Abstract
Active repression of protein synthesis protects cells against protein malfolding during endoplasmic reticulum stress, nutrient deprivation and oxidative stress. However, long-term adaptation to these conditions requires synthesis of new stress-induced proteins. Phosphorylation of the α-subunit of translation initiation factor 2 (eIF2α) represses translation in diverse stressful conditions. GADD34 is a stress-inducible regulatory subunit of a holophosphatase complex that dephosphorylates eIF2α, and has been hypothesized to play a role in translational recovery. Here, we report that GADD34 expression correlated temporally with eIF2α dephosphorylation late in the stress response. Inactivation of both alleles of GADD34 prevented eIF2α dephosphorylation and blocked the recovery of protein synthesis, normally observed late in the stress response. Furthermore, defective recovery of protein synthesis markedly impaired translation of stress-induced proteins and interfered with programmed activation of stress-induced genes in the GADD34 mutant cells. These observations indicate that GADD34 controls a programmed shift from translational repression to stress-induced gene expression, and reconciles the apparent contradiction between the translational and transcriptional arms of cellular stress responses.
Keywords: protein phosphatases/protein synthesis/secretion/signal transduction
Introduction
Diverse upstream stress signals are coupled to a common effector step, the phosphorylation of serine 51 on the α subunit of the eukaryotic translation initiation factor 2 (eIF2). eIF2α phosphorylation inhibits nucleotide exchange on the eIF2 complex, attenuating translation of most mRNAs and reducing protein synthesis (Hinnebusch, 2000). This adaptation is believed to protect cells against potentially toxic malfolded or modified proteins that may accumulate under stressed conditions (Brostrom and Brostrom, 1998). eIF2α phosphorylation can also activate downstream gene expression programs by paradoxically promoting translation of special mRNAs encoding transcription factors such as yeast Gcn4p or mammalian ATF4 (Hinnebusch, 1996; Harding et al., 2000a). Phosphorylated eIF2α thus integrates diverse and seemingly unrelated forms of stress to initiate signaling in a common downstream stress-response pathway that we refer to as an integrated stress response.
The endoplasmic reticulum (ER) resident transmembrane eIF2α kinase PERK is activated by imbalance between the load of client proteins translocated into the ER lumen and the capacity of the organelle to process the load (so-called ER stress) (Kaufman, 1999; Mori, 2000; Patil and Walter, 2001). The resulting rapid decrease in protein synthesis reduces the load on the ER and plays an important role in cells’ ability to resist ER stress (Harding et al., 1999, 2000b, 2001a; Scheuner et al., 2001). In the erythropoietic lineage, a different eIF2α kinase, HRI, is activated by lack of heme. The resulting decrease in globin synthesis prevents accumulation of toxic free globin chains and protects developing erythrocytes from destruction under conditions of limited iron availability (Han et al., 2001). In cells exposed to arsenite or cadmium, eIF2α phosphorylation and translation repression, which are effected by unknown mechanisms, minimize modification of newly synthesized proteins by the reactive transition metal (Brostrom et al., 1989; Brostrom and Brostrom, 1998). In all three examples, the phosphorylation of eIF2α protects the stressed cell from the toxic potential of its own modified or unfolded proteins.
The ER unfolded protein response (UPR) that activates PERK also induces transcription of genes whose products increase the organelle’s capacity to process its client proteins (Kaufman, 1999; Mori, 2000; Patil and Walter, 2001). The implementation of this adaptation requires that the induced mRNAs be translated into proteins. Furthermore, signaling in two of the three pathways known to activate gene expression in the UPR requires new protein synthesis; both XBP-1, an effector of IRE1 signaling (Yoshida et al., 2001; Calfon et al., 2002), and ATF4, an effector of the eIF2α phosphorylation-mediated integrated stress response (Harding et al., 2000a; also see below) must be translated de novo. Some UPR-induced mRNAs have features that may protect them from translational repression (Macejak and Sarnow, 1990), and translation of some mRNAs, such as the ATF4 mRNA, is even induced by modest levels of eIF2α phosphorylation (Harding et al., 2000a); however translation of most mRNA, including those of important ER chaperones like GRP78 (BiP) and GRP94 are repressed by severe ER stress (Harding et al., 2001b; and see below). Therefore, profound and persistent translational repression is seemingly at odds with cells’ ability to effect a more long-term adaptation to ER stress. In arsenite-treated cells also, eIF2α phosphorylation-mediated shut down in protein synthesis should interfere with synthesis of new proteins that promote long-term resistance to the toxin (Levinson et al., 1980; Sok et al., 2001; and see below).
It has long been known that translational repression in response to both ER stress and exposure to arsenite is transient, and that expression of stress-induced mRNAs and their encoded proteins coincides with a phase of translational recovery (Brostrom et al., 1989; Brostrom and Brostrom, 1998; Kaufman, 1999). Yet the cellular mechanisms underlying translational recovery remain poorly understood.
GADD34 is a stress-induced gene encoding a regulatory subunit of a protein phosphatase 1 (PP1c)-containing complex that can dephosphorylate eIF2α in vitro and in vivo (He et al., 1996; Novoa et al., 2001). Herpes simplex virus has evolved a GADD34-like activity to evade the consequences of eIF2α phosphorylation that are induced by the eIF2α kinase PKR in virally infected cells (Roizman, 1999). GADD34 is itself a target of the eIF2α phosphorylation-mediated integrated stress response (Novoa et al., 2001), suggesting that it may promote eIF2α dephosphorylation in stressed cells. Therefore, we decided to study GADD34’s role in switching between an early phase of translational repression and a later phase of derepression in the course of stress responses.
Results
eIF2α phosphorylation plays a role in GADD34 induction by the integrated stress response
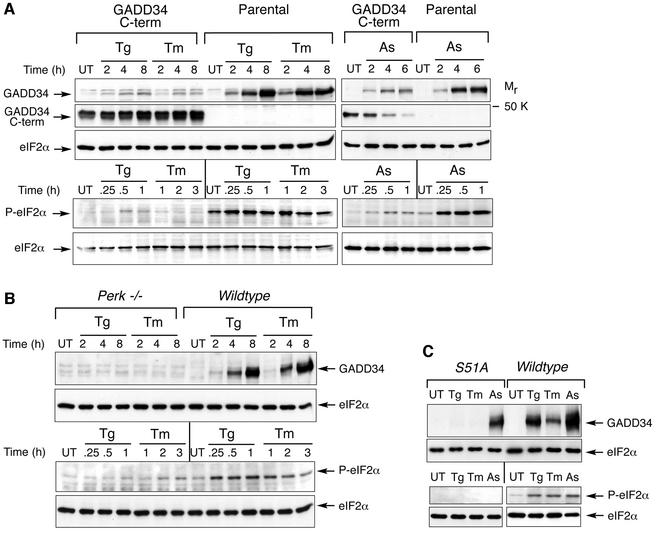
eIF2α phosphorylation at serine 51 promotes expression of stress-induced genes, such as the transcription factors ATF4 and CHOP (Scheuner et al., 2001). Expression of these genes, as well as GADD34, under conditions of ER stress or amino acid deprivation, requires activity of the upstream eIF2α kinases PERK and GCN2 (Harding et al., 2000a; Novoa et al., 2001). To confirm that induction of GADD34 protein in stressed cells proceeds via signaling through eIF2α phosphorylation, we compared endogenous GADD34 protein levels in stressed cells that overexpress a C-terminal fragment of GADD34 that constitutively dephosphorylates eIF2α with their level in similarly stressed wild-type cells (Novoa et al., 2001). Inhibition of eIF2α phosphorylation attenuated endogenous GADD34 expression in cells treated with agents that cause ER stress (thapsigargin and tunicamycin) or oxidative stress (arsenite) (Figure 1A). PERK–/– cells that are impaired in eIF2α phosphorylation in response to ER stress also failed to express GADD34 protein when challenged with thapsigargin or tunicamycin (Figure 1B), as predicted by our previous studies (Novoa et al., 2001). In mutant mouse embryonic fibroblasts in which both alleles of eIF2α encode a mutant protein that can not be phosphorylated by stress-activated kinases (S51A substitution; Scheuner et al., 2001), activation of GADD34 by ER stress is abolished completely and activation by arsenite is attenuated (Figure 1C). These observations support a role for eIF2α phosphorylation in promoting expression of GADD34 protein in stressed cells.
Fig. 1. eIF2α phosphorylation promotes GADD34 expression. (A) Immunoblot of GADD34, eIF2α phosphorylated on serine 51 (P-eIF2α) and total eIF2α from untreated (UT), thapsigargin (Tg)-, tunicamycin (Tm)-, or arsenite (As)-treated NIH-3T3 (Parental) cells or cells expressing a C-terminal fragment of GADD34 that constitutively dephosphorylates eIF2α (GADD34 C-term) (Novoa et al., 2001). (B) Immunoblot of GADD34, phosphorylated eIF2α and total eIF2α from untreated, thapsigargin and tunicamycin treated wild-type and PERK–/– mouse fibroblasts. (C) Immunoblot of GADD34 and total eIF2α from untreated (UT), thapsigargin (Tg; 8 h)-, tunicamycin (Tm; 8 h)- and arsenite (As; 4 h)-treated mouse embryonic fibroblasts with the indicated eIF2α genotype (upper panels). eIF2α phosphorylation was measured by immunoblot in the same cells following thapsigargin (Tg; 2 h), tunicamycin (Tm; 2 h) and arsenite (As; 2 h) treatment (lower panels).
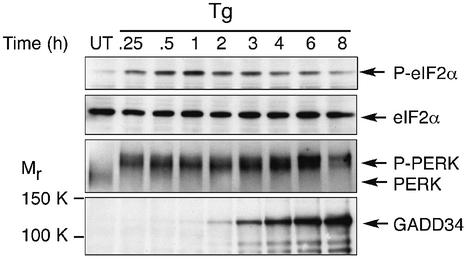
Levels of phosphorylated eIF2α have been shown to decrease late during the stress response (Brostrom et al., 1989; Brostrom and Brostrom, 1998; Kaufman, 1999), but the underlying mechanism remained obscure. We decided to examine the temporal relationship between changes in eIF2α phosphorylation levels during an ER stress response and the activity of the upstream kinase PERK and expression of the putative stress-induced phosphatase GADD34. Cells were treated continuously with the ER stress-inducer thapsigargin, and the phosphorylation state of eIF2α was measured at intervals by immunoblot with an antibody specific to the phosphorylated form of the protein. Activation of the upstream eIF2α kinase PERK was monitored by following the characteristic shift in mobility of the phosphorylated active form of the protein by SDS–PAGE (Harding et al., 1999), whereas GADD34 expression in the same lysates was measured by immunoblot. eIF2α dephosphorylation was noted despite persistent activation of the upstream kinase PERK (Figure 2). GADD34 protein is expressed at very low levels in unstressed cells, and its induction by thapsigargin correlated temporally with the phase of the stress response in which levels of phosphorylated eIF2α are observed to decline (Figure 2). While the temporal profile of GADD34 expression did not obviously precede eIF2α dephosphorylation, the near coincidence of the two events is consistent with a role for GADD34 in modulating phospho-eIF2α levels.
Fig. 2. Time course of GADD34 expression, eIF2α phosphorylation and PERK activation in ER stressed cells. Shown are immunoblots of P-eIF2α, total eIF2α, activated phosphorylated PERK (P-PERK), inactive PERK and GADD34 from untreated (UT) and thapsigargin (Tg)-treated NIH 3T3 cells.
Generation of GADD34ΔC-targeted cells
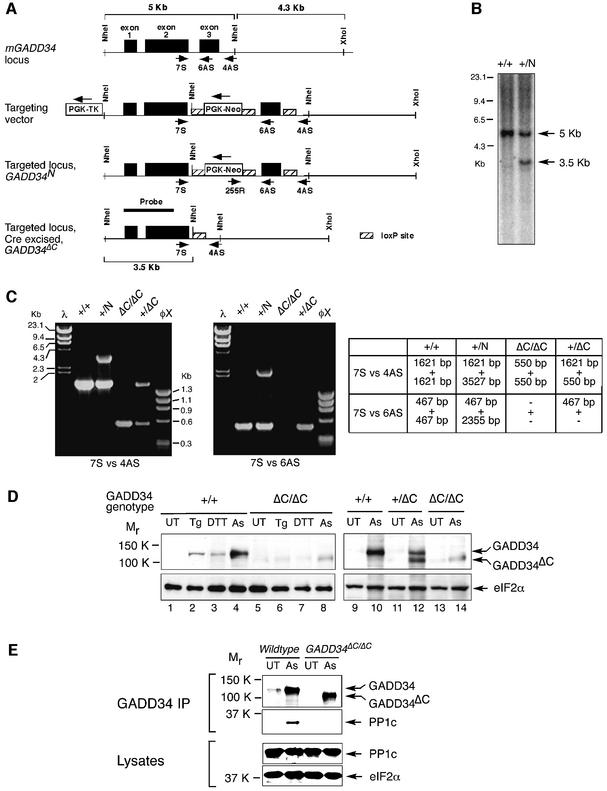
To further explore the role of GADD34 in eIF2α dephosphorylation in stressed cells, we introduced a targeted mutation into the GADD34 gene to delete the third exon, which encodes the domain of GADD34 protein (AA 549–657) that interacts with PP1c, the catalytic subunit of the phosphatase (Novoa et al., 2001). The targeting vector was designed to first introduce two loxP sites into the introns surrounding exon 3 and a neomycin resistance gene surrounded by loxP sites (Figure 3A). Mouse embryonic stem cell (ES) clones containing this GADD34N-targeted allele were obtained after selection with G418 and gancyclovir, and their genotype was confirmed by PCR and Southern blot (Figure 3B and C). Cre recombinase was transiently expressed to isolate ES clones that excised exon 3 and the neomycin resistance cassette (GADD34ΔC; Figure 3C). The mutant allele was passed through the germline of chimeric mice, and homozygous mutant GADD34ΔC/ΔC mouse embryonic fibroblasts were prepared from mutant embryos. Immunoblot analysis showed that the mutant allele encoded a truncated stress-induced protein, GADD34ΔC, of the predicted size, which was detected in the heterozygous (+/ΔC) and homozygous mutant cells (ΔC/ΔC) but was absent in the wild-type cells (Figure 3D).
Fig. 3. Targeted mutagenesis of GADD34. (A) Scheme of the genomic organization of mouse GADD34 and targeting strategy to delete exon 3 encoding the PP1c-interacting domain (amino acid residues 549–657). From the top down: the wild-type locus; the targeting vector with the position of the oligonucleotides used to genotype the derivative alleles (arrows) and the loxP-sites (hatched rectangles) showing; the targeted GADD34N locus before Cre-mediated excision of the Neo-cassette and exon 3; and the mutant GADD34ΔC allele after Cre-mediated excision of exon 3 and the Neo cassette. (B) Southern blot analysis of NheI-digested genomic DNA of the indicated GADD34 genotypes. The position of the radiolabeled probe (HinDIII cDNA fragment containing exon 1 and part of exon 2) and the predicted genomic GADD34 NheI fragments (5 and 3.5 Kb) are indicated in (A) above. (C) Detection of the various GADD34 alleles by PCR. The primers 7S versus 4AS, and 7S versus 6AS are shown in (A). The table indicates the expected PCR products for each GADD34 genotype. +, wild-type locus; N, targeted locus; ΔC, targeted locus after CRE-mediated excised of exon 3 and the neo cassette. (D) Immunoblot analysis of GADD34 gene product in untreated (UT), thapsigargin (Tg)-, dithiothreitol (DTT)- and arsenite (As)-treated wild-type (+/+), GADD34ΔC/+ and GADD34ΔC/ΔC fibroblasts. eIF2α immunoblotting serves as a control for loading. (E) GADD34 and protein phosphatase 1 (PP1c) immunoblot of GADD34–PP1c complexes immunoprecipitated with anti-GADD34 antiserum from untreated (UT) and arsenite (As)-treated wild-type and GADD34ΔC/ΔC cells (upper panels). Immunoblot of PP1c and total eIF2α in the lysate that served as the input for the GADD34–PP1c complex immunoprecipitation (two lower panels).
To verify that the truncated protein encoded by the mutant allele lacked eIF2α phosphatase activity, we analyzed the interaction between GADD34ΔC and the catalytic subunit of the protein phosphatase 1. Immunoprecipitation with GADD34 antibodies showed that GADD34ΔC did not form a complex with PP1c (Figure 3E), a result consistent with previous observations whereby overexpressing the GADD34ΔC truncated protein in transfected wild-type cells had no measurable impact on their stress response (Novoa et al., 2001). This, together with the normal stress response of heterozygous (+/ΔC) mutant cells (data not shown), suggests that the mutant allele has pure loss-of-function features and is devoid of significant dominant-negative effects on the stress response.
GADD34 is required for eIF2α dephosphorylation and translational recovery in response to ER stress and oxidative stress
Next, we examined the impact of the GADD34 mutation on eIF2α phosphorylation and protein synthesis in stressed cells. Wild-type and GADD34ΔC/ΔC mouse embryonic fibroblasts were continuously exposed to thapsigargin (an agent that causes persistent ER stress). At various time-points the rate of protein synthesis was measured by incorporation of radiolabeled methionine and cysteine into newly synthesized proteins, and eIF2α phosphorylation was analyzed in the same samples by immunoblotting with antibodies specific for the phosphorylated form of the protein.
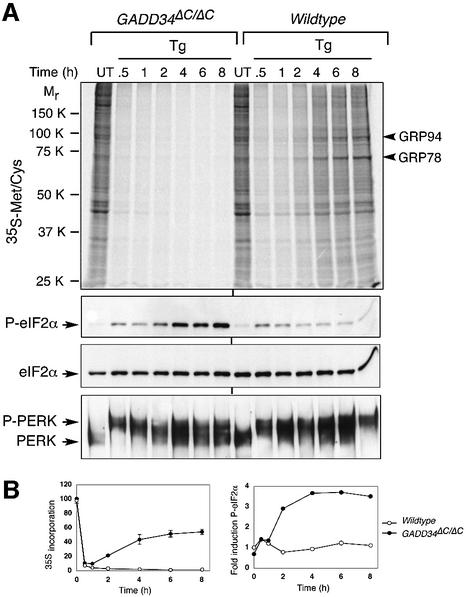
In wild-type cells, thapsigargin treatment was associated with a profound but transient eIF2α phosphorylation and repression of new protein synthesis that was maximal between 0.5 and 2 h of treatment. This was followed by a partial decrease in eIF2α phosphorylation and partial recovery of protein synthesis later in the course of the stress response (Figure 4). These observations are consistent with previouse studies (Prostko et al., 1993). Translation of the major ER stress-inducible chaperones, GRP78 and GRP94, occurred during this later phase of recovery from translational repression (Figure 4A). In contrast, thapsigargin-treated GADD34ΔC/ΔC cells experienced a progressive increase in eIF2α phosphorylation and persistent repression of protein synthesis, which interfered with translation of ER chaperones (Figure 4A and B). Activation of the stress-inducible kinase PERK was similar in both genotypes, suggesting that the mutation impacted on the dephosphorylation of eIF2α and not on stress-induced phosphorylation.
Fig. 4. GADD34 is required for eIF2α dephosphorylation and translational recovery during ER stress. (A) Upper panel, autoradiogram of an SDS–PAGE gel showing 35S-methionine/cysteine incorporation into newly synthesized proteins in untreated (UT) and thapsigargin (Tg)-treated wild-type and GADD34ΔC/ΔC fibroblasts. Arrowheads to the right of the autoradiogram indicate the ER stress-inducible chaperones GRP78 and GRP94. Lower panels, immunoblots of P-eIF2α and total eIF2α, and immunoblots of PERK immunoprecipitates from the same cells. (B) Graphic presentation of 35S-methionine/cysteine incorporation into newly synthesized proteins (left graph), and fold induction of phosphorylated eIF2α (right graph) in untreated and thapsigargin-treated wild-type and GADD34ΔC/ΔC fibroblasts. The level of 35S incorporation in untreated cells is set at 100% whereas the signal of phosphorylated eIF2α from untreated wild-type cells is set as 1. Shown are mean ± SEM of experiments performed in duplicate and reproduced twice.
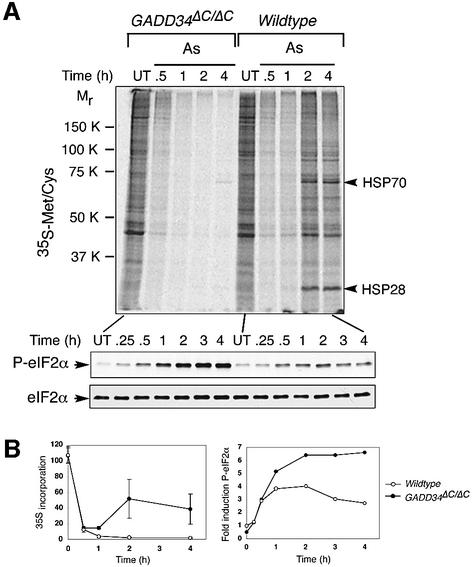
Similar differences between wild-type and mutant cells were observed in response to oxidative stress. In arsenite-treated wild-type cells, eIF2α phosphorylation was followed by dephosphorylation, translational recovery and synthesis of major stress-induced proteins HSP70 and HSP28, whereas persistent phosphorylation and translational repression were noted in arsenite-treated GADD34ΔC/ΔC mutant cells (Figure 5). The observations described above were made in mouse fibroblasts explanted from wild-type and mutant embryos. We observed similar effects of the GADD34 mutation in ER stressed and arsenite-treated ES cells (data not shown). These results show that GADD34-mediated eIF2α dephosphorylation plays a major role in stress responses as it is required for decreasing eIF2α phosphorylation levels late during the stress response. Inability to decrease the levels of phosphorylated eIF2α in the GADD34 mutant cells impairs translational recovery and stress-induced protein synthesis.
Fig. 5. GADD34 is required for eIF2α dephosphorylation and translational recovery during arsenite treatment. (A) Upper panel, autoradiogram of an SDS–PAGE gel showing 35S-methionine/cysteine incorporation into newly synthesized proteins in untreated (UT) and arsenite (As)-treated wild-type and GADD34ΔC/ΔC fibroblasts. Arrowheads to the right of the autoradiogram indicate the arsenite-inducible chaperones HSP70 and HSP28. Lower panels, immunoblots of P-eIF2α and total eIF2α from the same cells. (B) Graphic presentation of 35S-methionine/cysteine incorporation into newly synthesized proteins (left graph), and fold induction of phosphorylated eIF2α (right graph) in untreated and arsenite-treated wild-type and GADD34ΔC/ΔC fibroblasts. The level of 35S incorporation in untreated cells is set at 100% whereas the signal of phosphorylated eIF2α from untreated wild-type cells is set as 1. Shown are mean ± SEM of experiments performed in duplicate and reproduced twice.
GADD34-mediated eIF2α dephosphorylation is required for stress-induced gene expression
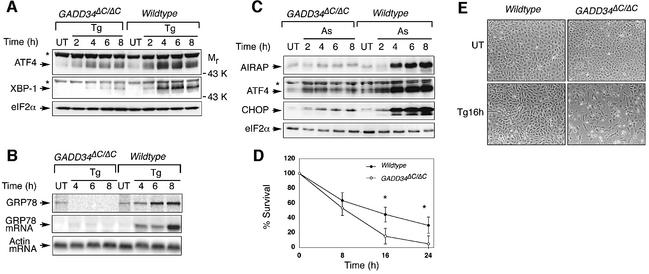
Expression of UPR target proteins is preceded by stress-induced transcription of their mRNAs (Kaufman, 1999; Patil and Walter, 2001). However, some of the transcription factors that mediate this induction, such as ATF4 and XBP-1, must be synthesized de novo (Harding et al., 2000a; Yoshida et al., 2001; Calfon et al., 2002). Accumulation of these upstream activators of stress-induced gene expression was impaired in GADD34ΔC/ΔC cells, most notably later in the course of the stress response (Figure 6A). Therefore, we wished to determine if GADD34-mediated eIF2α dephosphorylation also impacted on transcriptional induction of downstream UPR target genes. Northern blot analysis and pulse-labeling followed by immunoprecipitation showed that GADD34ΔC/ΔC cells were impaired not only in biosynthesis of GRP78 protein (Figures 4A and 6B) but also in induction of the encoding stress-induced mRNA (Figure 6B). In arsenite-treated GADD34ΔC/ΔC mutant cells, persistent repression of protein synthesis blocked the accumulation of arsenite-inducible proteins such as ATF4, CHOP and AIRAP (Figure 6C). Therefore, inability to restore protein synthesis in GADD34ΔC/ΔC cells affected not only the translation of the downstream targets of the stress response but also impaired activation of stress-induced gene expression programs.
Fig. 6. GADD34 promotes stress-induced gene expression. (A) Immunoblot of the UPR-induced transcription factors ATF4 and XBP-1 in untreated (UT) and thapsigargin-treated (Tg) wild-type and GADD34ΔC/ΔC fibroblasts. The asterisks mark irrelevant proteins detected by the antisera. (B) Upper panel, autoradiogram of an SDS–PAGE gel of GRP78 immunoprecipitated from untreated (UT) and thapsigargin (Tg)-treated wild-type and GADD34ΔC/ΔC fibroblasts labeled with 35S-methionine/cysteine. Middle and lower panels are northern blots of GRP78 and β-actin mRNA from the same cells. (C) Immunoblot of the arsenite-induced proteins AIRAP (Sok et al., 2001), ATF4 and CHOP from untreated and arsenite-treated wild-type and GADD34ΔC/ΔC fibroblasts. (D) Survival of thapsigargin-treated wild-type and GADD34ΔC/ΔC fibroblasts, measured by their ability to reduce MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide). Shown are the mean ± SEM of measurements carried out in duplicate on two different clones of cells from each genotype and reproduced 3 times. The asterisks denote P < 0.0001 by two-tailed t-test. 100% refers to MTT reduction by the untreated cells. (E) Photomicrographs of untreated and thapsigargin-treated wild-type and GADD34ΔC/ΔC fibroblasts.
The gene expression programs activated during stress responses impart stress resistance (Morimoto, 1998; Kaufman, 1999). Therefore, we compared the ability of wild-type and GADD34ΔC/ΔC cells to survive exposure to thapsigargin, an agent that causes ER stress. Mutant cells experienced decreased survival compared with wild-type cells (Figure 6D and E). Thus, GADD34, which is required for full induction of stress-induced gene expression, also protects stressed cells from death.
Discussion
The experiments described here, suggest a scenario whereby the integrated stress response, a signaling pathway activated by eIF2α phosphorylation of diverse causes, induces GADD34, which binds the catalytic subunit of a holophosphatase complex, directing it to phosphorylated eIF2α. GADD34-mediated eIF2α dephosphorylation thereby forms the effector arm of a negative-feedback loop that limits the impact of stress-activated eIF2α kinases such as PERK. Because of the inherent delay associated with induction of GADD34 mRNA and translation of the protein, this negative-feedback loop has built-in latency that accounts for the biphasic profile of translational repression in stressed cells.
Previous studies have documented the ability of GADD34 to promote eIF2α dephosphorylation, and have revealed the role of stress-inducible eIF2α kinases in activating GADD34 gene expression (He et al., 1996; Novoa et al., 2001). The experiments described in this paper establish a crucial role for GADD34-mediated eIF2α dephosphorylation in translational recovery during diverse stress responses. Analysis of GADD34 mutant cells that are incapable of effecting such translational recovery have revealed, for the first time, the essential role this process plays in stress-induced gene expression. GADD34 activity thus reconciles the apparent contradiction between the translational and transcriptional arms of cellular stress responses.
As GADD34 is a target of the integrated stress response and the encoded protein is a proximal agent of translational recovery, it must be synthesized under relatively repressive conditions of eIF2α phosphorylation. The features that allow GADD34 mRNA to be translated when eIF2α is phosphorylated are not known. The GADD34ΔC/ΔC cells also reveal some of the complexity involved in activating proximal targets of the integrated stress response. Accumulation of ATF4 protein is strictly dependent on stress-induced eIF2α phosphorylation and on the uORFs in its mRNA (Harding et al., 2000a; Novoa et al., 2001). Yet, in stressed GADD34ΔC/ΔC cells, ATF4 protein is expressed at lower levels than in the wild type (Figure 6A and C), despite very high levels of eIF2α phosphorylation in the mutant cells (Figures 4 and 5). This finding is consistent both with previous observations whereby uORFs afford but limit immunity from translational repression (Dever et al., 1993), and with the possible role of transcriptional feed-forward loops that reinforce ATF4 expression (Harding et al., 2000a) but are disrupted by the GADD34 mutation. It is interesting to note that stressed GADD34ΔC/+ cells express more of the truncated protein encoded by the mutant allele than the homozygous mutant GADD34ΔC/ΔC cells (Figure 3D, compare lanes 12 and 14). This observation suggests that GADD34-mediated eIF2α dephosphorylation may also participate in a feed-forward loop that supports its own expression.
The significance of GADD34 as an agent of translational recovery is most apparent under conditions of severe stress, with high levels of eIF2α phosphorylation and marked repression of protein synthesis. Such conditions are found in ischemic neurons in developing brain infarcts (Doutheil et al., 1997; Kumar et al., 2001; DeGracia et al., 2002), and it is likely that under these circumstances GADD34 may play an important role in the ultimate recovery of protein synthesis. Another circumstance in which GADD34 may play an important role in translational recovery is during arousal from the torpor of hibernation, as the tissues of hibernating mammals exhibit very high levels of eIF2α phosphorylation and polysome deaggregation. Translational repression in hibernating animals is reversed immediately upon arousal in a process that may involve GADD34 (Frerichs et al., 1998; Connor et al., 2001). Our studies suggest that in these circumstances, GADD34 could have a profound impact on the outcome of the stress response, as reflected in the decreased survival of ER stressed GADD34ΔC/ΔC cells (Figure 6D and E).
Our experiments show that in stressed cells, GADD34-mediated translational derepression is important not only for translation of the downstream effectors of long-term adaptations to stress, but also for the expression of essential intermediates in the activation of stress-induced gene expression programs. However, it is important to remember that signaling through eIF2α phosphorylation also occurs in physiological circumstances, such as in the β cells of the endocrine pancreas during glycemic excursions (Harding et al., 2001a), or in the liver in the course of normal metabolism (Scheuner et al., 2001). Under these circumstances, activation of GADD34 may be important in modulating the intensity of the integrated stress response. Limiting activity in the integrated stress response may promote cell survival as at least one of the effectors of this response, the transcription factor CHOP, has been implicated in promoting programmed cell death in ER stressed cells (Zinszner et al., 1998; McCullough et al., 2001; Oyadomari et al., 2001). GADD34 is an essential component of a negative-feedback loop operating in the integrated stress response. The physiological impact of this loop is likely to depend on the severity and rate at which stress develops.
Materials and methods
ES cell culture and gene targeting
The mouse GADD34 gene was targeted in W4 ES cells by deleting the third exon encoding residues 549–657 that contains the PP1c binding domain required for phosphatase activity of the complex (Novoa et al., 2001). The 3577 base pair NheI–NdeI and 6078 base pair XmaI–XhoI fragments constituted the 5′ and 3′ homology arms of the targeting vector. A PGK-Neor cassette surrounded by loxP sites was inserted into the intron between exon 2 and 3, and a third loxP site was inserted 3′ to the third exon. ES clones with the appropriately targeted locus were then electroporated with a CRE-expressing plasmid to remove the PGK-Neor cassette and delete the third exon of the gene. Selected clones were injected into blastocysts for germline transmission. Mouse embryonic fibroblasts were obtained at day 13.5 from embryos parented by heterozygous mutant animals.
Cell culture
NIH3T3 cells, wild-type, PERK–/– (Harding et al., 2000b) and GADD34ΔC/ΔC mouse embryonic fibroblasts were maintained in DMEM supplemented with 10% fetal bovine serum, and eIF2α Ser 51 to Ala mutant mouse fibroblasts (Scheuner et al., 2001) were grown in DMEM supplemented with 10% fetal bovine serum, β-mercaptoethanol (0.55 µM) and non-essential amino acids (10 µM). The constitutively active GADD34 C-terminal protein fragment was expressed in cells by transduction of the A1 retrovirus (Novoa et al., 2001). Cells were treated with tunicamycin (2 µg/ml), thapsigargin (0.2 µM), DTT (1 mM) and sodium arsenite (80 µM), as described previously (Harding et al., 1999, 2000b).
Immunoblot, immunoprecipitation and northern blot
Cell extracts for immunoblotting and immunoprecipitation were obtained by lysis in 0.5% Triton X-100-containing buffer as described previously (Harding et al., 2000a). GADD34–PP1c complexes were immunoprecipitated with GADD34 antibodies bound to protein A–Sepharose beads after incubation with cell extracts containing 1 mg of protein as described previously (Novoa et al., 2001).
Newly synthesized proteins were labeled in vivo by a 15 min pulse of 35S-Trans label (ICN; 50 µCi/ml) in DMEM lacking methionine but supplemented with 5% dialyzed calf serum. Cell extracts were prepared in 0.5% Triton X-100-containing buffer, and labeled proteins were resolved by SDS–PAGE and autoradiographed (Harding et al., 1999). Incorporation of 35S-methionine/cysteine into newly synthesized GRP78 was revealed by immunoprecipitation, SDS–PAGE and autoradiography.
Rabbit polyclonal antibodies against GADD34 were prepared by immunization with a fusion protein of the N-terminal 536 residues of mouse GADD34 to glutathione S-transferase. PERK (Harding et al., 2000b), ATF4 (Vallejo et al., 1993), AIRAP (Sok et al., 2001) and CHOP (Ron and Habener, 1992) were detected as described previously. The antiserum to GRP78 was a gift from Gert Kreibich, and those to PP1c and XBP-1 were purchased from Santa Cruz Biotechnology. The phosphorylated form of eIF2α was detected with an antibody specific to the protein phosphorylated on serine 51 (Research Genetics), and total eIF2α was detected with a monoclonal mouse antibody (Scorsone et al., 1987).
RNA preparation and northern blot analysis of GRP78 (BiP) and β-actin mRNA were carried out as described previously (Wang et al., 1996).
Acknowledgments
Acknowledgements
We thank David Levy, Robert Schneider and Joel Belasco for comments on the manuscript, NYU’s Kaplan Comprehensive Cancer Center’s transgenic mouse facility for producing chimeric mice, and Randal J.Kaufman and Donalyn Scheuner for generously providing the eIF2αS51A mutant cells. This work was supported by NIH grants (ES08681 and DK47119). D.R. is an Ellison Medical Foundation Senior Scholar in aging.
References
- Brostrom C.O. and Brostrom,M.A. (1998) Regulation of translational initiation during cellular responses to stress. Prog. Nucleic Acid Res. Mol. Biol., 58, 79–125. [DOI] [PubMed] [Google Scholar]
- Brostrom M.A., Lin,X.J., Cade,C., Gmitter,D. and Brostrom,C.O. (1989) Loss of a calcium requirement for protein synthesis in pituitary cells following thermal or chemical stress. J. Biol. Chem., 264, 1638–1643. [PubMed] [Google Scholar]
- Calfon M., Zeng,H., Urano,F., Till,J.H., Hubbard,S.R., Harding,H.P., Clark,S.G. and Ron,D. (2002) IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature, 415, 92–96. [DOI] [PubMed] [Google Scholar]
- Connor J.H., Weiser,D.C., Li,S., Hallenbeck,J.M. and Shenolikar,S. (2001) Growth arrest and DNA damage-inducible protein gadd34 assembles a novel signaling complex containing protein phosphatase 1 and inhibitor 1. Mol. Cell. Biol., 21, 6841–6850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGracia D.J., Kumar,R., Owen,C.R., Krause,G.S. and White,B.C. (2002) Molecular pathways of protein synthesis inhibition during brain reperfusion: implications for neuronal survival or death. J. Cereb. Blood Flow Metab., 22, 127–141. [DOI] [PubMed] [Google Scholar]
- Dever T.E., Chen,J.J., Barber,G.N., Cigan,A.M., Feng,L., Donahue,T.F., London,I.M., Katze,M.G. and Hinnebusch,A.G. (1993) Mammalian eukaryotic initiation factor 2 α kinases functionally substitute for GCN2 protein kinase in the GCN4 translational control mechanism of yeast. Proc. Natl Acad. Sci. USA, 90, 4616–4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doutheil J., Gissel,C., Oschlies,U., Hossmann,K.A. and Paschen,W. (1997) Relation of neuronal endoplasmic reticulum calcium homeostasis to ribosomal aggregation and protein synthesis: implications for stress-induced suppression of protein synthesis. Brain Res., 775, 43–51. [DOI] [PubMed] [Google Scholar]
- Frerichs K.U., Smith,C.B., Brenner,M., DeGracia,D.J., Krause,G.S., Marrone,L., Dever,T.E. and Hallenbeck,J.M. (1998) Suppression of protein synthesis in brain during hibernation involves inhibition of protein initiation and elongation. Proc. Natl Acad. Sci. USA, 95, 14511–14516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han A.P. et al. (2001) Heme-regulated eIF2α kinase (HRI) is required for translational regulation and survival of erythroid precursors in iron deficiency. EMBO J., 20, 6909–6918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding H., Zhang,Y. and Ron,D. (1999) Translation and protein folding are coupled by an endoplasmic reticulum resident kinase. Nature, 397, 271–274. [DOI] [PubMed] [Google Scholar]
- Harding H., Novoa,I., Zhang,Y., Zeng,H., Wek,R.C., Schapira,M. and Ron,D. (2000a) Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell, 6, 1099–1108. [DOI] [PubMed] [Google Scholar]
- Harding H., Zhang,Y., Bertolotti,A., Zeng,H. and Ron,D. (2000b) Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol. Cell., 5, 897–904. [DOI] [PubMed] [Google Scholar]
- Harding H., Zeng,H., Zhang,Y., Jungreis,R., Chung,P., Plesken,H., Sabatini,D. and Ron,D. (2001a) Diabetes mellitus and excocrine pancreatic dysfunction in Perk–/– mice reveals a role for translational control in survival of secretory cells. Mol. Cell., 7, 1153–1163. [DOI] [PubMed] [Google Scholar]
- Harding H.P., Novoa,I., Bertolotti,A., Zeng,H., Zhang,A., Urano,F., Jousse,C. and Ron,D. (2001b) Translational regulation in the cellular response to biosynthetic load on the endoplasmic reticulum. Cold Spring Harb. Symp. Quant. Biol., 66, 499–508. [DOI] [PubMed] [Google Scholar]
- He B., Chou,J., Liebermann,D.A., Hoffman,B. and Roizman,B. (1996) The carboxyl terminus of the murine MyD116 gene substitutes for the corresponding domain of the γ(1)34.5 gene of herpes simplex virus to preclude the premature shutoff of total protein synthesis in infected human cells. J. Virol., 70, 84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch A. (1996) Translational control of GCN4: gene-specific regulation by phosphorylation of eIF2. In Hershey,J., Mathews,M. and Sonenberg,N. (eds), Translational Control. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 199–244.
- Hinnebusch A.G. (2000) Mechanism and regulation of initiator methionyl-tRNA binding to ribosomes. In Sonenberg,N., Hershey, J.W.B. and Mathews,M.B. (eds), Translational control of gene expression. CSHL Press, Cold Spring Harbor, NY, pp. 185–243.
- Kaufman R.J. (1999) Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev., 13, 1211–1233. [DOI] [PubMed] [Google Scholar]
- Kumar R. et al. (2001) Brain ischemia and reperfusion activates the eukaryotic initiation factor 2α kinase, PERK. J. Neurochem., 77, 1418–1421. [DOI] [PubMed] [Google Scholar]
- Levinson W., Oppermann,H. and Jackson,J. (1980) Transition series metals and sulfhydryl reagents induce the synthesis of four proteins in eukaryotic cells. Biochim. Biophys. Acta, 606, 170–180. [DOI] [PubMed] [Google Scholar]
- Macejak D.G. and Sarnow,P. (1990) Translational regulation of the immunoglobulin heavy-chain binding protein mRNA. Enzymes, 44, 310–319. [DOI] [PubMed] [Google Scholar]
- McCullough K.D., Martindale,J.L., Klotz,L.O., Aw,T.Y. and Holbrook,N.J. (2001) Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol. Cell. Biol., 21, 1249–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K. (2000) Tripartite management of unfolded proteins in the endoplasmic reticulum. Cell, 101, 451–454. [DOI] [PubMed] [Google Scholar]
- Morimoto R.I. (1998) Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev., 12, 3788–3796. [DOI] [PubMed] [Google Scholar]
- Novoa I., Zeng,H., Harding,H. and Ron,D. (2001) Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphoryla tion of eIF2α. J. Cell Biol., 153, 1011–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyadomari S., Takeda,K., Takiguchi,M., Gotoh,T., Matsumoto,M., Wada,I., Akira,S., Araki,E. and Mori,M. (2001) Nitric oxide-induced apoptosis in pancreatic β-cells is mediated by the endoplasmic reticulum stress pathway. Proc. Natl Acad. Sci. USA, 98, 10845–10850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil C. and Walter,P. (2001) Intracellular signaling from the endoplasmic reticulum to the nucleus: the unfolded protein response in yeast and mammals. Curr. Opin. Cell Biol., 13, 349–355. [DOI] [PubMed] [Google Scholar]
- Prostko C.R., Brostrom,M.A. and Brostrom,C.O. (1993) Reversible phosphorylation of eukaryotic initiation factor 2 α in response to endoplasmic reticular signaling. Mol. Cell Biochem., 127, 255–265. [DOI] [PubMed] [Google Scholar]
- Roizman B. (1999) HSV gene functions: what have we learned that could be generally applicable to its near and distant cousins? Acta Virol., 43, 75–80. [PubMed] [Google Scholar]
- Ron D. and Habener,J.F. (1992) CHOP, a novel developmentally regulated nuclear protein that dimerizes with transcription factors C/EBP and LAP and functions as a dominant negative inhibitor of gene transcription. Genes Dev., 6, 439–453. [DOI] [PubMed] [Google Scholar]
- Scheuner D., Song,B., McEwen,E., Gillespie,P., Saunders,T., Bonner-Weir,S. and Kaufman,R.J. (2001) Translational control is required for the unfolded protein response and in-vivo glucose homeostasis. Mol. Cell, 7, 1165–1176. [DOI] [PubMed] [Google Scholar]
- Scorsone K.A., Panniers,R., Rowlands,A.G. and Henshaw,E.C. (1987) Phosphorylation of eukaryotic initiation factor 2 during physiological stresses which affect protein synthesis. J. Biol. Chem., 262, 14538–14543. [PubMed] [Google Scholar]
- Sok J., Calfon,M., Lu,J., Lichtlen,P., Clark,S. and Ron,D. (2001) Arsenite-inducible RNA-associated protein (AIRAP) protects cells from arsenite toxicity. Cell Stress Chaperones, 6, 6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo M., Ron,D., Miller,C.P. and Habener,J.F. (1993) C/ATF, a member of the activating transcription factor family of DNA-binding proteins, dimerizes with CAAT/enhancer-binding proteins and directs their binding to cAMP response elements. Proc. Natl Acad. Sci. USA, 90, 4679–4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.-Z. et al. (1996) Signals from the stressed endoplasmic reticulum induce C/EBP homologous protein (CHOP/GADD153). Mol. Cell. Biol., 16, 4273–4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Matsui,T., Yamamoto,A., Okada,T. and Mori,K. (2001) XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell, 107, 881–891. [DOI] [PubMed] [Google Scholar]
- Zinszner H., Kuroda,M., Wang,X., Batchvarova,N., Lightfoot,R.T., Remotti,H., Stevens,J.L. and Ron,D. (1998) CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev., 12, 982–995. [DOI] [PMC free article] [PubMed] [Google Scholar]