Abstract
The adenomatous polyposis coli (APC) protein is inactivated in most colorectal tumours. APC loss is an early event in tumorigenesis, and causes an increase of nuclear β-catenin and its transcriptional activity. This is thought to be the driving force for tumour progression. APC shuttles in and out of the nucleus, but the functional significance of this has been controversial. Here, we show that APC truncations are nuclear in colorectal cancer cells and adenocarcinomas, and this correlates with loss of centrally located nuclear export signals. These signals confer efficient nuclear export as measured directly by fluorescence loss in photobleaching (FLIP), and they are critical for the function of APC in reducing the transcriptional activity of β-catenin in complementation assays of APC mutant colorectal cancer cells. Importantly, targeting a functional APC construct to the nucleus causes a striking nuclear accumulation of β-catenin without changing its transcriptional activity. Our evidence indicates that the rate of nuclear export of APC, rather than its nuclear import or steady-state levels, determines the transcriptional activity of β-catenin.
Keywords: adenomatous polyposis coli/β-catenin/colorectal carcinomas/FLIP/nuclear export
Introduction
The majority of colorectal cancers have inactivating mutations in the adenomatous polyposis coli (APC) tumour suppressor (Kinzler and Vogelstein, 1996). Typically, these mutations produce protein truncations (‘APC cancer truncations’), and most of the somatic mutations map to the mutation cluster region (MCR) in the middle of the coding region (Nagase and Nakamura, 1993). Thus, there is a strong selection in cancer cells against the C-terminal sequences of APC, suggesting that the C-terminal half of APC harbours its tumour suppressor function(s). In addition, there may also be a selection to retain sequences upstream of the MCR during tumorigenesis (Fodde et al., 2001).
APC promotes the destabilization of β-catenin, a key Wnt signalling effector, by binding to the cytoplasmic Axin complex, which also contains glycogen synthase kinase-3β and casein kinase I (Polakis, 2000; Bienz, 2002). APC mutant cells show high levels of cytoplasmic and nuclear β-catenin; the latter binds to T-cell factor (TCF) and activates transcription of TCF target genes, which appears to drive tumorigenesis (van de Wetering et al., 2002). The Axin-binding sites in APC are located downstream of the MCR (Behrens et al., 1998) and are thus absent in almost all APC cancer truncations, arguing that the ability of APC to bind to Axin is critical for its tumour suppressor function. This notion is strongly supported by experimental evidence (Smits et al., 1999; von Kries et al., 2000; Rubinfeld et al., 2001).
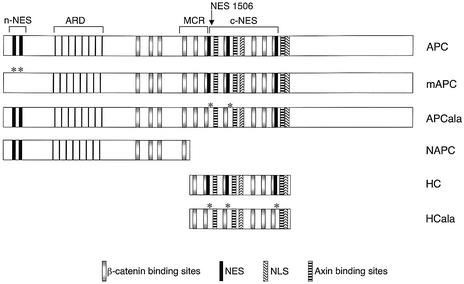
Recently, multiple nuclear export signals (NESs) have been discovered in the N-terminus (n-NESs) and in the central domain (c-NESs) of APC (Rosin-Arbesfeld et al., 2000; Henderson, 2000; Neufeld et al., 2000a,b) (Figure 1). These confer leptomycin B (LMB)-sensitive export from the nucleus, and evidence suggested that the nuclear export function of APC may be critical for the downregulation of nuclear β-catenin and of TCF-mediated transcription. The best conserved NESs are the c-NESs that are located immediately downstream of the MCR (Figure 1), and thus appear to be selected against in cancers. This suggested that the ability of APC to exit from the nucleus may be important for its tumour suppressor function (Rosin-Arbesfeld et al., 2000).
Fig. 1. Nuclear export and import signals in APC. Top: structure of APC (drawn roughly to scale), with NESs and NLSs and binding motifs for β-catenin (15Rs, 20Rs) and for Axin indicated; ARD, armadillo repeat domain; MCR, mutation cluster region in which most type I truncations end; type II truncations end upstream of the first Axin-binding motif, but retain NES1506. Below: APC constructs used in this study; asterisks indicate alanine substitutions that inactivate the corresponding NESs in the mutants.
However, various observations have challenged this notion (Bienz, 2002; Henderson and Fagotto, 2002). First, the n-NESs are retained in APC cancer truncations (Figure 1) yet they are more active than the c-NESs in minimal reporter constructs (Galea et al., 2001). Indeed, the APC truncation in the commonly used line SW480 cells retains some LMB-sensitive nuclear export activity (Henderson, 2000). Secondly, while the APC truncation in SW480 cells was observed in the nucleus by immunofluorescence (Rosin-Arbesfeld et al., 2000), cell fractionation has indicated that APC truncations may be entirely cytoplasmic (Smith et al., 1993; Neufeld and White, 1997; Galea et al., 2001). Thirdly, overexpression of truncated APC that lacks the c-NESs apparently reduced the nuclear β-catenin in transfected SW480 cells, while full-length APC with mutated n-NESs did not (Henderson, 2000; Neufeld et al., 2000b). Finally, β-catenin can exit from the nucleus by an LMB-insensitive mechanism that does not require an NES (Yokoya et al., 1999; Eleftheriou et al., 2001; Wiechens and Fagotto, 2001), so the nuclear export of β-catenin does not appear to depend on APC.
We thus sought to clarify whether, and how, the nuclear export function of APC controls β-catenin. We compared the subcellular locations and functions of endogenous wild-type and mutant APC in a range of colorectal cancer cells. Furthermore, we manipulated the nuclear export or import activity of APC constructs, and tested their function in reducing nuclear β-catenin and TCF-mediated transcription in APC mutant cancer cells. Finally, we performed FLIP (fluorescence loss in photobleaching) experiments to measure directly the nuclear export activity of APC constructs tagged with green fluorescent protein (GFP). Our results show that high steady-state levels of nuclear APC can cause nuclear accumulation of β-catenin without affecting its transcriptional activity. They indicate that the nuclear export rate of APC, rather than its nuclear import or steady-state levels, determines the function of β-catenin in transcription.
Results
APC truncations are nuclear in most colorectal cancer cells
Most colorectal cancer cell lines express APC truncations that retain the n-NESs but lack the c-NESs (‘type I truncations’; Figure 1). Type I truncations are also by far the most common type among sporadic colorectal tumours (e.g. Miyaki et al., 1994). However, some lines express APC truncations that retain the n-NES as well as the 5′ most c-NES (NES1506) (‘type II truncations’; Figure 1). Interestingly, this type is over-represented among the cell lines derived from colorectal tumours (nearly 20%; Rowan et al., 2000) since only a few percent of all sporadic colorectal tumours have mutations of this type (Rosin-Arbesfeld et al., 2000), suggesting that type II truncations may be more successful than type I truncations during cell line derivation. Even fewer cancers have truncating mutations downstream of the 5′ most Axin-binding site at codon 1572 (Nagase and Nakamura, 1993; for discussion of these mutations see Supplementary data available at The EMBO Journal Online). Finally, some colorectal cancer cell lines express wild-type APC, but carry activating mutations in β-catenin (Rowan et al., 2000).
We examined the nuclear–cytoplasmic distribution of endogenous APC in different colorectal cancer cell lines by immunofluorescence. To avoid bias due to genetic background, we included multiple lines of each type in our analysis. We used a highly specific antiserum against APC (anti-M-APC; Näthke et al., 1996) that does not show significant cross-reactivity in APC-null mutant cells (Rosin-Arbesfeld et al., 2001; Mogensen et al., 2002). This antibody produces only background staining of COLO320 cells (Figure 2A), which express high levels of a short APC truncation (Figure 4B) that does not overlap the central third of APC against which the antiserum was raised. We co-stained all cells with antibody against β-catenin, or lamin to mark their nuclear envelopes.
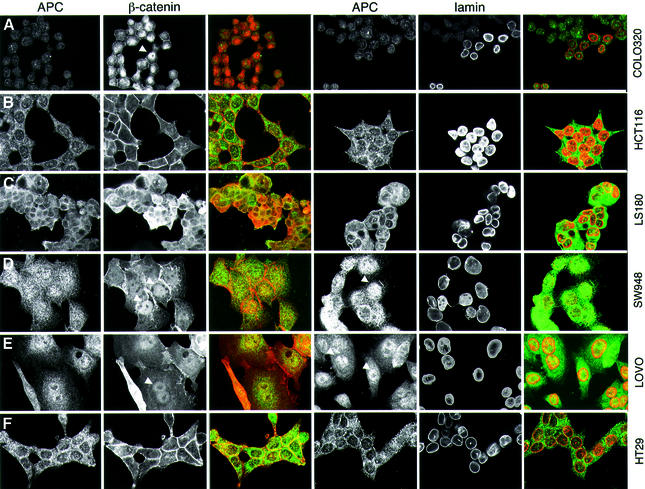
Fig. 2. Subcellular distribution of APC in colorectal cancer cell lines. Confocal sections through colorectal cancer cells from lines with a wild-type (B and C) or truncated APC (A and D–F), stained with anti-M-APC and anti-β-catenin as indicated; staining against lamin was used to mark the nuclear envelopes (merges: green, APC; red, β-catenin or lamin). Arrowheads indicate nuclear APC or β-catenin. APC is nuclear in cells with APC type I truncations (D and E), but excluded from nuclei in cells with wild-type APC (B and C) or with APC type II truncations that retain NES1506 (F). Note that the short type I truncation of COLO320 cells is not recognized by anti-M-APC (see also Figure 4B).
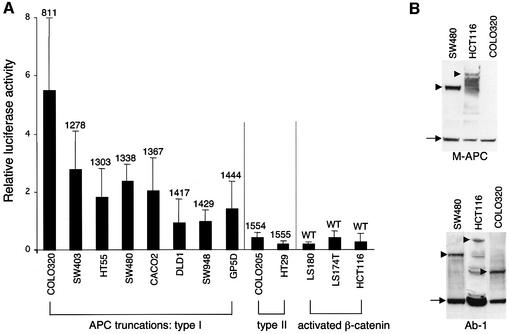
Fig. 4. Comparative TOPFLASH assays in colorectal cancer cell lines. (A) TCF-mediated transcription in colorectal cancer cell lines expressing wild-type or truncated APC, as indicated (codons of truncations are given above the bars); given are relative luciferase:Renilla values averaged from 3–6 independent experiments (error bars mark SDs; the Renilla values were obtained with pRL-CMV as an internal control, but essentially the same comparative values were obtained with pRL-TK and pRL-SV40). The TOPFLASH values are significantly lower in cell lines with activated β-catenin/wild-type APC or with APC type II truncations (retaining NES1506) compared with those with APC type I truncations (lacking NES1506). (B) Western blots of total cell extracts, probed with anti-M-APC (top) or with Ab-1 (bottom), to reveal full-length APC or APC truncations (arrowheads), and tubulin (arrows) as internal control. The short APC truncation in COLO320 cells is only detectable by Ab-1 (raised against an N-terminal peptide) but not by anti-M-APC (raised against the central third of APC). The levels of APC truncations in colorectal cancer cells are similar to one another, but appear to be generally higher than those of wild-type APC (though full-length APC is prone to degradation; see also Figure 5).
In APC wild-type cells (HCT116, LS174T and LS180), APC and β-catenin are excluded from the nucleus efficiently (Rosin-Arbesfeld et al., 2000, 2001) (Figure 2B and C). In contrast, each cell line with type I truncations examined (SW480, SW403, T84, GP5D, HT55, CACO2, DLD1, SW948 and LOVO) shows at least some nuclear APC. The levels of nuclear APC vary between lines, with SW480 showing the highest level (Rosin-Arbesfeld et al., 2000), though nuclear APC is also prominent in SW948 and LOVO (Figure 2D and E). Isolated cells tend to show the highest levels, whereas cells in the middle of confluent clusters show less nuclear APC, as previously demonstrated (Zhang et al., 2001). Each of these cell lines also shows considerable levels of nuclear β-catenin: typically, β-catenin is evenly distributed throughout the nucleus and cytoplasm, and also associated with the plasma membrane, but some cells (especially those at the periphery of clusters) show nuclear accumulation of β-catenin (Brabletz et al., 2001) (Figure 2D and E, arrowheads). Nuclear β-catenin is particularly prominent in COLO320 cells (Figure 2A).
Finally, we examined two lines with type II APC truncations (COLO205 and HT29). Interestingly, both show an APC staining pattern that closely resembles the wild-type in that APC is excluded from nuclei. Indeed, the staining patterns of HT29 and HCT116 cells look virtually indistinguishable (Figure 2B and F). Likewise, there is no detectable nuclear accumulation of β-catenin in these cells, which show predominantly membrane-associated β-catenin staining (Figure 2F).
Thus, the nuclear–cytoplasmic distributions of β-catenin correlate with those of APC. Importantly, in all colorectal cancer cell lines examined, APC truncations without the c-NESs are at least partly nuclear, whereas APC truncations that retain NES1506 exhibit nuclear exclusion. This indicates that NES1506 is critical for nuclear export of APC and β-catenin, and implies that the n-NESs are insufficient to mediate efficient nuclear export of either protein.
APC is partly nuclear in colorectal cancers
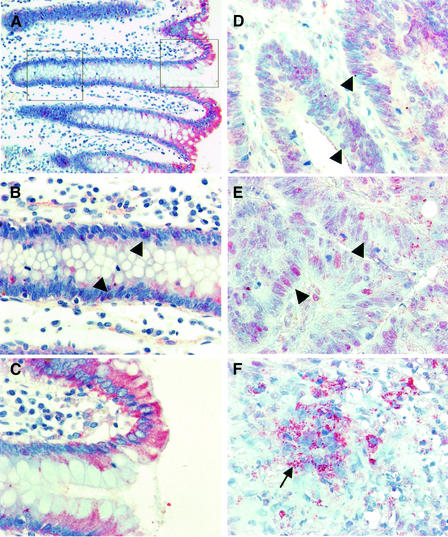
We also examined the subcellular distribution of endogenous APC truncations in a selection of well to moderately differentiated colorectal adenocarcinomas, 17 of which show loss of heterozygosity (LOH) at the APC locus (see Materials and methods). In the normal epithelium adjacent to these carcinomas, APC is more prevalent in the superficial mucosa compared with the basal crypt region (Näthke et al., 1996) (Figure 3A). Above the crypt, APC is predominantly cytoplasmic (Figure 3C), whereas in the crypt, we observe cytoplasmic APC as well as a low level of nuclear APC (Figure 3B, arrowheads). The latter may be a consequence of Wnt pathway activity in the crypt (van de Wetering et al., 2002). Notably, we do not see any accumulation of APC in the apical region of cells, as described previously (Miyashiro et al., 1995; Midgley et al., 1997; Reinacher-Schick and Gumbiner, 2001); however, the latter studies all used anti-APC peptide antibodies that are unreliable due to cross-reactivity with other antigens (Miyashiro et al., 1995; Rosin-Arbesfeld et al., 2001; Bienz, 2002; Mogensen et al., 2002; J.Wakeman, personal communication).
Fig. 3. Subcellular distribution of APC in colorectal carcinomas. Sections through colorectal carcinomas (D–F; two different tumours) and adjacent normal colonic mucosa (A–C), stained with anti-M-APC (red) and with hemalaun to mark the nuclei (blue); the image in (A) is taken at low magnification, to visualize the crypt axis. APC is cytoplasmic in normal cells above the crypt (A and C), but detectable in the crypt cell nuclei (B, arrowheads). In most carcinoma cells, APC is partly nuclear (D and E, arrowheads); high levels of granular cytoplasmic APC are seen in de-differentiated ‘mesenchymal’ cells at the invasive front (F, arrow).
In contrast, each of the 17 adenocarcinomas with APC LOH showed some nuclear APC, although the levels vary somewhat between and within carcinomas. Two typical staining patterns are shown in Figure 3D and E of type I APC mutant carcinomas (APC truncated at codons 1328 and 1356) in which we see mutant APC in the nuclei throughout the tumours. The only exceptions are the de-differentiated ‘mesenchymal’ cells at the invasive front: these contain considerably increased levels of cytoplasmic APC that appears granular (Figure 3F, arrow), perhaps reflecting aggregates similar to those observed with overexpressed APC truncations (Munemitsu et al., 1994) (Figure 5D).
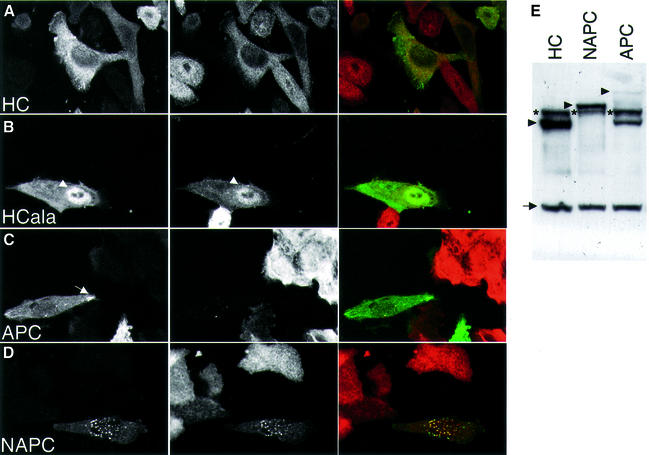
Fig. 5. Subcellular distribution of APC constructs in transfected SW480 cells. (A–D) Confocal sections through cells transfected with GFP-tagged APC constructs as indicated (green), stained with an antibody against β-catenin (red); one typical example of each construct is shown. HCala shows pronounced nuclear APC and β-catenin (arrowhead); the arrow indicates an APC microtubule tip cluster (seen only with full-length APC). Note the granular aggregates seen with NAPC (tagged with GFP, d; or with HA, not shown) that coincide with β-catenin staining. (E) Western blots of total cell extracts, probed with anti-M-APC and anti-tubulin, revealing relative expression levels of GFP-tagged APC constructs (arrowheads) and of endogenous APC (asterisks; in the case of full-length APC, a breakdown product is also observed); internal control, tubulin (arrow).
Loss of NES1506 correlates with high transcriptional activity of β-catenin
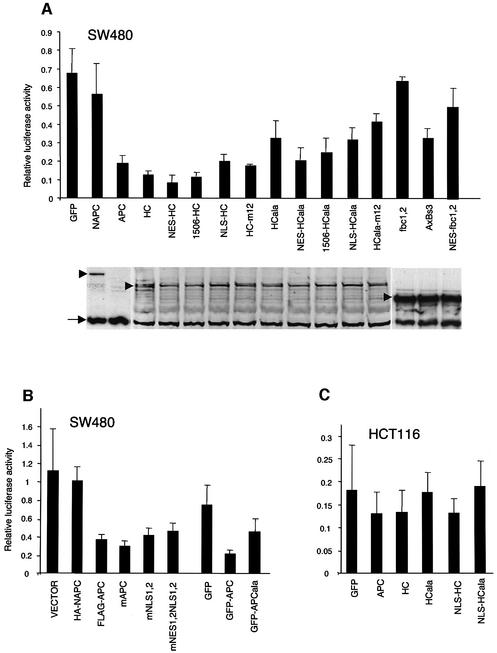
Next, we used TOPFLASH assays (Korinek et al., 1997) to compare the function of the different types of APC truncations. These assays have been used widely to measure the transcriptional activity of TCF and β-catenin (pTOPFLASH contains multimerized TCF-binding sites linked to a luciferase reporter; Korinek et al., 1997), but they have not been applied systematically to compare absolute levels of transcriptional activity between different colorectal cancer cell lines. We chose the TOPFLASH assay since it is highly quantitative and reproducible, and since it measures the most relevant function of APC in tumour suppression (i.e. its ability to reduce TCF/β-catenin transcription). Furthermore, it provides a direct read-out of APC function that is highly specific, unlike endogenous target genes whose expression levels reflect inputs from TCF and other signalling pathways. To avoid further bias due to genetic background, we used three different internal control Renilla genes based on different promoters. We succeeded in transfecting 13 different cell lines, and determined their TOPFLASH activity as a ratio of their luciferase:Renilla values. We also transfected these lines with pFOPFLASH (whose TCF-binding sites are mutated). All FOPFLASH values are exceedingly low (<0.04), confirming the specificity of the TCF/β-catenin input.
All eight lines with type I truncations show high TOPFLASH values (luciferase:Renilla values 1–5.5; Figure 4A). Among these lines, we see a rough inverse correlation between TOPFLASH activity and numbers of β-catenin-binding motifs (15R, 20R; Figure 1) retained in the truncations (Figure 4A): COLO320 with the shortest APC truncation (retaining none of these motifs) shows by far the highest activity (value of 5.5). Cell lines with truncations retaining all 15Rs but only one 20R (SW403, HT55, SW480, CACO2) show ∼2-fold lower values (2–2.5), while those retaining an additional 20R show the lowest values (1–1.5). The expression levels of these APC truncations are roughly comparable between lines (representative examples shown in Figure 4B). Thus, the activity of β-catenin may depend on its affinity for the APC truncations in these lines—the less binding of β-catenin, the more transcriptional activity. This suggests that the binding of β-catenin is competitive between APC and TCF.
Interestingly, lines with APC type II truncations show considerably less TOPFLASH activity, like lines with wild-type APC but mutant β-catenin (values <1; Figure 4A). These values are consistently 3- to 10-fold lower than the lowest values measured in lines with APC type I truncations (but they are still relatively high compared with cells with normal β-catenin and APC, e.g. 293T cells, whose TOPFLASH activity is barely measurable; not shown). Thus, the transcriptional activity of mutant β-catenin is roughly equivalent to the activity of wild-type β-catenin in the presence of an APC type II truncation.
These results indicate that the presence of NES1506 is a critical determinant of the transcriptional activity of β-catenin in colorectal cancer cells.
Functional contributions of c-NES versus n-NES
Our results implied that the n-NESs of APC truncations provide insufficient nuclear export activity for the reduction of TCF transcription. To confirm this experimentally, we carried out complementation assays of SW480 cells that lack endogenous APC function; this function can be restored if the cells are transfected with minimal APC fragments (Munemitsu et al., 1995). We thus compared the function of wild-type APC with that of two APC mutants without n-NESs (HC, the central third of APC; mAPC, full-length APC with mutated n-NESs) and of two mutants without c-NESs (NAPC, a type I truncation; APCala, full-length APC with mutated c-NESs) (Figure 1). We examined the subcellular distributions of these constructs in transfected SW480 cells, and compared their function in reducing nuclear β-catenin and TOPFLASH activity. Their expression levels were monitored by western blotting (Figure 5E).
HC is largely excluded from the nuclei of SW480 cells, correlating with low levels of nuclear β-catenin (Rosin-Arbesfeld et al., 2000) (Figure 5A). In contrast, HCala (whose NESs are mutated; Figure 1) is found in the nuclei of many transfected cells, correlating with β-catenin staining that is typically higher in the nucleus than in the cytoplasm (Figure 5B).
Full-length GFP-tagged APC is largely cytoplasmic, associated with microtubule tips, but also with the plasma membrane (Rosin-Arbesfeld et al., 2001), and essentially eliminates endogenous β-catenin staining (Munemitsu et al., 1995) (Figure 5C). We find the same APC and β-catenin staining patterns with mutated APC (see Supplementary figure 1), although Neufeld et al. (2000b) and Henderson (2000) reported high levels of nuclear β-catenin in cells transfected with mAPC. Possible explanations for this discrepancy are the variability of β-catenin staining (see above; Brabletz et al., 2001) and the scoring methods that are at best semi-quantitative (also in one study, scoring was carried out in the absence of construct tagging, and thus of unambiguous identification of transfected cells; Henderson, 2000). Likewise, we do not detect any obvious differences between APC and APCala in terms of GFP fluorescence (not shown), probably because of the strong tendency of full-length APC to associate with microtubule tips in motile cells (Näthke et al., 1996).
NAPC produces conspicuous cytoplasmic granules (Munemitsu et al., 1994), but can also be detected in the nuclei of some of the transfected SW480 cells (Figure 5D). Likewise, most transfected cells show strong granular β-catenin staining coinciding with the granular NAPC, and the typical diffuse cytoplasmic β-catenin staining is reduced (Figure 5D). It thus looks as though the apparent reduction of β-catenin reflects entirely its trapping by the NAPC granules, rather than genuine APC-mediated destabilization by the Axin complex.
Next, we used TOPFLASH assays to measure the ability of these APC constructs to restore APC function in transfected SW480 cells. Full-length APC and mAPC are equally active in reducing TOPFLASH values (Neufeld et al., 2000b; Henderson et al., 2002), while APCala is considerably less active (Figure 6B). Given that all three constructs are expressed at the same level (not shown), and show indistinguishable subcellular distributions (see above), this indicates that the c-NESs are functionally more important than the n-NESs. In support of this, HC is also highly active in reducing TOPFLASH values, while HCala is compromised in doing so (Rosin-Arbesfeld et al., 2000), and NAPC shows barely any activity (Figure 6A). The latter constructs are expressed at comparable levels (Figures 5E and 6A) so NAPC and HCala are genuinely less active than HC in reducing TCF transcription, indicating the functional importance of the c-NESs.
Fig. 6. TOPFLASH assays in colorectal cancer cells transfected with APC constructs. SW480 or HCT116 cells were transfected with APC constructs as indicated, and relative luciferase:Renilla values were determined as in Figure 4. (A and C) GFP-tagged APC constructs, compared with a GFP control; relative expression levels of constructs in (A) are shown underneath (western blots probed with anti-GFP; this antibody is less sensitive than anti-M-APC, so does not permit detection of full-length APC under these conditions). (B) APC constructs with various tags as indicated, and a vector control (containing HA). Note that the GFP derivatives produce generally lower TOPFLASH values than the HA- and Flag-tagged constructs, so have to be compared with the GFP control, and with GFP-tagged APC (see also A).
A minimal APC construct derived from the middle of APC (Axbs3, containing a 20R and an Axin-binding motif) reduces β-catenin activity in transfected SW480 cells, despite not bearing any apparent NESs (Rubinfeld et al., 2001). However, Axbs3 is less active than HC, despite being expressed at considerably higher levels; indeed, Axbs3 is no more active than HCala (Figure 6A). The activity of its parental construct (fbc1,2) can be increased by addition of either an Axin-binding site (Rubinfeld et al., 2001), or an NES (Figure 6A). This series of minimal constructs illustrates that an NES, like an Axin-binding site, can provide a functional improvement.
Nuclear import signals in APC proteins
Nuclear localization signals (NLSs) have been identified in APC (Zhang et al., 2000) (Figure 1), but these are not well conserved among APC proteins. Furthermore, they are deleted from APC cancer truncations, yet the latter are clearly able to get into the nucleus (see above), apparently due to their Armadillo repeat domain (ARD; Figure 1) (Rosin-Arbesfeld et al., 2000; Eleftheriou et al., 2001). Note that the ARD of β-catenin mediates its nuclear import, which requires neither an NLS nor the nuclear import machinery (Fagotto et al., 1998; Yokoya et al., 1999).
We re-examined the function of these NLSs in the context of HC, which lacks the ARD that may mask their nuclear import activity within full-length APC. We thus mutated the NLSs in HC and HCala and tested the mutants (HC-m12 and HCala-m12) in TOPFLASH assays of transfected SW480 cells.
The subcellular distributions of these mutants look indistinguishable from those of their parental constructs (not shown). Furthermore, there is little difference between wild-type and NLS mutant versions of HC, and of HCala, in their ability to reduce the TOPFLASH values (Figure 6A). We therefore also re-examined mNLS1,2 (i.e. full-length APC with mutant NLSs), which was reported to be inactive in reducing TOPFLASH values in transfected SW480 cells (Zhang et al., 2000). In our hands, this mutant is almost fully functional (Figure 6B). Likewise, mNES1,2NLS1,2 (APC with mutant n-NESs and NLSs; Zhang et al., 2000) is also similarly active as wild-type APC (Figure 6B), and its subcellular distribution is unchanged (Supplementary figure 1). The presence of the mutations in both constructs was confirmed by sequencing. Thus, we were unable to find any convincing evidence for the functional significance of these putative NLSs.
Nuclear targeting of APC constructs
It could be argued that the c-NES mutations affect an unknown function that coincides with these NES sequences, and that it is this other function rather than the nuclear export function that is selected against in APC cancer truncations (though the triple c-NES mutations in HC do not seem to decrease its ability to bind to β-catenin; Lee et al., 2002). In an attempt to rule this out, we linked HCala to a highly active heterologous NES (from Rev; Galea et al., 2001), or to NES1506, and asked whether this would restore its activity in TOPFLASH assays.
Indeed, both NES fusions are more active than HCala itself in reducing TOPFLASH values (Figure 6A). These increases in activity are small but consistent, and observed in every single experiment; moreover, the expression levels of the NES fusions are nearly the same as that of HCala (Figure 6A). Notably, linkage of the synthetic NES to HC also results in a small but consistent increase in its activity (Figure 6A). These results, like those from the Axbs3 series (see above), show that NES addition improves the function of APC constructs in reducing TCF transcription.
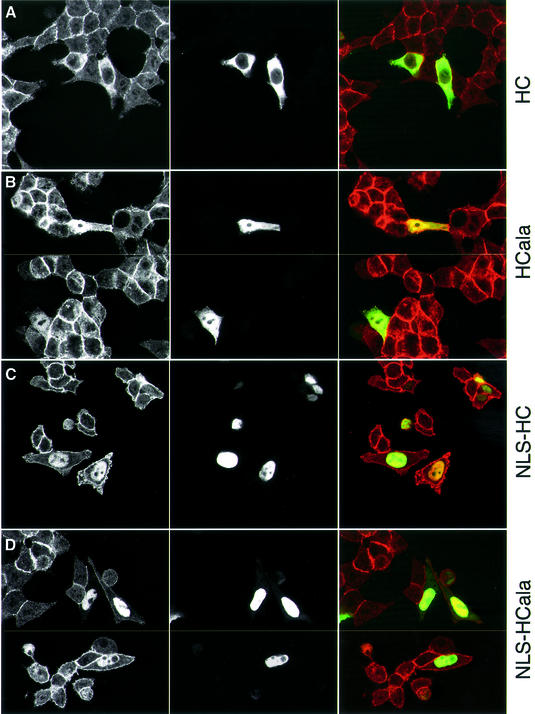
Next, we linked a synthetic NLS to HC and HCala, to see whether this also affects their function. Both NLS fusions accumulate to high levels in the nuclei of transfected cells (see below); their expression levels are the same as those of other HC constructs (Figure 6A). The nuclear targeting of HC only causes a moderate increase of the TOPFLASH values in transfected SW480 cells (Figure 6A).
We also tested NLS-HC and NLS-HCala in transfected HCT116 cells (which show little nuclear APC and β-catenin; Figure 2B), to see whether the nuclear targeting of HC affects the subcellular distribution of β-catenin. Interestingly, both NLS fusions promote a striking nuclear accumulation of β-catenin (Figure 7C and D). A slight accumulation of nuclear β-catenin is also detectable in HCala-transfected HCT116 cells (Figure 7B), though not with HC, which is excluded from the nucleus efficiently (Figure 7A). Evidently, nuclear targeting of HC or HCala causes nuclear accumulation of β-catenin, suggesting that APC can promote both nuclear export and import of β-catenin.
Fig. 7. Nuclear targeting of APC constructs causes nuclear accumulation of β-catenin. Confocal sections through HCT116 cells transfected with GFP-tagged APC constructs as indicated (green), stained with antibody against β-catenin (red); typical examples of each construct are shown (B and D show two fields each).
Despite this, nuclear targeting of HC does not affect its function in reducing TCF transcription in these cells: NLS-HC is as active as HC in TOPFLASH assays (Figure 6C). TOPFLASH values are reduced slightly though significantly by both constructs, and by full-length APC, as previously observed (Morin et al., 1997; Satoh et al., 2000) (Figure 6C). In contrast, neither HCala nor NLS-HCala affect TOPFLASH activity in these cells (Figure 6C). These results suggest that the transcriptional activity of β-catenin is determined predominantly by the rate of APC’s nuclear export, rather than by its nuclear import or its nuclear steady-state levels.
Direct measurements of nuclear export by FLIP
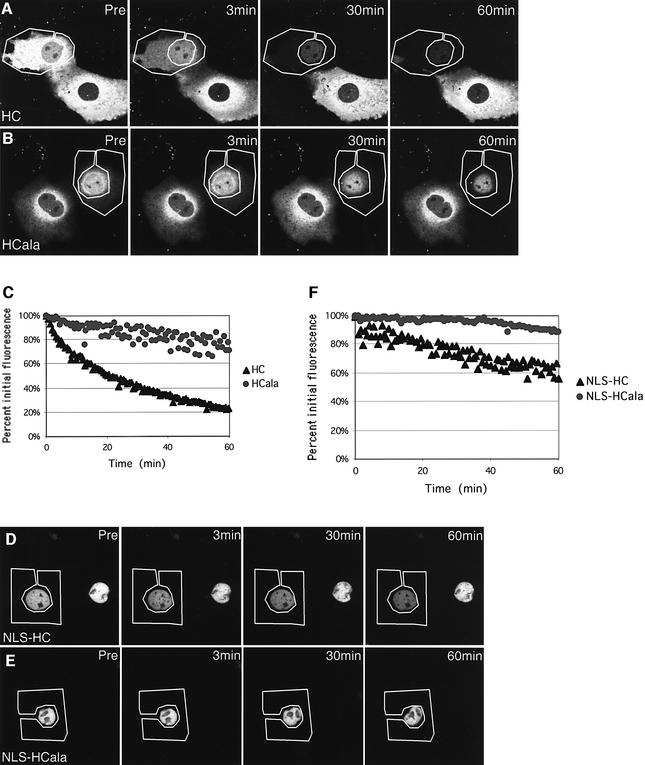
To date, the nuclear export activity of APC has been inferred entirely from its subcellular localization and its dependence on NES sequences and LMB. We thus performed FLIP experiments to measure directly the nuclear export of GFP-tagged HC and its derivatives. To obtain optimal quantitative results, we used simian COS cells since these are larger and flatter than colorectal cancer cells, but we also conducted some FLIP experiments with HC and HCala in SW480 cells to confirm our results (not shown). We selected individual healthy-looking transfected cells with moderate GFP levels, and we measured the loss of nuclear fluorescence while continually bleaching out the entire cytoplasmic fluorescence.
This revealed a clear difference of fluorescence loss between HC and HCala: in the case of HC, half of the nuclear fluorescence (NF1/2) is lost in ∼10–15 min, and an apparent plateau of ∼20% of the initial nuclear fluorescence is reached after ∼60 min (Figure 8A, and C, black triangles). This is somewhat slower than the loss of nuclear fluorescence of nucleoporin 98, the only protein to date whose rate of nuclear export has been determined by FLIP experiments: this protein shows an NF1/2 of ∼3 min, and reaches an apparent final plateau of ∼8% of the initial fluorescence after ∼20 min (Griffis et al., 2002). In contrast, the NF1/2 is much slower in the case of HCala, which reaches a plateau of ∼80% of its initial value after ∼40 min (Figure 8B, and C, grey dots). Again, this is comparable with the values determined by Griffis et al. (2002) for a nuclear control protein. These FLIP experiments indicate that HC exits from the nucleus fairly efficiently, whereas HCala exits much less.
Fig. 8. Nuclear export rates of GFP-tagged APC constructs measured by FLIP. Individual COS cells transfected with GFP-tagged HC constructs (as indicated) were selected, and their entire cytoplasm was subjected to repetitive photobleaching while their nuclear fluorescence was measured; graphs and images shown are of a representative cell from each group. (A, B, D and E) Confocal sections through transfected cells at various time points as indicated (pre, pre-bleaching). (C and F) Graphic representations of loss of nuclear fluorescence at each time point. Wild-type HC constructs (HC and NLS-HC) show significantly more loss of nuclear fluorescence than NES mutant constructs (HC-ala and NLS-HCala), demonstrating that their nuclear export activity depends on their NESs.
We also carried out FLIP experiments with NLS-HC and NLS-HCala. Because these constructs produce very high ratios of nuclear/cytoplasmic fluorescence, the NF1/2 values were intrinsically more difficult to determine, and are not directly comparable with the values obtained above. Nevertheless, there was a clear measurable difference of fluorescence loss between NLS-HC and NLS-HCala: in the case of NLS-HC, 40% of the initial fluorescence is lost after ∼60 min, and no plateau is reached yet at this stage (Figure 8D, and F, black triangles), whereas there is essentially no measurable loss of nuclear fluorescence in the case of NLS-HCala (Figure 8E, and F, grey dots). Thus, despite showing similar steady-state nuclear–cytoplasmic distributions, NLS-HC can exit from the nucleus, whereas NLS-HCala cannot.
These FLIP experiments confirm that the c-NESs determine the nuclear export activity of HC. They allow us to conclude the same for the NLS fusions whose nuclear export activity could not have been deduced from their subcellular locations.
Discussion
We have shown that typical APC cancer truncations which lack the c-NESs are detectable in the nucleus of all colorectal cancer cell lines examined. In contrast, the more rare APC type II truncations which retain c-NES1506 show considerable nuclear exclusion, similar to wild-type APC which is excluded from the nucleus efficiently. The same is essentially the case in the intestinal epithelium of the human colon: APC is excluded from the nuclei in normal tissue, but is detectable in the nuclei of APC mutant carcinoma cells. This points to the functional importance of the central NES1506 for efficient nuclear export activity of APC.
Our results do not rule out a function for the n-NESs which may contribute to the nuclear export of wild-type APC, and provide the residual nuclear export activity of APC truncations (Henderson, 2000). They may even help the tumour progression, which may need a minimal level of APC function (‘just right signalling’; Fodde et al., 2001). However, recent work on N-terminal attenuated APC (AAPC) mutations has demonstrated that these allow re-initiation of translation downstream at codon 184, producing an APC protein that is fully functional despite lacking all n-NESs (Heppner Goss et al., 2002). Similarly, Shih et al. (2000) have examined multiple functional read-outs of APC fragments in complementation assays of APC mutant cancer cells, and concluded that the central third of APC (similar to HC) is sufficient for tumour suppression.
The steady-state levels of nuclear β-catenin are irrelevant for its transcriptional activity
Our study confirms that the nuclear–cytoplasmic distributions of β-catenin often correlate with those of APC (e.g. Henderson, 2000; Neufeld et al., 2000b; Rosin-Arbesfeld et al., 2000). In addition, we found that HC targeted to the nucleus relocalizes β-catenin from the cytoplasm to the nucleus, indicating that the steady-state nuclear– cytoplasmic distribution of APC determines that of β-catenin.
Are the steady-state nuclear levels of these proteins functionally relevant? Perhaps the most critical functional consequence of APC and β-catenin activity in both normal and malignant development is the TCF-mediated transcription (van de Wetering et al., 2002), which we have measured in our study with TOPFLASH assays. Our results demonstrate that high steady-state levels of nuclear APC and β-catenin often do not correlate with high transcriptional activity. For example, among the APC mutant cell lines examined, SW480 cells have the highest nuclear β-catenin levels, but by no means the highest TOPFLASH activity. Moreover, targeting HC to the nucleus causes a striking nuclear accumulation of β-catenin without resulting in a large increase of TCF transcription. We conclude that the absolute levels of steady-state nuclear APC and β-catenin are largely irrelevant for their function in controlling TCF-mediated transcription.
The nuclear export function of APC is critical for the control of TCF/β-catenin transcription
Our FLIP experiments provide direct evidence for the rapid nuclear export of HC, a minimal APC fragment that is fully functional in complementation tests (see above). They further demonstrate that this export is reduced in NES mutant versions of HC, which are compromised in reducing TCF transcription. Significantly, some activity can be restored by linkage to a synthetic NES (Figure 6A). Taken together, these results indicate that the rate of nuclear export of APC is critical for its ability to reduce the transcriptional activity of β-catenin.
Support for this comes from our experiments in which HC was targeted to the nucleus by linkage to an NLS. Despite showing high steady-state nuclear levels, this NLS fusion still exits actively from the nucleus, as revealed by FLIP, and thus shuttles in and out. In contrast, FLIP demonstrated that an NLS-HCala no longer exits from the nucleus. Importantly, despite their similar steady-state subcellular distributions, the activity of the two NLS fusions in reducing TCF transcription is different (Figure 6A). Once again, the function of these APC fragments in reducing the transcriptional activity of β-catenin correlates with their ability to exit from the nucleus.
These experiments also indicate that the rate of nuclear import of APC may be not be critical for its function. Nuclear import of full-length APC appears to be mediated at least partly by the ARD (Rosin-Arbesfeld et al., 2000; Eleftheriou et al., 2001), but our experiments failed to provide any convincing evidence for the functional importance of the putative NLSs downstream of the MCR (Zhang et al., 2000). Therefore, we still do not know how HC gets into the nucleus, but we do know that a dramatic increase in its nuclear levels does not affect its function very much. So it appears that any level of nuclear import will suffice for the function of APC in reducing the activity of nuclear β-catenin.
Evidence that APC controls the free pool of nuclear β-catenin available for transcription
Our NLS fusions provide experimental evidence that the steady-state levels of nuclear APC determine the steady-state levels of nuclear β-catenin, and that APC can promote both nuclear export and import of β-catenin. The basis for this is likely to be the direct binding between APC and β-catenin. Two mechanisms have been envisaged by which APC may control the partitioning of β-catenin between nucleus and cytoplasm. Either APC could shuttle β-catenin directly, or it could determine the nuclear–cytoplasmic distribution of β-catenin indirectly by ‘anchorage’ (Bienz, 1999; Henderson and Fagotto, 2002).
The results from the NLS fusions argue against the formal possibility (Rosin-Arbesfeld et al., 2000) that the trapping of β-catenin by APC in the nuclei of colorectal cancer cells may explain their high levels of TCF transcription. Indeed, despite a massive increase of nuclear β-catenin (due to NLS-HC), we only observe a small increase in TCF transcription (Figure 6A). This implies that NLS-HC, and perhaps APC itself, may sequester β-catenin in the nucleus and keep it from binding to TCF, as suggested previously (Neufeld et al., 2000b). The comparative TOPFLASH values of the different colorectal cancer cells further support this notion: these values inversely correlate with the numbers of β-catenin-binding sites retained in their APC truncations, and thus presumably with the binding affinity between these truncations and β-catenin. Thus, the functionally relevant pool of nuclear β-catenin appears to be the ‘free’ pool, i.e. the β-catenin that is not complexed with APC, and APC may control the availability of nuclear β-catenin for transcription.
How does APC achieve this? We propose that APC accelerates the nuclear export of β-catenin, and that repeated cycling of APC through the nucleus results in pumping out of β-catenin from the nucleus, which lowers the concentration of free nuclear β-catenin and its availability to TCF. Once in the cytoplasm, APC targets β-catenin to the Axin complex for subsequent destruction, and perhaps to E-cadherin for incorporation into adherens junctions (Bienz, 1999, 2002). This important cytoplasmic function of APC thus removes β-catenin from the nuclear–cytoplasmic cycling pool. It provides effectively a drain for free β-catenin into which the nuclear β-catenin is directed by the nuclear export function of APC. Other possibilities can be envisaged, e.g. nuclear β-catenin may be released more readily from lingering APC that cannot exit from the nucleus than from APC–β-catenin complexes that are destined for nuclear export. Whatever the case, the affinity between full-length or truncated APC and β-catenin seems critical since it determines whether β-catenin is exported from the nucleus by APC (if bound tightly), or whether it is available to TCF (if bound less tightly).
NES-independent nuclear export of β-catenin?
Under conditions of overexpression, β-catenin can exit from the nucleus in a CRM1-independent way (Eleftheriou et al., 2001; Wiechens and Fagotto, 2001), and it has been proposed that the nuclear export of β-catenin is independent of APC in Wnt-stimulated and APC mutant cells (Henderson and Fagotto, 2002). One argument against this is that the mutant β-catenins in HCT116, LS180 and LS174T cells do not accumulate in the nuclei (Figure 2B and C), despite mutations in their GSK3 phosphorylation sites that allow them to avoid Axin- and APC-mediated degradation (mimicking the situation in Wnt-stimulated or APC mutant cells). Secondly, β-catenin is also excluded efficiently from nuclei in cells with APC type II truncations, but not in cells with APC type I truncations, the chief difference between these being the presence or absence of the c-NES1506.
Based on our evidence, we thus propose that the rate of the CRM1-independent nuclear export of β-catenin is relatively slow, and that APC accelerates this rate. We further suggest that the high levels and activity of nuclear β-catenin in APC type II mutant cells are at least partly due to the loss of the c-NES in type II cancer truncations. Together with the rarity of APC type II truncations in colon carcinomas (<3%; Rosin-Arbesfeld et al., 2000), this provides evidence that the loss of these c-NES, and thus the loss of accelerated nuclear export of β-catenin, is likely to contribute to the tumorigenesis.
Materials and methods
DNA constructs
The following APC plasmids were used: full-length APC (Smith et al., 1993) tagged with Flag epitope (Flag-APC), and mutants thereof (Flag-mNES1,2; Flag-mNLS1,2; Flag-mNES1,2NLS1,2; Neufeld et al., 2000b); GFP–APC, GFP–NAPC (Rosin-Arbesfeld et al., 2001), pCMV-APCmutantNES1+2 (Henderson, 2000; here called mAPC; Figure 1); and GFP-tagged HC and HCala (Rosin-Arbesfeld et al., 2000). We also generated a haemagglutinin (HA)-tagged version of NAPC; there were no significant differences in subcellular distributions between the differently tagged APC or NAPC constructs.
To generate NLS and NES fusions, the following sequences were linked to HC and HCala: TPPKKKRKVED (NLS consensus sequence); GQIDLLERLKELNLDSSN (NES from Rev; Galea et al., 2001); and SSLSALSLDEP (NES1506). The Rev NES was also linked to the N-terminus of Axbs3 and its precursor fbc1,2 (Rubinfeld et al., 2001). The same alanine substitutions as previously described (Zhang et al., 2000) were introduced into HC and HCala, to generate HC-m12 and HCala-m12. All derivatives of HC and of fbc1,2 were tagged with GFP at their N-termini. To generate APCala, the most active c-NESs (at 20R3 and 20R4; Galea et al., 2001) were mutated by alanine substitutions (as in HCala; Rosin-Arbesfeld et al., 2000) within full-length APC. Each plasmid was checked by sequencing.
Tissue culture, transfections and luciferase assays
Colorectal cancer cell lines were grown in Dulbecco’s modified Eagle’s medium (DMEM; supplemented with 10% fetal calf serum). Cells were transfected using LipofectAMINE (Gibco-BRL), except for LS180 and LS174T which were transfected by LipofectAMINE 2000 (Gibco-BRL). Cells were seeded at 1 × 105 cells per well in a 6-well plate 24 h prior to transfection, and transfected with 2 µg of effector plasmid (or with 0.35 µg for the fbc1,2 series), 0.5 µg of reporter (pFOPFLASH or pTOPFLASH; Korinek et al., 1997) and 0.05 µg of internal control plasmid (typically, pRL-CMV; Promega). In some experiments, pRL-TK and pRL-SV40 (Promega) were also used. At 24 h after transfection, total cell extracts were subjected to western blot analysis as described (Rosin-Arbesfeld et al., 2000, 2001) and assayed in parallel for luciferase activity according to the manufacturer’s instructions. Aliquots of cells were fixed for immunofluorescence.
The following antibodies were used: mouse Ab-1 (raised against an N-terminal peptide; 1:200; Oncogene Research Products); affinity-purified rabbit anti-M-APC (1:4000; Näthke et al., 1996); mouse anti-tubulin (1:500; Sigma T9026); and mouse anti-GFP (1:500; Santa Cruz SC-9996). The ratios between APC and tubulin levels (Figure 4B) indicate that the sensitivity of anti-M-APC is ∼10-fold higher than that of Ab-1, presumably reflecting a higher titre. Note that the levels of APC truncations and constructs indicated by the blots (in Figures 4 and 6) may not be directly comparable with those of full-length APC, since the unusually large size of APC may lower its transfer efficiency during blotting.
Immunofluorescence
Cells grown on coverslips were fixed for 20 min in phosphate-buffered saline (PBS) containing 4% paraformaldehyde, washed three times with PBS, permeabilized with 0.1% Triton X-100 and blocked with bovine serum albumin for 1 h. Subsequently, cells were incubated at room temperature with primary and secondary antibodies for 60 and 30 min, respectively.
The following antibodies were used: anti-M-APC (see above; 1:1000); mouse anti-β-catenin (1:500; Transduction Laboratories); mouse anti-lamin A/C (1:500; Santa Cruz); rat anti-HA (1:50; 3F10, Roche); and Alexa red and green (1:500; Molecular Probes). The specificity of anti-M-APC has been demonstrated previously (Näthke et al., 1996; Rosin-Arbesfeld et al., 2001; Mogensen et al., 2002) (see also Figures 2A and 4B). Also, pre-blocking with an ∼7-fold excess of APC4 protein fragment (overlapping the APC fragment used for immunisation; kindly provided by I.Näthke) completely inhibited APC but not simultaneous β-catenin staining. Images were collected on a Bio-Rad MRC 1024 confocal microscope.
For live cell imaging and FLIP analysis, see Supplementary data.
Analysis of colorectal carcinomas
Twenty-one well to moderately differentiated colorectal adenocarcinomas were chosen for analysis (Brabletz et al., 2001) since these are highly likely to show APC LOH as well as APC mutations. Genetic alterations at the APC locus were determined by LOH analysis, and by sequencing of the mutation cluster region after microdissection of formalin-fixed, paraffin-embedded tumour areas, as previously described (Brabletz et al., 2001). LOH was found in 17 of 21 cases (four cases were not informative); in seven cases, we were able to determine the precise mutation in the remaining allele (truncating mutations at codons 1321, 1350, 1328, 1356, 1358, 1365, 1476). We also examined 10 atypical adenocarcinomas (four mucinous, three medullary, one signet cell ring, two undifferentiated) with microsatellite instability, but without APC LOH; as expected, these showed essentially the same subcellular distribution of APC as the adjacent normal mucosa (with very little nuclear APC, or none at all; not shown).
For immunohistochemistry, anti-M-APC was used (see above; 1:300), using a staining protocol as described (Brabletz et al., 1999); biotinylated goat anti-rabbit Ig antiserum (1:50, Dako) was used as a secondary antibody. Sections were counterstained with hemalaun.
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We thank I.Näthke for antibody, P.Polakis, B.Henderson and K.Neufeld for plasmids, B.Nichols for imaging advice, and R.Fodde, R.Smits and H.Pelham for discussion. R.R.-A. was supported by an EMBO Long-term fellowship. T.B. was supported by a DFG grant (no. BR1399/4-1).
References
- Behrens J., Jerchow,B.A., Wurtele,M., Grimm,J., Asbrand,C., Wirtz,R., Kuhl,M., Wedlich,D. and Birchmeier,W. (1998) Functional interaction of an axin homolog, conductin, with β-catenin, APC and GSK3β. Science, 280, 596–599. [DOI] [PubMed] [Google Scholar]
- Bienz M. (1999) APC: the plot thickens. Curr. Opin. Genet. Dev., 9, 595–603. [DOI] [PubMed] [Google Scholar]
- Bienz M. (2002) The subcellular destinations of APC proteins. Nat. Rev. Mol. Cell Biol., 3, 328–338. [DOI] [PubMed] [Google Scholar]
- Brabletz T., Jung,A., Dag,S., Hlubek,F. and Kirchner,T. (1999) β-Catenin regulates the expression of the matrix metalloproteinase-7 in human colorectal cancer. Am. J. Pathol., 155, 1033–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabletz T., Jung,A., Reu,S., Porzner,M., Hlubek,F., Kunz-Schughart,L.A., Knuechel,R. and Kirchner,T. (2001) Variable β-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc. Natl Acad. Sci. USA, 98, 10356–10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eleftheriou A., Yoshida,M. and Henderson,B.R. (2001) Nuclear export of human β-catenin can occur independent of CRM1 and the adenomatous polyposis coli tumor suppressor. J. Biol. Chem., 276, 25883–25888. [DOI] [PubMed] [Google Scholar]
- Fagotto F., Gluck,U. and Gumbiner,B.M. (1998) Nuclear localization signal-independent and importin/karyopherin-independent nuclear import of β-catenin. Curr. Biol., 8, 181–190. [DOI] [PubMed] [Google Scholar]
- Fodde R., Smits,R. and Clevers,H. (2001) APC, signal transduction and genetic instability in colorectal cancer. Nat. Rev. Cancer, 1, 55–67. [DOI] [PubMed] [Google Scholar]
- Galea M., Eleftheriou,A. and Henderson,B.R. (2001) ARM domain-dependent nuclear import of adenomatous polyposis coli protein is stimulated by the B56α sub-unit of protein phosphatase 2A. J. Biol. Chem., 276, 45833–45839. [DOI] [PubMed] [Google Scholar]
- Griffis E.R., Altan,N., Lippincott-Schwartz,J. and Powers,M.A. (2002) Nup98 is a mobile nucleoporin with transcription-dependent dynamics. Mol. Biol. Cell, 13, 1282–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson B.R. (2000) Nuclear–cytoplasmic shuttling of APC regulates β-catenin subcellular localization and turnover. Nat. Cell Biol., 2, 653–660. [DOI] [PubMed] [Google Scholar]
- Henderson B.R. and Fagotto,F. (2002) The ins and outs of APC and β-catenin nuclear transport. EMBO Rep., 3, 834–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson B.R., Galea,M., Schuechner,S. and Leung,L. (2002) Lymphoid enhancer factor-1 blocks APC-mediated nuclear export and degradation of β-catenin: regulation by histone deacetylase 1. J. Biol. Chem., 277, 24258–24264. [DOI] [PubMed] [Google Scholar]
- Heppner Goss K.H., Trzepacz,C., Tuohy,T.M. and Groden,J. (2002) Attenuated APC alleles produce functional protein from internal translation initiation. Proc. Natl Acad. Sci. USA, 99, 8161–8166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinzler K.W. and Vogelstein,B. (1996) Lessons from hereditary colorectal cancer. Cell, 87, 159–170. [DOI] [PubMed] [Google Scholar]
- Korinek V., Barker,N., Morin,P.J., van Wichen,D., de Weger,R., Kinzler,K.W., Vogelstein,B. and Clevers,H. (1997) Constitutive transcriptional activation by a β-catenin–Tcf complex in APC–/– colon carcinoma. Science, 275, 1784–1787. [DOI] [PubMed] [Google Scholar]
- Lee M.S., D’Amour,K.A. and Papkoff,J. (2002) A yeast model system for functional analysis of β-catenin signaling. J. Cell Biol., 158, 1067–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midgley C.A., White,S., Howitt,R., Save,V., Dunlop,M.G., Hall,P.A., Lane,D.P., Wyllie,A.H. and Bubb,V.J. (1997) APC expression in normal human tissues. J. Pathol., 181, 426–433. [DOI] [PubMed] [Google Scholar]
- Miyaki M. et al (1994) Characteristics of somatic mutation of the adenomatous polyposis coli gene in colorectal tumors. Cancer Res., 54, 3011–3020. [PubMed] [Google Scholar]
- Miyashiro I. et al (1995) Subcellular localization of the APC protein: immunoelectron microscopic study of the association of the APC protein with catenin. Oncogene, 11, 89–96. [PubMed] [Google Scholar]
- Mogensen M.M., Tucker,J.B., Mackie,J.B., Prescott,A.R. and Näthke,I.S. (2002) The adenomatous polyposis coli protein unambiguously localizes to microtubule plus ends and is involved in establishing parallel arrays of microtubule bundles in highly polarized cells. J. Cell Biol., 157, 1041–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin P.J., Sparks,A.B., Korinek,V., Barker,N., Clevers,H., Vogelstein,B. and Kinzler,K.W. (1997) Activation of β-catenin–Tcf signaling in colon cancer by mutations in β-catenin or APC. Science, 275, 1787–1790. [DOI] [PubMed] [Google Scholar]
- Munemitsu S., Souza,B., Muller,O., Albert,I., Rubinfeld,B. and Polakis,P. (1994) The APC gene product associates with microtubules in vivo and promotes their assembly in vitro. Cancer Res., 54, 3676–3681. [PubMed] [Google Scholar]
- Munemitsu S., Albert,I., Souza,B., Rubinfeld,B. and Polakis,P. (1995) Regulation of intracellular β-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc. Natl Acad. Sci. USA, 92, 3046–3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase H. and Nakamura,Y. (1993) Mutations of the APC (adenomatous polyposis coli) gene. Hum. Mutat., 2, 425–434. [DOI] [PubMed] [Google Scholar]
- Näthke I.S., Adams,C.L., Polakis,P., Sellin,J.H. and Nelson,W.J. (1996) The adenomatous polyposis coli tumor suppressor protein localizes to plasma membrane sites involved in active cell migration. J. Cell Biol., 134, 165–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld K.L. and White,R.L. (1997) Nuclear and cytoplasmic localizations of the adenomatous polyposis coli protein. Proc. Natl Acad. Sci. USA, 94, 3034–3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld K.L., Nix,D.A., Bogerd,H., Kang,Y., Beckerle,M.C., Cullen,B.R. and White,R.L. (2000a) Adenomatous polyposis coli protein contains two nuclear export signals and shuttles between the nucleus and cytoplasm. Proc. Natl Acad. Sci. USA, 97, 12085–12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld K.L., Zhang,F., Cullen,B.R. and White,R.L. (2000b) APC-mediated downregulation of β-catenin activity involves nuclear sequestration and nuclear export. EMBO Rep., 1, 519–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakis P. (2000) Wnt signaling and cancer. Genes Dev., 14, 1837–1851. [PubMed] [Google Scholar]
- Reinacher-Schick A. and Gumbiner,B.M. (2001) Apical membrane localization of the adenomatous polyposis coli tumor suppressor protein and subcellular distribution of the β-catenin destruction complex in polarized epithelial cells. J. Cell Biol., 152, 491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin-Arbesfeld R., Townsley,F. and Bienz,M. (2000) The APC tumour suppressor has a nuclear export function. Nature, 406, 1009–1012. [DOI] [PubMed] [Google Scholar]
- Rosin-Arbesfeld R., Ihrke,G. and Bienz,M. (2001) Actin-dependent membrane association of the APC tumour suppressor in polarized mammalian epithelial cells. EMBO J., 20, 5929–5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan A.J., Lamlum,H., Ilyas,M., Wheeler,J., Straub,J., Papadopoulou,A., Bicknell,D., Bodmer,W.F. and Tomlinson,I.P. (2000) APC mutations in sporadic colorectal tumors: A mutational ‘hotspot’ and interdependence of the ‘two hits’. Proc. Natl Acad. Sci. USA, 97, 3352–3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinfeld B., Tice,D.A. and Polakis,P. (2001) Axin-dependent phosphorylation of the adenomatous polyposis coli protein mediated by casein kinase 1ε. J. Biol. Chem., 276, 39037–39045. [DOI] [PubMed] [Google Scholar]
- Satoh S. et al. (2000) AXIN1 mutations in hepatocellular carcinomas and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Nat. Genet., 24, 245–250. [DOI] [PubMed] [Google Scholar]
- Shih I.M., Yu,J., He,T.C., Vogelstein,B. and Kinzler,K.W. (2000) The β-catenin binding domain of adenomatous polyposis coli is sufficient for tumor suppression. Cancer Res., 60, 1671–1676. [PubMed] [Google Scholar]
- Smith K.J. et al. (1993) The APC gene product in normal and tumor cells. Proc. Natl Acad. Sci. USA, 90, 2846–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits R. et al. (1999) Apc1638T: a mouse model delineating critical domains of the adenomatous polyposis coli protein involved in tumorigenesis and development. Genes Dev., 13, 1309–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Wetering M. et al. (2002) The β-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell, 111, 241–250. [DOI] [PubMed] [Google Scholar]
- von Kries J.P., Winbeck,G., Asbrand,C., Schwarz-Romond,T., Sochnikova,N., Dell’Oro,A., Behrens,J. and Birchmeier,W. (2000) Hot spots in β-catenin for interactions with LEF-1, conductin and APC. Nat. Struct. Biol., 7, 800–807. [DOI] [PubMed] [Google Scholar]
- Wiechens N. and Fagotto,F. (2001) CRM1- and Ran-independent nuclear export of β-catenin. Curr. Biol., 11, 18–27. [DOI] [PubMed] [Google Scholar]
- Yokoya F., Imamoto,N., Tachibana,T. and Yoneda,Y. (1999) β-Catenin can be transported into the nucleus in a Ran-unassisted manner. Mol. Biol. Cell, 10, 1119–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F., White,R.L. and Neufeld,K.L. (2000) Phosphorylation near nuclear localization signal regulates nuclear import of adenomatous polyposis coli protein. Proc. Natl Acad. Sci. USA, 97, 12577–12582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F., White,R.L. and Neufeld,K.L. (2001) Cell density and phosphorylation control the subcellular localization of adenomatous polyposis coli protein. Mol. Cell. Biol., 21, 8143–8156. [DOI] [PMC free article] [PubMed] [Google Scholar]