Abstract
An important theme in molecular cell biology is the regulation of protein recruitment to the plasma membrane. Fundamental biological processes such as proliferation, differentiation or leukocyte functions are initiated and controlled through the reversible binding of signaling proteins to phosphorylated membrane components. This is mediated by specialized interaction modules, such as SH2 and PH domains. Cytohesin-1 is an intracellular guanine nucleotide exchange factor, which regulates leukocyte adhesion. The activity of cytohesin-1 is controlled by phospho inositide-dependent membrane recruitment. An interacting protein was identified, the expression of which is upregulated by cytokines in hematopoietic cells. This molecule, CYTIP, is also recruited to the cell cortex by integrin signaling via its PDZ domain. However, stimulation of Jurkat cells with phorbol ester results in re-localization of CYTIP to the cytoplasm, and membrane detachment of cytohesin-1 strictly requires co-expression of CYTIP. Con sequently, stimulated adhesion of Jurkat cells to intracellular adhesion molecule-1 is repressed by CYTIP. These findings outline a novel mechanism of signal chain abrogation through sequestration of a limiting component by specific protein–protein interactions.
Keywords: cell adhesion/CYTIP/cytohesin-1/integrins/lymphocytes
Introduction
Intracellular signal transduction events in eukaryotic cells are often initiated at the inner leaflet of the plasma membrane. Cell surface receptors can sense extracellular cues by binding to soluble, cell-based or matrix ligands, and the resulting information for, for example, growth, migration, proliferation or differentiation is transmitted to the cytoplasm via receptor oligomerization or conformational changes. Intracellular proteins are subsequently recruited to the activated receptors or to proximal downstream elements of the respective signaling cascade. Prominent examples of this type of regulation are the tyrosine kinase-dependent events triggered by epidermal growth factor (EGF), platelet-derived growth factor (PDGF), cytokines or insulin (Hunter, 1998, 2000). Other examples are the cell activation mechanisms induced by antigen receptors of the immune system (Germain, 2001; Reth, 2001). The recruited signal transduction intermediates may be protein or lipid kinases and phosphatases (Pawson et al., 2001, 2002), STAT transcription factors (Leonard, 2001) or other enzymes, e.g. guanine nucleotide exchange factors (GEFs) or GTPase-activating proteins (GAPs) (Pawson et al., 2001), or may belong to the growing family of multifunctional adaptor proteins (Geng and Rudd, 2002; Leo et al., 2002).
Protein interaction domains have evolved to serve these purposes. These are compact modules present in many signal transduction polypeptides. Some of them have specialized in interacting with phosphorylated membrane components, which may be either proteins or lipids. Of these, the so-called SH2 and PTB domains have a prominent function in recognizing phospho-tyrosines in a context-specific fashion (Pawson et al., 2002), whereas PH domains can bind phosphatidylinositides or the soluble phospho-inositols (Maffucci and Falasca, 2001).
Cell adhesion mediated by integrins is essential for many biological functions such as development, blood clotting, or in the immune response (Hynes, 1992, 1999). Binding of integrins to their counter-receptors or ligands is often not constitutive, but depends on ‘activation’ events that may be triggered by other cell surface receptors, which in turn initiate intracellular signal transduction cascades (Woodside et al., 2001). In the immune system, cells are particularly dependent on a fast and efficient regulation of their contacts with the extracellular milieu (van Kooyk and Figdor, 2000; Woodside et al., 2001). Cells have to be omnipresent in organismic transport systems such as blood and lymph to be able to access all possible sites of foreign invasion. However, these cells must also be capable of sensing an infection, and of leaving the transport vessels. They have to migrate in tissues, and to interact with infected cells in order to activate proper effector programs.
β2 integrins are expressed exclusively on cells of the immune system, where they play essential or highly important roles in leukocyte and lymphocyte attachment to the endothelium, in diapedesis or in T-cell effector functions (Diamond and Springer, 1994). Cytohesin-1 is an intracellular protein that binds to the β2 integrin leukocyte function antigen-1 (LFA-1) and has been implicated in activation of the receptor (Kolanus et al., 1996; Kolanus and Zeitlmann, 1998; Geiger et al., 2000; Weber et al., 2001). The molecule also acts as a GEF for ARF-GTPases, which are known to have important functions in cellular vesicle transport (Chardin et al., 1996). However, ARFs recently have been implicated in actin cytoskeletal remodeling during cell migration (D’Souza-Schorey et al., 1997; Palacios et al., 2001; Santy and Casanova, 2001). Congruently, the GEF function of cytohesin-1 has been shown to play a role in spreading of lymphocytes, which also involves the actin cytoskeleton, and in transendothelial migration (Geiger et al., 2000; Mayer et al., 2001). Furthermore, cytohesin-1 has been demonstrated to mediate F-actin-mediated shape changes and transformation events triggered by the human herpesvirus 8 protein kaposin A (Kliche et al., 2001).
Leukocytes and lymphocytes must be capable of controlling their interactions with other cells or with the matrix in a fast and efficient manner. Cytohesin-1 and other members of the cytohesin family have been shown to be recruited to the plasma membrane due to binding of their PH domains to phosphatidylinositol phospholipids, such as phosphatidylinositol-4,5-bisphosphate (PIP2) or phosphatidylinositol-3,4,5-trisphosphate (PIP3) (Klarlund et al., 1997; Nagel et al., 1998b; Venkateswarlu et al., 1998). However, we have no knowledge of how membrane recruitment of these proteins is reversed, or how leukocyte integrin activation is modulated and attenuated.
Here we report the characterization of a protein, the expression of which is regulated prominently in hematopoietic cells. This polypeptide, CYTIP, interrupts information flow in cell adhesion regulation in lymphocytes by actively sequestering its binding partner, the integrin-activating protein cytohesin-1, to the cytoplasm.
Results
Identification of CYTIP, a molecule upregulated in mature dendritic cells which interacts with cytohesin-1
In a differential display of PCR-amplified mRNA comparing in vitro generated monocyte-derived dendritic cells (DCs) with the same cells induced to mature by a cocktail containing cytokines [interleukin (IL)-1β, IL-6 and tumor necrosis factor-α (TNF-α)] and prostaglandin E2, we identified a remarkable increase of CYTIP mRNA transcription in mature DCs. This was confirmed by northern blotting (Figure 1B, left panel). The organ-derived mRNAs used to determine the expression pattern of CYTIP (Figure 1B, right panel) exhibit a very strong expression in lymph nodes, a strong signal in spleen and peripheral blood leukocytes, a weak expression in thymus- and bone marrow-derived RNA and no expression in fetal liver.
Fig. 1. Expression of CYTIP and cytohesin-1 in immature and mature DCs. (A) Schematic representation of the domain structures of CYTIP and cytohesin-1; the box and arrows highlight the coiled-coil domain-mediated oligomerization of the molecules. (B) Left panel: differential expression of CYTIP during maturation of human DCs. Northern blot containing 15 mg of total RNA per lane from human macrophages (M), day 7/immature human DCs and day 10/mature human DCs. The isolated PCR product from the differential display PCR was used as a probe. The positions of the 18S (1.8 kb) and 28S (4.7 kb) rRNA bands are indicated. The blot was reprobed with a human GAPDH PCR-amplified probe to estimate the relative amounts of RNA. Right panel: human tissue expression of CYTIP. A northern blot containing 2 mg of poly(A)+ RNA per lane from different human tissues was probed with a PCR-amplified product specific for CYTIP. The blot was reprobed with a 2 kb human β-actin cDNA probe as an internal control. PBL: peripheral blood lymphocytes. (C) Recognition specificity of anti-CYTIP monoclonal antibodies 1G1 and 2F9. Ig fusions of human cytohesin-1(E157K) and CYTIP were precipitated on protein A–Sepharose. In the subsequent immunoblot analysis, CYTIP but not cytohesin-1(E157K) was detected by 1G1 and 2F9. (D) Total lysate was prepared from immature (iDC) and mature (mDC) DCs, and endogenous CYTIP protein was monitored by immunoblot analysis (upper panel). Expression of CYTIP is strongly increased in mature DCs. As a control for the protein amount loaded in total, an anti-actin immunoblot was performed (lower panel). (E) Total lysates from 721, PBMCs and Jurkat cells were prepared, and endogenous CYTIP protein was monitored by immunoblot analysis using total lysate from 3 × 105 cells per lane.
Sequence (submitted to DDBJ/EMBL/GenBank; accession No. AF068836) analyses showed two highly conserved protein–protein interaction domains: a leucine-rich coiled-coil domain and a so-called PDZ domain (Fanning and Anderson, 1999) (see schematic in Figure 1A).
To identify potential interaction partners, we performed yeast two-hybrid screenings of a lymph node library, which resulted in nine positive clones, all coding for the B2-1 cDNA of cytohesin-1 (Liu and Pohajdak, 1992). The shortest of these clones codes for the N-terminal amino acids 13–57 of cytohesin-1, which therefore corresponds to the putative binding region of cytohesin-1 to CYTIP. These residues match very well with the coiled-coil domain of cytohesin-1 (see schematic in Figure 1A). To map the cytohesin-1 interaction site on the CYTIP molecule, we constructed baits containing the PDZ domains or the coiled-coil domains of CYTIP, and performed a two-hybrid mapping analysis with full-length cytohesin-1, using two independent two-hybrid subsystems. These analyses identified the coiled-coil region of CYTIP to be sufficient and necessary for interaction with cytohesin-1. Thus, the molecules appear to interact via coiled-coil-mediated hetero-oligomerization (not shown, see schematic in Figure 1A; see Supplementary data, available at The EMBO Journal Online). Rat monoclonal antibodies were then generated which recognize CYTIP specifically. Figure 1C demonstrates the binding specificity of two independently raised antibodies against CYTIP (1G1 and 2F9, respectively), using immunoglobulin fusion constructs of CYTIP and cytohesin-1: CYTIP but not cytohesin-1 is recognized by the reagent, using immunoblotting as the method of detection.
We then analyzed the regulation of CYTIP during maturation of DCs. Using lipopolysaccharide (LPS) as the maturation stimulus (Figure 1D), we found that CYTIP is also strongly upregulated at the protein level. CYTIP protein is not expressed exclusively in DCs. Figure 1E shows that the protein is also present in total lysates of the lymphoblastoid cell line 721, which is also a rich source of LFA-1 and cytohesin-1 (Geiger et al., 2000); CYTIP is expressed further in human peripheral blood mononuclear cells (PBMCs), albeit to a much lower extent. Interestingly, we were not able to detect CYTIP in total lysates of Jurkat cells.
Expression of cytohesin-1 and CYTIP mRNA in DCs
Since CYTIP is upregulated during maturation of DCs and because cytohesin-1 is the (putative) binding partner of CYTIP, we monitored the expression level of cytohesin-1 during the maturation process of DCs by real-time PCR. As shown in Figure 2A, a cytohesin-1 amplification product is detected specifically in DCs, whereas a cDNA of the highly homologous molecule cytohesin-2 cannot be detected in these cells. We were able to monitor a 5- to 20-fold increase of cytohesin-1 mRNA expression if comparing mature versus immature DCs (Figure 2B). Correspondingly, a 20- to 30-fold increase of mRNA expression of CYTIP during DC maturation was detected by this method.
Fig. 2. Specific expression of cytohesin-1 in DCs. (A) RT–PCR analysis of cytohesin-1 and cytohesin-2 expression in mature DCs. A 5 µg aliquot (lanes 4 and 8) or a 2 µg aliquot (lanes 3 and 7) of cDNA was used for PCRs. Negative controls were performed by omitting cDNA (lanes 2 and 6). A 5 ng aliquot of plasmid DNA containing either a cytohesin-1 (lane 5) or a cytohesin-2 (lane 1) cDNA insert was used as positive control. A 1 kb size marker is shown in lane 9. (B) Quantitative analysis of cytohesin-1 and CYTIP expression levels in immature and mature DCs, measured by real-time PCR. The results represent an average of at least three independent experiments carried out in triplicate for each value. (C) Western blot analysis of wild-type cytohesin-1 in cell lysates of immature [DC(–)] and mature [DC(+)] DCs. Cytohesin-1 was detected by anti-cytohesin-1 antibody, and 7H2 was visualized by a peroxidase-conjugated antibody via chemiluminescence.
Upregulation of cytohesin-1 protein in mature DCs
To confirm an upregulation of cytohesin-1 in mature DCs at the protein level, we performed a western blot on lysates of immature DCs, or of DCs that had been incubated with either maturation cocktail, LPS, TNF-α or poly(I:C). All maturation stimuli tested result in a significantly higher abundance of cytohesin-1 protein, the best stimulus being the maturation cocktail consisting of cytokines and prostaglandin E2 (Figure 2C; data not shown).
Co-immunoprecipitation of CYTIP and cytohesin-1
To confirm interaction of the molecules in hematopoietic cells, a cytoplasmic immunoglobulin-tagged version of CYTIP (Ig-CYTIP) was expressed in Jurkat cells using electroporation. Cell lysates were then incubated with protein A–Sepharose, and the resulting precipitates were subjected to western blot analysis. Figure 3A shows expression of both an Ig-control construct and Ig-CYTIP in the tumor cell line. However, endogenous cytohesin-1 is detected exclusively in Ig-CYTIP precipitates. A reciprocal pull-down experiment is shown in Figure 3B: Ig fusion proteins of cytohesin-1, cytohesin-2 and cytohesin-3 co-immunoprecipitate a flag-tagged CYTIP construct, while an Ig-control protein fails to bind flag-CYTIP. Thus, CYTIP appears also to bind cytohesin-2 and, to a lesser extent, cytohesin-3. Taking the mRNA expression data into account, interaction specificity is probably regulated at the level of cell type-specific gene expression.

Fig. 3. Co-immunoprecipitation of CYTIP and cytohesin-1 from Jurkat cells. (A) Western blot analysis of wild-type cytohesin-1 and Ig fusion proteins derived from recombinant vaccinia viruses. Ig fusion proteins were precipitated on protein A–Sepharose and detected by peroxidase-conjugated anti-Ig antibody (right panel). Immunoprecipitated wild-type cytohesin-1 was detected by anti-cytohesin-1 antibody 7H2 (left panel). Protein bands were visualized by chemiluminescence. (B) Upper panel: western blot analysis of Ig-cytohesin-1 fusion protein immunoprecipitating a flag-tagged CYTIP construct. Ig-cytohesin-1 and -2, and to a small extent Ig-cytohesin-3, were capable of interacting with flag-CYTIP. Cytoplasmic Ig domains failed to bind to CYTIP (control, leftmost lane). Lower panel: expression control, anti-Ig blot.
Association of endogenous CYTIP and cytohesin-1 proteins is observed in several cell types
Using anti-CYTIP antibody 2F9 we were also able to demonstrate endogenous co-immunoprecipitation of CYTIP with cytohesin-1. The results are shown in Figure 4: immunoprecipitation of cytohesin-1 with antibody 7H2 (Geiger et al., 2000) from 721 cells and PBMCs results in specific co-precipitation of CYTIP (Figure 4A and B). Furthermore, co-immunoprecipitation revealed a weak expression of CYTIP in Jurkat cells (Figure 4A) which had escaped detection in total lysates (see Figure 1). The overall amounts of co-precipitated material correspond very well with the relative expression levels.
Fig. 4. Co-immunoprecipitation of CYTIP with cytohesin-1 from Jurkat, 721 and human PBMCs. Endogenous cytohesin-1 was immunoprecipitated from total lysates of (A) Jurkat E6.1 and 721 cells using Cyh-1-specific antibodies 7H2 or 2D7 and (B) human PBMCs using monoclonal antibody 7H2 and an unspecific isotype control monoclonal antibody CAD9. Precipitated Cyh-1 was detected with monoclonal antibody 2D7. Co-precipitated CYTIP was detected with monoclonal antibody 2F9.
Thus, we demonstrate here on various levels that cytohesin-1 and CYTIP are bona fide interactors in several immune cell types, and under physiological conditions.
Association of CYTIP with the cell cortex is induced by Jurkat cell adhesion to integrin ligands
Cytohesin-1 plays a prominent role in β2 integrin-mediated cell adhesion (Kolanus et al., 1996). This function is regulated through its interaction with the β2 cytoplasmic domain, and via PH domain-dependent recruitment of the molecule to the plasma membrane (Kolanus et al., 1996). To begin assessing CYTIP function, we performed subcellular localization analyses of CYTIP using transfection assays and confocal laser scanning microscopy. The results in Figure 5 show that Ig-CYTIP is localized to the cytoplasm of cells that have been attached to poly-l-lysine (Figure 5A). However, a significant portion of CYTIP is recruited to the cell cortex following binding to integrin ligands [Figure 5B, fibronectin; Figure 5C, intercellular adhesion molecule 1 (ICAM-1) or vascular cell adhesion molecule (VCAM; not shown)]. Thus, CYTIP may be recruited to the plasma membrane of Jurkat cells in an integrin-dependent fashion.
Fig. 5. CYTIP is recruited to the cell cortex, following integrin-dependent cell adhesion. Jurkat cells were infected with a recombinant vaccinia virus coding for Ig-CYTIP, and subsequently were allowed to adhere to (A) poly-l-lysine, (B) fibronectin and (C) ICAM-1–Fc. The Ig fusion protein was detected with an FITC-labeled anti-Ig antibody and visualized by confocal laser scanning microscopy. Unspecific reactivity of the anti-Ig antibody was not detectable (data not shown).
Cortical localization of CYTIP requires a functional PDZ domain
We next investigated the molecular requirement for cortical localization. One possible way of recruitment was through the PDZ domain, a module known to be involved in clustering of plasma membrane receptors by interacting with their C-termini (Sheng and Sala, 2001). The carboxylate-binding pocket of PDZ domains is well conserved (Sheng and Sala, 2001) and it was therefore possible to identify residues that are likely to be involved in PDZ domain-dependent interactions. Mutant versions of CYTIP were generated and expressed, which contained point mutations in the respective residues (K82E, F90A and I92A). Ig fusion proteins of these mutants or wild-type CYTIP were expressed, and the subcellular localization of the variants was assessed by confocal microscopy. Figure 6 shows that the three PDZ domain mutants, although equally well expressed as an Ig fusion protein of wild-type CYTIP, lacked all capability of localizing to the cell cortex if placed on fibronectin, and likewise did not display any membrane association if cells were adherent to ICAM-1, VCAM or poly-l-lysine (not shown). Thus, integrin-dependent recruitment of CYTIP to the cell cortex absolutely requires an intact PDZ domain.
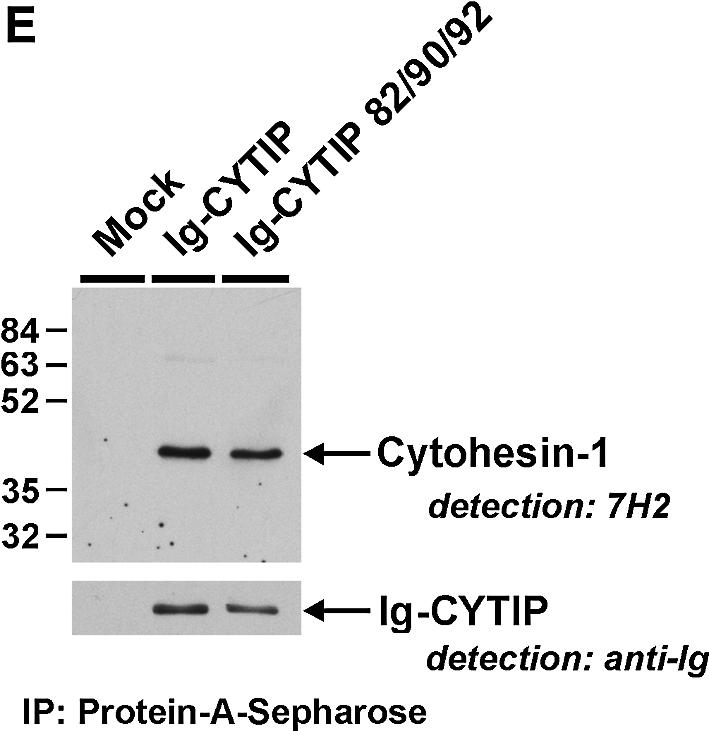
Fig. 6. The PDZ domain of CYTIP is required for cortical association of the protein in Jurkat cells. Cells had been infected with recombinant vaccinia virus, and were plated on fibronectin-coated microscope slides. Non-adherent cells were washed off with saline buffer. Cells expressing wild-type Ig-CYTIP chimera (A) display plasma membrane localization of CYTIP, while three independent PDZ domain mutants (B–D) lost their ability to interact with the membrane. Detection and analysis were performed as described in Figure 3 and in Materials and methods. (E) Upper panel: both wild-type Ig-CYTIP and Ig-CYTIP 82/90/92 (PDZ domain triple mutant) proteins interact with cytohesin-1. Recombinant vaccinia virus-derived Ig fusions were precipitated on protein A–Sepharose. Co-immunoprecipitated endogenous cytohesin-1 was recognized by anti-cytohesin-1 antibody 7H2. Lower panel: immuno blot analysis, showing comparable expression of Ig fusion proteins.
It was possible in principle that re-localization of Ig-CYTIP was mediated by endogenous cytohesin-1. However, our other findings would then suggest the PDZ domain also to play a role in the interaction of the molecules in cells. We therefore tested whether a PDZ domain triple mutant of CYTIP (Ig-CYTIP 82/90/92), in which all critical conserved residues were mutated, was affected in its ability to interact with cytohesin-1. Figure 6E shows that this was not the case. Therefore, PDZ domain-mediated recruitment of CYTIP is induced by a novel mechanism, possibly through as yet unknown, plasma membrane-localized ligands of CYTIP.
Phorbol ester treatment of Jurkat cells results in detachment of CYTIP from the cell cortex
We went on to probe CYTIP function in integrin affinity regulation, because cytohesin-1 had been shown to be important for the activity of β2 integrins in Jurkat cells. An important stimulus known to activate integrins is exerted by phorbol esters, proposed to occur through activation of members of the PKC family of protein kinases. We assessed subcellular localization of Ig-CYTIP in adherent Jurkat cells that had been treated with the phorbol ester phorbol 12-myristate 13-acetate (PMA) (Figure 7). Surprisingly, we discovered that the stimulus resulted in a profound alteration of CYTIP localization in cells that adhered through integrins. Cortical association of the molecule, detected in Jurkat cells adherent on fibronectin (Figure 7A), was completely abrogated in PMA-treated cells. Through co-localization of CYTIP with cellular F-actin, which in Jurkat cells is localized exclusively beneath the plasma membrane (Nagel et al., 1998a,b), we could demonstrate that phorbol ester triggered this re-localization in a quantitative fashion. Furthermore, we noticed that adhesion to ICAM-1 was markedly reduced in CYTIP-expressing cells that had been treated with PMA. Thus, phorbol ester treatment of Jurkat cells resulted in a re-localization of CYTIP to cytoplasmic compartments.
Fig. 7. Sequestration of CYTIP to the cytoplasm by PMA treatment. Jurkat cells were infected with a recombinant vaccinia virus expressing Ig-CYTIP, adhered to fibronectin and incubated with PMA as indicated. Ig-CYTIP is visualized with the help of an FITC-labeled anti-human IgG antibody (green). Cellular F-actin, which is associated with the plasma membrane, was detected by TRITC–phalloidin (red). Four independent samples are shown.
Membrane-associated cytohesin-1 re-localizes to the cytoplasm in phorbol ester-treated Jurkat cells expressing CYTIP
These findings prompted the investigation of a regulatory influence of CYTIP on cytohesin-1. To this end, we generated an enhanced green fluorescent protein (eGFP) fusion construct of CYTIP and first assessed the cellular localization of this molecule. Figure 8 and data not shown demonstrated that eGFP–CYTIP displayed a pattern of cortical or cytoplasmic localization identical to that of Ig-CYTIP used in the experiments above (Figure 8C and D). Co-expression of Ig-cytohesin-1 was then induced to investigate the localization of the exchange factor in the presence of CYTIP. As seen in the figure, membrane localization of cytohesin-1 alone was not altered by phorbol esters (Figure 8A2 and B2, respectively). However, in cells that co-expressed eGFP–CYTIP, cellular localization of cytohesin-1 was markedly shifted to the cytoplasm if cells were also treated with phorbol esters (Figure 8E and F). Thus, CYTIP appears responsible for re-localization of membrane-associated cytohesin-1 to the cytoplasm.
Fig. 8. Co-localization of CYTIP and cytohesin-1 in Jurkat cells. Cells were transfected by electroporation, using plasmids encoding either Ig-cytohesin-1 (A and B), eGFP–CYTIP (C and D) or both constructs (E and F). Equal amounts of total plasmid DNA were employed in all transfections. Following overnight incubation, cells were treated with PMA as indicated, and plated on fibronectin-coated microscope slides. For visualization of Ig-cytohesin-1, cells were subsequently permeabilized and incubated with a TRITC-labeled anti-Ig antibody. PMA treatment results in cytoplasmic localization of CYTIP (C1, 3; D1, 3). Importantly, a PMA-dependent shift of cytohesin-1 to the cytoplasm requires co-expression of CYTIP (B2, 3; F2, 3).
Phosphorylation of cytohesin-1 does not affect its interaction with CYTIP
We recently have described that cytohesin-1 is phosphorylated on C-terminal residues in phorbol ester-treated cells (Dierks et al., 2001). It has been demonstrated furthermore that this modification aids in association of cytohesin-1 with an insoluble cellular component, which is probably a component of the actin cytoskeleton (Dierks et al., 2001), but has no effect on membrane localization. It was therefore tempting to test whether phosphorylated cytohesin-1 was affected in its ability to interact with CYTIP. To this end, GST–cytohesin-1 was phosphorylated in vitro by incubation with PKCδ and ATP. Phosphorylation was monitored by electrophoretic mobility shift of the molecule, according to Dierks et al. (2001) (data not shown), and the phosphorylated form was probed for its ability to interact with the particular fraction, as described before (Dierks et al., 2001). However, as shown in Figure 9, interaction of GST–cytohesin-1 with Ig-CYTIP was not altered by PKC/ATP treatment. Thus, phosphorylation of cytohesin-1 appears at least not directly involved in the observed effects.

Fig. 9. Direct interaction of CYTIP and cytohesin-1 is not affected by phosphorylation of cytohesin-1. (A) Western blot analysis. Overexpressed Ig-CYTIP from Jurkat cells was complexed in vitro with phosphorylated GST–cytohesin-1 (+ATP/PKC lane) or unphos phorylated GST–cytohesin-1 (-ATP/PKC) in vitro. Protein-A-Seph., positive control, Ig-CYTIP has been immobilized directly on the Sepharose matrix; GSH-Seph., negative control, no GST–cytohesin-1 fusion protein was employed and, consequently, Ig-CYTIP was not detected. (B) Functional testing of the phosphorylation of cytohesin-1 in vitro, as described in the text, according to Dierks et al. (2001). GST–cytohesin-1 was incubated with cellular lysates from which nuclei and membranes had been depleted, and subsequently exposed to PKCδ and ATP as indicated. The normally soluble fusion protein (lane 1) associates with the insoluble cytoskeletal fraction (lane 4) in the presence of PKC and ATP in vitro. GST–Cyh-1 was detected by anti-cytohesin-1 antibody 7H2.
Jurkat cell adhesion to ICAM-1 is inhibited by CYTIP
Finally, we tested in a quantitative fashion whether β2 integrin-dependent adhesion of Jurkat cells was affected by CYTIP. The results are shown in Figure 10A. As described several times before, the controls show that Jurkat cell adhesion to ICAM-1 is stimulated by cytohesin-1 (Ig-cyh-1), and repressed by dominant-negative versions of cytohesin-1 (Ig-PHc, Ig-E157K) (Geiger et al., 2000). Importantly, expression of CYTIP also exerted a strong dominant inhibition of cell adhesion, which was absent if the ‘PDZ-null’ mutant Ig-CYTIP 82/90/92 or a construct lacking a coiled-coil domain was employed. The coiled-coil domain of CYTIP was also shown to be required for interaction with cytohesin-1 in co-immunoprecipitation assays (Figure 10C). Thus, our findings on the functional level were in full accordance with all results shown above. Detachment of the CYTIP–cytohesin-1 complex from the plasma membrane abrogated β2 integrin adhesion. Furthermore, this function requires intact PDZ and coiled-coil domains of CYTIP. It is important to note further that cell adhesion to ICAM-1 was inhibited specifically by CYTIP; no effect of the protein was seen on Jurkat adhesion to fibronectin (Figure 10B). This is in full accordance with our previous results, demonstrating a specific involvement of the cytohesin-1 signaling complex in β2 integrin-mediated adhesion events.

Fig. 10. Expression of CYTIP inhibits Jurkat cell adhesion to ICAM-1. Neither a triple PDZ domain mutant, CYTIP 82/90/92, nor a CYTIP mutant lacking the coiled-coil domain (Ig CYTIP Δ coiled-coil) have significant effects on β2 integrin activation. (A) Cytoplasmic Ig fusion constructs were expressed in Jurkat cells using recombinant vaccinia viruses, and stimulated for 30 min with PMA as indicated. Adhesion to ICAM-1–Fc was performed as described in Materials and methods. One experiment typical of eight independently performed experiments is shown. All employed viruses expressed the respective proteins at equal density (data not shown). (B) Analysis identical to that shown in (A), except that fibronectin was used as adhesion substrate. No effect on Jurkat cell adhesion to fibronectin was observed in this experiment. (C) Ig-CYTIP Δ coiled-coil (Δcc), Ig-CYTIP and Ig-control were overexpressed in Jurkat E6.1 cells, precipitated on protein A–Sepharose and detected by western blotting using horseradish peroxidase-conjugated anti-human IgG antibody. Co-precipitated endogenous Cyh-1 was detected with monoclonal antibody 2D7. Levels of endogenous Cyh-1 were examined by western blotting of total lysates before protein A precipitation.
Discussion
Membrane recruitment of cytoplasmic proteins for the purpose of signal transduction is a well established and thoroughly studied concept. Attenuation and downregulation of these responses is the other, equally important, face of the coin. In order to fine-tune their responses to biological stimuli, cells must be capable of specifically abrogating signaling events in progress. Protein- or lipid-phosphatases are the important general counteractors of kinase-driven responses (Hunter, 2000; Hermiston et al., 2002). An interesting specific example of negative signal attenuation has been reported on the function of the calcium flux effector enzyme phospholipase Cδ (PLCδ) which is membrane localized through a PH domain-mediated binding of PIP2. PLCδ-catalyzed PIP2 breakdown results in the generation of inositol-1,4,5-trisphosphate (IP3), which acts as a competitive inhibitor of PIP2. IP3 can therefore displace PLCδ from the membrane and attenuates the response through a negative feedback mechanism (Ferguson et al., 1995; Lemmon et al., 1996).
In this study, we describe a novel mechanism of interfering with a signal flow that depends on membrane recruitment of a critical component. A protein (CYTIP) that interacts with cytohesin-1 was identified. CYTIP displays an interesting distribution of mRNA expression. It is expressed predominantly in cells of the hematopoietic system, and we find that it is strongly upregulated during cytokine-dependent maturation of DCs. CYTIP was found to be almost identical to the previously described B3-1 mRNA that has been shown to be highly expressed in cytotoxic T-cell clones (Dixon et al., 1993). Furthermore, a protein termed Cybr, which corresponds to the GenBank database entry of CYTIP, was described recently to be upregulated by cytokines in several hematopoietic cell types (Tang et al., 2002). This intriguing pattern of regulation suggests a prominent role for the protein in cell activation and differentiation events in the immune system. CYTIP has a close relative, the recently described protein GRASP which interacts with cytohesin-3 (grp1). GRASP mRNA was detected in many cell types (Nevrivy et al., 2000); however, its expression is also strongly regulated, namely by retinoic acid (Nevrivy et al., 2000). Interestingly, in the course of retinoic acid-dependent differentiation of HL60 cells to neutrophil granulocytes, cytohesin-1 was found to be upregulated prominently (Garceau et al., 2001; C.Geiger and W.Kolanus, unpublished observations).
The interaction of CYTIP with cytohesin-1 prompted us to investigate a possible role for the protein in cell adhesion regulation. Since the expression profile of CYTIP also encompasses lymphocytes, we attempted a functional analysis in Jurkat cells. The major findings of these studies may be summarized as follows: (i) cellular localization of CYTIP is regulated by leukocyte integrins; (ii) membrane attachment of the protein is reversible, and re-localization of CYTIP to the cytoplasm was found to be inducible by phorbol ester treatment; (iii) membrane localization of CYTIP requires an intact PDZ domain; (iv) co-expression of CYTIP results in co-localization of cytohesin-1 and CYTIP to the cytoplasm; and (v) overexpression of CYTIP results in dominant inhibition of β2 integrin-mediated adhesion of phorbol ester-treated cells. From these findings, a novel mechanism of interference with eukaryotic signal transduction events may be postulated, namely the sequestration of a signaling protein from its active compartment through specific protein–protein interactions. These findings, furthermore, underscore the previously postulated importance of membrane association of cytohesin-1 (Nagel et al., 1998a) for the maintenance of β2 integrin binding to ICAM-1.
The fact that CYTIP and cytohesin-1 are upregulated during maturation of DCs and by cytokines in other hematopoietic cell types suggests a strong physiological significance of the regulation at cell level in the model system described here. DCs constantly monitor the organism for foreign invasion by sampling antigens. During an inflammation, these cells are mobilized and migrate to lymph nodes in order to present these antigens to recirculating T cells (Lipscomb and Masten, 2002). This mobilization is an important aspect of the ‘maturation’/differentiation process, besides the well-studied de novo expression of co-stimulatory molecules, which may directly aid in T-cell activation. We have thus found that DCs upregulate proteins that can either activate or repress β2 integrin-dependent adhesion. This suggests an important requirement of DCs for dynamic and flexible interaction with the extracellular milieu in the course of their travel to lymphatic organs.
This very notion also suggests that the interaction of cytohesin-1 and CYTIP inside the cell is not constitutive but may also be regulated. We have no direct evidence for this, but a number of observations support this hypothesis. Overexpression of CYTIP does not induce adhesion, which suggests that it does not support membrane association of cytohesin-1. This is further strengthened by the finding that CYTIP only associates with the plasma membrane in cells that adhere to integrin ligands. Thirdly, membrane association of CYTIP strictly requires a functional PDZ domain, so it is therefore unlikely that CYTIP is co-recruited through the PH domain of cytohesin-1. Thus, CYTIP appears to interact with the plasma membrane through independent routes where it might remain poised for interaction with cytohesin-1. Future studies using probes capable of detecting both cytohesin-1 and CYTIP in tissues during an immune response should help in further elucidating and elaborating this novel pathway.
Materials and methods
Generation of monocyte-derived DCs
Human DCs were prepared from PBMCs essentially as described (Romani et al., 1996). Briefly, 2 × 106 cells/well were plated in 6-well tissue culture plates in 3 ml of complete culture medium (RPMI-1640; PAA Laboratories GmbH, Linz, Austria), supplemented with 50 µg/ml gentamycin, 10% fetal calf serum (FCS; Biological Industries or Seromed Biochrom, Berlin, Germany) containing 800 U/ml granulocyte– macrophage colony-stimulating factor (GM-CSF; Leukomax™, sp. act. 1.1 × 106 U/mg; Novartis, Basel, Switzerland) and 1000 U/ml IL-4. Culture medium was renewed every other day by removing 1 ml of the medium and adding back 1.5 ml of fresh medium containing 1600 U/ml GM-CSF and 1000 U/ml IL-4. On day 7, non-adherent cells were harvested and analyzed or fed again in the 6-well plates for a further culture period of 2 or 3 days in the presence of 1.5 ml per well of monocyte-conditioned medium, supplemented with 20 ng/ml TNF-α (sp. act. 6 × 107 U/mg, a generous gift of Dr G.R.Adolf, Bender, Vienna, Austria) to generate mature DCs.
Differential display of PCR-amplified cDNAs
Differential display was performed according to Liang and Pardee (1998) with slight modifications. A 2 µg aliquot of total RNA was reverse transcribed in a total volume of 20 µl using an anchored (T12MG) primer. The same primer in combination with a random primer (GATCGAATGGA) was used to perform the differential display PCR. The amplification (40 cycles, annealing temperature 40°C) was carried out in a Perkin Elmer Thermocycler (GeneAmp PCR System 9600, Norwalk, CT). PCR products were analyzed on a 6% polyacrylamide gel under standard conditions, and detected by autoradiography. Differentially expressed fragments were recovered from the gel and reamplified with the same PCR primers and conditions (30 cycles) as described above. A 1 µl aliquot of the re-amplification PCR was used for cloning vector ligation.
cDNA library construction
A day 10 (mature) human DC cDNA library was constructed using 5 µg of poly(A)+ RNA according to the ZAP-express cDNA synthesis kit and ZAP express cDNA Gigapack III Gold Cloning Kit (Stratagene).
Isolation of a CYTIP full-length clone
A day 10 DC cDNA library was screened for a CYTIP full-length clone using the isolated re-amplified PCR fragment identified with differential display of PCR-amplified mRNA, which primes in the C-terminus of CYTIP, and a second PCR-amplified probe (primer pair: 5′-GATTC AAATGCTAGCAGACAC-3′ and 5′-GTCCACACCATTGATATTT GC-3′) priming more N-terminally. We screened 106 p.f.u. from the day 10 cDNA library using standard hybridization techniques. After three rounds of plaque purification in vivo, excision was performed according to the manufacturer’s protocol. The obtained plasmids were examined by restriction enzyme analyses and sequenced.
Yeast two-hybrid screening
To identify binding partners of CYTIP, a full-length clone was used to screen a lymph node cDNA library (Clontech, Palo Alto, CA) with the GAL4-based Matchmaker Two-hybrid system 2 (Clontech, Palo Alto, CA) according to the manufacturer’s protocol. A total of 2.6 × 106 transformants were screened; positive colonies were verified by restreaking.
To determine a more restricted region of CYTIP for protein–protein interaction, the two putative binding domains, the leucine-rich region and the PDZ domain, were amplified separately by PCR using the following primers: 5′-GAATTCGAGACTCTTAATGGAACAATGATTCT-3′ as sense primer and 5′-TCGGATCGGCTGGGCCTGGCCC-3′ as antisense primer for the leucine-rich region, and 5′-CTGAATTCAAGCTTG TTACTGTGGAGAAGC-3′ as sense primer and 5′-TGGGATCCTG TCCATTAAGAGTCTCTATCGTTAG-3′ as antisense primer for the PDZ domain. The PCR products were cloned into the EcoRI and BamHI sites of vector pGBT9. These bait constructs were co-transformed into yeast strain CG-1945 together with cytohesin-1 constructs in order to identify the interacting domains.
Quantitative PCR analysis was performed using real-time PCR (Abi Prism 7700 sequence detector, Applied Biosystems, Vienna, Austria). Sequences for probes (FAM label) and primers (synthesized by Microsynth) specific for cytohesin-1 were selected using the Primer Express software (Applied Biosystems): 5′-GGAGCTGCATGAGTT CACTGATC-3′ (sense), 5′-GGGTAGCCGGAAGCTCCA-3′ (antisense), 5′-ATCTCGTCCAGGCACTACGGCAGTTC-3′ (probe).
For PCR, the TaqMan PCR master mix from Applied Biosystems was used. Random primed cDNAs were prepared (Superscript II RNase H– reverse transcriptase, Life Technologies, Vienna, Austria) from total RNA isolated from immature and mature DCs.
Generation of rat monoclonal antibodies that recognize CYTIP
Rat monoclonal antibodies were raised against His6 fusion proteins of CYTIP subdomains, exactly as described previously (Geiger et al., 2000).
Co-immunoprecipitation of Ig-CYTIP fusion protein with cytohesin-1
A PCR product of a CYTIP cDNA was inserted into the MluI and NotI sites of an intracellular immunoglobulin fusion cassette based on the pRK5 vector as described by Geiger et al. (2000). The resulting genetic fusion yielded a chimera in which the CYTIP portion is attached to the C-terminus of the leaderless CH2 and CH3 domains encoding sequences of human IgG1 for convenient cytoplasmic expression and detection (Geiger et al., 2000).
The Ig-CYTIP chimera or control construct was then introduced into Jurkat cells using standard electroporation techniques. Following overnight incubation in complete medium [RPMI/10% fetal bovine serum (FBS)], the cells were lysed in buffer containing 100 mM Tris–HCl pH 7.5, 150 mM NaCl, 2 mM EDTA, 1 mM phenylmethylsulfonyl fluoride (PMSF) and 1% (v/w) Triton X-100. Ig fusion proteins subsequently were bound to protein A–Sepharose (6MB; Pharmacia, San Diego, CA) and washed repeatedly with lysis buffer. Co-immunoprecipitated cytohesin-1 was detected by standard western blot analysis following SDS–PAGE. Specific detection was facilitated through the use of monoclonal antibody 7H2, which recognizes human cytohesin-1 exclusively, and a peroxidase-conjugated affinity-purified anti-rat IgG preparation (Jackson ImmunoResearch, West Grove, PA).
Adhesion assay
Jurkat E6 cells (2 × 106) were infected with recombinant vaccinia viruses and incubated for 5 h at 37°C. After centrifugation, cells were resuspended in RPMI medium, labeled with 12 µg/ml bisbenzimide H33342 fluorochrome trihydrochloride (Calbiochem) and incubated for 30 min at 37°C with or without the addition of 40 ng/ml PMA. After centrifugation and resuspension in Hanks’ buffered saline solution (HBSS), the cells were delivered to 96-well plates (Nunc, Maxisorp) at 1.5 × 105 cells/well. Prior to adhesion, plates were coated with anti-human IgG (Fcγ-specific) antibody at 0.85 µg/well for 90 min at 25°C, blocked with 1% (w/v) bovine serum albumin (BSA) in phosphate-buffered saline (PBS), and incubated for 2 h at 25°C with culture supernatants from CV-1 cells expressing soluble ICAM-1–Rg fusion protein. Cells were allowed to adhere for 1 h at 37°C; unbound cells were washed off carefully with 3 × 200 µl of HBSS. Bound cells were assayed in 100 µl of 2% (v/v) formaldehyde in PBS using a fluorescence plate reader (Cytofluor II; PerSeptive). The signal of 1.5 × 105 cells/well at 490 nm corresponds to 100% adhesion. The results represent an average of at least four independent experiments carried out in quadruplicate for each value.
Confocal laser scanning microscopy
Microscope slides were pre-incubated with anti-human IgG (Fcγ-specific; Jackson ImmunoResearch) antibody at 1:100 dilution in 50 mM Tris pH 9.5 for 1.5 h at room temperature. Subsequently, the slides were blocked with 1% (w/v) BSA in PBS for 1 h and incubated with culture supernatants from CV-1 cells expressing ICAM-1–Fc fusion protein for 2 h at room temperature. Microscope slides (Marienfeld, Germany) were incubated directly with 100 ng/µl fibronectin (Sigma, Germany) in PBS for 2 h at room temperature, and blocked with 1% (w/v) BSA in PBS for 1 h. Poly-l-lysine-coated coverslips were purchased from Marienfeld (Germany). Jurkat cells (2 × 106) were infected with recombinant vaccinia viruses, incubated for 6 h at 37°C or electroporated (240 V/1200 µF; EasyJect Plus D2000, Searing, Belgium) with 20 µg of plasmid DNA in 400 µl of RPMI-medium + 30% FCS, and incubated for 16 h at 37°C. The cells were delivered and stimulated with or without the addition of 40 ng/ml PMA. After centrifugation and resuspension in HBSS, the cells were placed on the prepared slides (see above) for 30 min at 37°C. Non-adherent cells were washed off with HBSS, and adherent cells were fixed and immobilized with freshly prepared 2% (w/v) paraformaldehyde in PBS for 15 min. Subsequently, cells were blocked with 2% glycine in PBS, permeabilized for 10 min with 0.2% (v/v) Triton X-100 in PBS, and incubated with a fluorescein isothiocyanate (FITC)-labeled goat anti-human (Fcγ-specific) antibody (Dianova) or a tetramethylrhodamine isothiocyanate (TRITC)-labeled goat anti-human (Fcγ-specific) antibody (Dianova) in PBS for 2 h at room temperature. After a final wash with PBS, slides were mounted with mounting medium (Vector Laboratories, Burlingame, CA) and samples were examined by laser scanning microscopy (Leica TCS-NT systems, Leica). Confocal images were collected as 512 × 512 pixeled files and processed with the help of the Photoshop program (Adobe).
Constructs, expression plasmids
PCR products were derived from existing expression plasmids and inserted into the MluI and NotI sites of the vaccinia expression vector pcIgTkg, which encodes cytoplasmic Ig fusion proteins as described (Kolanus et al., 1996). Specifically, the following primers were used: GCCGCGACGCGTGCCACCATGTCTTTACAAAGGCTCCTG and GCCGCTGCGGCCGCTCAAAAGCGACTTTCTTCCTC (CYTIP). Mutants were generated by PCR from expression plasmids by using the primers: GTTACTGTGGAGGAGCAGGATAATG and CATTATCC TGCTCCTCCACAGTAAC (CYTIP K82E); GAAACATTTGGAGC TGAAATTCAG and CTGAATTTCAGCTCCAAATGTTTC (CYTIP F90A); GGATTTGAAGCTCAGTCTTACAGG and CCTGTAAGAC TGAGCTTCAAATCC (CYTIP I92A).
For eGFP expression, the constructs were subcloned into the expression vector pEGFP N1 (Clontech).
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We thank members of the labs for discussion and advice. This work was supported by the Deutsche Forschungsgemeinschaft (DFG, SFB 455 and SFB 576) and the Austrian Science Fund (FWF) project P13794-Med.
References
- Chardin P., Paris,S., Antonny,B., Robineau,S., Beraud-Dufour,S., Jackson,C.L. and Chabre,M. (1996) A human exchange factor for ARF contains Sec7- and pleckstrin-homology domains. Nature, 384, 481–484. [DOI] [PubMed] [Google Scholar]
- Diamond M.S. and Springer,T.A. (1994) The dynamic regulation of integrin adhesiveness. Curr. Biol., 4, 506–517. [DOI] [PubMed] [Google Scholar]
- Dierks H., Kolanus,J. and Kolanus,W. (2001) Actin cytoskeletal association of cytohesin-1 is regulated by specific phosphorylation of its carboxyl-terminal polybasic domain. J. Biol. Chem., 276, 37472–37481. [DOI] [PubMed] [Google Scholar]
- Dixon B., Sahely,B., Liu,L. and Pohajdak,B. (1993) Cloning a cDNA from human NK/T cells which codes for an unusual leucine zipper containing protein. Biochim. Biophys. Acta, 1216, 321–324. [DOI] [PubMed] [Google Scholar]
- D’Souza-Schorey C., Boshans,R.L., McDonough,M., Stahl,P.D. and Van Aelst,L. (1997) A role for POR1, a Rac1-interacting protein, in ARF6-mediated cytoskeletal rearrangements. EMBO J., 16, 5445–5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning A.S. and Anderson,J.M. (1999) PDZ domains: fundamental building blocks in the organization of protein complexes at the plasma membrane. J. Clin. Invest., 103, 767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson K.M., Lemmon,M.A., Schlessinger,J. and Sigler,P.B. (1995) Structure of the high affinity complex of inositol trisphosphate with a phospholipase C pleckstrin homology domain. Cell, 83, 1037–1046. [DOI] [PubMed] [Google Scholar]
- Garceau V., Houle,M.G., Chouinard,F., Gagnon,S., Harbour,D., Naccache,P.H. and Bourgoin,S.G. (2001) Characterization of cytohesin-1 monoclonal antibodies: expression in neutrophils and during granulocytic maturation of HL-60 cells. J. Immunol. Methods, 249, 121–136. [DOI] [PubMed] [Google Scholar]
- Geiger C. et al. (2000) Cytohesin-1 regulates β-2 integrin-mediated adhesion through both ARF-GEF function and interaction with LFA-1. EMBO J., 19, 2525–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng L. and Rudd,C.E. (2002) Signalling scaffolds and adaptors in T-cell immunity. Br. J. Haematol., 116, 19–27. [DOI] [PubMed] [Google Scholar]
- Germain R.N. (2001) The T cell receptor for antigen: signaling and ligand discrimination. J. Biol. Chem., 276, 35223–35226. [DOI] [PubMed] [Google Scholar]
- Hermiston M.L., Xu,Z., Majeti,R. and Weiss,A. (2002) Reciprocal regulation of lymphocyte activation by tyrosine kinases and phosphatases. J. Clin. Invest., 109, 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. (1998) The role of tyrosine phosphorylation in cell growth and disease. Harvey Lect., 94, 81–119. [PubMed] [Google Scholar]
- Hunter T. (2000) Signaling—2000 and beyond. Cell, 100, 113–127. [DOI] [PubMed] [Google Scholar]
- Hynes R.O. (1992) Integrins: versatility, modulation and signaling in cell adhesion. Cell, 69, 11–25. [DOI] [PubMed] [Google Scholar]
- Hynes R.O. (1999) Cell adhesion: old and new questions. Trends Cell Biol., 9, M33–M37. [PubMed] [Google Scholar]
- Klarlund J.K., Guilherme,A., Holik,J.J., Virbasius,J.V., Chawla,A. and Czech,M.P. (1997) Signaling by phosphoinositide-3,4,5-trisphosphate through proteins containing pleckstrin and Sec7 homology domains. Science, 275, 1927–1930. [DOI] [PubMed] [Google Scholar]
- Kliche S., Nagel,W., Kremmer,E., Atzler,C., Ege,A., Knorr,T., Koszinowski,U., Kolanus,W. and Haas,J. (2001) Signaling by human herpesvirus 8 kaposin A through direct membrane recruitment of cytohesin-1. Mol. Cell, 7, 833–843. [DOI] [PubMed] [Google Scholar]
- Kolanus W. and Zeitlmann,L. (1998) Regulation of integrin function by inside-out signaling mechanisms. Curr. Top. Microbiol. Immunol., 231, 33–49. [DOI] [PubMed] [Google Scholar]
- Kolanus W., Nagel,W., Schiller,B., Zeitlmann,L., Godar,S., Stockinger,H. and Seed,B. (1996) αLβ2 integrin/LFA-1 binding to ICAM-1 induced by cytohesin-1, a cytoplasmic regulatory molecule. Cell, 86, 233–242. [DOI] [PubMed] [Google Scholar]
- Lemmon M.A., Ferguson,K.M. and Schlessinger,J. (1996) PH domains: diverse sequences with a common fold recruit signaling molecules to the cell surface. Cell, 85, 621–624. [DOI] [PubMed] [Google Scholar]
- Leo A., Wienands,J., Baier,G., Horejsi,V. and Schraven,B. (2002) Adapters in lymphocyte signaling. J. Clin. Invest., 109, 301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard W.J. (2001) Role of Jak kinases and STATs in cytokine signal transduction. Int. J. Hematol., 73, 271–277. [DOI] [PubMed] [Google Scholar]
- Liang P. and Pardee,A.B. (1998) Differential display. A general protocol. Mol. Biotechnol., 10, 261–267. [DOI] [PubMed] [Google Scholar]
- Lipscomb M.F. and Masten,B.J. (2002) Dendritic cells: immune regulators in health and disease. Physiol. Rev., 82, 97–130. [DOI] [PubMed] [Google Scholar]
- Liu L. and Pohajdak,B. (1992) Cloning and sequencing of a human cDNA from cytolytic NK/T cells with homology to yeast SEC7. Biochim. Biophys. Acta, 1132, 75–78. [DOI] [PubMed] [Google Scholar]
- Maffucci T. and Falasca,M. (2001) Specificity in pleckstrin homology (PH) domain membrane targeting: a role for a phosphoinositide–protein co-operative mechanism. FEBS Lett., 506, 173–179. [DOI] [PubMed] [Google Scholar]
- Mayer G., Blind,M., Nagel,W., Bohm,T., Knorr,T., Jackson,C.L., Kolanus,W. and Famulok,M. (2001) Controlling small guanine-nucleotide-exchange factor function through cytoplasmic RNA intramers. Proc. Natl Acad. Sci. USA, 98, 4961–4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel W., Schilcher,P., Zeitlmann,L. and Kolanus,W. (1998a) The PH domain and the polybasic c domain of cytohesin-1 cooperate specifically in plasma membrane association and cellular function. Mol. Biol. Cell, 9, 1981–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel W., Zeitlmann,L., Schilcher,P., Geiger,C., Kolanus,J. and Kolanus,W. (1998b) Phosphoinositide 3-OH kinase activates the β2 integrin adhesion pathway and induces membrane recruitment of cytohesin-1. J. Biol. Chem., 273, 14853–14861. [DOI] [PubMed] [Google Scholar]
- Nevrivy D.J., Peterson,V.J., Avram,D., Ishmael,J.E., Hansen,S.G., Dowell,P., Hruby,D.E., Dawson,M.I. and Leid,M. (2000) Interaction of GRASP, a protein encoded by a novel retinoic acid-induced gene, with members of the cytohesin family of guanine nucleotide exchange factors. J. Biol. Chem., 275, 16827–16836. [DOI] [PubMed] [Google Scholar]
- Palacios F., Price,L., Schweitzer,J., Collard,J.G. and D’Souza-Schorey,C. (2001) An essential role for ARF6-regulated membrane traffic in adherens junction turnover and epithelial cell migration. EMBO J., 20, 4973–4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson T., Gish,G.D. and Nash,P. (2001) SH2 domains, interaction modules and cellular wiring. Trends Cell Biol., 11, 504–511. [DOI] [PubMed] [Google Scholar]
- Pawson T., Raina,M. and Nash,P. (2002) Interaction domains: from simple binding events to complex cellular behavior. FEBS Lett., 513, 2–10. [DOI] [PubMed] [Google Scholar]
- Reth M. (2001) Oligomeric antigen receptors: a new view on signaling for the selection of lymphocytes. Trends Immunol., 22, 356–360. [DOI] [PubMed] [Google Scholar]
- Romani N., Reider,D., Heuer,M., Ebner,S., Kampgen,E., Eibl,B., Niederwieser,D. and Schuler,G. (1996) Generation of mature dendritic cells from human blood. An improved method with special regard to clinical applicability. J. Immunol. Methods, 196, 137–151. [DOI] [PubMed] [Google Scholar]
- Santy L.C. and Casanova,J.E. (2001) Activation of ARF6 by ARNO stimulates epithelial cell migration through downstream activation of both Rac1 and phospholipase D. J. Cell Biol., 154, 599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M. and Sala,C. (2001) PDZ domains and the organization of supramolecular complexes. Annu. Rev. Neurosci., 24, 1–29. [DOI] [PubMed] [Google Scholar]
- Tang P. et al. (2002) Cybr, a cytokine-inducible protein that binds cytohesin-1 and regulates its activity. Proc. Natl Acad. Sci. USA, 99, 2625–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kooyk Y. and Figdor,C.G. (2000) Avidity regulation of integrins: the driving force in leukocyte adhesion. Curr. Opin. Cell Biol., 12, 542–547. [DOI] [PubMed] [Google Scholar]
- Venkateswarlu K., Oatey,P.B., Tavare,J.M. and Cullen,P.J. (1998) Insulin-dependent translocation of ARNO to the plasma membrane of adipocytes requires phosphatidylinositol 3-kinase. Curr. Biol., 8, 463–466. [DOI] [PubMed] [Google Scholar]
- Weber K.S., Weber,C., Ostermann,G., Dierks,H., Nagel,W. and Kolanus,W. (2001) Cytohesin-1 is a dynamic regulator of distinct LFA-1 functions in leukocyte arrest and transmigration triggered by chemokines. Curr. Biol., 11, 1969–1974. [DOI] [PubMed] [Google Scholar]
- Woodside D.G., Liu,S. and Ginsberg,M.H. (2001) Integrin activation. Thromb. Haemost., 86, 316–323. [PubMed] [Google Scholar]











