Abstract
An HLF (HIF-1α-like factor)/HIF-2α-knockout mouse is embryonic lethal, preventing investigation of HLF function in adult mice. To investigate the role of HLF in adult pathological angiogenesis, we generated HLF-knockdown (HLFkd/kd) mice by inserting a neomycin gene sandwiched between two loxP sequences into exon 1 of the HLF gene. HLFkd/kd mice expressing 80–20% reduction, depending on the tissue, in wild-type HLF mRNA were fertile and apparently normal. Hyperoxia–normoxia treatment, used as a murine model of retinopathy of prematurity (ROP), induced neovascularization in wild-type mice, but not in HLFkd/kd mice, whereas prolonged normoxia following hyperoxic treatment caused degeneration of retinal neural layers in HLFkd/kd mice due to poor vascularization. Cre-mediated removal of the inserted gene recovered normal HLF expression and retinal neovascularization in HLFkd/kd mice. Expression levels of various angiogenic factors revealed that only erythropoietin (Epo) gene expression was significantly affected, in parallel with HLF expression. Together with the results from intraperitoneal injection of Epo into HLFkd/kd mouse, this suggests that Epo is one of the target genes of HLF responsible for experimental ROP.
Keywords: erythropoietin/HLF/neovascularization/retinopathy/VEGF
Introduction
Retinal neovascularization is the most common cause of retinopathy of prematurity (ROP), diabetic retinopathy and age-related macular degeneration, which eventually leads to visual loss (Prost, 1988; Moss et al., 1994). Numerous clinical and experimental observations have indicated that ischemia or hypoxia triggers retinal neovascularization through an excessive production of one or more angiogenic factors. Identification of these factors is an important step toward understanding the mechanism of pathological angiogenesis and development of specific treatments for diseases involving angiogenesis, such as proliferative retinopathy, tumor growth and atherosclerosis. Among multiple factors known to be responsible for retinal neovascularization, many groups have reported vascular endothelial growth factor (VEGF) to be important (Aiello et al., 1995; Alon et al., 1995; Pierce et al., 1995; Seo et al., 1999). These groups have shown that VEGF is upregulated in a mouse model of retinal neovascularization (Pierce et al., 1995) and that overexpression of VEGF in transgenic mice stimulates retinal neovascularization (Okamoto et al., 1997; Tobe et al., 1998). They have also shown that VEGF antagonists or kinase inhibitors of the VEGF receptor prevent retinal neovascularization (Aiello et al., 1995; Seo et al., 1999).
The transcription factors HIF-1α (hypoxia-inducible factor-1α) (Wang et al., 1995) and HLF (Ema et al., 1997) [HIF-1α-like factor, also known as EPAS1 (Tian et al., 1997) and HIF-2α (Wenger and Gassmann, 1997)] play important roles in embryonic vascularization, and HIF-1α and HLF also activate the expression of genes such as VEGF (Liu et al., 1995; Forsythe et al., 1996), erythropoietin (Epo) (Wang and Semenza, 1993) and a series of glycolytic enzymes (Firth et al., 1994) in response to ischemic or hypoxic conditions. The two factors are substantially similar in their primary structures and belong to a growing superfamily of transcription factors characterized by structural motifs such as basic helix– loop–helix and PAS (a conserved sequence among Per, Arnt and Sim) domains. Under normoxic conditions, HIF-1α is negatively regulated by ubiquitylation and proteasomal degradation (Kallio et al., 1999). Prolyl hydroxylation is required for the interaction between HIF-1α and von Hippel-Lindau (VHL) protein, which plays a critical role in the ubiquitylation of HIF-1α (Epstein et al., 2001). When the oxygen concentration is reduced, these transcription factors are stabilized and form a heterodimer with another bHLH/PAS protein, Arnt. The heterodimer activates genes encoding angiogenic and hematopoietic factors by binding to the hypoxia responsive element (HRE) in their promoter (Semenza and Wang, 1992; Wang et al., 1995; Ema et al., 1997; Wenger and Gassmann, 1997). However, the modes of expression of HLF and HIF-1α differ substantially in various tissues of adult mice and during developmental processes (Ema et al., 1997; Jain et al., 1998), indicating that they have their own specific physiological functions in vivo.
Gene targeting technology has been utilized to investigate the function of HLF and HIF-1α, and has revealed that their complete deficiency results in developmental arrest and embryonic lethality. Histopathological analyses of homozygotic mutant embryos showed that HLF deficiency either causes severe vascular defects in both the yolk sac and embryo proper (Peng et al., 2000) or displays pronounced bradycardia due to defective catecholamine production (Tian et al., 1998). In contrast, HIF-1α-deficient mice manifested neural tube defects and cardiovascular malformations (Iyer et al., 1998; Ryan et al., 1998). Although the functions of these two transcription factors appear distinct, embryonic lethality prevented a detailed analysis of their contribution to angiogenesis in adult animals.
In this study, we generated HLF knockdown mice by inserting a neomycin resistance gene, sandwiched between two loxP sequences, into exon 1 of the HLF gene that encodes the untranslated region (5′-UTR) of HLF mRNA. In these mice, HLF mRNA was expressed at a much lower level by leaky transcription through a double poly(A) signal of the neo gene. These mice are viable and fertile without any evident pathological abnormality. Using these mutant mice, we report the essential roles of HLF in the mouse model of ROP by regulating the expression of the Epo gene.
Results
Targeting of the HLF gene by homologous recombination
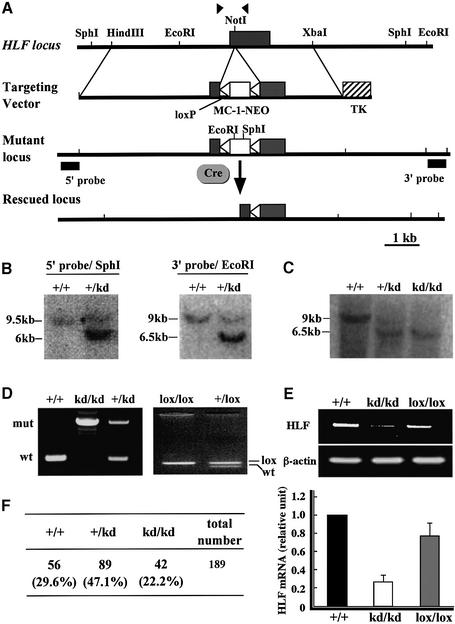
To investigate the role of HLF in mice, a targeting vector was designed so that the mouse HLF gene may be disrupted by inserting a PGK promoter–neomycin gene cassette, which is sandwiched between two loxP sequences, into exon 1 that encodes the 5′-UTR sequence by homologous recombination (Figure 1A). If the double poly(A) signal downstream of the neo gene does not work completely, the mRNA encoding HLF in the sequence continuing from the neo sequence can be produced because of leaky transcription through the double poly(A) signal at the end of the neo gene. The embryonic stem (ES) cell line (clone 144) heterozygous for the recombinant allele (HLFkd/+) was established by positive–negative selection (Figure 1B) and used to generate chimeric mice by blastocyst injection. Resulting chimeric mice were crossed with wild-type C57BL/6J mice to obtain mice heterozygous for the mutated HLF gene (HLFkd/+).
Fig. 1. Mutation of mouse HLF gene. Outline of targeting vector and restriction maps around the first exon (closed box) (A). Arrowheads indicate the sites of primers used to distinguish the wild-type HLF gene from the mutant. MC-1-NEO (open box) indicates the neomycin resistance (NEO) gene under the control of the MC-1 promoter and TK is the thymidine kinase gene under the control of the herpes simplex virus promoter. The NEO gene sandwiched between loxP sequences was inserted into exon 1 at the NotI site. DNA blot analysis of DNAs from recombinant ES cells (B) or mouse tails (C). Genomic DNAs were digested with SphI or EcoRI, and hybridized with the 5′ or 3′ external probe, respectively. Genotyping of the HLF locus from offspring (D). PCR analyses were carried out with genomic DNAs from offspring generated by mating heterozygous HLFkd mice (left panel). PCR analysis of the rescued HLFlox gene is also shown (right panel). The inserted NEO gene was removed by mating HLFkd mice with Cre-transgenic mice. RT–PCR analyses of HLF mRNA expression (E). RNAs were prepared from the eyes of wild-type, HLFkd/kd and HLFlox/lox mice, and subjected to RT–PCR analyses. Real-time quantitative PCR (bottom) was performed, and the amount of HLF mRNA from eyeballs of wild-type mice was designated as 1.0. Genotyping of littermates from the intercrosses of HLFkd/+ heterozygotes 2 weeks after birth (F).
Mice heterozygous for the HLFkd gene were phenotypically indistinguishable from their wild-type littermates and were interbred to yield mice homozygous for the mutated allele (HLFkd/kd). Genotyping of the newborn mice revealed that the HLFkd/kd mice were born in the ratio expected from Mendelian inheritance (Figure 1C and F) and were viable and fertile without any evident histopathological abnormalities. By using real-time quantitative PCR analysis, we have shown that the retinas of HLFkd/kd mice expressed HLF mRNA at about one-fifth that of wild type (Figure 1E). To investigate whether the reduced expression of HLF is due to insertion of the neo gene containing the double poly(A) signal, we deleted the neo gene cassette by mating HLFkd/kd mice with AYU-1-Cre mice that ubiquitously express the Cre protein (Niwa et al., 1993). Genotyping of the resulting offspring revealed that Cre recombinase successfully deleted the neo gene from the HLFkd gene, leaving one remnant loxP in the first exon (HLFlox) (Figure 1D). RT–PCR revealed that HLFlox/lox mice recovered a normal level of HLF mRNA expression comparable to that of the wild-type mice (Figure 1E).
Expression of HLF in mice homozygous for the HLFkd gene
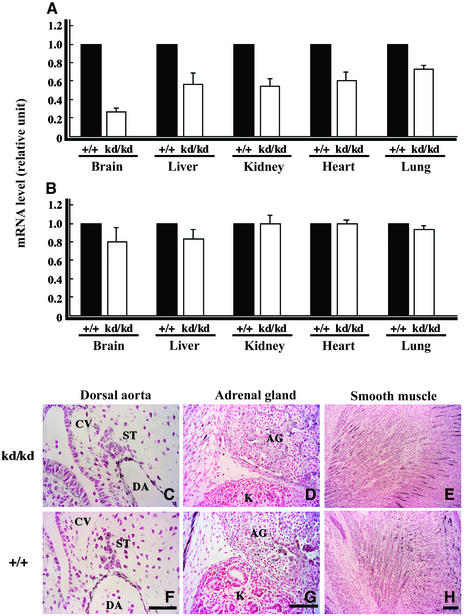
In HLFkd/kd mice, the expression of HLF mRNA was examined in various organs using real-time quantitative PCR (Figure 2A) and was reduced to 20–80%, depending on the organs, of wild type. The reason for this tissue-dependent variability in the reduced expression of HLF mRNA remains unknown. In contrast, the expression of HIF-1α mRNA in HLFkd/kd mice and wild-type mice did not differ in these organs (Figure 2B).
Fig. 2. Comparison of HLF expression between wild-type and HLFkd/kd mice. Expression levels of HLF (A) and HIF-1α (B) mRNA were examined under normoxic conditions in various organs (brain, liver, kidney, heart and lung) by real-time quantitative PCR. Immunohistochemistry was undertaken by staining with anti-HLF antibody in the dorsal aorta, adrenal gland and smooth muscle of HLFkd/kd (C–E) and wild-type (F–H) mice at E18.5. Note that HLF was similarly expressed in HLFkd/kd and wild-type mice in each organ. Bars indicate 25 µm (F) and 100 µm (G and H). DA, dorsal aorta; CV, cardinal vein; ST, sympathetic trunk; AG, adrenal gland; K, kidney.
Hemorrhaging was not observed during the embryonic stages in HLFkd/kd mice (data not shown), and heart rate did not differ significantly between HLFkd/kd and wild-type mice at E18.5 (HLFkd/kd: 28.3 ± 2.8, n = 3; wild type: 32.2 ± 6.6, n = 5).
Immunohistochemistry revealed that endothelial cells of the dorsal aorta and sympathetic cells at E10.5 (Figure 2C and F), adrenal gland at E15.5 (Figure 2D and G) and smooth muscle cells (Figure 2E and H) were stained positively with anti-HLF antibody in HLFkd/kd mice to a similar extent as in wild-type mice under the normoxic conditions.
Expression pattern of HLF in the mouse model of ROP
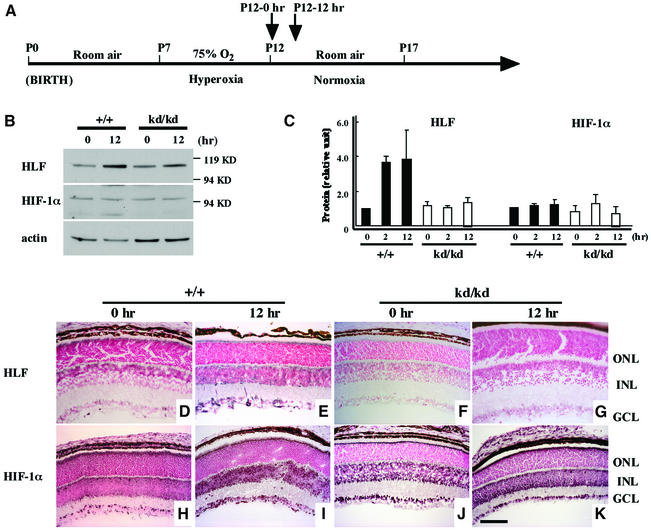
Immediately after hyperoxic treatment [postnatal day 12 (P12)–0 h] in the ROP model (Smith et al., 1994) (Figure 3A), eyeballs were removed from the wild-type and HLFkd/kd mice. First, expression of HLF and HIF-1α was examined by western blotting of the eyes of wild-type and HLFkd/kd mice at different time points after a shift from hyperoxic to normoxic conditions. Induction of HLF was detected even at 2 h after the normoxia in wild-type mice, while essentially no induction of HLF was observed in HLFkd/kd mice during 12 h of normoxia (Figure 3C). Unexpectedly, HIF-1α expression displayed only a slight variation under hypoxic and normoxic conditions in wild-type mice, or in HLFkd/kd mice (Figure 3C).
Fig. 3. Expression of protein and immunohistochemical staining of HLF and HIF-1α in retinas. The time course of the experiment is indicated (A). Samples were taken immediately after 75% O2 treatment (P12–0 h) and 2 and 12 h (P12–12 h) after the shift from 75% O2 to normal air. Whole-cell extracts were obtained at the appropriate time point (0, 2 and 12 h) and subjected to SDS–PAGE to detect the expression of HLF and HIF-1α (B). Actin antibody was used for detecting the expression of HLF and HIF-1α protein, and HLF and HIF-1α expressions in wild-type mice at P12–0 h were designated as 1.0 (C). Sections of eyeballs from wild-type (+/+) (D, E, H and I) and HLFkd/kd mice (F, G, J and K) at P12–0 h or P12–12 h were incubated with anti-HLF (D–G) and anti-HIF-1α antibody (H–K), and visualized as described in Materials and methods. GCL, ganglion cell layer; ONL, outer nuclear layer; INL, inner nuclear layer.
Secondly, immunohistochemistry was performed to examine HLF and HIF-1α expression in the retinas of wild-type and HLFkd/kd mice. The normoxic treatment following the hypoxic conditions moderately enhanced the expression of HIF-1α in the inner nuclear layer (INL) and ganglion cell layer (GCL) of both the wild-type and the mutant mice (Figure 3H–K). On the other hand, the expression of HLF was induced in the wild type immediately after shift to normoxia (Figure 3D and E) whereas it was not induced in HLFkd/kd mice under subsequent normoxic conditions (Figure 3F and G). Although HLF and HIF-1α share many biochemical and transcriptional properties (Ema et al., 1997; Wenger and Gassmann, 1997), their expression levels were independently regulated. The HLFlox/lox mice recovered enhanced HLF expression under relative hypoxic conditions, as observed in the wild type (data not shown).
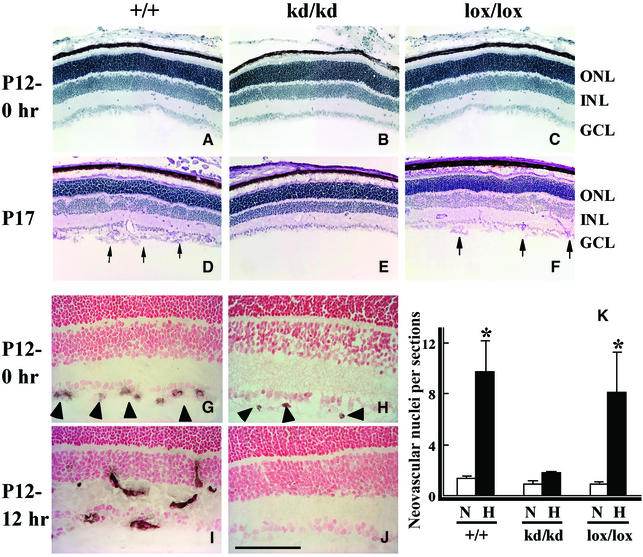
Suppression of retinal neovascularization of HLFkd/kd mice
Under hyperoxic conditions in the ROP model, retinal capillary obliteration is observed (Smith et al., 1994), and when mice are returned to room air (P12), they are considered to be exposed to relatively hypoxic conditions. Subsequently, retinal neovascularization occurs in 100% of animals under these conditions with the increase in VEGF mRNA (Pierce et al., 1995). In this ROP model, neovascularization in retinas was also reported to be maximal after 5 days of hyperoxic treatment followed by 5 days of relative hypoxia (P17) (Smith et al., 1994). For reference, when kept under normal conditions, wild-type and HLFkd/kd mice showed essentially no difference in their retinal structures at P12 (Figure 4A and B) and thereafter (data not shown). However, when wild-type mice were treated in the ROP model, multiple neovascularizations were induced at P17 on the vitreous side of the inner limiting membrane, with prominent neovascular tufts extending into the vitreous body (Figure 4D). In sharp contrast, there was essentially no neovascularization in the HLFkd/kd mice (Figure 4E). In total, 250 retinal cross-sections from each experimental group (three animals per group) were counted for neovascular buds to assess the extent of neovascularization, and the number was averaged per cross-section (Figure 4K). It was confirmed that neovascularization was remarkably induced in extent as well as in number in wild-type retinas by relative hypoxic treatment. Strikingly, in HLFkd/kd mice, retinal neovascularization was almost completely suppressed on the inner retinal surface (Figure 4E and K).
Fig. 4. Neovascularization in the retinas of mice with various genotypes of HLF treated in the model of ROP. Frozen retinal sections from wild-type (+/+), HLFkd/kd (kd/kd) and Cre-recombinase-expressing HLFkd/kd mice (lox/lox) were stained with PAS immediately after the shift from hyperoxia to normoxia (P12–0 h) (A–C). Retinas of HLF+/+, HLFkd/kd and HLFlox/lox mice were stained with hematoxylin at P17 (D–F). Arrows indicate numerous clumps of endothelial cells on the surface of the retinas (D and F). Retinas from the HLF+/+ and HLFkd/kd mice at P12–0 h and 12 h after shifting from hyperoxia to normoxia (P12–12 h) were subjected to immunostaining with anti-CD34 antibody (G–J). Arrowheads indicate neovascular buds detected in the GCL (G and H). Note that neovascular buds were elongated and infiltrated into the INL (I). Bar indicates 100 µm. Quantification of retinal neovascularization of HLF+/+, HLFkd/kd and HLFlox/lox mice at P17 (K). The numbers of neovascular nuclei on the retinal surfaces of HLF+/+, HLFkd/kd and HLFlox/lox mice were counted in 250 sections for each of the treated mice and averaged per section. *P < 0.01.
To investigate whether the suppressed neovascularization is due to a lowered expression of HLF, the HLFlox/lox mice were subjected to the model of ROP. Interestingly, susceptibility to massive proliferative neovascularization, as found in wild-type mice, was recovered in the treated HLFlox/lox mice (Figure 4F and K). These results clearly demonstrated that the level of HLF expression is critical for ROP, and that the effects of the reduced expression of HLF cannot be compensated by HIF-1α. In the ROP model, neovascularization was investigated by staining with an antibody against CD34, a membrane glycoprotein specific for vascular endothelial cells (Young et al., 1995). The formation of neovascular buds was observed on the surface of the GCL of retinas from both wild-type and mutant mice at P12–0 h immediately after shifting from hyperoxic to normoxic conditions, although neovascular buds were clearly fewer in the mutants (Figure 4G and H). After 12 h of normoxic conditions, the neovascular buds were elongated and appeared to infiltrate into the wild- type INL, while in mutant mice they totally disappeared (Figure 4I and J).
Expression of angiogenesis-related genes in the model of ROP
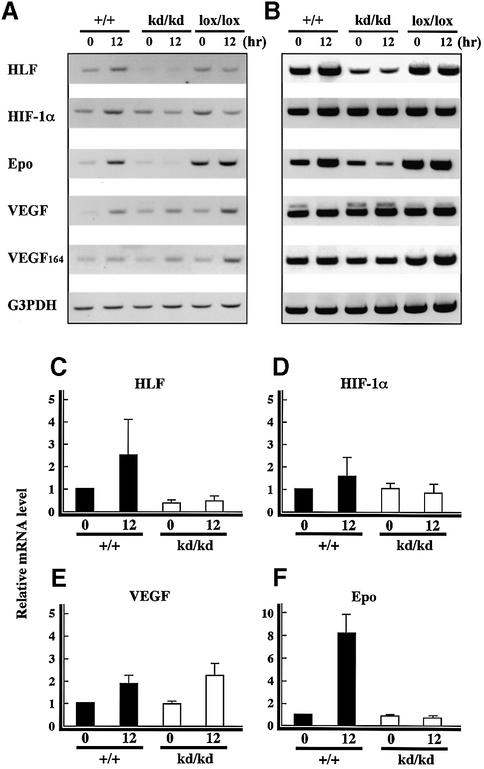
It has been reported that HLF and HIF-1α mRNAs and their proteins are synthesized at almost the same rate under normal O2 (21%) conditions as they are under hypoxic conditions (Ema et al., 1997). On the other hand, while their proteins were rapidly degraded by the ubiquitin– proteasome system under normoxic conditions, both HLF and HIF-1α proteins were stabilized and accumulated in response to low oxygen concentrations, leading to the enhanced expression of angiogenesis-related genes such as VEGF, VEGFR-1 (Flt-1) (Gerber et al., 1997), VEGFR-2 (Flk-1) (Kappel et al., 1999) and Tie-2 (Tian et al., 1997). We investigated the expression of such genes by RT–PCR and immunohistochemistry. The results from the RT–PCR analysis of various mRNAs were displayed at 25 and 30 reaction cycles, and real-time quantitative PCR was also performed (Figure 5). RT–PCR analysis using total RNA from the mutant mouse eyeballs confirmed the attenuated expression of HLF mRNA as compared with the wild type, even after relative hypoxia treatment. However, no significant elevation of HIF-1α expression was detected in the wild-type or HLFkd/kd mice after relative hypoxia treatment in the real-time quantitative PCR analysis.
Fig. 5. Expression of angiogenic factors analyzed by RT–PCR. RT–PCR analysis of mRNAs from retinas of HLF+/+, HLFkd/kd and HLFlox/lox mice in the model of ROP (P12–0 h, P12–12 h) was undertaken using specific pairs of primers for HLF, HIF-1α, Epo and two forms of VEGF. The results of the PCR analysis are shown at 25 (A) and 30 cycles (B). G3PDH was used as an internal control. Real-time quantitative PCR analysis was also performed for HLF (C), HIF-1α (D), VEGF (E) and Epo (F) in retinas of HLF+/+ and HLFkd/kd mice kept in the model of ROP (C–F). The relative mRNA level in HLF+/+ mice at P12–0 h is designated as 1.0.
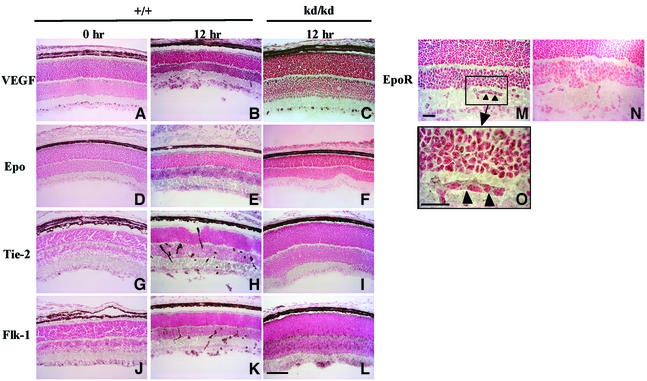
Expression of VEGF mRNA was somewhat upregulated in mice of both genotypes under relative hypoxic conditions as compared with hyperoxic conditions (Figure 5A and E). Immunohistochemistry showed that the protein level of VEGF was significantly elevated by relative hypoxic treatment in both wild-type and HLFkd/kd mice (Figure 6A–C). Thus, it was clearly shown that the mode of VEGF expression was not substantially different between wild-type and HLFkd/kd mice. Immunostainings of VEGF, Epo, Tie-2 and Flk-1 of HLFkd/kd mice at the 0 h point are the same as those of the wild type and deleted from Figure 6.
Fig. 6. Immunohistochemical staining with anti-angiogenic factor antibodies. Frozen retinal sections from HLF+/+ (A, D, G and J at P12–0 and B, E, H, K, M, N and O at P12–12) and HLFkd/kd mice (C, F, I and L at P12–12) treated in the model of ROP were immunostained with antibodies against VEGF (A–C), Epo (D–F), Tie-2 (G–I), Flk-1 (J–L) and EpoR (M–O). Magnified inserts of the enclosed area (M) are also presented in (O). Arrowheads show endothelial tubes expressing EpoR (M and O). Bars indicate 100 µm (L) and 25 µm (M and O).
In sharp contrast, Epo mRNA was enhanced by the relative hypoxia in wild-type mice, while its expression was markedly attenuated even by the successive normoxic treatment in HLFkd/kd mice (Figure 5A and F). This reduced expression of Epo mRNA paralleled that of HLF mRNA in the mutant mice (Figure 5A, C and F). Immunohistochemistry also demonstrated that HLFkd/kd mouse retinas were very weakly stained with anti-Epo antibody compared with the wild-type mice under the relative hypoxic conditions (Figure 6D–F). Other angiogenic factors, such as Flk-1, Flt-1, Tie-1, Tie-2, angiopoietin 1 and angiopoietin 2, were subjected to RT–PCR analysis and essentially no significant difference in their expression levels was observed between HLFkd/kd and wild-type mice (data not shown). In addition, immunostaining showed that the levels of these factors were significantly enhanced under relative hypoxic conditions in HLFkd/kd mice as well as in wild-type mice. Immunohistochemistry of VEGF, Flk-1 and Tie-2 displayed different regional expressions, because VEGF is a secretory protein, while Flk-1 and Tie-2 are receptor proteins associated with the plasma membranes of vascular endothelial cells. The expression of VEGF was uniformly enhanced in the GCL and INL of wild-type and mutant retinas exposed to relative hypoxia (Figure 6A–C). In contrast, Tie-2 displayed a rather clustered expression along the extending vascular tubes of wild-type retinas (Figure 6H), while it dispersed rather evenly in HLFkd/kd retinas, probably because no vascular formation developed (Figure 6I). Immunostaining using anti-Flk-1 antibody revealed a similar difference in the distribution of Flk-1 between wild-type and HLFkd/kd mouse retinas to that of Tie-2 (Figure 6K and L). Epo receptor (EpoR) was also expressed in the neovascular tubes of wild-type retinas under relative hypoxic conditions (Figure 6M and O), while EpoR immunostaining was eliminated in the presence of EpoR peptide as a control experiment (Figure 6N).
Association of Epo with neovascularization in the ROP
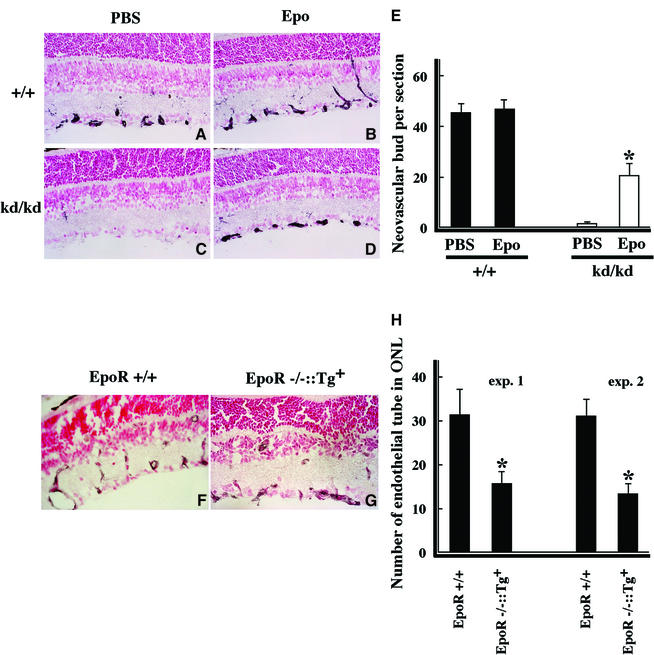
In order to confirm that Epo is involved in the neovascular process, Epo was injected intraperitoneally into HLFkd/kd mice during relative hypoxia treatment (Figure 7). Neovascular buds appeared in the GCL at P12–12 h in wild-type mice (Figure 7A and B) regardless of Epo administration, whereas neovascular buds were observed in HLFkd/kd mice only when Epo was injected exogenously (Figure 7C and D). Comparison between HLFkd/kd mice treated with or without Epo revealed a significant increase in the number of neovascular buds in the GCL of Epo-treated HLFkd/kd mice [control phosphate-buffered saline (PBS): 1.3 ± 0.2; Epo: 20.4 ± 4.5]. This demonstrated that Epo plays an important role in the formation or maintenance of neovascular buds following a shift from hyperoxic to normoxic conditions. The number of neovascular buds in Epo-injected HLFkd/kd mice did not fully recover to the level of wild type, probably due to the same reasons that administered Epo may not work effectively and/or that some factors other than Epo may also be needed for the formation of neovascular buds.
Fig. 7. Effects of Epo on the formation of neovascularization in retinas. HLF+/+ and HLFkd/kd mice were kept in the model of ROP and examined for the formation of neovascular buds after administration of control PBS (A and C) and Epo (B and D) at P12–24 h. CD34-positive neovascular buds were scored under the microscope (E). *P < 0.01. After the shift from hyperoxia to normoxia, wild-type (EpoR+/+) mice (F) and EpoR–/–::HG1-EpoR (Tg) mice (G) were examined at P17. The number of neovascular tube formations infiltrated into the INL was counted after the staining with anti-CD34 antibody (H). *P < 0.05.
To further clarify the effect of Epo on neovascularization elongating from the GCL towards the INL, EpoR-deficient mice rescued by intercrossing with transgenic mice expressing EpoR transgene under the control of the GATA-1 promoter (EpoR–/–::Tg+) (Suzuki et al., 2002) were utilized for the ROP model (Figure 7F–H). In these transgenic mice, the expression of EpoR was only detected in hematopoietic organs, such as the spleen and bone marrow, but not in the retina. The number of vascular tubes reaching into the INL of EpoR–/–::Tg+ (Figure 7G and H) at P17 was clearly less than that of the wild type (Figure 7F and H) in which EpoR is expressed normally, suggesting that the Epo/EpoR system is also necessary for neovacularization after relative hypoxic conditions in the retina.
Retinal damage in HLFkd/kd mice during relative hypoxic treatment
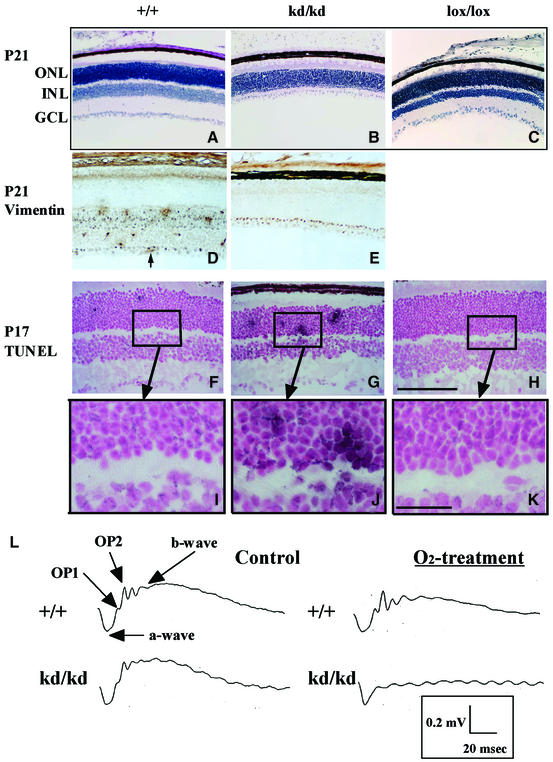
At P21 in the ROP model, we found degenerative changes in the inner retinal layer of the HLFkd/kd mice (Figure 8B), which could not be observed at P12 (Figure 4A and B) or P17 (Figure 4D and E), regardless of the HLF genotype. Hematoxylin staining of retinal sections from HLFkd/kd mice revealed markedly abnormal retinal structures (Figure 8B), probably resulting from a loss of the INL, as revealed by immunostaining analysis using an anti-vimentin antibody (Figure 8D and E). The expression of vimentin was restricted to the INL, GCL and new vessels of wild-type mice treated with relative hypoxia (Figure 8D). On the other hand, in hypoxia-treated HLFkd/kd mice, immunoreactivity against vimentin was observed only in the GCL and the monolayer (Müller cells) immediately above the GCL, showing that the INL cells almost completely disappeared in these mice (Figure 8B and E). TUNEL analyses revealed continual apoptosis in the retinal nuclear layers of the HLFkd/kd mice during relative hypoxic treatment. TUNEL analyses of retinas from animals immediately after transfer from hypoxia to normoxia showed frequent induction of apoptosis in both HLFkd/kd and wild-type retinas (data not shown). However, while apoptosis in the wild-type and HLFlox/lox mice was suppressed along with proliferative neovascularization (Figures 8F and H, 4D and F), it was persistently observed in HLFkd/kd mice at P17 (Figure 8G and J). The retinal damage observed in the HLFkd/kd mice was caused at least in part by continued apoptosis due to poor neovascularization.
Fig. 8. Structural and functional alterations induced in retinas from HLFkd/kd mice (P21) by prolonged normoxia treatment after hyperoxia. Hematoxylin-stained retinas of HLF+/+, HLFkd/kd and HLFlox/lox mice (P21) exposed to prolonged normoxia treatment following hyperoxia (A–C). Immunohistochemical staining of retinas from HLF+/+ and HLFkd/kd mice (P21) with an anti-vimentin antibody (D and E). Note that loss of the INL from HLFkd/kd retinas is presented (B). An arrow shows newly formed blood vessel in the retina (D). TUNEL reactions of retinal sections from HLF+/+, HLFkd/kd and HLFlox/lox mice at P17 (F–H). Magnified inserts of the enclosed areas are also presented (I–K). Retinal sections were subjected to the TUNEL reaction and followed by counterstaining with Nuclear Fast Red. Bars indicate 100 µm (H) and 25 µm (K). ERGs of HLF+/+ and HLFkd/kd mice at P21 in the model of ROP (L). Three animals from each experimental group were used.
To examine the physiological function of degenerated retinas in visual synaptic transmission in vivo, we analyzed single-flash electroretinogram (ERG) patterns of mouse retinas subjected to the model of ROP. The ERG response to a light flash can be divided into three waves: a-wave, b-wave and OPs (oscillatory potentials consisting of three to four wavelets, OP1–OP4), arising from photoreceptors, bipolar cells and amacrine/inner plexiform cells, respectively (Harada et al., 1998). ERG analyses revealed that while the wild-type mice subjected to the model of ROP had a normal flash-light response with a-wave, b-wave and OPs, the HLFkd/kd mice with the degenerative retinas showed a complete loss of b-wave and OPs, but had a normal a-wave amplitude (Figure 8L). These results indicate that HLFkd/kd mice in the model of ROP suffered from functional damage in the neural cells of the INL, but retained functional photoreceptor cells.
Discussion
In order to investigate the functional roles of HLF in adult animals, we produced HLFkd/kd mice by inserting the neo gene cassette sandwiched between the two loxP sequences into the first exon encoding the 5′-UTR. The double poly(A) addition signals of the inserted neo gene did not work completely, such that leaky transcription reduced the synthesis of HLF mRNA to approximately one-fifth of that in the retinas of wild-type mice.
Under normal conditions, HLFkd/kd newborn mice grow well with apparently normal retinal structures (data not shown), suggesting that this reduced level of HLF production was not harmful to the animals under normal conditions.
The model of ROP is initiated by hyperoxia-induced obliteration of newly formed blood vessels in the retinas of newborn mice (Alon et al., 1995). Subsequently, the retinas of newborn mice become relatively hypoxic when exposed to normal air, because of obliteration of the retinal vessels and induction of abnormal vasoproliferation (Ozaki et al., 1999). In contrast to wild-type mice, proliferative vascularization was not induced in the retinas of HLFkd/kd mice during normoxic conditions preceded by hyperoxic treatment. Although RT–PCR analyses revealed no prominent differences between wild-type and HLFkd/kd mice in their expression of the angiogenic factors VEGF, Flt-1, Flk-1, Tie-1 and Tie-2, only Epo mRNA was notably reduced in HLFkd/kd mice, in parallel with the attenuated expression of HLF (Figure 5A, B and F). In HLFlox/lox mice that had the inserted neo gene removed by Cre enzyme, the expression of Epo and HLF mRNAs recovered to normal levels, resulting in proliferative neovascularization in response to hypoxic conditions (Figure 4C, F and K). Immunochemical staining of Epo protein displayed similar changes to that of Epo mRNA in wild-type and HLFlox/lox mice, suggesting that Epo is one of the target genes of HLF that is responsible for ROP (Figures 5A, B, F and 6E). This suggestion was strongly supported by the results of the ROP experiments showing that intraperitoneal injection of Epo recovered significant susceptibility in the HLFkd/kd mice to proliferative retinopathy and, conversely, that the EpoR-engineered mice lost the susceptibility to retinopathy to a significant level (Figure 7).
Considering that Epo has been reported to function not only as a hematopoietic, but also as an angio genic factor (Anagnostou et al., 1990; Ribati et al., 1999), it is very likely that its reduced expression is not sufficient to induce proliferative neovascularization in HLFkd/kd mice. Needless to say, we cannot rigorously rule out the possibility that factors other than Epo, which are specifically regulated by HLF, are involved in proliferative vascularization. Despite its normal expression in HLFkd/kd mice, HIF-1α cannot functionally compensate for HLF in this process, while Epo gene expression is known to be regulated by both HIF-1α and HLF through the HRE located downstream of the gene by DNA transfection assays (Wanner et al., 2000). It remains to be elucidated why only HLF, but not HIF-1α, transactivates the expression of the Epo gene in the retinas of ROP.
During prolonged exposure to normoxia following hyperoxic treatment, the structure of the HLFkd/kd retinas displayed not only suppression of neovascularization, but also a prominent degenerative change in the INL at P21, probably resulting from prolonged hypoxia due to poor neovascularization. It was recently reported that Epo plays a protective role in neurons against apoptosis in cerebral ischemia (Digicaylioglu and Lipton, 2001; Siren et al., 2001). Indeed, expression of the EpoR was detected in the INL and neovasculature of wild-type mice (Figure 6M and N), suggesting that reduced Epo synthesis in HLFkd/kd retinas exposed to relative hypoxia may aggravate degeneration of the INL due to defective neural protection and poor neovascular formation. Localized expression of Epo in the retina is thought to be important in protecting nuclear layers, because Epo concentrations in the blood sera did not differ between HLFkd/kd and wild-type mice under normoxic conditions (data not shown).
Single-flash ERG analyses revealed that HLFkd/kd mice lost b-wave and OPs mainly derived from bipolar cells and the amacrine/interplexiform during the prolonged relative hypoxic treatment (Figure 8L). In contrast, wild-type mice undergoing the same treatment retained normal neurotransmission of light response, indicating that at least the Epo gene under the control of HLF may play a critical role in protection against degeneration of the INL in the ROP model.
VEGF is thought to represent a major factor triggering neovascularization in the retina of relative hypoxia (Aiello et al., 1995; Seo et al., 1999). Indeed, VEGF was expressed in the INL and GCL in response to hypoxia, and its receptor Flk-1 was also expressed in the elongated endothelial cells (Figure 6K), confirming that the VEGF/Flk-1 interaction induces proliferation of the retinal vasculature. Although neovascular bud formation was transiently observed immediately after hypoxia treatment, HLFkd/kd mice did not develop any vascular formation from these buds during treatment with hypoxia, even with a production of VEGF equivalent to that in wild-type mice (Figures 5E and 6). The results indicated that some factors other than VEGF are also necessary for induction of neovascularization and that Epo may be one of the factors that plays a protective or anti-apoptotic role in endothelial cell buds (Carlini et al., 1999) during neovasculogenesis in the ROP. Therefore, it would be interesting to investigate how Epo and VEGF interplay in the development of neovascularization in response to hypoxia. Recently, inhibitory PAS (IPAS) protein, which acts as a dominant-negative regulator of HIFs, was isolated by Makino et al. (2001). IPAS is expressed in response to hypoxia and inhibits the activities of HLF and HIF-1α in both corneal epithelium and retinal GCL and INL, suggesting a more complicated regulatory mechanism for neovascularization observed in the ROP.
Consequently, in addition to VEGF, Epo was found to be a key factor in the ROP, especially in the development of neovascularization, suggesting a therapeutic possibility of Epo and VEGF inhibitors in the ROP. HLFkd/kd mice have turned out to be very useful for investigation of the physiological roles of HLF and might also shed light on the function of HLF that is distinct from that of HIF-1α in terms of vascularization and angiogenesis under hypoxic conditions.
Materials and methods
HLF gene targeting
A genomic DNA sequence extending from the first exon to 5.1 and 2.7 kb in the 5′ and 3′ directions, respectively, was cloned from a 129/SV mouse genomic library and used for construction of a targeting vector. A neomycin resistance gene (neo) cassette sandwiched between loxP sequences was inserted into the first exon of the HLF gene. The constructed chimeric DNA was then cloned into pUC18. The targeting vector was amplified, linearized by NotI digestion and electroporated into E14 cells. The electroporated cells were subjected to double selection in G418 (300 µg/ml) and gancyclovir (1 µg/ml), and amplified. After 10 days, colonies were isolated, expanded and screened for homologous recombination by the PCR method. DNA from an isolated double-resistant clone was further confirmed for homologous recombination by DNA blot analysis. Double-resistant ES cells were injected into C57BL/6J blastocysts that were surgically implanted into the uterus of pseudopregnant ICR foster mothers. Chimeric mice were then intercrossed with C57BL/6J mice and the offspring were genotyped.
Murine model of retinal neovascularization
The murine hyperoxia–normoxia-induced proliferative retinopathy model established by Smith (1994) was used in this study. Briefly, at P7, 20 littermates from each group of HLFkd/kd and wild-type mice, along with nursing mothers, were exposed to 75% oxygen for 5 days, and at P12 the animals were returned to room air (21% O2). Following exposure to room air, a certain number of mice (the number is indicated in parentheses for each time point) were killed at one of the following time points: 0 h (3), 2 h (3), 12 h (3), 24 h (3), 36 h (2), 5 days (P17) (3) and 9 days (P21) (3). An oxygen-enriched atmosphere was obtained by mixing air with oxygen, and oxygen levels were monitored using a polarographic oxygen sensor (Teijin, Tokyo, Japan). Eyeballs were rapidly removed and embedded in an optimal cutting medium (O.C.T. Compound; Sakura, Tokyo, Japan) for histochemical and immunostaining analyses. The treated mice were kept with nursing mothers under normal conditions and eyes were removed at P17 under Nembutal anesthesia for histochemical and immunochemical analyses. Frozen retinal sections were cut at 5 µm for immunohistochemistry and periodic and acid Schiff stain (PAS) and hematoxylin staining.
Immunohistochemistry and electroretinogram analysis
Frozen sections of eyes were immunohistochemically stained using anti-HIF-1α, Epo, Tie-2, VEGF, Flk-1 EpoR (Santa Cruz Biotechnology, Santa Cruz, CA), vimentin (Nichirei, Tokyo, Japan) and CD34 (Young et al., 1995) antibodies. To obtain anti-HLF antibody, two rabbits were immunized with the synthetic 16 amino acids of the C-terminus of mouse HLF (656– 671 amino acids), and the resulting bleed was affinity purified (Sawady Technology Inc., Tokyo, Japan). The specificity of the anti-HLF antibody was examined by ELISA and western blot analyses.
The sections were fixed in 4% paraformaldehyde/PBS for 15 min, washed twice with PBS and incubated for 30 min with 10% normal goat serum. The slides were then incubated overnight at 4°C with respective specific antibodies or non-immune serum. After washing with PBS, the slides were further incubated with biotinylated goat anti-rabbit IgG antibody (Vector Laboratories, Inc., Burlingame, CA), followed by streptavidin-conjugated ABC complex (Vector Laboratories) or horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG antibody (Biosource, Camarillo, CA). Specific staining was visualized by incubation with 3,3′-diaminobenzidine. Counterstaining was carried out using Nuclear Fast Red.
Electroretinogram analysis was performed as described previously (Abe et al., 1999).
RT–PCR
Total RNA was prepared from eyeballs of mice with various genotypes of the HLF gene using RNA isolation reagent (Trizol, Invitrogen). cDNAs were synthesized using a RT–PCR kit (Clontech Laboratories) in a reaction solution (20 µl) containing total RNA (1 µg) prepared from eyeballs of mice treated in various ways. An aliquot (1 µl) of synthesized cDNA was amplified (Clontech Laboratories) in a total volume of 20 µl containing 150 mM dNTP, 0.2 U of Taq polymerase and 0.12 µg of each pair of primers. The PCR procedure consisted of 25 or 30 cycles of the reaction: 95°C for 30 s and 68°C for 1 min. The PCR products were electrophoretically separated on 2% agarose gels. In addition, cDNA was amplified and quantified using an ABI Prism 7700 Sequence Detector (Parkin Elmer, Foster City, CA). The primers used for the PCR reaction are listed in Table I.
Table I. Primers used for the PCR.
| VEGF | 5′ primer | 5′-CTTCCTACAGCACAGCAGATGTGAA-3′ |
| 3′ primer | 5′-TGGTGAATGGTTAATCGGTCTTTC-3′ | |
| VEGF164 | 5′ primer | 5′-CTTTACTGCTGTACCTTCACCATGC-3′ |
| 3′ primer | 5′-AACAAGGCTCACAAGTGATTTTCTGG-3 | |
| Epo | 5′ primer | 5′-TGGCACCCTGCTGCTTTTACTCTCCTT-3′ |
| 3′ primer | 5′-CTGGAGGCGACATCAATTCCTTCTGAG-3′ | |
| EpoR | 5′ primer | 5′-TCTTCACCACCCACAAGGGTAACTTCCA-3′ |
| 3′ primer | 5′-AGATGCCAGAATCGGACACCACAAGGTA-3′ | |
| Tie2 | 5′ primer | 5′-CGTCAACAAGCATCCTTCCTACCTGCTA-3′ |
| 3′ primer | 5′-TGCATCCTTCTGGTCCACTACACCTTTC-3′ | |
| HLF | 5′ primer | 5′-CAGCTCAGAGCTGAGGAAGG-3′ |
| 3′ primer | 5′-GTTGTAGACTCACTTGCC-3′ | |
| HIF-1α | 5′ primer | 5′-CAAGATCTCGGCGAACGAAAGAGTCTGA-3′ |
| 3′ primer | 5′-GAAGCACCTTCCACGTTGCTGACTTGAT-3′. | |
| Flt-1 | 5′ primer | 5′-TCTTGCTCACCATGGTCAGC-3′ |
| 3′ primer | 5′-GTCATGTGCACAAGTTTGGG-3′ | |
| Flk-1 | 5′ primer | 5′-TCGCTCTGTGGTTCTGCGTG-3′ |
| 3′ primer | 5′-GGTTTGAAATCGACCCTCGG-3′ | |
| G3PDH | 5′ primer | 5′-TGAAGGTCGGTGTGAACGGATTTGGC-3′ |
| 3′ primer | 5′-CATGTAGGCCATGAGGTCCACCAC-3′. |
Western blot analysis
Mouse eyeballs were lysed with lysis buffer [10 mM HEPES pH 7.9, 10 mM potassium chloride, 0.1 mM EDTA, 1 mM dithiothreitol, 1% NP-40 and protease inhibitor cocktail (Roche, Mannheim, Germany)]. Whole-cell extracts (100 µg) were resolved in 10% SDS– polyacrylamide gels and electrophoretically transferred onto a polyvinylidene difluoride membrane (Millipore, Bedford, MA). For HLF detection, rabbit anti-HLF antibody was used and, for HIF-1α, anti-HIF-1α antibody (H1α67; Lab Vision, Fremont, CA) was used. Detection was performed with HRP-conjugated goat anti-rabbit or rabbit anti-goat immunoglobulins (Biosource) and enhanced chemiluminescence (ECL; Amersham Biosciences, UK). After analysis, goat anti-actin antibody (I-19; Santa Cruz Biotechnology, Santa Cruz, CA) was used to verify equal protein loading and transfer.
TUNEL assay
The TUNEL assay was performed using an in situ apoptosis detection kit (TaKaRa, Code No. MK500) as specified by the manufacturer. Frozen eye sections (5 µm) were fixed in 4% paraformaldehyde/PBS for 30 min and washed with PBS for 10 min at room temperature (RT). Slides were treated with 0.3% hydrogen peroxide in methanol for 30 min at RT and incubated with permeabilization buffer for 5 min on ice. Subsequently, the samples were incubated with reaction buffer overnight at 4°C. After several washings with PBS, sections were incubated with anti-FITC–HRP conjugate for 30 min at 37°C and washed with PBS. Staining was performed using diaminobenzidine and samples were counterstained with Nuclear Fast Red.
Epo injection
To investigate the role of Epo in neovascularization, Epo (a generous gift from Chugai Pharmaceutical Co., Tokyo, Japan) was intraperitoneally injected into neonates (5000 IU/kg) (Grimm et al., 2002) at P12–0 h and P12–12 h. The effect of Epo on the formation of neovascular buds was evaluated by scoring the number of buds observed in the GCL at P12–24 h after staining with anti-CD34 antibody.
EpoR–/–::HG1-EPOR (Tg) mice were obtained by crossing EpoR+/–::Tg mice with EpoR+/– mice, as reported previously (Suzuki et al., 2002), and newborn mice were exposed to 75% oxygen for 5 days and subsequently returned to room air. Mice were analyzed at P17 for neovascularization by staining with anti-CD34 antibody, and the number of tube formations reaching the INL were scored under the microscope.
Acknowledgments
Acknowledgements
The authors would like to thank Drs K.Gradin (Tohoku University) and T.O’Connor (University of Tsukuba) for critical reading of the manuscript, Drs K.Araki and K.Yamamura (Kumamoto University) for the Cre-expressing transgenic mice, K.Yasumoto (Tohoku University) for kind help and suggestions and Ms N.Kaneko (University of Tsukuba) for histological analyses. Our hearty thanks are also due to Ms S.Suzuki, M.Irie and Mr H.Abe for secretarial and graphic work. This work was supported in part by a Grant-in-Aid for Scientific Research of Priority Area (A) of the Ministry of Education, Science, Sports and Culture of Japan, funds for Research for the Future Program of the Japan Society for the Promotion of Science and for Core Research for Evolution Science of Japan Science and Technology Corporation, for Program for Promotion of Basic Research Activities for Innovative Biosciences, for Project of Exploratory Research for Advanced Technology of JST and a fund from Sankyo Co.
References
- Abe T. et al. (1999) Functional analysis after auto iris pigment epithelial cell transplantation in patients with age-related macular degeneration. Tohoku J. Exp. Med., 189, 295–305. [DOI] [PubMed] [Google Scholar]
- Aiello L.P., Pierce,E.A., Foley,E.D., Takagi,H., Chen,H., Riddle,L., Ferrara,N., King,G.L. and Smith,L.E. (1995) Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF–receptor chimeric proteins. Proc. Natl Acad. Sci. USA, 92, 10457–10461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alon T., Hemo,I., Itin,A., Pe’er,J., Stone,J. and Keshet,E. (1995) Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat. Med., 10, 1024–1028. [DOI] [PubMed] [Google Scholar]
- Anagnostou A., Lee,E.S., Kessimian,N., Levinson,R. and Steiner,M. (1990) Erythropoietin has a mitogenic and positive chemotactic effect on endothelial cells. Proc. Natl Acad. Sci. USA, 87, 5978–5982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlini R.G., Alonzo,E.J., Dominguez,J., Blanca,I., Weisinger,J.R., Rothstein,M. and Bellorin-Font,E. (1999) Effect of recombinant human erythropoietin on endothelial cell apoptosis. Kidney Int., 55, 546–553. [DOI] [PubMed] [Google Scholar]
- Digicaylioglu M. and Lipton,S.A. (2001) Erythropoietin-mediated neuroprotection involves cross-talk between Jak2 and NF-κB signaling cascades. Nature, 412, 641–647. [DOI] [PubMed] [Google Scholar]
- Ema M., Taya,S., Yokotani,N., Sogawa,K., Matsuda,Y. and Fujii-Kuriyama,Y. (1997) A novel bHLH–PAS factor with close sequence similarity to hypoxia-inducible factor 1α regulates the VEGF expression and is potentially involved in lung and vascular development. Proc. Natl Acad. Sci. USA, 94, 4273–4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein A.C. et al. (2001) C.elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell, 107, 43–54. [DOI] [PubMed] [Google Scholar]
- Firth J.D., Ebert,B.L., Pugh,C.W. and Ratcliffe,P.J. (1994) Oxygen-regulated control elements in the phosphoglycerate kinase 1 and lactate dehydrogenase A genes: similarities with the erythropoietin 3′ enhancer. Proc. Natl Acad. Sci. USA, 91, 6496–6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe J.A., Jiang,B.H., Iyer,N.V., Agani,F., Leung,S.W., Koos,R.D. and Semenza,G.L. (1996) Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell. Biol., 16, 4604–4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber H.P., Condorelli,F., Park,J. and Ferrara,N. (1997) Differential transcriptional regulation of the two vascular endothelial growth factor receptor genes. Flt-1, but not Flk-1/KDR, is up-regulated by hypoxia. J. Biol. Chem., 272, 23659–23667. [DOI] [PubMed] [Google Scholar]
- Grimm C., Wenzel,A., Groszer,M., Mayser,H., Seeliger,M., Samardzija,M., Bauer,C., Gassmann,M. and Reme,C.E. (2002) HIF-1-induced erythropoietin in the hypoxic retina protects against light-induced retinal degeneration. Nat. Med., 8, 718–724. [DOI] [PubMed] [Google Scholar]
- Harada T. et al. (1998) Functions of the two glutamate transporters GLAST and GLT-1 in the retina. Proc. Natl Acad. Sci. USA, 95, 4663–4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer N.V. et al. (1998) Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1α. Genes Dev., 12, 149–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S., Maltepe,E., Lu,M.M., Simon,C. and Bradfield,C.A. (1998) Expression of ARNT, ARNT2, HIF-1α, HIF-2α and Ah receptor mRNAs in the developing mouse. Mech. Dev., 73, 117–123. [DOI] [PubMed] [Google Scholar]
- Kallio P.J., Wilson,W.J., O’Brien,S., Makino,Y. and Poellinger,L. (1999) Regulation of the hypoxia-inducible transcription factor 1α by the ubiquitin–proteasome pathway. J. Biol. Chem., 274, 6519–6525. [DOI] [PubMed] [Google Scholar]
- Kappel A., Ronicke,V., Damert,A., Flamme,I., Risau,W. and Breier,G. (1999) Identification of vascular endothelial growth factor (VEGF) receptor-2 (Flk-1) promoter/enhancer sequences sufficient for angioblast and endothelial cell-specific transcription in transgenic mice. Blood, 93, 4284–4289. [PubMed] [Google Scholar]
- Liu Y., Cox,S.R., Morita,T. and Kourembanas,S. (1995) Hypoxia regulates vascular endothelial growth factor gene expression in endothelial cells. Identification of a 5′ enhancer. Circ. Res., 77, 638–643. [DOI] [PubMed] [Google Scholar]
- Makino Y., Cao,R., Svensson,K., Bertilsson,G., Asman,M., Tanaka,H., Cao,Y., Berkenstam,A. and Poellinger,L. (2001) Inhibitory PAS domain protein is a negative regulator of hypoxia-inducible gene expression. Nature, 414, 550–554. [DOI] [PubMed] [Google Scholar]
- Moss S.E., Klein,R. and Klein,B.E. (1994) Ten-year incidence of visual loss in a diabetic population. Ophthalmology, 101, 1061–1070. [DOI] [PubMed] [Google Scholar]
- Niwa H., Araki,K., Kimura,S., Taniguchi,S., Wakasugi,S. and Yamamura,K. (1993) An efficient gene-trap method using poly A trap vectors and characterization of gene-trap events. J. Biochem., 113, 343–349. [DOI] [PubMed] [Google Scholar]
- Okamoto N., Okamoto,N., Tobe,T., Hackett,S.F., Ozaki,H., Vinores,M.A., LaRochelle,W., Zack,D.J. and Campochiaro,P.A. (1997) Transgenic mice with increased expression of vascular endothelial growth factor in the retina: a new model of intraretinal and subretinal neovascularization. Am. J. Pathol., 151, 281–291. [PMC free article] [PubMed] [Google Scholar]
- Ozaki H. et al. (1999) Hypoxia inducible factor-1α is increased in ischemic retina: temporal and spatial correlation with VEGF expression. Invest. Ophthalmol. Vis. Sci., 40, 182–189. [PubMed] [Google Scholar]
- Peng J., Zhang,L., Drysdale,L. and Fong,G.H. (2000) The transcription factor EPAS-1/hypoxia-inducible factor 2α plays an important role in vascular remodeling. Proc. Natl Acad. Sci. USA, 97, 8386–8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce E.A., Avery,R.L., Foley,E.D., Aiello,L.P. and Smith,L.E. (1995) Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc. Natl Acad. Sci. USA, 92, 905–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prost M. (1988) Experimental studies on the pathogenesis of retinopathy of prematurity. Br. J. Ophthalmol., 72, 363–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribati D., Presta,M., Vacca,A., Ria,R., Giuliani,R., Dell’Era,P., Nico,B., Roncali,L. and Dammacco,F. (1999) Human erythropoietin induces a pro-angiogenic phenotype in cultured endotherial cells and stimulates neovascularization in vivo. Blood, 93, 2627–2636. [PubMed] [Google Scholar]
- Ryan H.E., Lo,J. and Johnson,R.S. (1998) HIF-1α is required for solid tumor formation and embryonic vascularization. EMBO J., 17, 3005–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza G.L. and Wang,G.L. (1992) A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol. Cell. Biol., 12, 5447–5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo M.S. et al. (1999) Dramatic inhibition of retinal and choroidal neovascularization by oral administration of a kinase inhibitor. Am. J. Pathol., 154, 1743–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siren A.-L. et al. (2001) Erythropoietin prevents neural apoptosis after cerebral ischemia and metabolic stress. Proc. Natl Acad. Sci. USA, 98, 4044–4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L.E., Wesolowski,E., McLellan,A., Kostyk,S.K., D’Amato,R., Sullivan,R. and D’Amore,P.A. (1994) Oxygen-induced retinopathy in the mouse. Invest. Ophthalmol. Vis. Sci., 35, 101–111. [PubMed] [Google Scholar]
- Suzuki N., Ohneda,O., Takahashi,S., Higuchi,M., Mukai,H.Y., Nakahata,T., Imagawa,S. and Yamamoto,M. (2002) Erythroid-specific expression of the erythropoietin receptor rescued its null mutant mice from lethality. Blood, 100, 2279–2288. [DOI] [PubMed] [Google Scholar]
- Tian H., McKnight,S.L. and Russell,D.W. (1997) Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev., 11, 72–82. [DOI] [PubMed] [Google Scholar]
- Tian H., Hammer,R.E., Matsumoto,A.M., Russell,D.W. and McKnight,S.L. (1998) The hypoxia-responsive transcription factor EPAS1 is essential for catecholamine homeostasis and protection against heart failure during embryonic development. Genes Dev., 12, 3320–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobe T., Okamoto,N., Vinores,M.A., Derevjanik,N.L., Vinores,S.A., Zack,D.J. and Campochiaro,P.A. (1998) Evolution of neovascularization in mice with overexpression of vascular endothelial growth factor in photoreceptors. Invest. Ophthalmol. Vis. Sci., 39, 180–188. [PubMed] [Google Scholar]
- Wang G.L. and Semenza,G.L. (1993) General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc. Natl Acad. Sci. USA, 90, 4304–4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G.L., Jiang,B.H., Rue,E.A. and Semenza,G.L. (1995) Hypoxia-inducible factor 1 is a basic-helix–loop–helix PAS heterodimer regulated by cellular O2 tension. Proc. Natl Acad. Sci. USA, 92, 5510–5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner R.M. et al. (2000) Epolones induce erythropoietin expression via hypoxia-inducible factor-1α activation. Blood, 96, 1558–1565. [PubMed] [Google Scholar]
- Wenger R.H. and Gassmann,M. (1997) Oxygen(es) and the hypoxia-inducible factor-1. Biol. Chem., 378, 609–616. [PubMed] [Google Scholar]
- Young P.E., Baumhueter,S. and Lasky,L.A. (1995) The sialomucin CD34 is expressed on hematopoietic cells and blood vessels during murine development. Blood, 85, 96–105. [PubMed] [Google Scholar]