Abstract
UV irradiation and other stress-activated signals activate the Jun N-terminal kinase (JNK, SAPK) pathway. The induction of JNK activity results in the activation of proto-oncogene c-Jun and activator protein-1 (AP-1) transcriptional activity. Data presented here show that UV mediated the activation of JNK correlated with UV-induced apoptosis and that overexpression of a dominant negative JNK blocked UV-induced apoptosis. However, the molecular events that lead to JNK activation in response to UV treatment are not clear. In this report, we provide evidence that a Fas receptor binding protein, Daxx, mediates UV-induced JNK activation and apoptosis. A dominant negative Daxx, coding for the C-terminal region (112 amino acids) of Daxx, was constructed and used in the experiments. Our data show that overexpression of the dominant negative Daxx partially inhibits UV-induced JNK phosphorylation in 293 cells. Inhibition of JNK phosphorylation resulted in the inhibition of c-Jun activation upon UV irradiation. Our data also show that the inhibition of JNK activation by dominant negative Daxx correlates with the reduced rate of apoptotic death of 293 cells after UV irradiation. Surprisingly, overexpression of wild-type Daxx also inhibited UV-induced apoptosis, suggesting that Daxx competes for Fas receptor binding sites with other proapoptotic factors such as FADD. In addition, overexpression of a dominant negative mutant of FADD did not affect UV-induced JNK activation but does inhibit UV-induced apoptosis. These results suggest that UV-induced JNK activation is not sufficient but required for induction of apoptosis.
Abbreviations: JNK, stress-activated protein kinase; DaxxC, C-terminal region (112 amino acids) of Daxx; SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis
Introduction
Apoptosis is a morphological phenomenon characterized by nuclear chromatin condensation, cellular shrinkage, membrane blebbing, margination of the nuclear matrix, and degradation of nuclear DNA into oligonucleosomal length fragments [1]. Exposure of certain eukaryotic cells to UV irradiation results in their apoptotic death, thereby reducing the risk of cancer. UV irradiation of mammalian cells results in initiation of two key signaling cascades due to activation of cell surface receptors such as the Fas and TNF receptors [2,3]. This includes the Fas-FADD-caspase-8-axis and the Jun N-terminal kinase (JNK) cascade. Both these signaling events are required for the initiation of the apoptotic machinery. Inhibition of JNK activation by overexpressing a dominant negative JNK (DN-JNK) has been shown to block UV-induced apoptosis [4]. In addition, UV-induced aggregation of the Fas and TNF receptors has been demonstrated to initiate the caspase cascade by activating caspase-8 [2,5]. Although the mechanism by which UV initiates the apoptotic cascade is now well studied, the pathway for UV-induced JNK activation and its correlation to apoptosis is still not clear. Goillot et al. [6] proposed that JNK activation mediates Fas-induced apoptosis. In an effort to better define the molecular events that result in JNK activation after UV irradiation, we proposed the involvement of the Fas binding protein Daxx [7]. Overexpression of Daxx has been shown to result in JNK activation independent of receptor activation and potentiates Fas-induced apoptosis [7]. The Fas-binding domain of Daxx, which is located at the C-terminal region (112 amino acids), acts as a dominant negative inhibitor of Fas-induced JNK activation and apoptosis [7]. Recent studies also suggest that Daxx is a nuclear protein [8]. Upon stimulation, Daxx translocates from the nucleus to the cytoplasm. The cytoplasmic Daxx then interacts with Fas receptor and Ask1, which induces apoptosis [9,10]. These results together suggest that Daxx plays a critical role in the Fas-mediated JNK activation as well as apoptosis. In the present study, we provide evidence that Daxx mediates UV-induced JNK activation. However, we also demonstrate that Daxx-mediated JNK activation is not sufficient to induce apoptosis upon UV irradiation.
Materials and Methods
Cell Lines and Culture Conditions
The 293 cells (human kidney) were maintained in Dulbecco's modified essential medium (DMEM; Life Technologies, Gaithersburg, MD) containing 10% fetal calf serum, 1% l-glutamine, 100 U/ml penicillin, and 100 µg/ml streptomycin at 37°C in an atmosphere of 5% CO2. The BJAB and BJAB (dominant negative FADD, DN-FADD) cells were maintained in RPMI 1640 containing 10% fetal calf serum, 1% l-glutamine, 100 U/ml penicillin, and 100 µg/ml streptomycin at 37°C in an atmosphere of 5% CO2. The BJAB (DN-FADD) was grown in media containing 3 mg/ml G418 (Life Technologies).
hDaxx and N-Terminal Deleted hDaxx (hDaxxC) Expression Vector Construction
The open reading frames of human Daxx (hDaxx; amino acids 1–740) and hDaxxC (amino acids 626–740) were amplified from a HeLa cDNA library using PCR. A high-fidelity DNA polymerase (Pwo; Roche Molecular Biochemicals, Indianapolis, IN) was used for the PCR. A T7 tag (MASMTGGQQMG) or Flag tag (MDYKDDDDK) was added to the N-terminal of hDaxx and hDaxxC. The primers used for PCR were: T7-hDaxx 5′ primer: GCGAATTCCACCATGGCTAGCATGACTGGTGGACAGCAAATGGGTGCCACCGCTAACAGCATC Flag-hDaxxC 5′ primer: GCGAATTCCACCATGGACTACAAGGACGACGATGACAAGGGTCCCCCCTGCAAAAAA3′ primer: GCGAATTCGCGGCCGCTATTAATCAGAGTCTGAGAG.
The primers were designed so that the resulting fragment would contain a Kozak sequence (CCACC) followed by an ATG codon for initiation of protein translation. Two EcoRI and one NotI restriction sites were also added to the primers for subcloning the fragments. The PCR products were subcloned into the mammalian expression vector pZ, which contains the adenovirus major late promoter and simian virus 40 (SV40) enhancer element for transcription initiation. In addition, the vector contains the SV40 origin for replication in COS-1 and 293 cells. A unique feature of pZ expression vector is that it utilizes the encephalomyelocarditis (ELC) internal ribosomal entry site to initiate translation of neoR. This feature allowed us to use this expression vector to establish stable cell lines overexpressing interested genes. The sequences of the inserted genes were determined by the DNA Sequencing Core of University of Michigan.
DNA Transfection
At 24 hours before transfection, the 293 human kidney cells were seeded into gelatin-coated six-well culture plates. The 293 cells were transfected by the Ca-phosphate procedure. In brief, 1.5 ml of culture media containing 33 µM chloroquine was added to each plate. Then 0.5 ml of Caphosphate mixture containing 5 µg DNA was slowly dropped into the media. The cells were incubated for 7 hours before changing to regular culture media.
Immunoblotting of Daxx and DaxxC Expressed in 293 Cells
We performed Western blot analysis with the cell extracts prepared from pZ-T7-hDaxx and pZ-Flag-hDaxxC transfected 293 cells. The expression vectors were transiently transfected into 293 cells using Ca-phosphate method as described above. After 48 hours, the transfected cells were lysed using NP-40 lysis buffer. The protein concentration was determined using the Bio-Rad Protein Assay Reagent (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer's procedure. The total cell extracts (15 µg) were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and electroblotted to nitrocellulose membrane. The membrane was probed with anti-Daxx polyclonal antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) and analyzed by SuperSignal Chemiluminescent detection system as recommended by the supplier (Pierce, Rockford, IL).
UV Irradiation
At 24 hours posttransfection, the cells were irradiated with UVC using a germicidal lamp (UVP, Upland, CA). The intensity of the UV light was measured prior to each experiment with a UVX radiometer (UVP) and was set at 1.5 or 3 W/m2 depending on the dosage used for irradiation. For adherent cells, the culture media was drained before the irradiation and fresh media was added to the plates after irradiation. Suspension cells was pelleted by centrifugation (1000g), resuspended in 0.5 ml of media, and plated on a 60-mm plate for irradiation. After UV irradiation, 2 ml of media was added to each plate.
JNK Activation Assay
The UV-induced JNK activation was determined by measuring the phosphorylation of JNK using a SAPK/JNK Assay Kit (New England Biolaboratories, Beverly, MA) according to manufacturer's procedure. Briefly, equal amounts of cell extract prepared form the transfected 293 cells were resolved on 12% SDS-PAGE and electroblotted onto nitrocellulose membrane. The membrane was probed with anti-phospho-JNK antibody (1 µg/ml) and analyzed by SuperSignal Chemiluminescent immunoblotting detection system as described by the supplier (Pierce). The amount of Flag-tagged JNK was determined by the same method using anti-Flag antibody.
In Vitro c-Jun Kinase Assay
In vitro c-Jun kinase assay was performed using the SAPK/JNK Assay Kit (New England Biolaboratories) with a modified procedure. Flag-JNK expression vector (0.5 µg) was cotransfected with pZ vector (4.5 µg) or with pZ-Flag-hDaxxC (4.5 µg). After 48 hours, the transfected cells were irradiated with 0 or 100 J/m2 UVC as indicated. At 30 minutes postirradiation, the cells were lysed and the expressed Flag-tagged JNK was immunoprecipitated from the cell extracts using anti-Flag antibodies. The immunoprecipitates were incubated with 0.2 µg of c-Jun protein (Promega, Madison WI) in kinase assay buffer provided with the kit at 30°C for 30 minutes. The samples were then subjected to 12% SDS-PAGE and c-Jun phosphorylation was detected using anti-phospho-c-Jun antibody provided with kit.
Morphological Analysis
The mammalian expression vectors (1 µg) containing hDaxxC or DN-JNK were cotransfected with EGFP expression vector, pEGFP-1 (0.5 µg; Clontech Laboratories, Palo Alto, CA), into 293 cells using Lipofactamine Plus reagent (Life Technologies) according to manufacturer's procedure. At 24 hours posttransfection, the cells were UV-irradiated (15 J/m2). At 48 hours postirradiation, the percentage of apoptotic cells was determined by the number of green cells with apoptotic morphology divided by the total number of blue cells. The images of EGFP expression were captured by an Olympus IX70 fluorescence microscope (Olympus America, Melville, NY) using a Kodak M290 camera (Eastman Kodak, Rochester, NY) with an exposure time of 0.25 seconds.
Results
UV Irradiation of 293 Cells Results in a Time- and Dose-Dependent Activation of JNK
To first establish that UV irradiation results in activation of JNK, 293 cells were irradiated with various doses of UV. Using an antibody specific for the phosphorylated form of JNK, we demonstrated that there was a detectable increase in expressed JNK phosphorylation 30 minutes after irradiation at 15 J/m2 (Figure 1A). With increasing doses of UV, there was a corresponding increase in the phosphorylated form of JNK but no corresponding increase in JNK levels was detected (Figure 1A). Using 100 J/m2, a dose of UV that resulted in maximal stimulation of JNK in 30 minutes (Figure 1A), we demonstrated that JNK activation as determined by the presence of the phosphorylated form of JNK was detectable immediately after irradiation (0 minute) and peaked at 30 minutes, after which there was a gradual decrease in the levels of phosphorylated JNK. Because the levels of total JNK were not significantly varied at these time points (Figure 1B, right panel), differential reactivity to the antibody specific for the phosphorylated form of JNK (at residues 183 and 185) is indicative of differential phosphorylation.
Figure 1.
UV irradiation induces JNK activation in a dose- and time-dependent manner. Total cell extracts (30 µg) from UV-irradiated 293 cells were resolved on 12% SDS-PAGE and electroblotted to nitrocellulose membrane. The phosphorylated JNK and total JNK were probed using anti-phospho-SAPK/JNK (Thr183/Tyr185) antibody and anti-JNK antibody.
Construction and Expression of Full-Length and Dominant Negative Form of hDaxx
To investigate if Daxx was involved in UV-mediated JNK activation, we isolated the hDaxx coding sequence from HeLa cell mRNA by RT-PCR. The oligonucleotides used to amplify the hDaxx coding sequence were derived from a published sequence (accession no. AF015956). Two expression plasmids were constructed: one that contained the entire coding sequence as well as an N-terminal T7 epitope tag (pZ-T7-hDaxx) and a second that contained the C-terminus of hDaxx (residues 626–740) as well as an N-terminal T7 epitope tag (pZ-T7-hDaxxC). The latter was constructed as a dominant negative based on the results of Yang et al. [7], that residues 136 to 242 of the mouse sequence act as a dominant negative. To confirm that the constructed plasmids resulted in the expression of expected polypeptides, transient transfection assays were done using 293 cells and the resulting cell extracts were resolved by SDS-PAGE followed by Western blot analysis using a Daxx-specific polyclonal antibody. The wild-type hDaxx expression construct yielded a 120-kDa polypeptide and the hDaxxC construct yielded a 16-kDa polypeptide as predicted from peptide sequences (Figure 2).
Figure 2.
Western blot analysis of overexpressed Daxx and DaxxC. 293 cells were transfected with pZ vector alone (lane 1), pZ-T7-Daxx (lane 2), and pZ-Flag-DaxxC (lane 3). After 48 hours, cell extracts were prepared from the transfected cells and resolved on 15% SDS-PAGE. The proteins were then electroblotted to nitrocellulose membranes. The membrane was probed with anti-Daxx polyclonal antibodies. The expression of Daxx and DaxxC was detected by the chemiluminescent detection system.
Daxx Mediates UV-Dependent JNK Activation
We as well as others have previously reported that UV-induced apoptosis is mediated by ligand-independent activation of the Fas receptor, which results in the recruitment of FADD to the activated (multimerized) receptor and subsequent activation of caspase-8 [2,5]. Based on the results presented here as well as others which state that Fas-induced JNK activation is mediated by Daxx [7,10,11], we investigated if UV-induced JNK activation may also be mediated by Daxx. To test this, 293 cells were transfected with an expression vector for JNK either in the presence of a control expression vector or in the presence of an expression vector for hDaxxC, or DN-JNK. To ensure that analysis of JNK phosphorylation status was limited to those cells that were transfected with and therefore expressed DN-JNK or hDaxxC (or vector control), all transfections included an expression vector for JNK at a 1:9 ratio. This would ensure that every cell that was transfected with JNK would also express hDaxxC or DN-JNK. Forty-eight hours after transfection, cells were UV-irradiated (100 J/m2) and cell extracts were prepared 30 minutes postirradiation to examine the phosphorylation status of JNK in the transfected cells. As shown in Figure 3, cells transfected with a control vector only had no detectable JNK (Figure 3, lane 1) whereas cells transfected with the control vector and the JNK expression vector contained detectable levels of phosphorylated (active) JNK (Figure 3, lane 2) as well as total JNK (Figure 3, lane 2). This confirms that JNK status was only being measured in the transfected cells and that endogenous JNK was not a confounding factor. When UV-dependent activation of JNK was measured in cells expressing DN-JNK, a 33-fold inhibition of JNK phosphorylation was observed (Figure 3, lane 3 vs 2) whereas only a two-fold difference in the level of JNK was observed in the two samples (Figure 3, lane 3 vs 2), indicating that DN-JNK expression resulted in a 16-fold inhibition of JNK activation after UV. Interestingly, expression of hDaxxC inhibited JNK activation four-fold (Figure 3, lane 4 vs 2) but the levels of total JNK varied by less than two-fold between the two samples (Figure 3, lane 4 vs 2). To further substantiate that hDaxxC expression resulted in inhibition of JNK activation in response to UV, 293 cells were transfected with a Flag-tagged JNK expression vector in the presence of a control vector or in the presence of hDaxxC. Forty-eight hours after transfection, JNK was immunoprecipitated from these cells and analyzed for its ability to phosphorylate c-Jun protein. Despite the presence of JNK, Jun-specific kinase activity was not detected in the absence of UV irradiation (Figure 4, lane 1) but was readily detected 30 minutes after UV irradiation (Figure 4, lane 2). Expression of hDaxxC resulted in a two-fold decrease in phosphorylation of c-Jun, which is in agreement with results in Figure 3.
Figure 3.
DN-JNK and DaxxC inhibit UV-induced JNK activation. Total cell extracts (10 µg) from transfected and UV-irradiated 293 cells were resolved on 12% SDS-PAGE and electroblotted to nitrocellulose membrane. The phosphorylated JNK (Panel A) and total JNK (Panel B) were probed using anti-phospho-SAPK/JNK (Thr183/Tyr185) antibody and anti-JNK antibody.
Figure 4.
In vitro c-Jun phosphorylation assay. The assay was performed using SAPK/JNK Assay Kit. Overexpressed Flag-tagged JNK was immunoprecipitated from the transfected cells using anti-Flag antibody. The precipitates were washed with lysis buffer and kinase assay buffer supplied by manufacturer. The precipitates were then incubated with c-Jun (0.2 µg; Promega) in the kinase assay buffer at 30°C for 30 minutes. The phosphorylation of c-Jun was detected using anti-phospho-c-Jun antibody according to manufacturer's procedure.
Daxx-Mediated JNK Activation is Not Sufficient for Induction of Apoptosis upon UV
Because previous studies have demonstrated a role for hDaxx-mediated JNK activation in Fas receptor-mediated apoptosis, we next investigated if hDaxx was also required for UV-induced apoptosis. 293 cells were transfected with a control vector, a DN-JNK expression vector, or an hDaxxC expression vector. These transfections also included an EGFP expression vector at one-fifth the concentration of the above vectors to identify transfected cells from nontransfected cells upon green fluorescence. As shown in Figure 5A, UV irradiation of control vector-transfected (in the presence of an EGFP plasmid) cells 48 hours posttransfection monitored by fluorescence microscope revealed a percentage (41%) of green cells that appeared apoptotic (rounded or floating). This morphology was reduced to 16% in cells that was transfected but not irradiated. When the expression vector for DN-JNK was transfected into 293 cells, only 20% of the green cells (transfected) appeared apoptotic upon UV irradiation (Figure 5B), indicating that DN-JNK expression resulted in inhibition of UV-induced apoptosis. Overexpression of wild-type JNK has no significant effect on UV-induced apoptosis (30%), confirming previous reports [4,12] that JNK activation is required for UV-induced apoptosis. Similarly, expression of hDaxxC also resulted in inhibition of UV-induced apoptosis because only 25% of the cells appeared apoptotic upon UV irradiation (Figure 5B). However, our data also show that overexpression of a wild-type hDaxx can also inhibit UV-induced apoptosis to 20% (Figure 5). Because expression of hDaxx does not inhibit UV-induced JNK activation (data not shown), our data suggest that hDaxx and hDaxxC may inhibit UV-induced apoptosis by competing with other apoptotic factor(s), such as FADD, for binding of Fas receptor. These results also suggest that UV-induced JNK activation alone is not sufficient to induce apoptosis.
Figure 5.
Determination of UV-induced apoptosis of 293 cells. Panel A: Morphology of blue cells without UV irradiation and blue apoptotic cells (pointed) with UV irradiation. Panel B: The percentage of apoptotic cells (average of four individual experiments) and the percentage of blue floaters (average of two individual experiments).
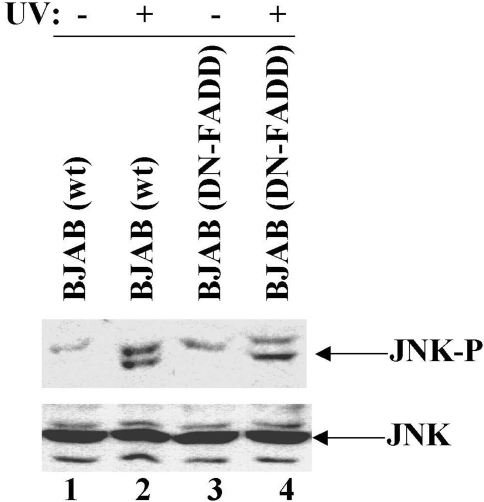
To further confirm the hypothesis that JNK activation is not essential for UV-induced apoptosis, we analyzed JNK activation in UV-treated BJAB cells, which expresses a DN-FADD. Our previous observation is that UV irradiation results in the recruitment of FADD to the Fas receptor due to ligand-independent aggregation of the receptor [2]. The UV-induced apoptosis is inhibited in the BJAB cells that stably overexpress a dominant negative form of FADD, which displaces endogenous FADD from Fas [13]. Our data show that UV irradiation of BJAB-DN-FADD cells resulted in activation of JNK (Figure 6, lane 4) in a manner analogous to BJAB cells (Figure 6, lane 2). Because BJAB-DN-FADD cells are resistant to UV-induced apoptosis, the result agrees that JNK activation is not enough to induce apoptosis upon UV irradiation.
Figure 6.
DN-FADD does not inhibit UV-induced JNK activation. BJAB (wild type) and BJAB (DN-FADD) cells were irradiated with 0 (lanes 1 and 3) or 100 J/m2 (lanes 2 and 4) of UVC. At 30 minutes postirradiation, the cells were lysed and JNK activation was analyzed by Western blot using anti-phospho-SAPK/JNK (Thr183/Tyr185) antibody and anti-JNK antibody. As shown in Panel A, UV irradiation induced JNK activation in both BJAB (lane 2) and BJAB (DN-FADD) (lane 4) cells. Total loaded amount of JNK was monitored using anti-JNK antibody (Panel B).
Discussion
The molecular events that lead to death receptor-induced apoptosis have been well characterized. Upon Fas activation, an adapter molecule termed FADD is recruited to the cytoplasmic segment of the Fas receptor through a homophilic interaction between a stretch of 60 and 80 amino acids, dubbed the death domain that is present in both molecules. An equivalent death domain is present within the prodomain of caspase-8 (FLICE, MACH, Mch5). Interaction between the death domains allows for the recruitment of the death protease caspase-8 to the receptor signaling complex. Following conversion to the active dimeric species, caspase-8 is free to proteolytically activate caspase-3 (CPP32, Yama, apopain), leading to cleavage of death substrates such as the nuclear enzyme poly (ADP-ribose) polymerase (PARP).
We previously reported that UV irradiation results in aggregation of Fas. Upon UV irradiation (15 and 30 J/m2), Fas aggregated within 15 minutes in BJAB cells. Increasing amounts of Fas-FADD interaction were also detected by co-immunoprecipitation of FADD with Fas. We also demonstrated that overexpression of DN-FADD blocked UV-induced apoptosis of BJAB cells. Furthermore, it was reported that UV irradiation induced caspase-3 activation and PARP cleavage in HaCaT cells [5]. These results suggest that UV irradiation utilizes the Fas-FADD-caspase-8-caspase-3 pathway to induce apoptosis. However, UV irradiation also activates the JNK (SAPK) pathway. The induction of JNK activity results in the activation of proto-oncogene c-Jun and activator protein-1 (AP-1) transcriptional activity. JNK activation has been proposed to play a major role in UV-induced apoptosis [6]. These studies demonstrated that activation of JNK in response to UV irradiation correlated with UV-induced apoptosis. Overexpression of a DN-JNK blocked UV-induced cell death.
In this study, we attempted to further delineate the molecular events that lead to JNK activation in response to UV irradiation. Utilizing Daxx and its dominant negative form DaxxC, we tested the hypothesis that Daxx, though its recruitment to the activated Fas receptor, mediates UV-induced activation of JNK. It has been reported that the N-terminal deleted form of mouse Daxx (DaxxC) inhibits Fas-induced JNK activation and apoptosis of 293 cells. In our study, a human hDaxx was cloned from HeLa cells and an N-terminal deleted form of human hDaxx was constructed. Our results demonstrate that overexpression of hDaxxC partially inhibits UV-induced JNK activation, suggesting that hDaxx is involved in UV-induced JNK activation. To further analyze whether UV-induced JNK activation is critical for induction of apoptosis, we transiently transfected 293 cells with JNK, DN-JNK, hDaxx, and hDaxxC, and studied the efficiency with which UV induced apoptosis in the transfected cells. Our data show that reduction of JNK activation by DN-JNK, as well as hDaxxC, inhibits UV-induced apoptosis. This result suggests that JNK activation is required for UV-induced apoptosis. Interestingly, overexpression of the wild-type hDaxx also inhibits apoptosis of 293 cells upon UV irradiation. We hypothesize that hDaxx, and possibly hDaxxC, may inhibit UV-induced apoptosis by competing with proapoptotic factors (such as FADD) for Fas binding. Because overexpression of hDaxx does not affect UV-induced JNK activation, the results also suggest that JNK activation alone is not enough to induce apoptosis after UV irradiation. To confirm the hypothesis that UV-induced JNK activation is not sufficient for induction of apoptosis, we determined JNK activation in BJAB cells stably transfected with a DN-FADD. We have previously shown that overexpression of DN-FADD inhibits UV-induced apoptosis by blocking the Fas-FADD-caspase-8 axis. These results demonstrate that expression of DN-FADD does not inhibit UV-induced JNK activation, further demonstrating that UV-induced JNK activation is not sufficient to initiate apoptosis.
Based on our results and the fact that caspase-8-mediated apoptosis was amplified through mitochondrial release of cytochrome c [14], which is upregulated by JNK activation [12], a model was proposed (Figure 7). We propose that UV-induced activation of the Fas-FADD-caspase-8 pathway results in a death signal and that UV-induced activation of JNK is required to amplify the death signal through cytochrome c release, such that apoptosis occurs.
Figure 7.
Model of UV-induced apoptotic signaling pathway.
Acknowledgements
We would like to give our thanks to Roger J. Davis, University of Massachusetts Medical School, for providing us the expression vectors of JNK and DN-JNK. This research was partially supported by the Michigan Memorial-phoenix Project (S.W.) and the NCI with CA78041 (A.R.) and CA86926 (S.W.).
References
- 1.Wyllie AH, Kerr JF, Currie AR. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- 2.Rehemtulla A, Hamilton CA, Chinnaiyan AM, Dixit VM. Ultraviolet radiation-induced apoptosis is mediated by activation of cd-95 (fas/apo-1) J Biol Chem. 1997;272(41):25783–25786. doi: 10.1074/jbc.272.41.25783. [DOI] [PubMed] [Google Scholar]
- 3.Chatterjee M, Wu S. Involvement of fas receptor and not tumor necrosis factor-alpha receptor in ultraviolet-induced activation of acid sphingomyelinase. Mol Carcinog. 2001;30(1):47–55. doi: 10.1002/1098-2744(200101)30:1<47::aid-mc1012>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 4.Chen YR, Wang X, Templeton D, Davis RJ, Tan TH. The role of c-jun n-terminal kinase (jnk) in apoptosis induced by ultraviolet c and gamma radiation. Duration of jnk activation may determine cell death and proliferation. J Biol Chem. 1996;271(50):31929–31936. doi: 10.1074/jbc.271.50.31929. [DOI] [PubMed] [Google Scholar]
- 5.Aragane YKD, Metze D, Wilkes G, Poppelmann B, Luger TA, Schwarz T. Ultraviolet light induces apoptosis via direct activation of cd95 (fas/apo-1) independently of its ligand cd95l. J Cell Biol. 1998;140(1):171–182. doi: 10.1083/jcb.140.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goillot E, Raingeaud J, Ranger A, Tepper RI, Davis RJ, Harlow E, Sanchez I. Mitogen-activated protein kinase-mediated fas apoptotic signaling pathway. Proc Natl Acad Sci USA. 1997;94(7):3302–3307. doi: 10.1073/pnas.94.7.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang X, Khosravi-Far R, Chang HY, Baltimore D. Daxx, a novel fas-binding protein that activates jnk and apoptosis. Cell. 1997;89(7):1067–1076. doi: 10.1016/s0092-8674(00)80294-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michaelson JS, Bader D, Kuo F, Kozak C, Leder P. Loss of daxx, a promiscuously interacting protein, results in extensive apoptosis in early mouse development. Genes Dev. 1999;13(15):1918–1923. doi: 10.1101/gad.13.15.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charette SJ, Landry J. The interaction of hsp27 with daxx identifies a potential regulatory role of hsp27 in fas-induced apoptosis. Ann NY Acad Sci. 2000;926:126–131. doi: 10.1111/j.1749-6632.2000.tb05606.x. [DOI] [PubMed] [Google Scholar]
- 10.Charette SJ, Lavoie JN, Lambert H, Landry J. Inhibition of daxx-mediated apoptosis by heat shock protein 27. Mol Cell Biol. 2000;20:7602–7612. doi: 10.1128/mcb.20.20.7602-7612.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torii S, Egan DA, Evans RA, Reed JC. Human daxx regulates fas-induced apoptosis from nuclear pml oncogenic domains (pods) EMBO J. 1999;18(21):6037–6049. doi: 10.1093/emboj/18.21.6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tournier C, Hess P, Yang DD, Xu J, Turner TK, Nimnual A, Bar-Sagi D, Jones SN, Flavell RA, Davis RJ. Requirement of jnk for stress-induced activation of the cytochrome c-mediated death pathway. Science. 2000;288(5467):870–874. doi: 10.1126/science.288.5467.870. [DOI] [PubMed] [Google Scholar]
- 13.Chinnaiyan AM, Tepper CG, Seldin MF, O'Rourke K, Kischkel FC, Hellbardt S, Krammer PH, Peter ME, Dixit VM. Fadd/mort1 is a common mediator of cd95 (fas/apo-1) and tumor necrosis factor receptor-induced apoptosis. J Biol Chem. 1996;271(9):4961–4965. doi: 10.1074/jbc.271.9.4961. [DOI] [PubMed] [Google Scholar]
- 14.Kuwana T, Smith JJ, Muzio M, Dixit V, Newmeyer DD, Kornbluth S. Apoptosis induction by caspase-8 is amplified through the mitochondrial release of cytochrome c. J Biol Chem. 1998;273(26):16589–16594. doi: 10.1074/jbc.273.26.16589. [DOI] [PubMed] [Google Scholar]