Abstract
Expression of TERT, the reverse transcriptase component of telomerase, is necessary to convert normal human cells to cancer cells. Despite this, “telomerization” by hTERT does not appear to alter the normal properties of cells. In a cell transplantation model in which bovine adrenocortical cells form vascularized tissue structures beneath the kidney capsule in scid mice, telomerization does not perturb the functional tissue-forming capacity of the cells. This cell transplantation model was used to study the cooperation of hTERT with SV40 T antigen (SV40 TAg) and oncogenic Ras in tumorigenesis. Only cells expressing all three genes were tumorigenic; this required large T, but not small t, antigen. These cells produced a continuously expanding tissue mass; they were invasive with respect to adjacent organs and eventually destroyed the kidney. Cells expressing only hTERT or only Ras produced minimally altered tissues. In contrast, SV40 TAg alone produced noninvasive nodules beneath the kidney capsule that had high proliferation rates balanced by high rates of apoptosis. The use of cell transplantation techniques in a cell type that is able to form tissue structures with or without full neoplastic conversion allows the phenotypes produced by individual cooperating oncogenes to be observed.
Keywords: telomerase, cell transplantation, adrenal cortex, SV40 T antigen, oncogenic Ras
Introduction
Cells from long-lived mammals show a limited capacity for replication in culture; this phenomenon, termed cellular senescence, has been shown to result from progressive shortening of telomeres [1–3]. This was first demonstrated for human cells, but bovine cells also exhibit telomere shortening, leading to replicative senescence [4,5]. In both human and bovine cells, telomere shortening and replicative senescence are prevented by the ectopic expression of the reverse transcriptase component of telomerase (TERT) [2,5]. In contrast, although primary rodent cells undergo a cessation of proliferation in culture, this does not result from telomere shortening [6]. Moreover, human cells and bovine cells are resistant to neoplastic transformation by both chemical carcinogens and oncogenic viruses, whereas rodent cells readily transform to full tumorigenicity following exposure to these agents [7]. Although the mechanisms by which cells from long-lived mammals demonstrate this resistance to neoplastic conversion have not been elucidated, one candidate mechanism for the difference is that prevention of replicative senescence is required for neoplastic transformation in cells from these species. This concept is supported by results of experiments on the genetic modifications required to convert normal human cells to a fully tumorigenic state [8–13]. Successive transduction of normal cells by three retroviruses encoding SV40 T antigen (SV40 TAg), oncogenic RasG12V, and hTERT produced cells that were fully tumorigenic [8]. When cells were transduced with retroviruses encoding SV40 TAg and Ras only, without hTERT, cells did not produce tumors [8,9,11–13].
The mechanism by which known oncoproteins, such as SV40 TAg and Ras, cooperate with hTERT in neoplastic conversion remains to be clarified. Despite the evidence that hTERT is required for tumorigenic conversion of human cells, ectopic expression of hTERT by itself without cooperating oncoproteins does not appear to alter the normal properties of the cells. This was first shown in cell culture; hTERT-expressing cells retain normal cell cycle check points and do not show karyotypic instability [14,15]. The apparently normal behavior of such cells prompted the coining of the term “telomerization,” implying immortalization without the abnormalities commonly found in immortalized cell lines [16,17]. Subsequently, in this laboratory, we extended these findings by showing that telomerized cells also retain completely normal characteristics following transplantation into a host animal. We used clonal bovine adrenocortical cells, which form vascularized tissue structures when transplanted beneath the capsule of the kidney; these tissues replace the essential functions of the host animal's adrenal glands and maintain the life of adrenalectomized animals [18]. When we made telomerized clones by ectopic expression of hTERT, we found that these cells formed normal functional vascularized tissue, essentially identical to tissue formed from nongenetically modified clonal cells [5]. Like the normal cells, the telomerized cells displayed a profound decrease in cell proliferation on being transferred from the cell culture to the in vivo environment [5,18]. In other experiments, telomerization of fibroblasts prevented the changes in gene expression associated with replicative senescence and permitted the production of a normal skin equivalent after transplantation [19]. Although telomerized keratinocytes exhibited a delay in terminal differentiation in organotypic raft culture [20], this may have been due to inadequate culture conditions for the keratinocytes. Thus, the paradox remains that hTERT is required for neoplastic transformation, yet does not disturb normal differentiated cell function, at least as evidenced by the ability of telomerized cells to form functional tissues by cell transplantation.
In the present experiments, we continued the approach of using cell transplantation as a technique for investigating the phenotypes of genetically modified cells. Following the paradigm of neoplastic transformation of human cells by the combination of hTERT, SV40 TAg, and RasG12V [8], we show that a similar approach using bovine adrenocortical cells also results in full neoplastic transformation in this cell transplantation model. However, cells transduced with retroviruses encoding only hTERT or only RasG12V produced tissues that did not have the properties of a malignant tumor; in particular, these tissues were able to maintain the life of adrenalectomized animals. In contrast, the expression only of SV40 TAg produced tissues that exhibited high proliferation rates yet did not result in full neoplastic transformation.
Materials and Methods
Growth of Bovine Adrenocortical Cells in Culture
Bovine adrenocortical cells were derived by enzymatic and mechanical dispersion from the adrenal cortex of 2-year-old steers, as previously described [21]. Cells were plated in Dulbecco's modified Eagle's medium/Ham's F-12 1:1 with 10% fetal bovine serum, 10% horse serum, and 0.1 ng/ml recombinant FGF-2 (R&D Systems, Minneapolis, MN) [21]. The gas phase was 5% O2, 5% CO2, and 90% N2.
Retroviral Transduction
Retroviral vector plasmids were transfected into PT67 cells (Clontech, Palo Alto, CA), which produce a virus type (10A1) that efficiently infects most human cells [22]; we found that it also infects bovine adrenocortical cells. Adrenocortical cells were infected by co-culture with PT67 cells in a Transwell system (Corning, Corning, NY) [23]. Following 4 days of co-culture, transduced adrenocortical cells were grown in the appropriate selective condition (2 µg/ml puromycin, 400 µg/ml G418, or 50 µg/ml hygromycin), until the culture was devoid of nonresistant cells. Retroviral plasmids were generously donated by J. Campisi (pBabe-puro-hTERT [24]), C. Cepko (ZIPneoSV40X1/T [25]), and J. Shay (pBabe-hygro-RasG12V [15]).
In different experiments, primary cells were transduced with a single retrovirus, or were successively infected with two or three viruses. In this case, the second and third infections were performed on cells after the culture was completely antibiotic-resistant following the previous infection, but cells were not grown extensively between infections.
Biochemical Characterization of Retrovirally Transduced Cells
Western blotting was performed using standard techniques. Antibody Ab-1 (PAb419; Oncogene Science, Cambridge, MA) detects both large T and small t antigens [26]. For immunoprecipitation of Ras, whole cell extract (100 µg of protein) was incubated with 5 µg of anti-H-Ras rat monoclonal antibody (Y13-259; Oncogene Science) and 1 µl of protein G-agarose beads (Oncogene Science) in 100 µl of RIPA buffer for 30 minutes. Beads were washed in the same buffer and the adsorbed protein was electrophoresed and blotted. Blots were incubated with pan-Ras-Val12 mouse monoclonal (clone DWP; Oncogene Science) and bound antibody was detected by anti-mouse peroxidase conjugate and chemiluminescence.
Telomerase activity in detergent extracts of cells was assessed by the telomerase repeat amplification protocol (TRAP) assay as previously described [23].
Transplantation of Cells Beneath the Kidney Capsule of scid Mice
ICR scid mice originally purchased from Taconic (Germantown, NY) were maintained in an animal barrier facility as a breeding colony. Animals (both males and females) at an age greater than 6 weeks (∼25 g body weight) were used in these experiments. Procedures were approved by the Institutional Animal Care Committee and were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals. The cell transplantation procedure was performed under tribromoethanol anesthesia. In some experiments, in which the ability of transplanted cells to functionally replace the animals' adrenal glands was tested, the animals were also adrenalectomized in the same surgical procedure. The methods have been described in detail previously [27]. In all experiments, we transplanted 2x106 adrenocortical cells together with 4x105 lethally irradiated FGF-secreting 3T3 cells [28]. The left kidney was exteriorized. Adrenocortical cells and 3T3 cells were introduced into the subrenal capsule space using a transrenal injection with a 50-µl Hamilton syringe fitted with a blunt 22-gauge needle. In some experiments, to confine the transplanted adrenocortical cells within a defined space so that the growth, vascularization, and function of the cells could be readily studied, we used a small cylinder to create a virtual space beneath the kidney capsule into which the cells were located [18,27]. Following transplantation of the cells, the kidney was returned to the retroperitoneal space and the incisions in the body wall and skin were closed. Postoperative care for the animals, including the administration of analgesics and antibiotics, was as previously described [27]. Animals were sacrificed at various times following transplantation, as described in the Results section.
Histological and Immunohistochemical Analysis
The fixation, paraffin embedding, and histological examination of tissue formed from transplanted cells were carried out using standard techniques. Immunocytochemistry was performed with several antibodies as follows: the Ki-67 proliferation-associated antigen was detected using monoclonal antibody MIB-1 (Immunotech, Westbrook, ME). This antibody does not recognize the mouse homologue of Ki-67 but recognizes the bovine protein with staining equivalent to that observed with human tissues (our unpublished observations). SV40 TAg was detected with monoclonal antibody Ab-2 (PAb416; Oncogene Science), which is reactive for large T, but not small t, antigen [26]; Ras was detected with a mouse monoclonal anti-human H-Ras antibody (clone 18; BD Transduction Laboratories, Lexington, KY). Primary antibodies were detected with biotin-conjugated anti-mouse antibodies and avidin-biotin-peroxidase complex (Vector Laboratories, Burlingame, CA), as recommended by the manufacturer. Sections were lightly counterstained with hematoxylin.
Apoptotic cells were visualized in tissue sections using in situ oligonucleotide labeling (ISOL) [29]. In this procedure, a hairpin oligonucleotide is ligated to DNA double-strand breaks, as found in apoptotic cells. Biotin-containing oligonucleotides were used as supplied in the ApopTag (ISOL) in situ oligo ligation kit (Intergen, Purchase, NY). Bound biotin on the section was visualized with avidin-biotin-peroxidase complex as for antibody staining.
Results
In these experiments, we used cell transplantation techniques to investigate the properties of bovine adrenocortical cells expressing combinations of hTERT, SV40 TAg, and RasG12V. Table 1 provides an overview of the combinations we tested and the results obtained.
Table 1.
Summary of Results.
| Genetic modification | Characteristics of cells transplanted in scid mice |
| hTERT→SV40 TAg→RasV12G | Tumorigenic; rapid proliferation; uncontrolled growth; invasive; kidney ultimately destroyed |
| hTERT | Small tissue; very low proliferation rate; normal histological appearance; no invasive properties |
| hTERT→SV40 TAg | Small tissue; relatively high proliferation rate accompanied by cell death; tissue does not expand over time; no invasive properties |
| SV40 TAg | Properties same as hTERT→SV40 TAg |
| RasV12G | Small tissue; very low proliferation rate; abnormal histological appearance; no invasive properties |
| SV40 Tag→RasV12G | No tissue or tumor formed at site of cell transplantation |
| Nongenetically modified cells and normal clones of cells* | Small tissue; very low proliferation rate; normal histological appearance; no invasive properties |
| Telomerized clones (hTERT+low level of SV40 TAg)* | Same as nongenetically modified cells |
These results were previously published [5,18].
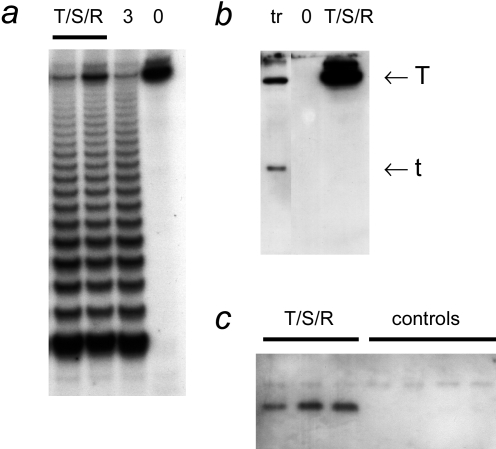
When cells were successively infected with retroviruses expressing hTERT, SV40 TAg, and RasG12V, the transduced cells were tumorigenic when transplanted in scid mice. hTERT→SV40 TAg→ Ras cells were characterized biochemically in culture before transplantation. They had strong telomerase activity (Figure 1). This activity was shown to be caused by transduction with the hTERT retrovirus, and not the SV40 TAg or Ras viruses, because cells transduced with retroviruses without hTERT were completely telomerase-negative (not shown). hTERT→SV40 TAg→Ras cells expressed high levels of SV40 large T antigen, but did not express small t antigen. As expected, cells transfected with a plasmid containing the early region of SV40 expressed both large T and small t antigens. This difference is addressed further in the Discussion section. Expression of RasG12V was demonstrated by immunoprecipitation.
Figure 1.
Biochemical characterization of bovine adrenocortical cells transduced with hTERT, SV40 TAg, and Ras retroviruses. (a) Telomerase activity. TRAP assays were performed on extracts of hTERT→SV40 TAg→Ras cells (T/S/R; 0.25 and 0.5 µg of protein) and on an extract of 3T3 cells (“3”; 0.5 µg, positive control). “0” indicates the reaction performed with buffer only. (b) SV40 TAg. Western blot probed with an antibody that recognizes both large T and small t antigens. Extracts (100 µg of protein each) from hTERT→SV40 TAg→Ras cells and from clone 47 (Ref. [5]; cells transfected with hTERT and SV40 plasmids; labeled “tr”). (c) Ras. Extracts of separately grown cultures of hTERT→SV40 TAg→Ras cells and from separate batches of primary nongenetically modified cells (controls) were used for immunoprecipitation with an anti-Ras antibody, followed by Western blotting using an antibody against RasV12, as described in Materials and Methods section.
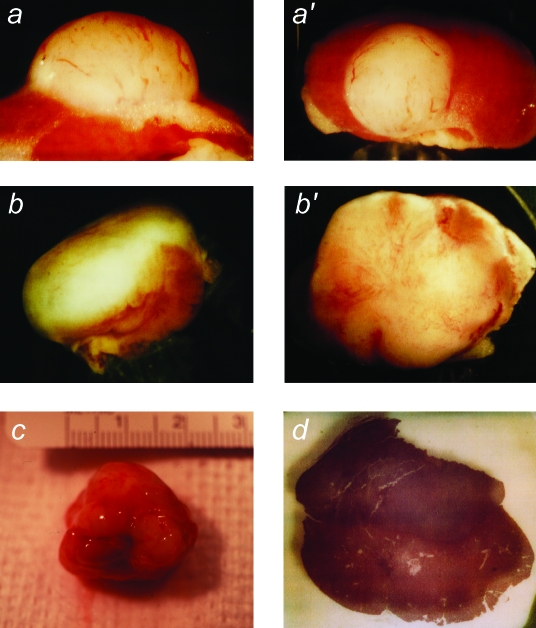
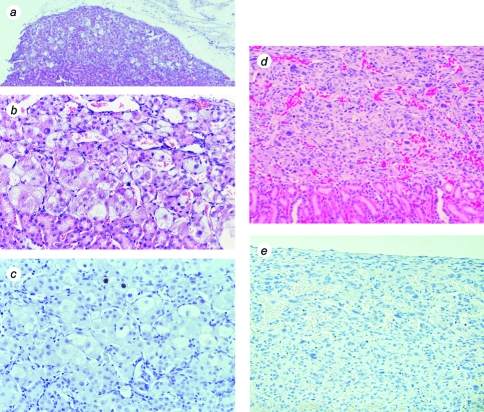
When hTERT→SV40 TAg→Ras cells were transplanted under the kidney capsule of scid mice, they produced continuously expanding tissue masses (Figure 2). Vascularization of these masses was evidenced by the presence of many prominent blood vessels on their surfaces. At early times (30–40 days), the masses protruded from the side of the kidney. Later (60–75 days), the masses surrounded and invaded the kidney, ultimately destroying the organ. As the tumor masses expanded, it was evident on gross inspection that the cells were invading surrounding organs, including the liver and spleen. By 60 days, as shown in Figure 2, tumors had attained dimensions of >2.5 cm and weighed 1.5 to 2.5 g.
Figure 2.
Macroscopic appearance of tumors produced from transplanted hTERT→SV40 TAg→Ras cells. After growth in culture, cells were transplanted under the kidney capsule of scid mice. Animals were sacrificed at intervals of 30 to 75 days following cell transplantation. The tumor masses found to have resulted from growth of the transplanted cells were photographed, together with the host animal kidney. (a,a′) Example of kidney and tissue mass removed from an animal at 30 days (magnification, x12, x8). (b,b′) Tissue mass removed at 45 days (magnification, x4, x6). (c) Tumor and kidney removed at 60 days (magnification, x2). (d) Gross appearance of a part of a similar tumor and kidney removed at 60 days and cut transversely (the tumor mass is the darker tissue above the kidney) (magnification, x8).
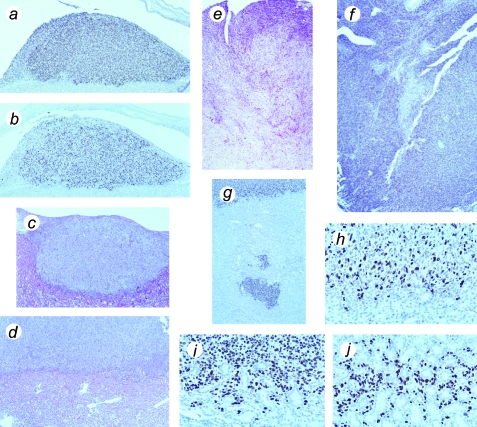
Histological identification of the transplanted cells at the edge of the expanding tumor mass was aided by immunocytochemical staining with a monoclonal antibody against SV40 TAg. Examination of Ki-67 expression in serial sections showed that the transplanted cells have an extremely high proliferation rate (Figure 3). SV40 TAg labeling demonstrated that the tissue expands continuously over time and is invasive. Invasion into the kidney was shown both by detection of groups of cells at remote distances from the primary tumor mass and by detection of many individual cells located between kidney tubules (Figure 3).
Figure 3.
Histological appearance of tumors formed from hTERT→SV40 TAg→Ras cells, showing proliferation and invasive behavior. (a) Tumor mass above mouse kidney, removed at 30 days, stained with an antibody against SV40 TAg. (b) Serial section from same specimen stained for Ki-67 proliferation-specific antigen. (c) Hematoxylin and eosin-stained section of tumor mass in relation to kidney, removed at 30 days. (d) Hematoxylin and eosin-stained section of tumor mass above kidney, removed at 60 days. (e) Hematoxylin and eosin-stained section of late tumor, removed at 75 days, showing advanced invasion and destruction of mouse kidney. (f) Similar stage of tumor stained for SV40 TAg. (g) Part of kidney at 60 days, stained for SV40 TAg, showing detached mass of tumor cells within kidney. (h) Similar specimen showing border of kidney and tumor, stained for Ki-67 antigen (note that mouse Ki-67 is not recognized by this antibody). (i,j) Similar specimen, stained for SV40 TAg, showing invasion of tumor cells into kidney; note cells surrounding kidney tubules in (j). Magnification (a–d), x100; (e–g), x50; (h–j), x400.
We used the cell transplantation model to examine the properties of cells that had been genetically modified in ways other than hTERT→SV40 TAg→Ras. As noted above, all cells genetically modified by transduction with hTERT, with or without other genes, had telomerase activity, whereas all cells without hTERT were telomerase-negative. However, when they were transplanted, all non-hTERT cells had an extensive remaining replicative potential. All were capable of growth for >20 population doublings in culture beyond the population doubling level at which they were transplanted. Control nongenetically modified bovine adrenocortical cells undergo a total of approximately 100 population doublings before becoming senescent [30]. As we observed previously, bovine adrenocortical cells expressing SV40 TAg, with or without Ras but without hTERT, eventually entered crisis [30]. Cells with SV40 TAg were used at a population doubling level well before crisis (at least 20 population doublings).
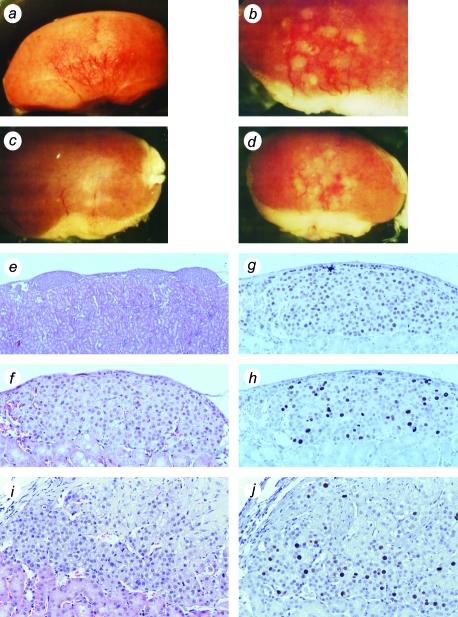
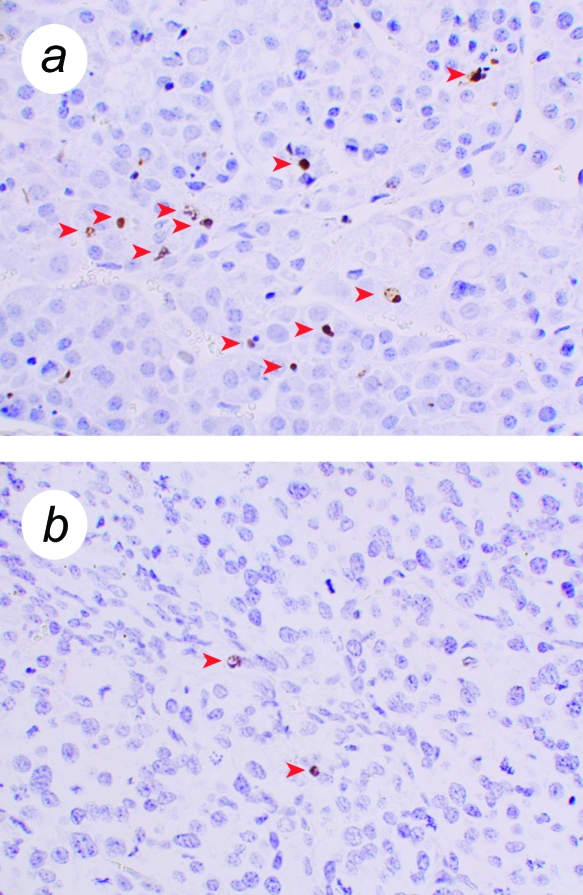
First, we studied cells that had not been transduced with the Ras retrovirus. Both hTERT→SV40 TAg cells and SV40 TAg cells formed tissue following cell transplantation that was clearly abnormal, yet not tumorigenic. These tissues had high proliferation rates (Figure 4). Nevertheless, they remained small over the duration of these experiments (up to 70 days following cell transplantation) and showed no invasive properties with respect to the kidney. These properties — retaining a small size despite high proliferation rates — suggested that there must also be a high rate of cell death. This was confirmed using ISOL staining (in situ oligo ligation; a method that specifically detects double-strand breaks in DNA that are characteristic of apoptosis) (Figure 5). Nuclei that showed a positive reaction by ISOL staining were present in tissues formed from SV40 TAg tissues, but were uncommon in tissues formed from primary cells or hTERT→SV40 TAg→Ras cells. ISOL-stained cells in SV40 TAg tissues showed typical apoptotic morphology, such as nuclear shrinkage and condensed chromatin; stained apoptotic bodies were also observed. In SV40 TAg tissues, 2% to 5% of cells were in apoptosis as determined by ISOL staining, whereas in hTERT→SV40 TAg→Ras tissues, and in other tissues formed from cells not expressing SV40 TAg, the frequency was variable but very low (∼0.1%).
Figure 4.
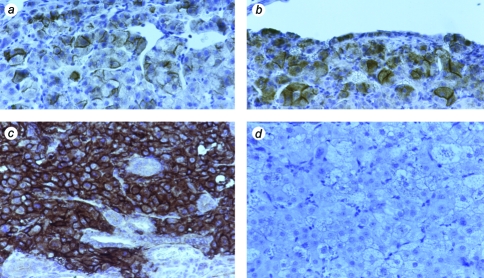
Tissues formed from SV40 TAg cells and hTERT→SV40 TAg cells. Bovine adrenocortical cells were transduced with SV40 TAg retrovirus (a–h) or were successively infected with hTERT and SV40 TAg retroviruses (i,j). (a–d) Gross appearance of SV40 TAg tissues formed on surface of mouse kidney; four examples, removed from host animals at 45 to 60 days (magnification, x10, x20, x10, x10). (e) Hematoxylin and eosin-stained section of kidney showing transplant tissue beneath capsule as a series of small nodules (magnification, x40). (f) Enlarged view of one nodule (x200). (g) Section stained for SV40 TAg (x200). (h) Section stained for Ki-67 (x200). (i) Hematoxylin and eosin-stained section of hTERT→SV40 TAg tissue (x200). (j) hTERT→SV40 TAg tissue stained for Ki-67 (x200).
Figure 5.
Cell death in tissue formed from SV40 TAg cells. Tissue was formed from bovine adrenocortical cells transduced with SV40 TAg retrovirus, or hTERT→SV40 TAg→Ras. Sections were used for ISOL detection of apoptotic cells. (a) Tissue formed from SV40 TAg cells. (b) Tissue formed from hTERT→SV40 TAg→Ras cells. Stained cells are marked with arrowheads. (b) shows the only two such cells found in an entire section of hTERT→SV40 TAg→Ras tumor. Magnification, x500.
The differences in behavior between hTERT→SV40 TAg cells and hTERT→SV40 TAg→Ras cells clearly indicate the critical role of oncogenic Ras in tumorigenicity. We therefore transplanted cells that had been transduced with the Ras retrovirus alone. Surprisingly, cells expressing RasG12V produced functional tissue following cell transplantation; the tissue formed permitted the survival of the host animals when the animals were adrenalectomized at the time of cell transplantation. Whereas the low proliferation rate resembles that in tissue formed from normal, nongenetically modified cells, or from telomerized cells, the histological appearance was abnormal. In tissues formed from Ras cells, many cells were enlarged. Figure 6 shows the contrast between the appearance of tissue formed from Ras cells and tissues formed from hTERT cells. Tissue formed from hTERT cells was small and had a very low proliferation rate (Figure 6), in agreement with previous data on tissue formation by telomerized cell clones created by transfection rather than retroviral infection. High levels of Ras protein were detected by immunocytochemistry both in hTERT→SV40 TAg→Ras tissues and tissues formed from cells transduced with Ras only, but not in tissues formed from normal nongenetically modified cells (Figure 7).
Figure 6.
Tissues formed from Ras and hTERT cells. Bovine adrenocortical cells were infected with retroviruses encoding either RasG12V (a–c) or hTERT (d,e). (a,b,d) Hematoxylin and eosin-stained sections; magnification, x40, x200, x150. (c,e) Proliferation visualized by staining with antibody against Ki-67 antigen; magnification, x200, x150.
Figure 7.
Expression of Ras in tissues formed from bovine adrenocortical cells infected with RasG12V retrovirus; tissues stained with anti-Ras antibody. (a,b) Two examples of tissues formed from cells infected with RasG12V retrovirus only. (c) Tissue formed from hTERT→SV40 TAg→Ras cells. (d) Tissue formed from control bovine adrenocortical cells, not genetically modified; magnification, x200.
In contrast to these findings, cells transduced with the combination of SV40 TAg and Ras produced no detectable tissue or tumor (Table 1). The site of injection of the cells through the kidney to the cell transplantation site under the kidney capsule was positively identified, as evidenced by damage caused by entry and withdrawal of the needle used. Nevertheless, no adrenocortical cells were observed at the injection site.
Discussion
We investigated the genetic requirements for the production of tumorigenicity in bovine adrenocortical cells. Telomere biology is similar in bovine and human cells; cells from both species senesce by telomere shortening, are immortalized by telomerase, and have stable karyotypes both before and after telomerization [4,5]. Specifically, we previously showed that telomerized bovine adrenocortical cell clones are apparently immortalized and do not undergo telomere shortening [5]; additionally, bovine adrenocortical cells transduced with a retroviral vector encoding hTERT, as used in these experiments, are apparently immortalized (unpublished observations). Although bovine adrenocortical cells exhibit a transient telomerase activity when freshly isolated from the animal and first placed in culture [23], all long-term cultures are completely devoid of telomerase activity, like other bovine and human cells. Here, we show that telomerase activity is necessary for tumorigenicity in bovine cells, as previously demonstrated for human cells [8]. Only the combination of hTERT, SV40 large T antigen, and RasG12V produces cells that are tumorigenic (continuously expanding tissue that was invasive). Small t antigen is not required. The use of cell transplantation techniques permits the phenotypes of individual genes and combinations to be observed in the context of transplant tissues in a host animal. Thus, we show that none of the single genes or combinations, other than all three, produces tissue that is either continuously expanding or invasive.
These results contrast dramatically with those of previous cell transplantation experiments using normal or telomerized bovine adrenocortical cells. Nongenetically modified cells or telomerized cells form functional vascularized tissue that permits the survival of adrenalectomized animals; these tissues remain at a constant size over long periods and have a low rate of cell division [5,18]. In agreement with our previous results, tissue formed from hTERT-transduced cells was normal in appearance and had a low proliferation rate. However, perhaps surprisingly, cells transduced singly with either SV40 TAg or RasG12V also produced small tissue structures that maintained the life of adrenalectomized animals.
In this cell transplantation model of oncoprotein cooperation, SV40 TAg by itself produced a distinctive phenotype. Although the effect of SV40 TAg is well known in rodent tissues, especially the ectopic expression of SV40 TAg under the control of tissue-specific promoters, the effects of SV40 TAg are much less well established in tissues of long-lived mammals that undergo telomere shortening and senescence. This issue is of relevance to the question of the health risks associated with human SV40 exposure [31]. SV40 TAg tissues, and also hTERT→SV40 TAg tissues, had a high proliferation rate that appeared to be balanced by a high rate of cell death. Clearly, SV40 TAg, as a powerful oncoprotein that interrupts the normal restraints on growth imposed by Rb and p53 [32,33], exerts considerable effects by itself. Many differentiated cells in tissues divide very rarely, even though they are not postmitotic. The mechanism by which this quiescence is enforced is not known, but likely involves negative signals through integrins and other interactions with the extracellular matrix [34]. We surmise that by forcing cells in tissues to enter the cell cycle, SV40 TAg causes apoptosis, perhaps resulting from conflicting positive and negative growth signals [35,36].
When hTERT and Ras were expressed together with SV40 TAg, there was little cell death, thus permitting continuous expansion of the tissue; these cells were also invasive. Yet neither one of these genes alone conferred these properties when combined with SV40 TAg; tissues formed from hTERT→SV40 TAg cells remained small and noninvasive, whereas SV40 TAg→Ras cells did not survive cell transplantation. The latter result is curious, although it is in agreement with previous data using fibroblasts and kidney epithelial cells [8]. More work will be required to determine the causes of this failure of cell survival. It may be that the abnormalities conferred by the genes individually (high proliferation and cell death for SV40 TAg; abnormal tissue structure for Ras) produce more severe abnormalities in combination, which do not permit cell survival in vivo. This also suggests that TERT is able to counteract this effect and so confer tumorigenic properties on these abnormal cells. These experiments do not address the mechanism by which TERT can have this effect, but it is noteworthy that a variety of recent data suggest that TERT has effects other than telomere maintenance [37–39]. Moreover, when they were transplanted, the SV40 TAg→Ras cells had a remaining replicative potential of at least 20 population doublings, as did all the cell populations used in these studies. Therefore, the failure of cells other than hTERT→SV40 TAg→Ras to form tumors is unlikely to be the result of insufficient remaining replicative potential of these cells.
Other features of hTERT→SV40 TAg→Ras cells that we do not present in detail here are in agreement with findings on tumorigenic conversion of human cells. At least some clonal populations of bovine hTERT→SV40 TAg→Ras cells maintain a diploid karyotype, but are as tumorigenic as others that do not. For human kidney epithelial cells, it was shown that diploid lines cloned from the original population are tumorigenic [10]. Additionally, tumors formed from hTERT→SV40 TAg→Ras cells may be serially transplanted, either as cells dissociated from the tumor tissue or as tissue fragment inserted subcutaneously.
The tumorigenicity of bovine adrenocortical cells in this model did not require the activity of SV40 small t antigen. Recently, it has been shown that the cooperation of hTERT, SV40, and Ras in tumorigenic conversion of human cells requires both large T and small t antigens [13]. In those experiments, retroviruses that separately encode large T and small t antigens were used, whereas in earlier experiments [8], the retroviral construct pZIPneoSV40X1/T [25] was used — the same one that we used to express SV40 TAg in our experiments. Because this retrovirus includes the genomic SV40 early region, virions produced by packaging cell lines may include both spliced and unspliced forms [40]. If there is no selective pressure for cells to express SV40 small t antigen, then it would be expected that cells infected with this retrovirus would mostly express only large T antigen, encoded by normally spliced viral RNA. When small t antigen is required for tumorigenicity [13], there will be a strong selection pressure for tumors to grow from cells that were infected by virions that encode both large T and small t antigens. The finding that tumorigenesis in bovine adrenocortical cells does not require small t antigen requires further elucidation. There are at least three possibilities: 1) a species difference between the behavior of bovine and human cells; 2) a tissue-specific difference; or 3) the use of cell transplantation techniques versus traditional subcutaneous or intramuscular injection. If this last possibility is considered, it may be that small t antigen is not required for tumorigenicity but for cell survival at some stage of the experimental protocol used for tumorigenicity testing.
The ability to form functional tissues from cells expressing RasG12V is perhaps surprising, in view of some of the previously reported effects of expression of this oncogene in normal cells [41]. We did not observe accelerated senescence when cells were infected with this retrovirus, but it should be noted that the senescence-promoting effect of Ras is prevented by growth of cells under low (physiological) oxygen tensions [42].We routinely grow adrenocortical cells under 5% O2. We did not investigate the effects of Ras at “normal” (20%) oxygen because adrenocortical cells do not grow well at this concentration [21]. The ability to create tissues expressing RasG12V should aid in determining the role of Ras in oncoprotein cooperation.
Cell transplantation, because it can be used to form tissues from normal as well as abnormal cells, offers some advantages in the study of the roles of genes in tumorigenicity. Differences in the genetic requirements for tumorigenicity when cells are tested by cell transplantation versus conventional subcutaneous and intramuscular injection (such as the requirement for small t antigen) may indicate that some genetic modifications in conventional systems are required for cells to survive the process by which tumorigenicity is tested, rather than for tumorigenicity per se. The ability of cell transplantation to create tissues expressing specific genes and gene combinations enables greater insight into the mode by which oncoproteins cooperate in full neoplastic transformation. Telomerase activity is clearly required for tumorigenicity, yet hTERT alone produces almost no change in tissue properties. These findings also suggest that the potential for telomerized cells to evolve to a tumorigenic state should be carefully evaluated before telomerized cells are used in human cell therapy [2,15,43].
Footnotes
This work was supported by National Institute on Aging grants AG12287, AG13663, and AG20752, and by a Senior Scholar Award from the Ellison Medical Foundation.
References
- 1.Greider CW. Telomeres and senescence: the history, the experiment, the future. Curr Biol. 1998;8:R178–R181. doi: 10.1016/s0960-9822(98)70105-8. [DOI] [PubMed] [Google Scholar]
- 2.Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichsteiner S, Wright WE. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 3.Wright WE, Shay JW. Cellular senescence as a tumor-protection mechanism: the essential role of counting. Curr Opin Genet Dev. 2001;11:98–103. doi: 10.1016/s0959-437x(00)00163-5. [DOI] [PubMed] [Google Scholar]
- 4.Lanza RP, Cibelli JB, Blackwell C, Cristofalo VJ, Francis MK, Baerlocher GM, Mak J, Schertzer M, Chavez EA, Sawyer N, Lansdorp PM, West MD. Extension of cell life-span and telomere length in animals cloned from senescent somatic cells. Science. 2000;288:665–669. doi: 10.1126/science.288.5466.665. [DOI] [PubMed] [Google Scholar]
- 5.Thomas M, Yang L, Hornsby PJ. Formation of normal functional tissue from transplanted adrenocortical cells expressing telomerase reverse transcriptase. Nat Biotechnol. 2000;18:39–42. doi: 10.1038/71894. [DOI] [PubMed] [Google Scholar]
- 6.Wright WE, Shay JW. Telomere dynamics in cancer progression and prevention: fundamental differences in human and mouse telomere biology. Nat Med. 2000;6:849–851. doi: 10.1038/78592. [DOI] [PubMed] [Google Scholar]
- 7.Macieira-Coelho A. Relevance for In Vivo Aging. 1988. Biology of Normal Proliferating Cells In Vitro; p. 17. Karger, Basel. [Google Scholar]
- 8.Hahn WC, Counter CM, Lundberg AS, Beijersbergen RL, Brooks MW, Weinberg RA. Creation of human tumour cells with defined genetic elements. Nature. 1999;400:464–468. doi: 10.1038/22780. [DOI] [PubMed] [Google Scholar]
- 9.Elenbaas B, Spirio L, Koerner F, Fleming MD, Zimonjic DB, Donaher JL, Popescu NC, Hahn WC, Weinberg RA. Human breast cancer cells generated by oncogenic transformation of primary mammary epithelial cells. Genes Dev. 2001;15:50–65. doi: 10.1101/gad.828901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zimonjic D, Brooks MW, Popescu N, Weinberg RA, Hahn WC. Derivation of human tumor cells in vitro without widespread genomic instability. Cancer Res. 2001;61:8838–8844. [PubMed] [Google Scholar]
- 11.Rich JN, Guo C, McLendon RE, Bigner DD, Wang XF, Counter CM. A genetically tractable model of human glioma formation. Cancer Res. 2001;61:3556–3560. [PubMed] [Google Scholar]
- 12.Sonoda Y, Ozawa T, Hirose Y, Aldape KD, McMahon M, Berger MS, Pieper RO. Formation of intracranial tumors by genetically modified human astrocytes defines four pathways critical in the development of human anaplastic astrocytoma. Cancer Res. 2001;61:4956–4960. [PubMed] [Google Scholar]
- 13.Hahn WC, Dessain SK, Brooks MW, King JE, Elenbaas B, Sabatini DM, DeCaprio JA, Weinberg RA. Enumeration of the simian virus 40 early region elements necessary for human cell transformation. Mol Cell Biol. 2002;22:2111–2123. doi: 10.1128/MCB.22.7.2111-2123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang XR, Jimenez G, Chang E, Frolkis M, Kusler B, Sage M, Beeche M, Bodnar AG, Wahl GM, Tlsty TD, Chiu CP. Telomerase expression in human somatic cells does not induce changes associated with a transformed phenotype. Nat Genet. 1999;21:111–114. doi: 10.1038/5056. [DOI] [PubMed] [Google Scholar]
- 15.Morales CP, Holt SE, Ouellette M, Kaur KJ, Yan Y, Wilson KS, White MA, Wright WE, Shay JW. Absence of cancer-associated changes in human fibroblasts immortalized with telomerase. Nat Genet. 1999;21:115–118. doi: 10.1038/5063. [DOI] [PubMed] [Google Scholar]
- 16.Shay JW, Wright WE. The use of telomerized cells for tissue engineering. Nat Biotechnol. 2000;18:22–23. doi: 10.1038/71872. [DOI] [PubMed] [Google Scholar]
- 17.Yang J, Nagavarapu U, Relloma K, Sjaastad MD, Moss WC, Passaniti A, Herron GS. Telomerized human microvasculature is functional in vivo. Nat Biotechnol. 2001;19:219–224. doi: 10.1038/85655. [DOI] [PubMed] [Google Scholar]
- 18.Thomas M, Northrup SR, Hornsby PJ. Adrenocortical tissue formed by transplantation of normal clones of bovine adrenocortical cells in scid mice replaces the essential functions of the animals' adrenal glands. Nat Med. 1997;3:978–983. doi: 10.1038/nm0997-978. [DOI] [PubMed] [Google Scholar]
- 19.Funk WD, Wang CK, Shelton DN, Harley CB, Pagon GD, Hoeffler WK. Telomerase expression restores dermal integrity to in vitro-aged fibroblasts in a reconstituted skin model. Exp Cell Res. 2000;258:270–278. doi: 10.1006/excr.2000.4945. [DOI] [PubMed] [Google Scholar]
- 20.Farwell DG, Shera KA, Koop JI, Bonnet GA, Matthews CP, Reuther GW, Coltrera MD, McDougall JK, Klingelhutz AJ. Genetic and epigenetic changes in human epithelial cells immortalized by telomerase. Am J Pathol. 2000;156:1537–1547. doi: 10.1016/S0002-9440(10)65025-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hornsby PJ, McAllister JM. Culturing steroidogenic cells. In: Waterman MR, Johnson EF, editors. Methods in Enzymology. Vol. 206. San Diego: Academic Press; 1991. pp. 371–380. [DOI] [PubMed] [Google Scholar]
- 22.Miller AD, Chen F. Retrovirus packaging cells based on 10A1 murine leukemia virus for production of vectors that use multiple receptors for cell entry. J Virol. 1996;70:5564–5571. doi: 10.1128/jvi.70.8.5564-5571.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suwa T, Yang L, Hornsby PJ. Telomerase activity in primary cultures of normal adrenocortical cells. J Endocrinol. 2001;170:677–684. doi: 10.1677/joe.0.1700677. [DOI] [PubMed] [Google Scholar]
- 24.Kim SH, Kaminker P, Campisi J. TIN2, a new regulator of telomere length in human cells. Nat Genet. 1999;23:405–412. doi: 10.1038/70508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jat PS, Cepko CL, Mulligan RC, Sharp PA. Recombinant retroviruses encoding simian virus 40 large T antigen and polyomavirus large and middle T antigens. Mol Cell Biol. 1986;6:1204–1217. doi: 10.1128/mcb.6.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harlow E, Crawford LV, Pim DC, Williamson NM. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981;39:861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas M, Hornsby PJ. Transplantation of primary bovine adrenocortical cells into scid mice. Mol Cell Endocrinol. 1999;153:125–136. doi: 10.1016/s0303-7207(99)00070-2. [DOI] [PubMed] [Google Scholar]
- 28.Forough R, Xi Z, MacPhee M, Friedman S, Engleka KA, Sayers T, Wiltrout RH, Maciag T. Differential transforming abilities of non-secreted and secreted forms of human fibroblast growth factor-1. J Biol Chem. 1993;268:2960–2968. [PubMed] [Google Scholar]
- 29.Didenko VV, Tunstead JR, Hornsby PJ. Biotin-labeled hairpin oligonucleotides. Probes to detect double-strand breaks in DNA in apoptotic cells. Am J Pathol. 1998;152:897–902. [PMC free article] [PubMed] [Google Scholar]
- 30.Hornsby PJ, Cheng CY, Lala DS, Maghsoudlou SS, Raju SG, Yang L. Changes in gene expression during senescence of adrenocortical cells in culture. J Steroid Biochem Mol Biol. 1992;43:951–960. doi: 10.1016/0960-0760(92)90323-B. [DOI] [PubMed] [Google Scholar]
- 31.Butel JS, Lednicky JA. Cell and molecular biology of simian virus 40: implications for human infections and disease. J Natl Cancer Inst. 1999;91:119–134. doi: 10.1093/jnci/91.2.119. [DOI] [PubMed] [Google Scholar]
- 32.Pipas JM, Levine AJ. Role of T antigen interactions with p53 in tumorigenesis. Semin Cancer Biol. 2001;11:23–30. doi: 10.1006/scbi.2000.0343. [DOI] [PubMed] [Google Scholar]
- 33.Ali SH, DeCaprio JA. Cellular transformation by SV40 large T antigen: interaction with host proteins. Semin Cancer Biol. 2001;11:15–23. doi: 10.1006/scbi.2000.0342. [DOI] [PubMed] [Google Scholar]
- 34.Huang S, Ingber DE. The structural and mechanical complexity of cell-growth control. Nat Cell Biol. 1999;1:E131–E138. doi: 10.1038/13043. [DOI] [PubMed] [Google Scholar]
- 35.Naik P, Karrim J, Hanahan D. The rise and fall of apoptosis during multistage tumorigenesis: down-modulation contributes to tumor progression from angiogenic progenitors. Genes Dev. 1996;10:2105–2116. doi: 10.1101/gad.10.17.2105. [DOI] [PubMed] [Google Scholar]
- 36.Tzeng YJ, Zimmermann C, Guhl E, Berg B, Avantaggiati ML, Graessmann A. SV40 T/t-antigen induces premature mammary gland involution by apoptosis and selects for p53 missense mutation in mammary tumors. Oncogene. 1998;16:2103–2114. doi: 10.1038/sj.onc.1201733. [DOI] [PubMed] [Google Scholar]
- 37.Fu W, Begley JG, Killen MW, Mattson MP. Anti-apoptotic role of telomerase in pheochromocytoma cells. J Biol Chem. 1999;274:7264–7271. doi: 10.1074/jbc.274.11.7264. [DOI] [PubMed] [Google Scholar]
- 38.Zhu H, Fu W, Mattson MP. The catalytic subunit of telomerase protects neurons against amyloid β-peptide-induced apoptosis. J Neurochem. 2000;75:117–124. doi: 10.1046/j.1471-4159.2000.0750117.x. [DOI] [PubMed] [Google Scholar]
- 39.Oh H, Taffet GE, Youker KA, Entman ML, Overbeek PA, Michael LH, Schneider MD. Telomerase reverse transcriptase promotes cardiac muscle cell proliferation, hypertrophy, and survival. Proc Natl Acad Sci USA. 2001;98:10308–10313. doi: 10.1073/pnas.191169098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kriegler M, Perez CF, Hardy C, Botchan M. Transformation mediated by the SV40 T antigens: separation of the overlapping SV40 early genes with a retroviral vector. Cell. 1984;38:483–491. doi: 10.1016/0092-8674(84)90503-8. [DOI] [PubMed] [Google Scholar]
- 41.Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16 INK4A. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 42.Lee AC, Fenster BE, Ito H, Takeda K, Bae NS, Hirai T, Yu ZX, Ferrans VJ, Howard BH, Finkel T. Ras proteins induce senescence by altering the intracellular levels of reactive oxygen species. J Biol Chem. 1999;274:7936–7940. doi: 10.1074/jbc.274.12.7936. [DOI] [PubMed] [Google Scholar]
- 43.Weinberg RA. Telomeres. Bumps on the road to immortality. Nature. 1998;396:23–24. doi: 10.1038/23825. [DOI] [PubMed] [Google Scholar]