Abstract
The development of genomic instability is an important step in generating the multiple genetic changes required for cancer. One consequence of genomic instability is the overexpression of oncogenes due to gene amplification. One mechanism for gene amplification is the breakage/fusion/bridge (B/F/B) cycle that involves the repeated fusion and breakage of chromosomes following the loss of a telomere. B/F/B cycles have been associated with low-copy gene amplification in human cancer cells, and have been proposed to be an initiating event in high-copy gene amplification. We have found that spontaneous telomere loss on a marker chromosome 16 in a human tumor cell line results in sister chromatid fusion and prolonged periods of chromosome instability. The high rate of anaphase bridges involving chromosome 16 demonstrates that this instability results from B/F/B cycles. The amplification of subtelomeric DNA on the marker chromosome provides conclusive evidence that B/F/B cycles initiated by spontaneous telomere loss are a mechanism for gene amplification in human cancer cells.
Keywords: chromosome fusion, breakage/fusion/bridge cycles, DNA amplification, telomere, chromosome instability
Abbreviations: ES, embryonic stem; HSV-tk, herpes simplex virus thymidine kinase; bp, base pair; PNA, peptide nucleic acid; B/F/B, breakage/fusion/bridge; DAPI, 4,6-diamino-2-phenylindole; BAC, bacterial artificial chromosome; DM, double minute
Introduction
Genomic instability is an important step in generating the multiple genetic changes required for cancer [1–3]. Genomic instability can result in both the loss of tumor suppressor genes through deletions or chromosome loss, or the overexpression of oncogenes through gene amplification [4,5]. Gene amplification can occur through a number of different mechanisms. In human tumor cells, high-copy gene amplification most often involves the formation of doubleminute (DM) chromosomes [6,7]. Gene amplification can also result from the breakage/fusion/bridge (B/F/B) cycles, which were first described in maize [8]. B/F/B cycles are initiated when broken chromosomes fuse and then break during anaphase when the two centromeres are pulled in opposite directions. The B/F/B cycle is then continued in the next cell cycle when sister chromatids fuse following DNA replication. B/F/B cycles have been found to be the primary mechanism for gene amplification in hamster cells [9–12]; however, the importance of B/F/B cycles in genomic instability in human cancer is unclear.
The ends of chromosomes, called telomeres, play an important role in maintaining chromosome stability and preventing chromosome fusion. Telomeres are composed of a 6-bp repeat sequence and associated proteins that form a cap that protects chromosome ends [13,14]. Telomeric repeat sequences are added on by the enzyme telomerase, which compensates for the loss of the ends of chromosomes during cell division. In humans, telomerase activity is expressed in germ line cells, but not in most somatic cells [15]. As a result, in somatic cells, telomeres continue to shorten with each cell division, which has been proposed to be the mechanism for mediating cell senescence [16]. Consistent with this hypothesis, expression of the gene for the catalytic subunit of telomerase is capable of extending the lifespan of primary human fibroblasts [17]. Thus, to overcome telomere shortening, immortalized cells invariably regain the ability to maintain telomeres, either through the activation of telomerase activity [16,18] or through the activation of an alternative mechanism that appears to involve recombination [19,20]. The importance of telomeres in preventing chromosome fusion is illustrated by the massive increase in chromosome fusion observed in somatic cells that fail to senesce and therefore continue to undergo telomere shortening [18]. Increased chromosome fusion is also seen in cells with mutations in genes that affect telomere function [21–25]. The ability of cells to properly maintain telomeres is therefore a critical factor in maintaining chromosome stability.
Although telomeres must be maintained for cells to become immortal, cancer cells commonly have problems in maintaining telomeres and preventing chromosome fusion. Many cancer cells demonstrate a high frequency of telomere associations involving chromosomes joined at or near their telomeres, which has been proposed to result from a failure to properly maintain telomere length [15]. Consistent with this observation, many early passage tumor cells in culture demonstrate telomere instability leading to chromosome fusion [26]. A variety of mechanisms may be involved in spontaneous telomere loss. Fluctuations in telomerase activity in some cancer cell lines have been demonstrated to result in global changes in telomere length and increased chromosome fusion [27,28]. However, other cancer cell lines can demonstrate a high frequency of chromosomes without detectable telomeres, which is independent of average telomere length [26,29], suggesting that stochastic mechanisms can also result in telomere loss. Stochastic mechanisms of spontaneous telomere loss could include a failure to replicate telomeres during DNA synthesis or double-strand breaks occurring within or near telomeric repeat sequences, which has been shown to result in telomere loss in mouse ES cells [30].
As first proposed by McClintock [8], the addition of telomeres to the ends of broken chromosomes can promote chromosome stability by preventing or terminating B/F/B cycles. Broken chromosomes can acquire telomeres by a variety of mechanisms. Telomeres can be added on directly to the ends of broken chromosomes by telomerase, as demonstrated in Tetrahymena [31] and yeast [32]. Direct addition of telomeres to the ends of broken chromosomes has been associated with human genetic disease [33–35], and has been observed at the location of double-strand breaks in mouse embryonic stem (ES) cells [36]. However, it is unclear whether telomerase is involved in the direct addition of telomeres in mammalian cells. Telomeres can also be acquired through the translocation of the ends of other chromosomes, as has been observed in human cancer cells [37,38] and in mouse ES cells [30]. Finally, telomeres can be acquired through break-induced replication, as has been observed in yeast [39,40]. In break-induced replication, the broken end of the chromosome invades a region of homology on another chromosome and replicates the end of the chromosome. Whether break-induced replication occurs in mammalian cells is not known.
Evidence that telomere loss and B/F/B cycles play an important role in human cancer is provided by the presence of a high frequency of anaphase bridges in many early passage tumor cells [26] and tumors [41]. In addition, structures consistent with B/F/B cycles have been observed in low-copy gene amplification in some human tumor cells lines [42,43]. B/F/B cycles could also be an early step in high-copy gene amplification. Although human tumor cells in culture selected for gene amplification usually contain DM chromosomes, one clone with low-copy amplification contained structures consistent with B/F/B cycles [44]. When placed under more stringent selection conditions, the amplified genes in this clone converted to DM chromosomes, leading to the proposal that B/F/B cycles are an early step in the formation of DM chromosomes. Consistent with this hypothesis, regions amplified by B/F/B cycles in hamster cells have also been shown to form DM chromosomes [11].
We previously investigated the consequences of spontaneous telomere loss in a human tumor cell line that has a herpes simplex virus thymidine kinase (HSV-tk) selectable marker gene integrated immediately adjacent to a telomere [38]. Loss of the HSV-tk gene was found to be associated with the formation of inverted repeats, large duplications, and prolonged periods of instability in the “marker” chromosome containing the telomeric plasmid sequences. These results suggested that chromosome instability resulting from telomere loss involved sister chromatid fusion and B/F/B cycles. To confirm the presence of B/F/B cycles, we have now analyzed the cells containing the unstable marker chromosome for the presence of anaphase bridges. The extent of amplification of subtelomeric DNA on the marker chromosome was also investigated to determine whether B/F/B cycles initiated by spontaneous telomere loss are a mechanism for gene amplification in human cancer cells.
Materials and Methods
Cell Culture
The EJ-30 cell line (obtained from Dr. William Dewey, University of California, San Francisco) was subcloned from the EJ bladder cell carcinoma cell line, which is also named MGH-U1 [45]. Clones A3 and B3 were isolated from EJ-30 following transfection with the pNCT-tel plasmid, and contain a single copy of the plasmid integrated at the end of a chromosome [38]. After isolation, the clones were expanded to approximately 107 cells prior to Southern blot analysis and isolation of subclones deficient in HSV-tk. HSV-tk-deficient subclones were selected from multiple experiments using medium containing both ganciclovir (50 µM) and G418 (400 µg/ml) to ensure that some portion of the plasmid was present to allow for analysis of the recombination events involved.
Southern Blot Analysis
Genomic DNA purified as previously described [46] was digested with restriction enzymes according to the manufacturer's instructions. For analysis of terminal fragments, the DNA was digested with BAL31 following the manufacturer's recommendations (Promega, Madison, WI), extracted with phenol/chloroform, precipitated, resuspended, and digested with BamHI. Genomic DNA was fractionated by agarose gel electrophoresis using standard protocols, depurinated by treatment with 0.25 M HCl for 30 minutes, and transferred in 0.5 M NaOH onto a charged nylon Hybond-N+ membrane (Amersham, Piscataway, NJ) using a vacuum transfer apparatus (Amersham). Prehybridization for 3 hours and hybridization overnight were performed at 658C in 5x SSPE, 5x Denhardt's solution, 0.5% sodium dodecyl sulfate (SDS), and 0.25 mg/ml salmon sperm DNA. Probes were labeled with [α32P]dCTP (New England Nuclear, Chicago, IL) using a High Prime labeling kit (Roche, Indianapolis, IN). Filters were washed three times in 2x SSPE with 0.1% SDS at room temperature, twice in 1x SSPE with 0.1% SDS at 658C, and twice in 0.1x SSC with 0.1% SDS at 658C.
Chromosome Analysis
For analysis of anaphase cells, cultures were grown as monolayers in chamber slides and fixed with ice-cold methanol for 15 minutes followed by ice-cold methanol: acetone (1:1 vol/vol) for 30 minutes. FISH analysis was performed using Spectrum Green-labeled chromosome 16-specific painting probe (Vysis, Downers Grove, IL) following the manufacturer's protocol. DNA was counterstained with 4,6-diamino-2-phenylindole (DAPI). Anaphase cells were identified by their characteristic chromosomal configurations.
Preparation of metaphase chromosomes and hybridizations were performed as previously described [47,48]. Cosmid clones RT99 (Genbank accession no. AC004653) and 317H7 (Genbank accession no. AC005569) have been mapped to the end of chromosome 16p [49]. These cosmids were isolated from partially digested DNA libraries made from flow-sorted human chromosome 16 [50]. To improve separation of FISH signals, chromosomes were stretched. Cells were treated with colcemid (0.4 µg/ml, 30 minutes), harvested by trypsinisation, swollen with a hypotonic solution of 0.075 M KCl/serum (5:1 vol/vol), and sedimented onto glass slides for 5 minutes at 1000 rpm (Cytospin; Shandon, Pittsburgh, PN). Chromosomes were fixed overnight in methanol/acetic acid (3:1 vol/vol). The BAC probes that were used consisted of GS-121-I4, which is located a maximum of 160 kb from the telomere on 16p, GS-240-G10 for the long arm of chromosome 16 (16q), and BAC GS-121-I4, which is located a maximum of 200 kb from the telomere on 16q [51].
Telomere analysis was performed as previously described [52] using telomere-specific peptide nucleic acid (PNA) probes labeled with Cy3 (Perseptive Biosystems, Foster City, CA).
Results
Chromosome Instability Associated with Spontaneous Telomere Loss in a Human Tumor Cell Line
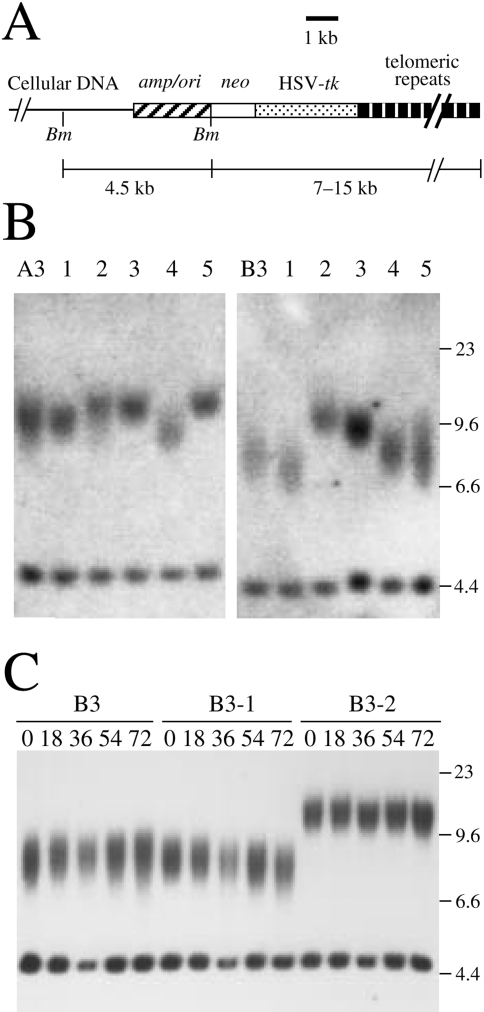
The relationship between telomere loss and chromosome instability in human cells was investigated using clone B3 of the human EJ-30 bladder cell carcinoma cell line [38]. Clone B3 contains a selectable HSV-tk gene located immediately adjacent to a telomere on chromosome 16 (Figure 1A). Cell clones containing selectable marker genes adjacent to a telomere are established by transfection with linearized plasmids containing telomeric repeat sequences on one end. The integration of the plasmid sequences on the end of a broken chromosome results in a new telomere “seeded” from the telomeric repeat sequences within the plasmid [53–55]. The seeded telomeres on these marker chromosomes are elongated in culture, and their length and dynamics become similar to the other telomeres in the cell [29,56]. In the EJ-30 clones A3 and B3, the terminal BamHI restriction fragments average approximately 7.5 and 9.5 kb, respectively, indicating telomeres that average approximately 3 and 5 kb in length (Figure 1B). Subclones of A3 and B3 selected at random demonstrated variability in the length of the terminal restriction fragments, ranging from 7 to 10 kb in clone B3 and from 9 to 12 kb in clone A3 (Figure 1B). With increasing time in culture, clone B3 showed no change in the length of the seeded telomere, whereas subclones of B3 showed gradual changes in the length of the seeded telomere (Figure 1C). These results are similar to those obtained with other tumor cell lines, in which the length of the seeded telomeres is at equilibrium in the original clones, whereas telomere length in subclones is often not at equilibrium but returns to equilibrium with time in culture [29,56]. Thus, the seeded telomeres in clones of EJ-30 are relatively stable, although some variation in telomere length is observed in different cells in the population.
Figure 1.
Structure of the integrated plasmid sequences and length of seeded telomeres in EJ-30 clones A3 and B3. (A) Clones A3 and B3 contain a single copy of the pNCT-tel plasmid integrated on the end of a chromosome. The locations of the cellular DNA, plasmid vector (amp/ori), neo gene, HSV-tk gene, and telomeric repeat sequences are shown. The location of BamHI restriction sites (Bm) used for Southern blot analysis and the size of the BamHI restriction fragments are shown. (B) Southern blot analysis of BamHI-digested genomic DNA from clones A3 and B3, and subclones 1 through 5 selected at random from each clone. Hybridization was performed with the pNCT-Δ probe, which is identical to pNCT-tel except that it does not contain telomeric repeat sequences. (C) Southern blot analysis of BamHI-digested genomic DNA from clone B3 and its subclones B3-1 and B3-2 after different numbers of cell doublings using the pNCT-Δ plasmid as a probe. The internal BamHI fragments in clones A3 and B3 are both approximately 4.5 kb in length. The terminal fragments containing 4.4 kb of plasmid sequences are heterogeneous in length due to variability in the length of the telomeres in different cells in the population. Molecular size markers consisting of Lambda bacteriophage HindIII restriction fragments are shown.
Selection with ganciclovir for cells that had lost the HSV-tk gene provides a method for following the consequences of spontaneous telomere loss on a specific chromosome without selection for gene amplification. Clones of EJ-30 containing telomeric integration sites showed a high rate of spontaneous loss of the HSV-tk gene (10-4 events/cell per generation) as compared to clones with interstitial integration sites [38]. In contrast, the seeded telomeres in mouse ES cells are relatively stable and spontaneous loss of the HSV-tk gene is below the level of detection [30]. Thus, the seeded telomeres in tumor cell lines are relatively unstable, consistent with studies indicating telomere instability in tumor cells and tumor cell lines [26,29]. The analysis of four HSV-tk-deficient (HSV-tk-) subclones of clone B3, G45, G55, G60, and G71 demonstrated the presence of inverted repeats within the plasmid sequences, whereas cytogenetic analysis showed the presence of large duplications on the end of the marker chromosome [30,38]. The heterogeneity in the structure of the marker chromosome in different cells in the population and the continued instability of the marker chromosome in many second-generation subclones demonstrated that this instability continues for many cell generations. The combined results from these experiments strongly indicated that sister chromatid fusion was the first event leading to chromosome instability resulting from spontaneous telomere loss.
Anaphase Bridges Associated with Chromosome Instability Resulting from Spontaneous Telomere Loss
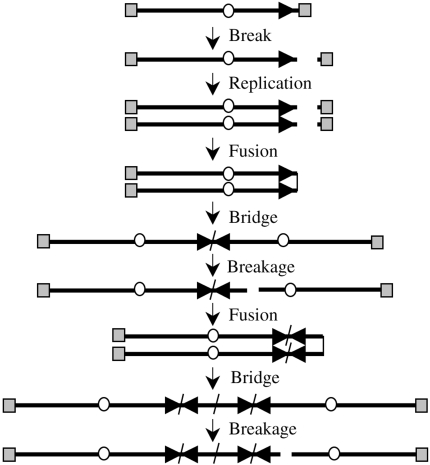
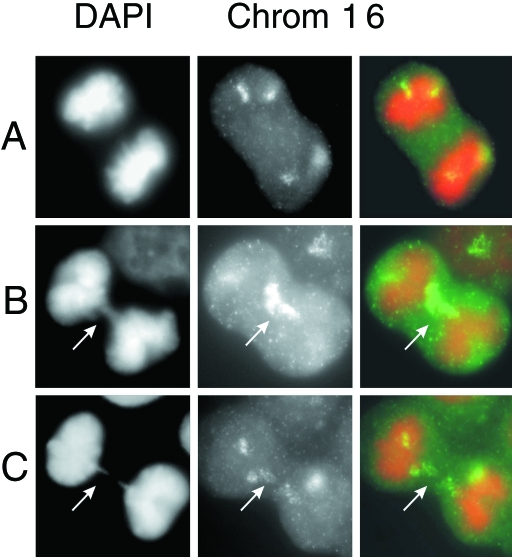
The presence of inverted repeats, large duplications, and prolonged periods of chromosome instability following the loss of a telomere are consistent with a mechanism involving B/F/B cycles (Figure 2). To determine whether B/F/B cycles are involved in the instability in the marker chromosome, we examined subclone G71 for the presence of anaphase bridges, which are considered a hallmark of chromosome fusion resulting from telomere loss [8,26,41]. Anaphase bridges were observed in 10% (21 of 200) of the anaphase cells in subclone G71. In contrast, the parental B3 cell line had significantly fewer bridges in anaphase cells (1 of 200). In addition, hybridization with a chromosome 16-specific probe showed that the anaphase bridges in subclone G71 consisted of chromosome 16 in 9 of 11 anaphase bridges that were analyzed (Figure 3). The presence of a high frequency of anaphase bridges involving chromosome 16 provides compelling evidence that the marker chromosome in subclone G71 is involved in B/F/B cycles.
Figure 2.
The mechanism of gene amplification involving B/F/B cycles. B/F/B cycles are initiated when sister chromatids fuse following the loss of a telomere. The resulting dicentric chromosome forms a bridge during anaphase and breaks again, continuing the cycle until the chromosome obtains a new telomere. Breakage at locations other than the site of fusion results in amplification of sequences in one daughter cell and deletions in the other daughter cell. The distance between the amplified arrays is dependent upon the distance of the break from the site of fusion. The telomeres (gray squares), centromeres (circles), and orientation of the subtelomeric sequences (arrow) are shown.
Figure 3.
Anaphase bridges specific to chromosome 16 in subclone G71. The staining of total chromatin with DAPI (left panel, pseudo-colored orange) is used to identify anaphase cells without (A) and with (B,C) chromosome bridges (arrows). Hybridization with a chromosome 16-specific painting probe (center panel, green) demonstrates that most anaphase bridges in subclone G71 involve chromosome 16. The merged image showing both DAPI staining and hybridization with the chromosome 16-specific probe is also shown (right panel).
DNA Amplification Resulting from B/F/B Cycles Initiated by Spontaneous Telomere Loss
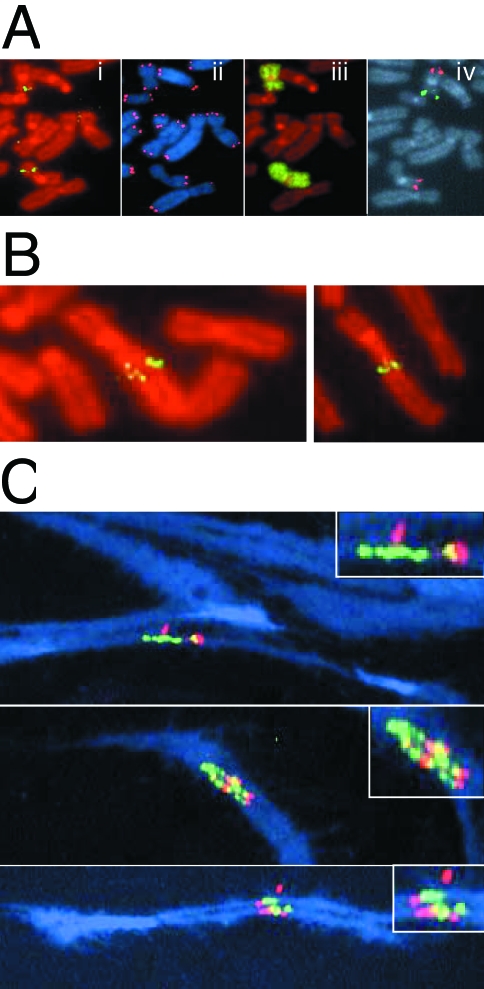
B/F/B cycles can result in amplification of DNA (Figure 2), and amplified regions consistent with B/F/B cycles have been observed in a number of mammalian cells [9–12]. To determine whether B/F/B cycles in clone B3 result in gene amplification, metaphase chromosomes from subclone G71 were hybridized with cosmid RT99 that contains sequences subtelomeric to the plasmid on the end of the marker chromosome (Figure 4Ai). The same metaphase chromosome preparations were also hybridized with a telomeric probe to monitor the presence of a telomere (Figure 4Aii) and chromosome 16-specific probes to identify the marker chromosome (Figure 4, Aiii and iv). A chromosome 16-specific painting probe was used to identify both chromosome 16 homologs (Figure 4Aiii), whereas hybridization with two bacterial artificial chromosome (BAC) clones specific for the p and q arms of chromosome 16 was used to distinguish between the two homologs (Figure 4Aiv). Due to the deletion of approximately 4Mb of DNA from the end of the p arm upon integration of the telomeric plasmid in clone B3, the marker chromosome does not hybridize with the p arm-specific BAC clone GS-121-I4. In many metaphase spreads, the hybridization signal with the RT99 cosmid probe was located internally within the marker chromosome (Figure 4Ai), consistent with previous results demonstrating duplications on the end of chromosome 16 in subclones containing inverted repeats [38]. The lack of a detectable telomere and a rounded end on the p arm in approximately 20% of the cells in subclone G71 were consistent with continued sister chromatid fusion in many cells in the population (Figure 4Aii). In addition, of 72 metaphase spreads analyzed, all but one showed an increase in hybridization intensity of the subtelomeric RT99 probe in the marker chromosome over that observed in the other chromosome 16 homologue. In approximately half of the metaphases examined (35 of 72), the increase in hybridization intensity with RT99 was small and localized in single spots (Figure 4Ai). In most of the other cells in the population (32 of 72), the increase in hybridization was more pronounced and multiple spots were observed (Figure 4B). Only a small fraction of the cells (4 of 72) contained multiple bands typical of the hybridization patterns previously reported for genes amplified by B/F/B cycles in hamster [9–12] or human [42,43] cells. In contrast to the marker chromosome, the corresponding region in the homologous chromosome in all of the metaphase spreads examined appeared normal with no indication of amplification.
Figure 4.
Gene amplification resulting from the spontaneous telomere loss. (A) Metaphase chromosomes from subclone G71 hybridized with (i) a subtelomeric cosmid probe RT99 and counterstained with propidium iodide; (ii) a telomere-specific PNA probe and counterstained with DAPI; (iii) a chromosome 16-specific painting probe and counterstained with propidium iodide; and (iv) both a chromosome 16q-specific BAC clone GS-240-G10 (red) and a chromosome 16p-specific BAC clone GS-121-I4 (green). The marker chromosome is identified by the absence of hybridization with the GS-121-I4 BAC clone due to the terminal deletion associated with integration of the pNCT-tel plasmid. (B) Two additional metaphase chromosomes of subclone G71 demonstrating increased hybridization with the subtelomeric cosmid probe RT99 and variability in the size of the fragment joined onto the end of the marker chromosome. (C) Three metaphase spreads containing stretched chromosomes were hybridized with fluorescein-labeled cosmid RT99 (green) located immediately adjacent to the telomere and rhodamine-labeled cosmid 317H7 (red) located 1 Mb from the telomere. Overlapping RT99 and 317H7 hybridization signals appear yellow. Chromosomes were counterstained with DAPI (blue). The inserts are magnified views of the amplified regions.
To investigate the structure of the amplified region at higher resolution, stretched metaphase chromosomes from subclone G71 were hybridized simultaneously with a fluorescein-labeled cosmid RT99 located adjacent to the integration site, and a rhodamine-labeled cosmid 317H7 located 1 Mb from the integration site. Multiple hybridization signals involving both cosmid probes were commonly observed, demonstrating amplification of the subtelomeric region (Figure 4C). However, the amplified arrays did not consist of alternating signals from the two cosmids, which would result if the breaks occurred outside of the amplified region (see Figure 2). Instead, most hybridization signals involved cosmid RT99 adjacent to the site of fusion, with only occasional signals involving the more distal cosmid 317H7. This observation demonstrates that most breaks occurred within 1 Mb of the site of fusion and, as a result, the sequences in clone 317H7 are often excluded from the amplified region. The presence of as many as six separate hybridization signals with cosmid RT99 in some metaphase spreads demonstrated that the marker chromosome had been involved in at least three B/F/B cycles.
Discussion
The results presented with EJ-30 [38] and from other studies involving human tumor cells in culture [26–29] demonstrate that human tumor cells often have a high rate of spontaneous telomere loss. A variety of factors could contribute to this apparent inability to properly maintain functional telomeres in the EJ-30 tumor cell line. One possible mechanism is a periodic fluctuation in the level of telomerase, which has been reported to result in a dramatic shortening of telomeres in some cells in the population in some tumor cell lines [27,28]. Some fluctuation in telomere length is seen on the marker chromosome in clones of EJ-30 (Figure 1). However, analysis of telomeres in the HSV-tk-deficient subclones using PNA probes showed that the telomeres in these cells are not significantly shorter in length than the parental cell lines (data not shown). Therefore, the loss of the telomere on the marker chromosome in B3 results from either a transient shortening of all telomeres, or stochastic events occur that result in the complete loss of individual telomeres. Regardless of the mechanism, it is clear from our results that telomere loss can have a dramatic influence on chromosome stability.
Previous studies on the mechanism of gene amplification in mammalian cells have primarily focused on the analysis of the structure of highly amplified regions. In the present study, we have analyzed the mechanism of gene amplification by following the fate of a single chromosome after the loss of a telomere. As a result, we have been able to observe the early steps involved in gene amplification, providing several valuable insights into the role of B/F/B cycles in this process. One important insight is that when a telomere is not added to the end, the first event resulting from telomere loss is often sister chromatid fusion. All four of the HSV-tk- subclones of B3 that the telomere, had contained inverted repeats involving the plasmid sequences [30,38]. Similar inverted repeats were also seen in four HSV-tk- mouse ES cell subclones in which telomere loss was induced with the I-SceI endonuclease, although in the ES cells the addition of a telomere at the site of the break was the most common event observed [30]. The instability in the marker chromosome in all of the mouse and human subclones containing inverted repeats [30,38] also demonstrates that sister chromatid fusion is often followed by B/F/B cycles. Sister chromatid fusion is also an important mechanism in perpetuating B/F/B cycles, as demonstrated by the cytogenetic analysis showing the apparent fusion of the ends of sister chromatids lacking a telomere in subsequent cell cycles (Figure 4A).
The demonstration in our study of gene amplification and anaphase bridges in the same chromosome proves conclusively that B/F/B cycles can lead to gene amplification in human cancer cells. Previous studies have shown an increased rate of anaphase bridges in human cancer cells [26,57] and the presence of amplified regions consistent with B/F/B cycles [42–44]. However, the experimental systems used in these studies do not allow for the analysis of both anaphase bridges and gene amplification in the same cell line. The presence of bridges involving chromosome 16 in 10% of the anaphase cells in subclone G71 also demonstrates that B/F/B cycles can last for many generations after the initial sister chromatid fusion. This observation is consistent with the lack of detectable telomeres on the end of the marker chromosome in approximately 20% of the cells in subclone G71 [38]. In contrast, a similar study in mouse ES cells found that B/F/B cycles were generally much shorter in duration, with the marker chromosome acquiring a telomere through the translocation of fragments from other chromosomes [30]. In some instances, telomere acquisition in subclone G71 was also accompanied by translocation of fragments from other chromosomes [38]. However, in some cells, new telomeres were observed on the marker chromosome without detectable translocations, indicating that either microtranslocations or direct addition of telomeric repeats was involved. Both microtranslocations [37] and direct addition of telomeres [33–36] have been observed in human cells.
A third important observation in the present study is the small size of the amplified regions in subclone G71. Previous studies have observed that anaphase bridges usually break within preexisting fragile sites [12,43]. However, the results presented here demonstrate a tendency to break within 1 Mb of the original site of fusion. This breakage near the site of fusion could be due to the creation of a new fragile site caused by the presence of the large inverted repeats, which have been shown to be highly unstable [58–60]. A tendency to break near the site of fusion would also explain why the marker chromosome appears relatively “normal” in many cells in the population in subclone G71 despite having undergone one or more sister chromatid fusions [38]. As a result, the typical ladders seen in genes amplified by B/F/B cycles in hamster cells are uncommon in subclone G71. However, this could also be due to the lack of selection for gene amplification in our system, because the genes that are amplified are often far from the initial site of sister chromatid fusion. Regardless, it is clear from our studies that B/F/B cycles can result in the amplification of relatively small regions that are similar in size to the amplified regions commonly seen in human cancer cells [4].
Another interesting observation made in our study is the relatively modest amount of amplification that was observed despite the prolonged B/F/B cycles involving the marker chromosome. Despite the fact that there was no selection for gene amplification, the continuation of B/F/B cycles for more than 20 generations in many cells in the population would provide ample opportunity for extensive gene amplification. These results suggest that B/F/B cycles are not an efficient mechanism for generating high-copy gene amplification in human cells, which could explain why highly amplified genes in human cells are not found in structures typically associated with B/F/B cycles [6,7]. Despite this apparent inefficiency in generating high-copy DNA amplification, B/F/B cycles may be important in gene amplification in human cancer. Many cancer cell lines and early passage cancer cells in culture have high rates of telomere associations [15] and anaphase bridges associated with telomere instability [26,57], suggesting a high rate of chromosome fusion. In fact, low-copy amplified arrays typical of B/F/B cycles have been observed in some human cancer cell lines [42,43]. The presence of inverted repeats in amplified regions in human cancer cells [61] would also suggest that B/F/B cycles can play a role as an early event in the generation of highly amplified genes. Although most highly amplified genes in human cancer cells are located on DM chromosomes, this amplification may have been initiated by B/F/B cycles in some instances. Genes amplified initially through B/F/B cycles have been demonstrated to convert to DM chromosomes [11,44], which would be consistent with our observation that the region containing the inverted repeats appears to form a new fragile site. Cells that convert to DM chromosomes appear to have a selective advantage under more stringent selection conditions [44], possibly because DM chromosomes are a more efficient mechanism for generating high-copy gene amplification than B/F/B cycles.
B/F/B cycles can also lead to chromosome instability by promoting recombination with other chromosomes, which can promote nonreciprocal translocations, transfer of the amplified genes to other chromosomes, and complex chromosome rearrangements [11,38]. The types of translocations and chromosome rearrangements seen in the human tumor cells [38] and mouse ES cells [30] in our studies are typical of the rearrangements seen in tumors in telomerase-deficient mice [62] and human tumors [63]. B/F/B cycles resulting from spontaneous telomere loss are therefore likely be an important mechanism for generating both gene amplification and the chromosome rearrangements commonly found in human cancer.
Acknowledgements
We thank Luis Martins for his technical expertise. We thank Norman Doggett and Robert Sutherland of Los Alamos National Laboratory for providing the RT99 and 317H7 cosmids.
Footnotes
These authors contributed equally to the work.
The work of the J.P.M. laboratory was supported by grant number R01CA69044 from the National Cancer Institute, National Institutes of Health. The work of the L.S. laboratory was supported by contract numbers FigH-CT-1999-00002 and FigH-CT-1999-00009 from the Commission of European Communities.
References
- 1.Tlsty TD. Genomic instability and its role in neoplasia. Curr Top Microbiol Immunol. 1997;221:37–46. doi: 10.1007/978-3-642-60505-5_4. [DOI] [PubMed] [Google Scholar]
- 2.Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194:23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- 3.Loeb LA. Mutator phenotype may be required for multistage carcinogenesis. Cancer Res. 1992;51:3075–3079. [PubMed] [Google Scholar]
- 4.Schwab M. Oncogene amplification in solid tumors. Semin Cancer Biol. 1999;9:319–325. doi: 10.1006/scbi.1999.0126. [DOI] [PubMed] [Google Scholar]
- 5.Brodeur GM, Hogarety MD. Gene amplification in human cancers: biological and clinical significance. In: Vogelstein B, Kinzler KW, editors. The Genetic Basis of Human Cancer. New York: McGraw-Hill; 1998. pp. 161–172. [Google Scholar]
- 6.Brison O. Gene amplification and tumor progression. Biochim Biophys Acta. 1993;1155:25–41. doi: 10.1016/0304-419x(93)90020-d. [DOI] [PubMed] [Google Scholar]
- 7.Hahn PJ. Molecular biology of double-minute chromosomes. Bioessays. 1993;15:477–484. doi: 10.1002/bies.950150707. [DOI] [PubMed] [Google Scholar]
- 8.McClintock B. The stability of broken ends of chromosomes in Zea mays. Genetics. 1941;41:234–282. doi: 10.1093/genetics/26.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma C, Martin S, Trask B, Hamlin JL. Sister chromatid fusion initiates amplification of the dihydrofolate reductase gene in Chinese hamster cells. Genes Dev. 1993;7:605–620. doi: 10.1101/gad.7.4.605. [DOI] [PubMed] [Google Scholar]
- 10.Smith KA, Gorman PA, Stark MB, Groves RP, Stark GR. Distinctive chromosomal structures are formed very early in the amplification of CAD genes in Syrian hamster cells. Cell. 1990;63:1219–1227. doi: 10.1016/0092-8674(90)90417-d. [DOI] [PubMed] [Google Scholar]
- 11.Toledo F, Buttin G, Debatisse M. The origin of chromosome rearrangements at early stages of AMPD2 gene amplification in Chinese hamster cells. Curr Biol. 1993;3:255–264. doi: 10.1016/0960-9822(93)90175-n. [DOI] [PubMed] [Google Scholar]
- 12.Coquelle A, Pipiras E, Toledo F, Buttin G, Debatisse M. Expression of fragile sites triggers intrachromosomal mammalian gene amplification and sets boundaries to early amplification. Cell. 1997;89:215–225. doi: 10.1016/s0092-8674(00)80201-9. [DOI] [PubMed] [Google Scholar]
- 13.McEachern MJ, Krauskopf A, Blackburn EH. Telomeres and their control. Annu Rev Genet. 2000;34:331–358. doi: 10.1146/annurev.genet.34.1.331. [DOI] [PubMed] [Google Scholar]
- 14.Blackburn EH. Switching and signaling at the telomere. Cell. 2001;106:661–673. doi: 10.1016/s0092-8674(01)00492-5. [DOI] [PubMed] [Google Scholar]
- 15.de Lange T. Telomere dynamics and genome instability in human cancer. In: Blackburn EH, Greider CW, editors. Telomeres. Plainview, NY: Cold Spring Harbor Press; 1995. pp. 265–293. [Google Scholar]
- 16.Harley CB. Telomeres and aging. In: Blackburn EH, Greider CW, editors. Telomeres. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1995. pp. 247–263. [Google Scholar]
- 17.Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu C-P, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 18.Counter CM, Avilion AA, LeFeuvre CE, Stewart NG, Greider CW, Harley CB, Bacchetti S. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 1992;11:1921–1929. doi: 10.1002/j.1460-2075.1992.tb05245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murnane JP, Sabatier L, Marder BA, Morgan WF. Telomere dynamics in an immortal human cell line. EMBO J. 1994;13:4953–4962. doi: 10.1002/j.1460-2075.1994.tb06822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dunham MA, Neumann AA, Fasching CL, Reddel RR. Telomere maintenance by recombination in human cells. Nat Genet. 2000;26:447–450. doi: 10.1038/82586. [DOI] [PubMed] [Google Scholar]
- 21.van Steensel B, Smogorzewska A, de Lange T. TRF2 protects human telomeres from end-to-end fusions. Cell. 1998;92:401–413. doi: 10.1016/s0092-8674(00)80932-0. [DOI] [PubMed] [Google Scholar]
- 22.Hsu H-L, Gilley D, Galande SA, Hande MP, Allen B, Kim S-H, Li GC, Campisi J, Kohwi-Shigematsu T, Chen DJ. Ku acts in a unique way at the mammalian telomere to prevent end joining. Genes Dev. 2000;14:2807–2812. doi: 10.1101/gad.844000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bailey SM, Meyne J, Chen DJ, Kurimasa A, Li GC, Lehnert BE, Goodwin EH. DNA double-strand break repair proteins are required to cap the ends of mammalian chromosomes. Proc Natl Acad Sci USA. 1999;96:14899–14904. doi: 10.1073/pnas.96.26.14899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilley D, Tanaka H, Hande MP, Kurimasa A, Li GC, Oshimura M, Chen DJ. DNA-PKcs is critical for telomere capping. Proc Natl Acad Sci USA. 2001;98:15084–15088. doi: 10.1073/pnas.261574698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goytisolo FA, Samper E, Edmonson S, Taccioli GE, Blasco MA. The absence of the DNA-dependent protein kinase catalytic subunit in mice results in anaphase bridges and increased telomeric fusions with normal telomere length and G-strand overhang. Mol Cell Biol. 2001;21:3642–3651. doi: 10.1128/MCB.21.11.3642-3651.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gisselsson D, Jonson T, Petersen A, Strombeck B, Dal Cin P, Hoglund M, Mitelman F, Mertens F, Mandahl N. Telomere dysfunction triggers extensive DNA fragmentation and evolution of complex chromosome abnormalities in human malignant tumors. Proc Natl Acad Sci USA. 2001;98:12683–12688. doi: 10.1073/pnas.211357798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bryan TM, Englezou A, Dunham MA, Reddel RR. Telomere length dynamics in telomerase-positive immortal human cell populations. Exp Cell Res. 1998;239:370–378. doi: 10.1006/excr.1997.3907. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz JL, Jordan R, Liber H, Murnane JP, Evans HH. TP53-dependent chromosome instability is associated with transient reductions in telomere length in immortal telomerase-positive cell lines. Genes Chromosomes Cancer. 2001;30:236–244. [PubMed] [Google Scholar]
- 29.Sprung CN, Afshar G, Chavez EA, Lansdorp P, Sabatier L, Murnane JP. Telomere instability in a human cancer cell line. Mutat Res. 1999;429:209–223. doi: 10.1016/s0027-5107(99)00115-3. [DOI] [PubMed] [Google Scholar]
- 30.Lo AWI, Sprung CN, Fouladi B, Pedram M, Sabatier L, Ricoul M, Reynolds GE, Murnane JP. Chromosome instability as a result of double-strand breaks near telomeres in mouse embryonic stem cells. Mol Cell Biol. 2002;22:4836–4850. doi: 10.1128/MCB.22.13.4836-4850.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu G, Blackburn H. Developmentally programmed healing of chromosomes by telomerase in Tetrahymena. Cell. 1991;67:823–832. doi: 10.1016/0092-8674(91)90077-c. [DOI] [PubMed] [Google Scholar]
- 32.Diede SJ, Gottschling DE. Telomerase-mediated telomere addition in vivo requires DNA primase and DNA polymerase α and δ. Cell. 1999;99:723–733. doi: 10.1016/s0092-8674(00)81670-0. [DOI] [PubMed] [Google Scholar]
- 33.Varley H, Di S, Scherer SW, Royle NJ. Characterization of terminal deletions at 7q32 and 22q13. healed by de novo telomere addition. Am J Hum Genet. 2000;67:610–622. doi: 10.1086/303050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong AC, Ning Y, Flint J, Clark K, Dumanski JP, Ledbetter DH, McDermid HE. Molecular characterization of a 130-kb terminal microdeletion at 22q in a child with mild mental retardation. Am J Hum Genet. 1997;60:113–120. [PMC free article] [PubMed] [Google Scholar]
- 35.Flint J, Thomas K, Micklem G, Raynham H, Clark K, Doggett NA, King A, Higgs DR. The relationship between chromosome structure and function at a human telomeric region. Nat Genet. 1997;15:252–257. doi: 10.1038/ng0397-252. [DOI] [PubMed] [Google Scholar]
- 36.Sprung CN, Reynolds GE, Jasin M, Murnane JP. Chromosome healing in mouse embryonic stem cells. Proc Natl Acad Sci USA. 1999;96:6781–6786. doi: 10.1073/pnas.96.12.6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meltzer PS, Guan X-Y, Trent JM. Telomere capture stabilizes chromosome breakage. Nat Genet. 1993;4:252–255. doi: 10.1038/ng0793-252. [DOI] [PubMed] [Google Scholar]
- 38.Fouladi B, Miller D, Sabatier L, Murnane JP. The relationship between spontaneous telomere loss and chromosome instability in a human tumor cell line. Neoplasia. 2000;2:540–554. doi: 10.1038/sj.neo.7900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hackett JA, Feldser DM, Greider CW. Telomere dysfunction increases mutation rates and genomic instability. Cell. 2001;106:275–286. doi: 10.1016/s0092-8674(01)00457-3. [DOI] [PubMed] [Google Scholar]
- 40.Bosco G, Haber JE. Chromosome break-induced replication leads to nonreciprocal translocations and telomere capture. Genetics. 1998;150:1037–1047. doi: 10.1093/genetics/150.3.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rudolph KL, Millard M, Bosenberg MW, DePinho RA. Telomere dysfunction and evolution of intestinal carcinoma in mice and humans. Nat Genet. 2001;28:155–159. doi: 10.1038/88871. [DOI] [PubMed] [Google Scholar]
- 42.Shuster MI, Han L, Le Beau MM, Davis E, Sawicki M, Lese CM, Park NH, Colicelli J, Gollin SM. A consistent pattern of RIN1 rearrangements in oral squamous cell carcinoma cell lines supports a breakage-fusion-bridge cycle model for 11q23 amplification. Genes Chromosomes Cancer. 2000;28:153–163. [PubMed] [Google Scholar]
- 43.Hellman A, Ziotorynski E, Scherer SW, Cheung J, Vincent JB, Smith DI, Trakhtenbrot L, Kerem B. A role for common fragile site induction in amplification of human oncogenes. Cancer Cell. 2002;1:89–97. doi: 10.1016/s1535-6108(02)00017-x. [DOI] [PubMed] [Google Scholar]
- 44.Singer MJ, Mesner LD, Friedman CL, Trask BJ, Hamlin JL. Amplification of the human dihydrofolate reductase gene via double minutes is initiated by chromosome breaks. Proc Natl Acad Sci USA. 2000;97:7921–7926. doi: 10.1073/pnas.130194897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Toole CM, Povey S, Hepburn P, Franks LM. Identity of some human bladder cancer cell lines. Nature. 1983;301:429–430. doi: 10.1038/301429a0. [DOI] [PubMed] [Google Scholar]
- 46.Murnane JP, Fuller LF, Painter RB. Establishment and characterization of a permanent pSVori- transformed ataxia-telangiectasia cell line. Exp Cell Res. 1985;158:119–126. doi: 10.1016/0014-4827(85)90437-9. [DOI] [PubMed] [Google Scholar]
- 47.Dutrillaux B, Couturier J. La Pratique de l'Analysee Chromosomique Masson, Paris. 1981 [Google Scholar]
- 48.Lemieux N, Dutrillaux B, Viegas-Pequignot E. A simple method for simultaneous R-or G-banding and fluorescence in situ hybridization of small single-copy genes. Cytogenet Cell Genet. 1992;59:311–312. doi: 10.1159/000133277. [DOI] [PubMed] [Google Scholar]
- 49.Doggett NA, Goodwin LA, Tesmer JG, Meincke LJ, Bruce DC, Clark LM, Altherr MR, Ford AA, Chi H-C, Marrone BL, Longmire JL, Lane SA, Whitmore SA, Lowenstein MG, Sutherland RD, Mundt MO, Knill EH, Bruno WJ, Macken CA, Torney DC, Wu JR, Griffith J, Sutherland GR, Deaven LL, Callen DF, Moyzis RK. An integrated physical map of human chromosome 16. Nature. 1995;377(Supplement):335–365. doi: 10.1038/377335a0. [DOI] [PubMed] [Google Scholar]
- 50.Longmire JL, Brown NC, Meincke LJ, Campbell ML, Albright KL, Fawcett JJ, Campbell EW, Moyzis RK, Hildebrand CE, Evans GA, Deaven LL. Construction and characterization of partial digest DNA. Genet Anal Tech Appl. 1993;10:69–76. doi: 10.1016/1050-3862(93)90037-j. [DOI] [PubMed] [Google Scholar]
- 51.Knight SJL, Lese CM, Precht KS, Kuc J, Ning Y, Lucas S, Regan R, Brenan M, Nicod A, Lawrie NM, Cardy DLN, Nguyen H, Hudson TJ, Riethman HC, Ledbetter DH, Flint J. An optimized set of human telomere clones for studying telomere integrity and architecture. Am J Hum Genet. 2000;67:320–332. doi: 10.1086/302998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lansdorp PM, Verwoerd NP, van de Rijke FM, Dragowska V, Little M-T, Dirks RW, Raap AP, Tanke HJ. Heterogeneity in telomere length of human chromosomes. Hum Mol Genet. 1996;5:685–691. doi: 10.1093/hmg/5.5.685. [DOI] [PubMed] [Google Scholar]
- 53.Hanish JP, Yanowitz JL, De Lange T. Stringent sequence requirements for the formation of human telomeres. Proc Natl Acad Sci USA. 1994;91:8861–8865. doi: 10.1073/pnas.91.19.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barnett MA, Buckle J, Evans EP, Porter ACG, Rout D, Smith AG, Brown WRA. Telomere directed fragmentation of mammalian chromosomes. Nucleic Acids Res. 1993;21:27–36. doi: 10.1093/nar/21.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Farr C, Fantes J, Goodfellow P, Cooke H. Functional reintroduction of human telomeres into mammalian cells. Proc Natl Acad Sci USA. 1991;88:7006–7010. doi: 10.1073/pnas.88.16.7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sprung CN, Sabatier L, Murnane JP. Telomere dynamics in human cancer cell line. Exp Cell Res. 1999;247:29–37. doi: 10.1006/excr.1998.4293. [DOI] [PubMed] [Google Scholar]
- 57.Gisselsson D, Pettersson L, Hoglund M, Heidenblad M, Gorunova L, Wiegant J, Mertens F, Dal Cin P, Mitelman F, Mandahl N. Chromosomal breakage-fusion-bridge events cause genetic intratumor heterogeneity. Proc Natl Acad Sci USA. 2000;97:5357–5362. doi: 10.1073/pnas.090013497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Akgun E, Zahn J, Baumes S, Brown G, Liang F, Romanienko PJ, Lewis S, Jasin M. Palindrome resolution and recombination in the mammalian germ line. Mol Cell Biol. 1997;17:5559–5570. doi: 10.1128/mcb.17.9.5559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Waldman AS, Tran H, Goldsmith EC, Resnick MA. Long inverted repeats are an at-risk motif for recombination in mammalian cells. Genetics. 1999;153:1873–1883. doi: 10.1093/genetics/153.4.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gebow D, Miselis N, Liber H. Homologous and nonhomologous recombination resulting in deletion: effects of p53 status, microhomology, and repetitive DNA length and orientation. Mol Cell Biol. 2000;20:4028–4035. doi: 10.1128/mcb.20.11.4028-4035.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ford M, Fried M. Large inverted duplications are associated with gene amplification. Cell. 1986;45:425–430. doi: 10.1016/0092-8674(86)90328-4. [DOI] [PubMed] [Google Scholar]
- 62.Artandi SE, Chang S, Lee S-L, Alson S, Gottlieb GJ, Chin L, DePinho RA. Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature. 2000;406:641–645. doi: 10.1038/35020592. [DOI] [PubMed] [Google Scholar]
- 63.Atkin NB. Lack of nonreciprocal translocations in carcinomas. Cancer Genet Cytogenet. 1986;21:275–278. doi: 10.1016/0165-4608(86)90009-9. [DOI] [PubMed] [Google Scholar]