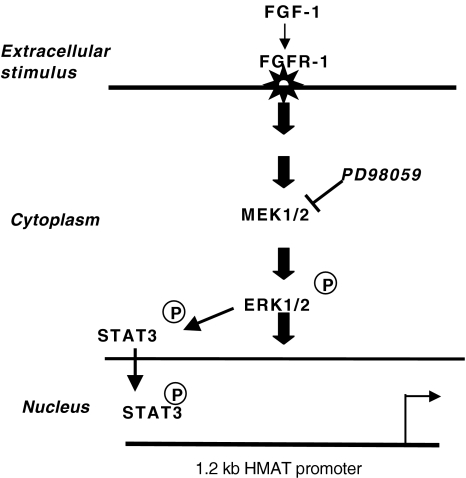
We have provided evidence in our proposed model (Figure 7) that ERK1/2 and STAT3 are involved in FGF-1-mediated promatrilysin expression. FGF-1 binds to FGF receptor and activates ERK1/2, which in turn activates STAT3. The transcription factor STAT3 translocates to the nucleus and binds specific cis elements in the 1.2-kb human matrilysin promoter and stimulates transcription of the matrilysin gene in LNCaP cells.
Figure 7.
Proposed mechanism of FGF-1-mediated activation of the MEK1/2, ERK1/2, and the transcription factor STAT3 in LNCaP cells. Based on our results, we propose the following pathway for FGF-1-induced promatrilysin expression in LNCaP cells. We have provided evidence for the role of MAP kinases (MEK1/2 and ERK1/2) and the involvement of the transcription factor STAT3 where it is activated in the cytoplasm by ERK1/2 and is translocated to the nucleus and binds to specific cis elements in the matrilysin promoter, thus inducing the transcription of the human matrilysin gene.
In conclusion, we have determined that FGF-1 induces promatrilysin expression in LNCaP cells in a time-dependent manner that involves ERK and STAT3. The MEK1/2 inhibitor (PD98059) blocked promatrilysin expression at the transcriptional level. Though basic FGF has been shown earlier to activate ERK and phosphorylate STAT3 (Tyr-705) in myoblasts [43], this is the first report to show that acidic FGF and STAT3 (Ser-727) are involved in the signaling leading to the expression of a MMP. Our findings support the idea that paracrine factors play an important role in the regulation of matrilysin in human prostate cancer cells.
Acknowledgements
This work was supported in part by NCI Grant SPO1 CA 56666 and the Cancer Center Core Grant 2 P30 CA23074.
Abbreviations
- MMP
matrix metalloproteinase
- STAT
signal transducer and activators of transcription
- ERK
extracellular signal-regulated kinases
- HMAT-LUC
human matrilysin luciferase
- MEK1/2
MAP kinase ERK kinase
- FGF
fibroblast growth factor
References
- 1.Parker ME. PSA thresholds for prostate cancer detection. JAMA, J Am Med Assoc. 1997;278:699–700. doi: 10.1001/jama.1997.03550090023014. [DOI] [PubMed] [Google Scholar]
- 2.Knox JD, Mack CF, Powell WC, Bowden GT, Nagle RB. Prostate tumor cell invasion: a comparison of orthotopic and ectopic models. Invasion Metastasis. 1993;13:325–331. [PubMed] [Google Scholar]
- 3.Noel A, Gilles C, Bajou K, Devy L, Kebers F, Lewalle JM, Maquoi E, Munaut C, Remacle A, Foidart JM. Emerging roles for proteinases in cancer. Invasion Metastasis. 1997;17:221–239. [PubMed] [Google Scholar]
- 4.Newell KJ, Witty JP, Rodgers WH, Matrisian LM. Expression and localization of matrix-degrading metalloproteinases during colorectal tumorigenesis. Mol Carcinogen. 1994;10:199–206. doi: 10.1002/mc.2940100404. [DOI] [PubMed] [Google Scholar]
- 5.Basset P, Bellocq JP, Wolf C, Stoll I, Hutin P, Limacher JM, Podhajcer OL, Chenard MP, Rio MC, Chambon P. A novel metalloproteinase gene specifically expressed in stromal cells of breast carcinomas. Nature. 1990;348:699–704. doi: 10.1038/348699a0. [DOI] [PubMed] [Google Scholar]
- 6.McDonnell S, Navre M, Coffey RJ, Matrisian LM. Expression and localization of the matrix metalloproteinase pump-1 (MMP-7) in human gastric and colon carcinomas. Mol Carcinogen. 1991;4:527–533. doi: 10.1002/mc.2940040617. [DOI] [PubMed] [Google Scholar]
- 7.Pajouh MS, Nagle RB, Breathnach R, Finch JS, Brawer MK, Bowden GT. Expression of metalloproteinase genes in human prostate cancer. J Cancer Res Clin Oncol. 1991;117:144–150. doi: 10.1007/BF01613138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson CL, Matrisian LM. Matrilysin: an epithelial matrix metalloproteinase with potentially novel functions. Int J Biochem Cell Biol. 1996;28:123–136. doi: 10.1016/1357-2725(95)00121-2. [DOI] [PubMed] [Google Scholar]
- 9.Kox JD, Wolf C, McDaniel K, Clark V, Loriot M, Bowden GT, Nagle RB. Matrilysin expression in human prostate carcinoma. Mol Carcinogen. 1996;15:57–63. doi: 10.1002/(SICI)1098-2744(199601)15:1<57::AID-MC8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 10.Klein RD, Maliner-Jongewaard MS, Udayakumar TS, Boyd JL, Nagle RB, Bowden GT. Promatrilysin expression is induced by fibroblast growth factors in the prostatic carcinoma cell line LNCaP but not in normal primary prostate epithelial cells. Prostate. 1999;41:215–223. doi: 10.1002/(sici)1097-0045(19991201)41:4<215::aid-pros1>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 11.Klint P, Claesson-Welsh L. Signal transduction by fibroblast growth factor receptors. Front Biosci. 1999;4:165–177. doi: 10.2741/klint. [DOI] [PubMed] [Google Scholar]
- 12.Johnson DE, Williams LT. Structural and functional diversity in the FGF receptor multigene family. Adv Cancer Res. 1993;60:1–41. doi: 10.1016/s0065-230x(08)60821-0. [DOI] [PubMed] [Google Scholar]
- 13.Burgess WH, Dionne CA, Kaplow J, Mudd R, Friesel R, Zilberstein A, Schlessinger J, Jaye M. Characterization and cDNA cloning of phospholipase C-gamma, a major substrate for heparin-binding growth factor 1 (acidic fibroblast growth factor)-activated tyrosine kinase. Mol Cell Biol. 1990;10:4770–4777. doi: 10.1128/mcb.10.9.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cobb MH, Boulton TG, Robbins DJ. Extracellular signal-regulated kinases: ERKs in progress. Cell Regul. 1991;2:965–978. doi: 10.1091/mbc.2.12.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 16.Marshall CJ. MAP kinase kinase kinase, MAP kinase kinase and MAP kinase. Curr Opin Genet Dev. 1994;4:82–89. doi: 10.1016/0959-437x(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 17.Kuroki M, O'Flaherty JT. Extracellular signal-regulated protein kinase (ERK)-dependent and ERK-independent pathways target STAT3 on serine-727 in human neutrophils stimulated by chemotactic factors and cytokines. Biochem J. 1999;341:691–696. [PMC free article] [PubMed] [Google Scholar]
- 18.Wu YY, Bradshaw RA. Activation of the Stat3 signaling pathway is required for differentiation by interleukin-6 in PC12-E2 cells. J Biol Chem. 2000;275:2147–2156. doi: 10.1074/jbc.275.3.2147. [DOI] [PubMed] [Google Scholar]
- 19.Lenormand P, Sardet C, Pages G, L'Allemain G, Brunet A, Pouyssegur J. Growth factors induce nuclear translocation of MAP kinases (p42mapk and p44mapk) but not of their activator MAP kinase kinase (p45mapkk) in fibroblasts. J Cell Biol. 1993;122:1079–1088. doi: 10.1083/jcb.122.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pang L, Sawada T, Decker SJ, Saltiel AR. Inhibition of MAP kinase kinase blocks the differentiation of PC-12 cells induced by nerve growth factor. J Biol Chem. 1995;270:13585–1388. doi: 10.1074/jbc.270.23.13585. [DOI] [PubMed] [Google Scholar]
- 21.Chung J, Uchida E, Grammer TC, Blenis J. STAT3 serine phosphorylation by ERK-dependent and-independent pathways negatively modulates its tyrosine phosphorylation. Mol Cell Biol. 1997;17:6508–6516. doi: 10.1128/mcb.17.11.6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hashimoto K, Yano A, Tanabe T, Usui T, Kihira Y, Matsuo Y. Localization and expression of matrix metalloproteinase-7 in human prostate. Nippon Hinyokika Gakkai Zasshi. 1997;88:852–857. doi: 10.5980/jpnjurol1989.88.852. [DOI] [PubMed] [Google Scholar]
- 23.Okamura K, Sato Y, Matsuda T, Hamanaka R, Ono M, Kohno K, Kuwano M. Endogenous basic fibroblast growth factor-dependent induction of collagenase and interleukin-6 in tumor necrosis factor-treated human microvascular endothelial cells. J Biol Chem. 1991;266:19162–1965. [PubMed] [Google Scholar]
- 24.Chua CC, Chua BH, Zhao ZY, Krebs C, Diglio C, Perrin E. Effect of growth factors on collagen metabolism in cultured human heart fibroblasts. Connect Tissue Res. 1991;26:271–281. doi: 10.3109/03008209109152444. [DOI] [PubMed] [Google Scholar]
- 25.Newberry EP, Willis D, Latifi T, Boudreaux JM, Towler DA. Fibroblast growth factor receptor signaling activates the human interstitial collagenase promoter via the bipartite Ets-AP1 element. Mol Endocrinol. 1997;11:1129–1144. doi: 10.1210/mend.11.8.9958. [DOI] [PubMed] [Google Scholar]
- 26.Miyagi N, Kato S, Terasaki M, Shigemori M, Morimatsu M. Fibroblast growth factor-2 and-9 regulate proliferation and production of matrix metalloproteinases in human gliomas. Int J Oncol. 1998;12:1085–1109. doi: 10.3892/ijo.12.5.1085. [DOI] [PubMed] [Google Scholar]
- 27.Miyake H, Yoshimura K, Hara I, Eto H, Arakawa S, Kamidono S. Basic fibroblast growth factor regulates matrix metalloproteinases production and in vitro invasiveness in human bladder cancer cell lines. J Urol. 1997;157:2351–2355. [PubMed] [Google Scholar]
- 28.Kurogi T, Nabeshima K, Kataoka H, Okada Y, Koono M. Stimulation of gelatinase B and tissue inhibitors of metalloproteinase (TIMP) production in co-culture of human osteosarcoma cells and human fibroblasts: gelatinase B production was stimulated via up-regulation of fibroblast growth factor (FGF) receptor. Int J Cancer. 1996;66:82–90. doi: 10.1002/(SICI)1097-0215(19960328)66:1<82::AID-IJC15>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 29.Udayakumar TS, Klein RD, Maliner MS, Nagle RB, Bowden GT. Aberrant expression of fibroblast growth factor receptor-1 in prostate epithelial cells allows induction of promatrilysin expression by fibroblast growth factors. Int J Cancer. 2001;91:187–192. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1023>3.3.co;2-n. [DOI] [PubMed] [Google Scholar]
- 30.Ornitz DM, Xu J, Colvin JS, McEwen DG, MacArthur CA, Coulier F, Gao G, Goldfarb M. Receptor specificity of the fibroblast growth factor family. J Biol Chem. 1996;271:15292–15297. doi: 10.1074/jbc.271.25.15292. [DOI] [PubMed] [Google Scholar]
- 31.Givol D, Yayon A. Complexity of FGF receptors: genetic basis for structural diversity and functional specificity. FASEB J. 1992;6:3362–3369. [PubMed] [Google Scholar]
- 32.McKeehan WL, Wang F, Kan M. The heparan sulfate-fibroblast growth factor family: diversity of structure and function. Prog Nucleic Acid Res Mol Biol. 1998;59:135–176. doi: 10.1016/s0079-6603(08)61031-4. [DOI] [PubMed] [Google Scholar]
- 33.Guillonneau X, Tassin J, Berrou E, Bryckaert M, Courtois Y, Mascarelli F. In vitro changes in plasma membrane heparan sulfate proteoglycans and in perlecan expression participate in the regulation of fibroblast growth factor 2 mitogenic activity. J Cell Physiol. 1996;166:170–187. doi: 10.1002/(SICI)1097-4652(199601)166:1<170::AID-JCP19>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 34.Mohammadi M, Honegger AM, Rotin D, Fischer R, Bellot F, Li W, Dionne CA, Jaye M, Rubinstein M, Schlessinger J. tyrosine-phosphorylated carboxy-terminal peptide of the fibroblast growth factor receptor (Flg) is a binding site for the SH2 domain of phospholipase C-gamma 1. Mol Cell Biol. 1991;11:5068–5078. doi: 10.1128/mcb.11.10.5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang J, Mohammadi M, Rodrigues GA, Schlessinger J. Reduced activation of RAF-1 and MAP kinase by a fibroblast growth factor receptor mutant deficient in stimulation of phosphatidylinositol hydrolysis. J Biol Chem. 1995;270:5065–5072. doi: 10.1074/jbc.270.10.5065. [DOI] [PubMed] [Google Scholar]
- 36.Dudley DT, Pang L, Decker SJ, Bridges AJ, Saltiel AR. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.L'Allemain G. Deciphering the MAP kinase pathway. Prog Growth Factor Res. 1994;5:291–334. doi: 10.1016/0955-2235(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 38.Davis RJ. The mitogen-activated protein kinase signal transduction pathway. J Biol Chem. 1993;268:14553–14556. [PubMed] [Google Scholar]
- 39.Wen Z, Zhong Z, Darnell JE. Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell. 1995;82:241–250. doi: 10.1016/0092-8674(95)90311-9. [DOI] [PubMed] [Google Scholar]
- 40.Zhang X, Blenis J, Li HC, Schindler C, Chen-Kiang S. Requirement of serine phosphorylation for formation of STAT-promoter complexes. Science. 1995;267:1990–1994. doi: 10.1126/science.7701321. [DOI] [PubMed] [Google Scholar]
- 41.Maliner-Stratton MS, Udayakumar TS, Klein RD, Nagle RB, Bowden GT. Interleukin-1β induced promatrilysin expression is mediated by NFkB-regulated synthesis of interleukin-6 in the prostate carcinoma cell line LNCaP. Neoplasia. 2001;3:1–12. doi: 10.1038/sj.neo.7900178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Megeney LA, Perry RLS, Lecouter JE, Rudnicki A. Basic FGF and LIF signaling activates STAT3 in proliferating myoblasts. Dev Genet. 1996;19:139–145. doi: 10.1002/(SICI)1520-6408(1996)19:2<139::AID-DVG5>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 43.Decker T, Kovarik P. Serine phosphorylation of STATs. Oncogene. 2000;19:2628–2637. doi: 10.1038/sj.onc.1203481. [DOI] [PubMed] [Google Scholar]
- 44.Rabinovitz I, Cress AE, Nagle RB. Biosynthesis and secretion of laminin and S-laminin by human prostate carcinoma cells lines. Prostate. 1994;25:97–107. doi: 10.1002/pros.2990250207. [DOI] [PubMed] [Google Scholar]
- 45.Hurtley SM, Helenius A. Protein oligomerization in the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:277–307. doi: 10.1146/annurev.cb.05.110189.001425. [DOI] [PubMed] [Google Scholar]
- 46.Pelham HR. Control of protein exit from the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:1–23. doi: 10.1146/annurev.cb.05.110189.000245. [DOI] [PubMed] [Google Scholar]
- 47.Brauchle M, Gluck D, Di Padova F, Han J, Gram H. Independent role of p38 and ERK 1/2 mitogen-activated kinases in the upregulating of matrix metalloproteinase-1. Exp Cell Res. 2000;258:135–144. doi: 10.1006/excr.2000.4913. [DOI] [PubMed] [Google Scholar]
- 48.Wan Y, Belt A, Wang Z, Voorhees J, Fisher G. Transmodulation of epidermal growth factor receptor mediates IL-1 beta-induced MMP-1 expression in cultured human keratinocytes. Int J Mol Med. 2001;7:329–334. [PubMed] [Google Scholar]
- 49.Cho A, Graves J, Reidy MA. Mitogen-activated protein kinases mediate matrix metalloproteinase-9 expression in vascular smooth muscle cells. Arterioscler, Thromb Vasc Biol. 2001;20:2527–2532. doi: 10.1161/01.atv.20.12.2527. [DOI] [PubMed] [Google Scholar]