Abstract
Malignant gliomas are refractory to conventional therapies, including surgery, radiotherapy and chemotherapy. Thus, a variety of therapies such as the inhibition of angiogenesis and signal transduction pathways have been attempted. In the present study, we have evaluated the combined effect of endostatin, an inhibitor of angiogenesis, and a DNA enzyme targeting the protein kinase Cα (PKCα) gene expression. Inhibition of PKCα by a nuclease-resistant DNA enzyme eliminated PKCα gene expression and induced apoptosis in most glioma cells. To assess the efficacy of endostatin and the PKCα DNA enzyme in vivo, rats bearing the intracranial tumor BT4C were given a combined treatment of endostatin and the PKCα enzyme. Survival was significantly enhanced by continuous delivery of endostatin (P<.0004) and rats treated with a single injection of the active DNA enzyme lived significantly longer than those treated with the inactive form (P<.045). Interestingly, a single injection of the PKCα DNA enzyme in combination with continuous delivery of endostatin significantly improved animal survival compared with PKCα (P<.0009) or endostatin (P<.025) alone. Thus, the combined treatment may represent an attractive therapeutic strategy against malignant gliomas.
Keywords: angiogenesis, DNA enzyme, endostatin, gliosarcoma, protein kinase Cα
Introduction
Resistance to chemotherapy and radiotherapy is a major obstacle for the treatment of malignant gliomas that are surgically incurable because of their diffusely infiltrative nature. Glioblastoma multiforme is the most frequent and most malignant glial neoplasm [1]. Thus, the search for novel therapeutic approaches is clearly warranted.
Regulatory proteins involved in tumor cell proliferation and angiogenesis are expected to be promising candidates for gene therapy [2]. Some studies have shown that the expression of Bcl-2-related proteins and the activation of the mitogen activated protein kinase (MAPK) signaling cascades are associated with malignant cell growth [2–6]. One of the protein kinases that was implicated in malignant glioma cell proliferation is the protein kinase C (PKC) [7–9]. PKC exists as a family of at least 12 closely related isoenzymes [10,11]. Because of the similarity in structure characteristics among the different isoenzymes, it has been difficult to develop isoenzyme-specific inhibitors and hence to establish the physiological roles for each isoenzyme member. One method to inhibit gene expression of related isoenzymes is to use ribozymes or DNA enzymes [12]. Inhibition of gene function by this technology relies primarily on Watson-Crick base pairing of nucleic acids. After binding, catalytic nucleic acids are able to cleave their corresponding target mRNAs [12–14]. In this respect, inhibition of protein kinase Cα (PKCα) gene expression by a nuclease-resistant ribozyme or DNA enzymes blocked glioma cell proliferation both in vitro and in vivo, leading to the conclusion that PKCα is a key modulator of malignant glioma growth [9,12,15]. In addition, some studies have supported the role of the PKCα signaling pathway in the tumorigenicity and metastasis of other malignancies such as breast and intestinal tumors [7,16,17].
The formation of the vascular network is essential for tumors to grow and metastasize [18]. Glioblastoma multiforme shows a high degree of proliferation of the microvasculature, thus serving as suitable targets for anti-angiogenic therapy [19,20]. To date, a number of inducers and inhibitors of angiogenesis have been described [21–24]. Endostatin is a 20-kDa cleavage product of the C-terminal domain of collagen XVIII and was originally purified from a murine hemangioendothelioma [24]. It has been shown to be a potent anti-angiogenic factor in various neoplasias [25].
We have recently found that subcutaneous and intracranial growth of gliosarcomas can be inhibited by endostatin [26]. This inhibition was characterized by a reduction in both tumor size, blood vessel volume and tumor blood flow as well as increased apoptosis within the tumors. Notably, endostatin was found to be more effective on subcutaneous tumor growth compared with its intracranial counterpart. In this respect, animal bearing intracranial tumors survived only 3 days longer than controls after daily administration of endostatin [26].
In addition to angiogenesis, several lines of evidence indicate that autonomous growth of cancer cells is a consequence of genetic alteration which affect signal transduction pathways, in particular those which are implicated in the control of cell proliferation [27]. We have previously shown that the PKCα is important for glioma cell survival and proliferation. Indeed, its targeting with nucleic acid enzymes induced apoptosis and inhibited subcutaneous tumor growth [9,12,15]. In the present paper, we explored the combined effects of a PKCα DNA enzyme and endostatin on intracranial tumor growth, both given locally.
Materials and Methods
Generation of Endostatin and the DNA Enzyme
Recombinant human endostatin expressed in the yeast Picia pastoris [28] was used in this study. We used a DNA enzyme (DRz4) targeting the translation start site AUG in human PKCα mRNA that has been previously characterized [12]. As control, a DNA enzyme with reversed antisense arms (DRz4r) was used.
Cell Line
The BT4C tumor was derived from serial in vivo passages of the BT4C tumor, originally induced from transformed fetal rat brain cells after exposure to ethyl-nitrosourea [29]. The BT4C cells were grown in RPMI medium with 10% heatinactivated newborn calf serum (FCS). The cell line was tested negative for viral agents in a rat antibody production test according to the Federation of Laboratory Animal Association recommendations [30].
In Vitro Activity of the DNA Enzyme
BT4C cells were seeded in 96-well tissue culture plates at a density of 103 cells per well in 100 µl complete RPMI medium. After incubation for at least 6 hours at 37°C, cells were transfected with the test molecules as described previously [9]. Briefly, the appropriate concentration of DNA enzyme was mixed with 2 µg cationic liposomes (DOTAP; Boehringer Mannheim) and transfection buffer (25 mM HEPES, pH 7.4), yielding a final volume of 10 µl. The mixture was vortexed and incubated at room temperature for at least 10 minutes before adding to cell cultures. After 40 hours transfection time, viable cell number was assessed by the MTT assay [12].
To analyze the kinetics of uptake of the DNA enzyme by glioma cells, BT4C cells were seeded 104 cells per well in 100 µl RPMI complete medium and then transfected with a fluorescein-tagged DRz4 (200 nM) as described above. At various time points, cells (within three wells) were trypsinated, washed and then analyzed by flow cytometry.
Western Blots
Protein extracts were prepared from control and test molecule-treated cells. Briefly, cells were washed twice with phosphate-buffered saline (PBS) and lysed at 4°C with lysing buffer (0.2% NP40 in PBS and protease inhibitors). After 20 to 30 minutes of incubation, cell lysates were centrifuged for 10 minutes at 15,000 rpm and supernatant was collected. Protein concentrations were determined using the protein assay Kit (Bio-Rad Laboratories, Hercules, CA). Equivalent amounts of cytoplasmic proteins (15 µg) were analyzed by 10% SDS-PAGE and transferred to nitrocellulose membranes. The membranes were blocked with 5% BSA in immunoblotting buffer (10 mM Tris, 150 mM NaCl and 0.1% Tween-20) overnight at 4°C, and subsequently incubated with rabbit IgG polyclonal anti-PKCα, anti-Bcl-xl or β-actin antibodies. After washing, membranes were incubated with horseradish peroxidase conjugated anti-rabbit IgG antibody. The immunoreactive proteins were visualized by an ECL detection Kit (Amersham).
Analysis of Apoptosis by the TUNEL-Method
A commercially available in situ cell death fluorescein detection kit based on terminal deoxynucleotidyl transferase (TdT)-mediated dUTP-FITC nick end labeling (TUNEL) was used (Boehringer, Ingelheim, Germany). In these experiments, glioma cells were transfected for 48 hours and then they were detached from the plates by gentle scraping. The cells were thereafter collected by centrifugation, washed with PBS, fixed with 4% paraformaldehyde and then permeabilized with 0.1% Triton X-100 in 0.1% sodium citrate for 2 minutes at 4°C. After washing with PBS, cells were incubated with the TUNEL-reagents for 30 minutes, washed with PBS and then analyzed by flow cytometry (Becton Dickinson). TUNEL positive cells were gated, and the results were analyzed by CELLQuest software.
Animals
Inbred BD-IX rats (Harlan Olac, Bicester, UK) of both sexes weighing at least 250 g were caged in pairs or groups of three animals in Macrolon III cages. The animal holding rooms were maintained at constant temperature and humidity on a 12-hour light and dark schedule at an air exchange rate of 18 changes/hour. Animal care and protocol were in accordance with national legislation and institutional guidelines. The animals were tested negative for parasitical, bacterial and viral agents according to the FELASA recommendations [30]. For the surgical procedures, the rats were anaesthetized with a combination of fentanyl / fluanisone and midazolam, and buphrenorphin was used as a postoperative analgetic agent. The rats were euthanized with a lethal dose of pentobarbitone i.p. (100 mg/kg). Treatment and control groups consisted of seven animals each.
The Rat Gliosarcoma Model
To establish intracranial tumors, the anaesthetized rats were fixed in a stereotactic frame (David Kopf Instruments, Tujunga, CA), before the skin was incised and a 1.0-mm burr hole was made to fit the coordinates (3.3 mm posterior to and 2.5 mm to the right of the bregma, depth 2.8 mm). The injection device consisted of a 30-G blunt needle connected through a catheter (Small Parts, Miami Lakes, FL) to an infusion pump (Harvard Apparatus, Holliston, MS). The needle was fixed in the electrode holder of the stereotactic frame, and then vertically introduced into the brain. A total of 50,000 cells in 5 µl of RPMI were injected into the brain during a period of 1 minute. The needle was kept in place for 2 minutes and then slowly retracted. Closure was done with bone wax and sutures.
Treatment Protocol
Human endostatin was administered using osmotic mini pumps (Alzet, Mountain View, CA). The pumps was positioned subcutaneous on the back of the animal, connected to a brain infusion kit delivering a continuous infusion of 5 µl/hour (3 mg endostatin/24 hours) for up to 28 days at the same location where the tumor cells were inoculated 3 days earlier. The DRz4 enzyme (100 µg), or its inactive form DRz4r (100 µg) in 5 µl saline, were administered as a single intracranial injection on day 3, using the stereotactic devise and injection apparatus at the same coordinates as described above. The control animals were treated with 0.9% NaCl during the same period. Thereafter, the rats were examined regularly for local tumor growth. The animals were euthanized with an overdose of pentobarbitone when they showed a weight reduction of 10% and/or clinical signs of raised intracranial pressure (reduced activity, impaired locomotoric activity, and lethargy) and were subsequently considered as death events in the survival curves.
Statistical Analysis
Statistical analysis of the data was performed with the Logrank test and significance was assumed for P<.05.
Results
Inhibition of Malignant Cell Growth by the DRz4 Enzyme
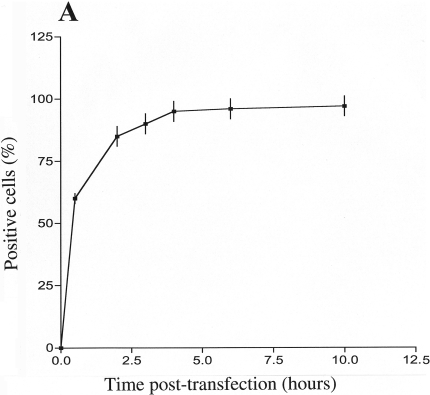
We have previously reported the development of a nuclease-resistant PKCα DNA enzyme (DRz4) capable of inducing apoptosis in sensitive cells such as human malignant gliomas [12]. To further investigate its potential therapeutic use, in vitro and in vivo studies with rat malignant gliomas were performed. We have assessed the uptake of the DNA enzyme DRz4 into BT4C cells. In these experiments, cells were transfected to a 5′-fluorescein-tagged DRz4 enzyme and samples were taken at various time points. To remove membrane-bound molecules, cells were trypsinated, washed and then analyzed by flow cytometry. Figure 1A shows that by 4 hours, almost all the cells had taken up the DNA enzyme.
Figure 1.
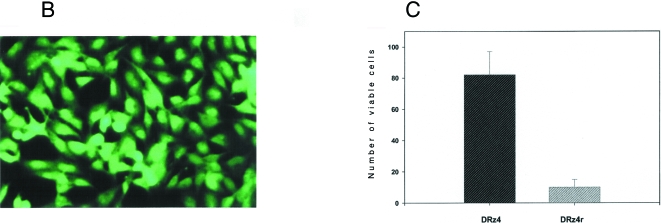
DRz4 uptake by glioma cells and its effect on viable cell number. (A) DNA enzyme uptake by BT4C cells. Cells were transfected with a fluorescein-tagged DRz4 (200 nM) using DOTAP (2 µg). Samples were taken at various time points and analyzed by flow cytometry. Values are means±SEM based on three experiments. (B) A fluorescent image of glioma cells transfected with a fluorescein-tagged DRz4 for 7 hours. Cells were transfected for 7 hours using the same conditions as in A, and then analyzed by an epifluorescent microscope. (C) BT4C glioma cells were transfected for 40 hours with the test molecules (200 nM) and viable cell number was estimated with the MTT assay. The reduction of viable cell number was expressed as a percentage of that obtained in untreated cells. DRz4= active DNA enzyme, DRz4r = inactive DNA enzyme. Values are means±SEM, n=4.
To establish whether the ribozyme crossed the cell membrane and entered the cytoplasm and/or the nucleus to inhibit PKCα gene expression, cells were analyzed by an epifluorescent microscope. In these experiments, BT4C cells were cultured on chamber slides and transfected with fluorescein-tagged DNA enzyme. Upon examination, these cells demonstrated almost 100% transfection efficiency (Figure 1B) as has been shown for human gliomas [12]. Notably, a high fraction of the DNA enzyme exhibited a nuclear localization. Taken together, these results would indicate that the experimental conditions in relation to DOTAP (15 µg/ml) and ribozyme concentration (100 to 200 nM) seems to be adequate for glioma cells.
To examine the effect of DRz4 on cell survival, BT4C cells were transfected with the DRz4 enzyme or its inactive form (DRz4r), and viable cell number was monitored by the MTT assay (Figure 1C). Notably, viable cell number was reduced by 80% compared with that of DOTAP-treated cells. In contrast, the inactive DNA enzyme (DRz4r) showed only 15% inhibition, hence significantly lower than its active form.
Effects of the DRz4 Enzyme on PKC_ Gene Expression
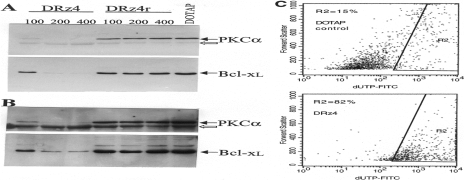
To evaluate whether the reduction in cell number after DNA enzyme treatment was reflected at the protein level, protein extracts from controls and DNA enzyme-treated cells were subjected to Western blot analysis (Figure 2A). In DNA enzyme-treated cells, the protein level of PKCα was nearly eliminated, whereas no significant effects were observed with the inactive DNA enzyme. The observed inhibitory effect was specific, because the protein level of a cross-reactive protein, indicated by the open arrow, was not affected by the DRz4 treatment (Figure 2B, lower panel). Additionally, the protein level of β-actin was not affected by the treatment (data not shown). The secondary band seen in Western blot is more likely to be a cross-reactive protein, because it reacted with only one polyclonal antibody preparation. No reactivity was seen with other antibodies specific for PKC-α [9]. As we have reported previously, the inhibition of PKCα gene expression by an RNA ribozyme or a DNA enzyme also affected the Bcl-xl gene expression.
Figure 2.
Effects of the DRz4 enzyme on gene expression and of apoptosis. After 40 hours transfection time, cytoplasmic protein extracts were prepared from control (DOTAP) and DRz4- and DRz4r-treated cells. Fifteen micrograms from each sample was analyzed by Western blots using specific antibodies (A). The final concentrations of the DRz4 and DRz4r are indicated. A potential cross-reactive protein is indicated by the open arrow. A higher exposure of the data is shown in B. (C) Quantification of cellular DNA fragmentation by the TUNEL method. Controls and DRz4-treated cells were analyzed by the TUNEL method as described in Materials and Methods section. Of the DNA enzyme-treated cells, 82% were apoptotic. Cells were transfected for 48 hours.
The downregulation of Bcl-xl may indicate that cells are killed by apoptosis. Thus, we analyzed the extent of this process in the presence and absence of the DNA enzyme (Figure 2C). In contrast to DOTAP-treated cells, where 15% of the cells were apoptotic, 82% of the DNA enzyme-treated cells were apoptotic. This result would confirm our previous data that the overexpression of PKCα is required for glioma cell survival and proliferation [12].
Therapeutic Effects of the Combined Treatment with the DRz4 Enzyme and Endostatin on Intracranial Tumor Growth
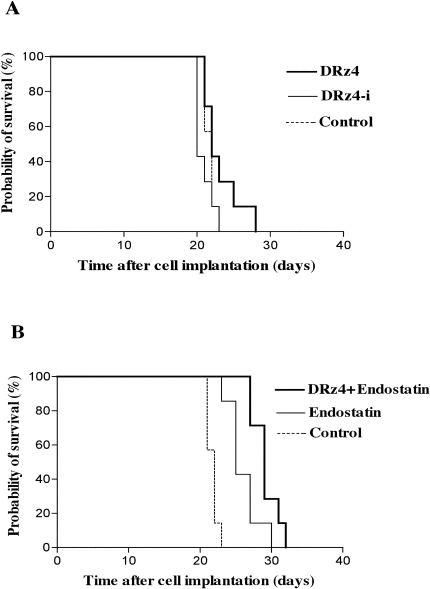
To test the efficacy of the DRz4 molecules in vivo, rats with intracerebrally implanted BT4C glioma cells were treated with a single intracranial injection of the test molecules 3 days after cell inoculation. As shown in Figure 3A, there was a significant difference in the survival times between animals that had been treated with the active DRz4 enzyme compared with those treated with the inactive enzyme DRz4r. Rats treated with a continuous intracranial delivery of endostatin lived significantly longer than untreated controls (Figure 3B).
Figure 3.
Kaplan-Meier survival curves of rats with BT4C brain tumors. Rats were inoculated with BT4C glioma cells. After 3 days, they were treated with either a single injection of DRz4 or DRz4r enzyme (A) (n=7). The effects of a continuous delivery of endostatin or a combination of the two treatment modalities are shown in B (n=7).
To evaluate the effects of the combined treatment, rats were given a single intracranial injection of the DRz4 enzyme (100 µg) on day 3 after inoculation in combination with continuous intracranial delivery of endostatin (3 mg/kg per 24 hours). As illustrated in Figure 3B, rats treated with both molecules lived longer (P<.025) (mean 29.1 days) than rats treated with endostatin only (mean 26.0 days).
Discussion
The failure of malignant gliomas to respond to current therapy prompted us to test the efficacy of a combined targeting of the PKCα gene expression and tumor angiogenesis. The designed DRz4 enzyme inhibited PKCα gene expression and reduced viable cell number. Animals treated with a single injection of the DRz4 survived longer than those treated with the inactive DNA enzyme. The combination of both endostatin and DRz4 improved animal survival compared with single treatments.
Most brain tumors are characterized by a high recurrence rate, in particular malignant gliomas that remain resistant to all current treatment strategies [31]. Suppression of programmed cell death was found to be one of the main mechanisms responsible for drug resistance [32]. In this regard, glioma cells overexpressed the anti-apoptotic protein Bcl-xl [9]. Thus, understanding the mechanisms underlying the overexpression of the Bcl-xl protein in glioma cells is important for the design of apoptotic agents. We have previously found that the expression and/or activity of Bcl-xl is under the control of the PKCα-signaling pathway in malignant gliomas [9]. As shown in this study, specific inhibition of PKCα by the DRz4 enzyme also inhibited Bcl-xl gene expression. Interestingly, both the PKCα and Bcl-xl were found to be localized in the mitochondrial membrane, thus suggesting a direct interaction between these proteins [12]. Similarly, PKCα was found to interact with the anti- apoptotic protein Bcl-2. In this respect, phosphorylation of Bcl-2 at Ser-70 by PKCα inhibited apoptosis induced by chemotherapeutic drugs such as VP16 [32]. Thus, these observations would define PKCα as a central regulator of both cell proliferation and survival.
With the potential to induce apoptosis in sensitive cells, the developed PKCα DNA enzyme should constitute an important therapeutic tool [12]. Consistent with its anti- proliferative effects on malignant gliomas growing in vitro, a single injection of the DRz4 enzyme produced a significant inhibitory effect on intracranial tumor growth in vivo. This finding is in agreement with the idea that constitutive activation of PKC, in particular the PKCα isoform, is required for malignant tumor growth [9,12,33,34].
Endostatin was found to be a potent inhibitor of angiogenesis [21,24]. In this respect, recombinant human endostatin was shown to induce regression and prevent growth of subcutaneous experimental tumors when administered in daily doses as high as 20 mg/kg [24]. Given the inhibitory effects on tumor growth in nude mice [21] and glioma cells growing subcutaneously in rats, it is possible that the inhibition of endothelial cell migration was inhibited [35]. In line with this, we recently have shown that the angiogenic process is indeed inhibited [36].
Although locally continuous administration with endostatin has prolonged survival among rats with intracranial tumors compared with daily subcutaneous injections, the observed effect is still not quite impressive. Endostatin-treated rats survived only 5 days longer than untreated controls. Various mechanisms might account for the low effect of endostatin on intracranial gliosarcoma growth. For example, other angiogenic pathways, which are independent of endostatin, may be involved. This points to the importance of targeting additional pathways responsible for cell proliferation and angiogenesis. Indeed, rats treated with both endostatin and DRz4 enzyme survived 9 days longer than controls (P<.025). In addition to being involved in glioma cell proliferation, PKCα was found to regulate the expression of bFGF [37], a potent angiogenic factor for gliomas. Therefore, the mechanism of PKCα may also be angiogenic inhibition, as well as the tumor effects previously demonstrated. In contrast to gene and cell therapy approaches [21,38], locally delivered drugs (e.g., recombinant proteins and nucleic acid enzymes) are expected to be safer. Injection of the ribozyme DOTAP/formulation into the brain of control rats (without tumors) had no apparent cytotoxic effects after 3 months observation period.
Taken together, the present data show that intracranial inoculated glioma cells respond to a combined treatment with locally applied DRz4 enzyme and endostatin. It will be of interest to explore if this combined treatment may inhibit the growth of other tumors.
Acknowledgements
We thank BKL Sim for the generous supply of endostatin. The work was supported in part by Norwegian Cancer Society and the Norwegian Research Council.
References
- 1.Kleihues P, Cavenee WK. Pathology and Genetics of Tumours of the Nervous System. Lyon, France: International Agency for Research on Cancer; 1997. [Google Scholar]
- 2.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 3.Plattner R, Gupta S, Khosravi-Far R, Sato KY, Perucho M, Der CJ, Stanbridge EJ. Differential contribution of the ERK and JNK mitogen-activated protein kinase cascades to Ras transformation of HT1080 fibrosarcoma and DLD-1 colon carcinoma cells. Oncogene. 1999;18:1807–1817. doi: 10.1038/sj.onc.1202482. [DOI] [PubMed] [Google Scholar]
- 4.Silvany RE, Eliazer S, Wolff NC, Ilaria RL., Jr Interference with the constitutive activation of ERK1 and ERK2 impairs EWS/FLI-1-dependent transformation. Oncogene. 2000;19:4523–4530. doi: 10.1038/sj.onc.1203811. [DOI] [PubMed] [Google Scholar]
- 5.Reed JC. Bcl-2 family proteins. Oncogene. 1998;17:3225–3236. doi: 10.1038/sj.onc.1202591. [DOI] [PubMed] [Google Scholar]
- 6.Zamzami N, Brenner C, Marzo I, Susin SA, Kroemer G. Subcellular and submitochondrial mode of action of Bcl-2-like oncoproteins. Oncogene. 1998;16:2265–2282. doi: 10.1038/sj.onc.1201989. [DOI] [PubMed] [Google Scholar]
- 7.Masur K, Lang K, Niggemann B, Zanker KS, Entschladen F. High PKC alpha and low E-cadherin expression contribute to high migratory activity of colon carcinoma cells. Mol Biol Cell. 2001;12:1973–1982. doi: 10.1091/mbc.12.7.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Couldwell WT, Antel JP, Apuzzo ML, Yong VW. Inhibition of growth of established human glioma cell lines by modulators of the protein kinase-C system. J Neurosurg. 1990;73:594–600. doi: 10.3171/jns.1990.73.4.0594. [DOI] [PubMed] [Google Scholar]
- 9.Sioud M, Sorensen DR. A nuclease-resistant protein kinase C alpha ribozyme blocks glioma cell growth. Nat Biotechnol. 1998;16:556–561. doi: 10.1038/nbt0698-556. [DOI] [PubMed] [Google Scholar]
- 10.Newton AC, Johnson JE. Protein kinase C: a paradigm for regulation of protein function by two membrane-targeting modules. Biochim Biophys Acta. 1998;1376:155–172. doi: 10.1016/s0304-4157(98)00003-3. [DOI] [PubMed] [Google Scholar]
- 11.Dekker LV, Parker PJ. Protein kinase C—a question of specificity. Trends Biochem Sci. 1994;19:73–77. doi: 10.1016/0968-0004(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 12.Sioud M, Leirdal M. Design of nuclease resistant protein kinase calpha DNA enzymes with potential therapeutic application. J Mol Biol. 2000;296:937–947. doi: 10.1006/jmbi.2000.3491. [DOI] [PubMed] [Google Scholar]
- 13.Haseloff J, Gerlach WL. Simple RNA enzymes with new and highly specific endoribonuclease activities. Nature. 1988;334:585–591. doi: 10.1038/334585a0. [DOI] [PubMed] [Google Scholar]
- 14.Santoro SW, Joyce GF. A general purpose RNA-cleaving DNA enzyme. Proc Natl Acad Sci USA. 1997;94:4262–4266. doi: 10.1073/pnas.94.9.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leirdal M, Sioud M. Ribozyme inhibition of the protein kinase C alpha triggers apoptosis in glioma cells. Br J Cancer. 1999;80:1558–1564. doi: 10.1038/sj.bjc.6690560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Brian C, Vogel VG, Singletary SE, Ward NE. Elevated protein kinase C expression in human breast tumor biopsies relative to normal breast tissue. Cancer Res. 1989;49:3215–3217. [PubMed] [Google Scholar]
- 17.Ways DK, Kukoly CA, deVente J, Hooker JL, Bryant WO, Posekany KJ, Fletcher DJ, Cook PP, Parker PJ. MCF-7 breast cancer cells transfected with protein kinase C-alpha exhibit altered expression of other protein kinase C isoforms and display a more aggressive neoplastic phenotype. J Clin Invest. 1995;95:1906–1915. doi: 10.1172/JCI117872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 19.Vajkoczy P, Schilling L, Ullrich A, Schmiedek P, Menger MD. Characterization of angiogenesis and microcirculation of high-grade glioma: an intravital multifluorescence microscopic approach in the athymic nude mouse. J Cereb Blood Flow Metab. 1998;18:510–520. doi: 10.1097/00004647-199805000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Johansson M, Bergenheim AT, Widmark A, Henriksson R. Effects of radiotherapy and estramustine on the microvasculature in malignant glioma. Br J Cancer. 1999;80:142–148. doi: 10.1038/sj.bjc.6690333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blezinger P, Wang J, Gondo M, Quezada A, Mehrens D, French M, Singhal A, Sullivan S, Rolland A, Ralston R, Min W. Systemic inhibition of tumor growth and tumor metastases by intramuscular administration of the endostatin gene. Nat Biotechnol. 1999;17:343–348. doi: 10.1038/7895. [DOI] [PubMed] [Google Scholar]
- 22.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 23.Beck L, Jr, D'Amore PA. Vascular development: cellular and molecular regulation. FASEB J. 1997;11:365–373. [PubMed] [Google Scholar]
- 24.O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 25.Boehm T, Folkman J, Browder T, O'Reilly MS. Antiangiogenic therapy of experimental cancer does not induce acquired drug resistance. Nature. 1997;390:404–407. doi: 10.1038/37126. [DOI] [PubMed] [Google Scholar]
- 26.Sorensen D, Read T-A, Porwol T, Olsen BR, Timpl R, Sasaki T, Iversen PO, Benestad HB, Sim BKL, Bjerkvig R. Endostatin reduces vascularization, blood flow, and growth in a rat gliosarcoma. Neuro-Oncol. 2002;4:1–8. doi: 10.1215/15228517-4-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sioud M. Nucleic acid enzymes as a novel generation of anti-gene agents. Curr Mol Med. 2001;1:575–588. doi: 10.2174/1566524013363366. [DOI] [PubMed] [Google Scholar]
- 28.Sim BKL, Fogler WE, Zhou XH, Liang H, Madsen JW, Luu K, O'Reilly M, Tomaszewski E, Fortier AH. Zinc ligand-disrupted recombinant human endostatin: potent inhibitor of tumor growth, safety and pharmacokinetic profile. Angiogenesis. 1999;3:41–51. doi: 10.1023/a:1009058931769. [DOI] [PubMed] [Google Scholar]
- 29.Mella O, Bjerkvig R, Schem BC, Dahl O, Laerum OD. A cerebral glioma model for experimental therapy and in vivo invasion studies in syngeneic BD IX rats. J Neurooncol. 1990;9:93–104. doi: 10.1007/BF02427829. [DOI] [PubMed] [Google Scholar]
- 30.Rehbinder C, Baneux P, Forbes D, van Herck H, Nicklas W, Rugaya Z, Winkler G. FELASA recommendations for the health monitoring of mouse, rat, hamster, gerbil, guinea pig and rabbit experimental units. Report of the Federation of European Laboratory Animal Science Associations (FELASA) Working Group on Animal Health accepted by the FELASA Board of Management, November 1995. Lab Anim. 1996;30:193–208. doi: 10.1258/002367796780684881. [DOI] [PubMed] [Google Scholar]
- 31.DeAngelis LM. Brain tumors. N Engl J Med. 2001;344:114–123. doi: 10.1056/NEJM200101113440207. [DOI] [PubMed] [Google Scholar]
- 32.Ruvolo PP, Deng X, Carr BK, May WS. A functional role for mitochondrial protein kinase Calpha in Bcl2 phosphorylation and suppression of apoptosis. J Biol Chem. 1998;273:25436–25442. doi: 10.1074/jbc.273.39.25436. [DOI] [PubMed] [Google Scholar]
- 33.Couldwell WT, Uhm JH, Antel JP, Yong VW. Enhanced protein kinase C activity correlates with the growth rate of malignant gliomas in vitro. Neurosurgery. 1991;29:880–886. doi: 10.1097/00006123-199112000-00013. discussion 886 – 87. [DOI] [PubMed] [Google Scholar]
- 34.Whelan RD, Parker PJ. Loss of protein kinase C function induces an apoptotic response. Oncogene. 1998;16:1939–1944. doi: 10.1038/sj.onc.1201725. [DOI] [PubMed] [Google Scholar]
- 35.Kim YM, Jang JW, Lee OH, Yeon J, Choi EY, Kim KW, Lee ST, Kwon YG. Endostatin inhibits endothelial and tumor cellular invasion by blocking the activation and catalytic activity of matrix metalloproteinase. Cancer Res. 2000;60:5410–5413. [PubMed] [Google Scholar]
- 36.Read TA, Sorensen DR, Mahesparan R, Enger PO, Timpl R, Olsen BR, Hjelstuen MH, Haraldseth O, Bjerkvig R. Local endostatin treatment of gliomas administered by microencapsulated producer cells. Nat Biotechnol. 2001;19:29–34. doi: 10.1038/83471. [DOI] [PubMed] [Google Scholar]
- 37.Leirdal M, Sioud M. Protein kinase Calpha isoform regulates the activation of the MAP kinase ERK1/2 in human glioma cells: involvement in cell survival and gene expression. Mol Cell Biol Res Commun. 2000;4:106–110. doi: 10.1006/mcbr.2000.0259. [DOI] [PubMed] [Google Scholar]
- 38.Ding I, Sun JZ, Fenton B, Liu WM, Kimsely P, Okunieff P, Min W. Intratumoral administration of endostatin plasmid inhibits vascular growth and perfusion in MCa-4 murine mammary carcinomas. Cancer Res. 2001;61:526–531. [PubMed] [Google Scholar]