Abstract
Type I cells have been defined to be independent of mitochondria for the induction of Fas death receptor-mediated apoptosis, whereas Type II cells are mitochondria-dependent. Knock-out studies in mice show that thymocytes are Type I and liver cells are Type II. We have previously shown that primary human hepatocytes and HCT116 human colon carcinoma cells behave like Type II cells because TRAIL-induced apoptosis can be blocked by the caspase 9 inhibitor, Z-LEHD-FMK. On the other hand, caspase 9 inhibition does not allow survival of TRAIL-treated SW480 colon cancer cells, which is predicted for Type I cells. Investigating the differences in TRAIL-induced apoptotic pathways in HCT116 and SW480 cells revealed that although FADD, BID, and procaspase 3 protein levels are higher in SW480 cells, and although procaspase 8 and FLIP processing is more efficient at the TRAIL-DISC of SW480 cells, BID, procaspase 3, XIAP, and PARP cleavages occur more rapidly in HCT116, despite the higher levels of BCL-2 and HSP70. Cytochrome c release from the mitochondria to the cytoplasm is more efficient in HCT116 cells. These results suggest BID cleavage as a possible limiting factor in the involvement of mitochondria in TRAIL-induced cell death. Thus, regulation of BID cleavage may define if a cell is mitochondria-dependent or-independent in response to TRAIL death receptor-induced apoptosis.
Keywords: caspase, DISC, FLIP, Bid, cancer therapy
Introduction
TRAIL is a membrane-incorporated trimeric ligand, which binds two proapoptotic death receptors, DR4 and KILLER/DR5, and two antiapoptotic decoy receptors, DcR1 (TRID) and DcR2 (TRUNDD). The interaction between the ligand and its death receptors leads to the assembly of a multicomponent death-inducing signaling complex (DISC) at the cell membrane. The adaptor FADD provides a bridge between the death receptors and the initiator procaspase 8 [1]. Procaspase 8 autocatalytically activates itself by cleaving the p10 and p20 subunits off the full-length zymogen. Active caspase 8 then can cleave and activate the effector caspases 3, 6, and 7. This axis from the cell membrane to apoptosis is referred to as the “extrinsic pathway.” Many kinds of cellular stress, including starvation, oxidative damage, and DNA damage, are known to initiate another death axis known as the “intrinsic pathway” where caspase activation follows signals transduced at the level of the mitochondria. The critical event appears to be the release of cytochrome c from the intermembrane space of mitochondria into the cytoplasm, where, together with Apaf 1 and procaspase 9, the apoptosome is formed. At the apoptosome, the initiator procaspase 9 becomes activated and further can cleave downstream effector caspases, including caspase 3, which can actually cleave procaspase 8 engaging the extrinsic cascade in an amplification loop. Another cross-talk between the two axes was found when it was discovered that BID, when cleaved by caspase 8, is capable of translocating into mitochondria and signaling cytochrome c release [2].
There are both positive and negative regulators of the intrinsic and extrinsic cell death pathways, emphasizing the importance of apoptosis regulation. At the level of initiator caspases, cellular FLIP is capable of binding FADD, generally accepted to be competing for DISC binding with procaspase 8 [3]. A large family of regulators is the BCL-2 family, which includes both positive regulators such as BAX, BAK, and BID and antiapoptotic molecules such as BCL-2 and BCL-XL [4]. These antiapoptotic molecules are capable of delaying or preventing cytochrome c release in response to both intrinsic or extrinsic death inducers, whereas proapoptotic members have the opposite effect. Recently, it was found that several heat shock proteins are capable of regulating apoptotic events positively or negatively. HSP27 has been shown to bind cytochrome c, thus lowering the levels of cytochrome c available for apoptosome formation [5]. HSP70 and HSP90 can interact with Apaf 1 directly, thereby obstructing apoptosome formation [6–8]. The inhibitor of apoptosis protein (IAP) family of antiapoptotic regulators includes IAP-1, IAP-2, XIAP, NIAP, BRUCE, and survivin. Cellular IAPs bind effector caspases [9] and block their activity [10]. The activity of IAPs is neutralized by SMAC/DIABLO, a small molecule released from mitochondria along with cytochrome c. SMAC/DIABLO binds IAPs and thus releases active caspases [11,12].
When the importance of mitochondria in death receptor-mediated apoptosis was investigated, it became clear that some cells, called Type I, are independent of mitochondria in Fas-induced apoptosis. On the other hand, in other cells, mitochondrial involvement is crucial; these cells are known as Type II [13]. In the initial paper defining these cell types, BCL-2 overexpression was used to group the mitochondriadependent and-independent cells [14]. Although the physiological relevance of this system is still debated, studies in knock-out mice have provided convincing evidence that there may be tissues geared to respond in a Type I versus Type II manner. For example, hepatocytes from Bid-/- mice are resistant to the Fas-activating antibody in vivo but their thymocytes are still sensitive to Fas [15]. Hepatocytes from Bax-/- Bak-/- mice are also resistant to Fas, with their thymocytes being sensitive to Fas but resistant to etoposide and radiation [16]. Thymocytes from Apaf 1-/- and caspase 9-/- mice are also sensitive to Fas but resistant to DNA-damaging agents, such as etoposide, dexamethasone, and γ-radiation [17,18]. Thus, in mice, thymocytes are Type I cells, whereas hepatocytes are prototypical mitochondria-dependent, Type II cells. The mechanism for the difference in use of the intrinsic and extrinsic pathways is not understood. It has been suggested that the levels of activated caspase 8 generated at the DISC may define the necessity for mitochondrial involvement [14].
We have shown that primary human hepatocytes behave in a Type II manner, such that inhibition of caspase 9 by ZLEHD-FMK leads to the prevention of TRAIL-induced apoptosis. Among the different cell lines tested, HCT116 cells responded similar to the hepatocytes, whereas SW480 cells did not survive after TRAIL treatment in the presence of the caspase 9 inhibitor [19]. Furthermore, HCT116 cells deficient for BAX are resistant to TRAIL-induced apoptosis [20]. Both SW480 and HCT116 come from the colon, and it is counterintuitive that the same tissue should develop different strategies of apoptosis induction to the same signal. Nevertheless, it is possible that the different conditions, under which the cells became cancerous, may have influenced the levels of different proapoptotic and antiapoptotic regulators, leading to the prevalence of the intrinsic or extrinsic pathways. Taking into account this limitation, we undertook to investigate the differences between the apoptotic pathways of SW480 and HCT116 cells. We report that endogenous FADD, BID, and procaspase 3 protein levels are higher in SW480 cells, and the processing of procaspase 8 and FLIP is more efficient at the DISC of SW480 cells. This is interesting because the timing of BID, procaspase 3, XIAP, and PARP depletion is faster in HCT116 cells, despite the higher levels of the negative regulators BCL-2 and HSP70. Further, cytochrome c release is slower in SW480 cells. Thus, it appears that the limiting step for mitochondrial involvement in SW480 cells upon TRAIL treatment may be the cleavage of BID.
Materials and Methods
Cell Lines
The human colon cancer cell line SW480 and the human lymphocytic cell line SKW6.4 were obtained from the ATCC (Manassas, VA). The human non small cell lung cancer cell line H460 was obtained from Dr. Stephen Baylin (Johns Hopkins University, Baltimore, MD) and the human colon cancer cell line HCT116 was obtained from Dr. Bert Vogelstein (Johns Hopkins University). HCT116 cells were grown in McCoy's 5A medium, SW480 in DMEM (high glucose), and H460 in RPMI 1640, each supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. All media were purchased from Gibco BRL-Life Technologies (Rockville, MD). The cells were grown at 37°C with 5% CO2.
NHH#2 (normal human hepatocytes, sample #2) were isolated from the liver of a 9-year-old boy, who died in a house fire, as described in Ref. [19]. NKNT-3 cells (normal human hepatocytes immortalized with the SV40 T antigen) were obtained from Dr. Ira Fox (University of Nebraska, Omaha, NE). NHH#2 and NKNT-3 cells were grown in CS Complete Serum Free Medium (Cell Systems Corp., Seattle, WA).
Western Analyses
Cell lysates were analyzed on 10% or 15% SDS-PAGE and probed with the following antibodies (Ab), at the given dilutions (vol/vol): anti-actin monoclonal mouse Ab-1/250 (Santa Cruz Biotechnology, Santa Cruz, CA); anti-BID rabbit polyclonal Ab-1/500 (BD PharMingen, San Diego, CA); anti-BID rabbit polyclonal-1500 or 1/1000 (Cell Signaling Technology, Beverly, MA); anti-BCL-2 mouse monoclonal Ab-1/500 (DAKO, Glostrup, Denmark); anti-BCL-XL mouse IgG-1/500 (BD PharMingen); anti-cIAP1 and anti-cIAP2 rabbit IgGs-1/1000 (R&D Systems, Minneapolis, MN); anti-XIAP(hILP) mouse IgG-1/250 (Transduction Laboratories, Lexington, KY); anti-FADD mouse IgG-1/2000 (Upstate Biotechnologies, Lake Placid, NY); anti-FLIP rat IgG-1/1000 (Alexis, San Diego, CA); anti-caspase 3 (E-8) mouse IgG-1/500 (Santa Cruz Biotechnology); anti-caspase 8(1C12) mouse IgG-1/1000 (Cell Signaling Technology); anti-caspase 9 rabbit polyclonal Ab-1/500 (BD PharMingen); anti-cleaved caspase 9 polyclonal rabbit Ab-1/500 (Cell Signaling Technology); anti-HSP27 mouse, anti-HSP70 mouse, and anti-HSP90 rat IgGs-1/1000 each (StressGen Biotechnologies, Victorian BC, Canada); anti-PARP polyclonal rabbit Ab-1/2000 (Roche, Mannheim, Germany); and anti-cytochrome c mouse monoclonal Ab-1/1000 (BD PharMingen).
Immunoprecipitation of TRAIL-DISC
Cells from confluent T75 flasks (>4x107) were collected by trypsinization and treated with 50 ng/ml 6x His-TRAIL (R&D Systems) in a total volume of 2 ml in the respective growth medium for the cells. The anti-6x Histidine mouse IgG (R&D Systems) was added to both control and test samples at 1 µg/ml. The cells were incubated at 37°C for 15 or 30 minutes, as indicated in the figure legends, and the reaction was stopped by the addition of ice-cold 1x PBS to the reaction tubes. The DISC immunoprecipitation buffers and protocols were modified from Sprick et al. [21]. The TRAIL-DISC buffer used consisted of 30 mM Tris (pH 7.5), 150 mM NaCl, 10% (vol/vol) glycerol, and 1% (vol/vol) Triton X-100. The cells were lysed on ice in 1 ml of ice-cold TRAIL-DISC buffer supplemented with Complete (protease inhibitor cocktail) as directed by the manufacturer (Roche) and 1 mM PMSF for greater than 30 minutes. The debris was pelleted twice after which 3% of the supernatant was saved for analysis as a total lysate control. To the rest of the supernatant, 30 µl of Protein A/G PLUS-Agarose (Santa Cruz Biotechnology) was added. The incubation of the beads with the lysates continued at 4°C for 48 hours in constant rotation. The beads were pelleted and washed four times with 700 µl of ice-cold TRAIL-DISC buffer, after which the beads were eluted in 175 µl of Immunopure Gentle Ab/Ag Elution Buffer (Pierce, Rockford, IL), supplemented with 0.1 M DTT, at room temperature for greater than 2 hours. The beads were pelleted and the proteins in the supernatant were precipitated using methanol-40% (vol/vol)/chloroform-10% (vol/vol) extraction. The protein precipitate was resuspended in 2x Laemmli buffer and analysed further by Western blotting.
TRAIL Treatment Time Course
A total of 1.5x106 cells were plated per T25 1 day ahead of treatment. Cells were treated with 50 ng/ml TRAIL (R&D Systems) in the presence of the cross-linking anti-6x His antibody at 1 µg/ml. The cross-linking antibody was present in the control sample as well. Where caspase inhibitors were used, their final concentration was 20 µM. For the combination of caspases 2 and 9 inhibitors, each was present at 10 µM. The caspase 8 inhibitor Z-IETD-FMK, the caspase 2 inhibitor Z-VDVAD-FMK, the caspase 9 inhibitor Z-LEHD-FMK, and the pan caspase inhibitor Z-VAD-FMK were purchased from R&D Systems. The incubation continued for 4, 8, or 24 hours at 37°C, after which the attached and the floating cells were collected and analyzed by Western blotting.
Cellular Fractionation
Confluent T75 flasks (>107 cells) of HCT116 and SW480 were treated with His-TRAIL (Alexis) at 100 ng/ml in the presence of the cross-linking anti-Histidine Ab at 1 µg/ml for 4 hours in a total volume of 5 ml per flask. The floating and the attached cells were collected through trypsinization. The cells were lysed in 68 mM sucrose, 200 mM d-mannitol, 50 mM KCl, 1 mM EGTA, 1 mM ETDA, 1 mM DTT, 1 mM PMSF, and Complete protease inhibitor cocktail as directed by the manufacturer (Roche). The cells were first lysed on ice for 80 minutes and then through 40 strokes of a Dounce homogenizer. The lysates were centrifuged at 2700 rpm for 5 minutes in a Beckman SS34 rotor to obtain the nuclear/whole cell fraction. The pellets were washed once in the same lysis buffer and collected in the same way. The supernatants, containing the heavy membrane and the cytosolic fractions, were centrifuged at 11,000 rpm for 10 minutes in the same rotor to obtain the heavy membrane fraction. The heavy membrane pellet was washed once and recovered under the same conditions. The cytosolic fraction was precipitated from the supernatant using 6x vol of 50% ethanol, 25% methanol, and 25% acetone, prechilled to -80°C. The precipitation was allowed to occur at -80°C overnight. The cytosolic fraction was pelleted by centrifugation in 1.5-ml tubes in a microfuge at 10,000 rpm for 10 minutes at 4°C. The pellet was rinsed once in the above described precipitation solution and recovered through centrifugation. The pellets were air-dried and resuspended in 2x Laemmli buffer. All the fractions were subjected to 15% SDS-PAGE and Western analysis.
Results
Endogenous Levels of FADD, BID, and Procaspase 3 are Higher in SW480 Versus HCT116 Cells
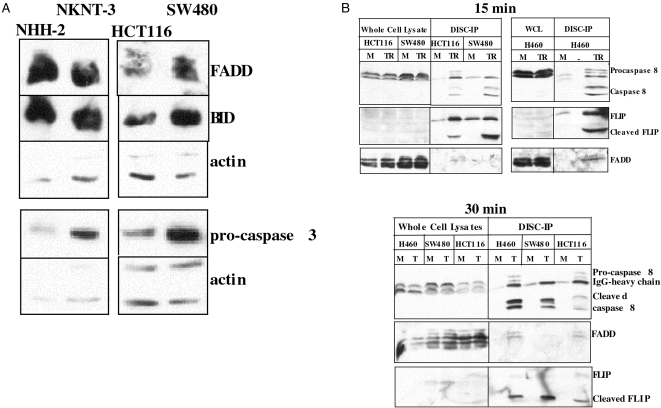
Semiquantitative RT-PCR analysis of DR4, KILLER/DR5, DcR1, and DcR2 levels in HCT116 and SW480 cells has shown that both cell lines express high levels of all four receptors, relative to other tested cell lines [22]. HCT116 and SW480 are highly sensitive to TRAIL and even at 20 ng/ml, greater than 50% Annexin V-positive cells can be observed after a 4-hour incubation time [19].We focused on the intracellular apoptotic pathway components to identify differences between the two cell lines. Western blot analysis (Figure 1A) shows that SW480 cells have higher endogenous levels of FADD, BID, and procaspase 3. The presence of high levels of FADD and procaspases 8 fits with the expectations for a Type I cell. High FADD and procaspase 8 levels would provide adequate substrates for the TRAIL-DISC (see below), giving rise to large amounts of active caspase 8, which could act directly on the abundant procaspase 3, thus minimizing the necessity for the mitochondrial amplification loop even if it does take part.
Figure 1.
Endogenous levels of FADD, BID, and procaspase 3 are higher in SW480 cells. Procaspase 8 and FLIP processing at the TRAIL-DISC. (A) Western analysis of the endogenous levels of the indicated proteins in NHH-2, NKNT-3, SW480 and HCT116 cells. (B) The cells were treated with His-TRAIL for 15 minutes (upper panel) or 30 minutes (lower panel) in the presence of the cross-linking antibody. The TRAIL-DISC complex was immunoprecipitated and tested for the presence of procaspase 8, FADD, and FLIP. “M” stands for mock, and “TR” or “T” for TRAIL treatment, as described in Materials and Methods.
Procaspase 8 and Flip Processing are More Efficient at the TRAIL-DISC of SW480 Versus HCT116 Cells
It had been suggested that low levels of procaspase 8 recruitment to the DISC in Type II cells make the mitochondrial loop necessary for generating enough active caspase 8 [14]. We tested if the difference in the apoptotic pathways in HCT116, SW480, and H460 could be accounted for by the relative levels of procaspase 8, FADD, and FLIP or by the processing of procaspase 8 and FLIP at the DISC. The protein levels of FADD and procaspase 8 being higher in SW480 cells, one would expect that there would be more of these proteins at the TRAIL-DISC of SW480 cells. By immunoprecipitating the TRAIL-DISC, we were able to show that FADD, procaspase 8, and FLIP are present at the TRAIL-DISC (Figure 1B). By comparing the relative amount of procaspase 8 in SW480, H460 (Type I-like) versus HCT116 (Type II-like) cells, it is not possible to conclude that there is more procaspase 8 recruited to the DISC of Type I-like cells. It may be expected that there should be more FLIP at the TRAIL-DISC of HCT116 cells to obstruct the generation of active caspase 8, but on the contrary, H460 and SW480 cells have larger amounts of FLIP and still the processed caspase 8 and FLIP bands are stronger (Figure 1B). It appears that the presence of full-length FLIP does not impede the efficiency with which the small subunit is cleaved off procaspase 8. Another interesting aspect of the SW480 DISC is that FADD levels are lower than observed in HCT116 cells. Higher FADD and lower FLIP levels may be expected to facilitate procaspase 8 activation; however, just the opposite appears to be the case. Low FADD and high FLIP levels in SW480 appear to coexist with the quick processing of both FLIP and procaspase 8 (Figure 1B). This suggests that while in its full-length form, FLIP may have a positive effect on caspase 8 activation. When the caspase 8 activity in TRAIL-treated cell lysates was compared, the IETD-pNA cleaving activity in SW480 cells was higher (data not shown).
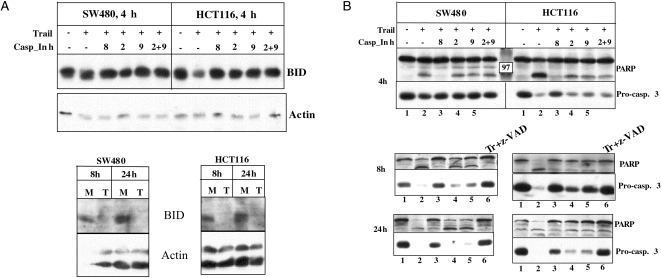
The Cleavage of BID, Procaspase 3, and PARP is Faster in HCT116 Versus SW480 Cells
Because the efficiency of caspase 8 activation appears higher at the TRAIL-DISC of SW480 versus HCT116, we hypothesized that procaspase 3 and PARP cleavages should be faster in SW480 cells. Surprisingly, the cleavages of BID (Figure 2A), procaspases 3, and PARP (Figure 2B) appear to occur more rapidly in HCT116 cells because at 4 hours after TRAIL treatment, there is a clear reduction in the levels of their native forms. SW480 cells deplete the same molecules substantially by 8 hours (Figure 2). Thus, the endogenous levels of the proteins or the efficiency of caspase 8 activation at the DISC does not correlate with the timing of BID, procaspase 3, and PARP cleavage. These results point to BID cleavage as the step where SW480 cells fail to activate mitochondria as effectively as HCT116 cells.
Figure 2.
Timing of BID, procaspase 3, and PARP cleavage in SW480 and HCT116 cells after TRAIL treatment. (A) The cells were treated with TRAIL in the presence of the different inhibitors for the indicated times — 4, 8, and 24 hours. The molecular composition of the different caspase inhibitors is given in Materials and Methods. BID cleavage was analyzed through Western analysis. (B) The cleavages of procaspase 3 and PARP were tested after 4, 8, and 24 hours of TRAIL treatment. Different caspase inhibitors were used to show the difference in their effects on procaspase 3 and PARP cleavage in HCT116 and SW480 cells. Caspases 9 and 2 inhibitors prevent procaspase 3 and PARP cleavages in HCT116 cells (see 8-hour time point). “M” stands for mock, and “T” or “Tr” for TRAIL treatment.
When the effect of the different caspase inhibitors is examined, it becomes clear that the caspase 8 inhibitor, ZIETD-FMK, is capable of blocking procaspase 3 and PARP cleavages in both cell lines even at 24 hours. The caspases 2 and 9 inhibitors can prevent procaspase 3 and PARP cleavage much better in HCT116 than SW480 cells (Figure 2B and Ref. [19]).
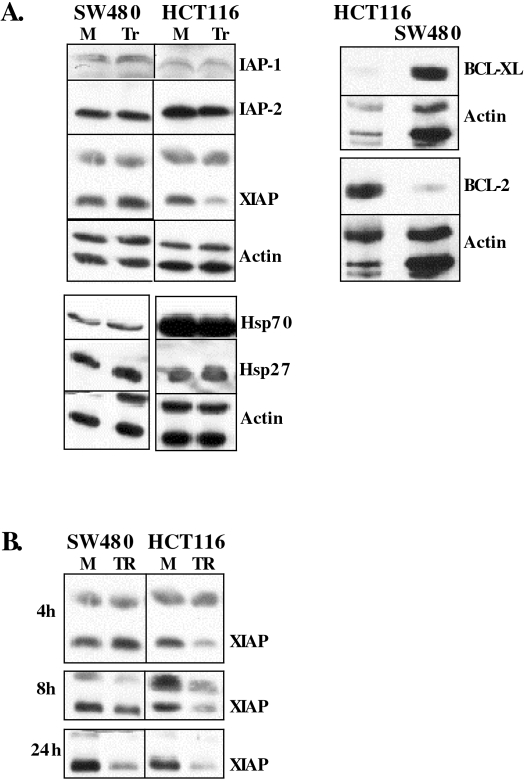
BCL-2 and HSP70 Levels are Higher in HCT116 Cells; XIAP is Cleaved Faster in TRAIL-Treated HCT116 Cells
Because apoptotic pathways are tightly regulated by positive and negative regulators, we checked if HCT116 and SW480 cells have different levels of the negative regulators acting on mitochondria: BCL-2 and BCL-XL; the caspase inhibitors IAP-1, IAP-2, and XIAP; and the apoptosome inhibitors HSP27, HSP70, and HSP90 (Figure 3). Among these, BCL-2 and HSP70 levels are higher in HCT116 cells. Like BID, procaspase 3, procaspase 9, and PARP, XIAP cleavage is also faster in HCT116 cells (Figure 3B). This suggests that once activated, the HCT116 mitochondria have the ability to initiate downstream events in spite of the higher levels of negative regulators BCL-2 and HSP70.
Figure 3.
Endogenous levels of antiapoptotic regulators and timing of XIAP cleavage. (A) Endogenous levels of the indicated antiapoptotic regulators were tested through Western analysis. (B) Timing of XIAP cleavage after TRAIL treatment. “M” stands for mock, and “Tr” for TRAIL treatment.
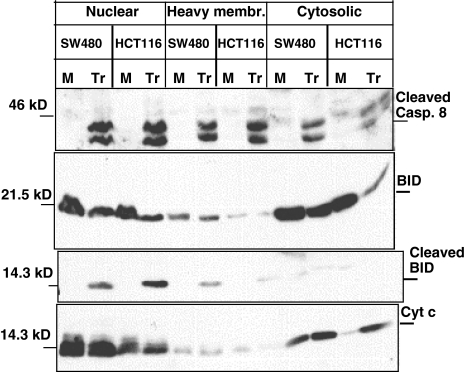
Cytochrome c Release is Faster in HCT116 Versus SW480 Cells
To test if the limiting event is BID cleavage, we checked the release of cytochrome c from the mitochondria upon TRAIL treatment. We observed a clear accumulation of cytochrome c in the cytosolic fraction of TRAIL-treated HCT116 cells, but not in SW480 cells (Figure 4). As expected, there was a dramatic reduction in the BID levels in the cytosolic fraction of HCT116 cells. The truncated form of BID accumulated in the heavy membrane fraction after TRAIL treatment but was not detectable in the cytosolic fraction. We also tested the levels of procaspase 8 and its cleaved form in the different fractions (Figure 4). It is clear that the levels of active caspase 8 are not lower in SW480 cells, and still BID cleavage is not efficient.
Figure 4.
Cytochrome c release from mitochondria after TRAIL treatment in SW480 and HCT116 cells. Nuclear, heavy membrane, and cytosolic fractions were obtained after TRAIL treatment as described in Materials and Methods. The different fractions were analyzed for procaspase 8, BID, and cytochrome c. “M” stands for mock, and “Tr” for TRAIL treatment.
Discussion
So far, the reason for the preferential use of the intrinsic or extrinsic pathways in different tissues in response to death receptor activation is not clearly understood. We find that although FADD, BID, and procaspase 3 levels are higher in SW480 cells and although the processing of procaspase 8 and FLIP is more efficient at the TRAIL-DISC of SW480 cells, the cleavages of BID, procaspase 3, XIAP, and PARP occur more rapidly in HCT116 cells, despite higher BCL-2 and HSP70 levels. These results suggest that BID cleavage is the limiting step in SW480 cells. This notion is supported by the slower rate of cytochrome c release observed in SW480 cells. Because the pNA-IETD cleaving activity in SW480 cells is higher in SW480 cells (data not shown), it is unlikely that the reason for the inefficient BID cleavage is the lack of caspase 8 enzymatic activity. However, it is possible that the interaction between the two molecules is different in the two cell lines. The interaction between caspase 8 and BID can be influenced by: 1) a posttranslational modification of BID, besides myristoylation; 2) the presence of a negative regulator in SW480 cells; 3) the presence of a positive regulator in HCT116 cells; and 4) a mutation in BID, so that the physical interaction is affected.
Besides the cleavage of BID by caspase 8 [2] and myristoylation of tBID [23], tBID has been reported to be ubiquitinated and targeted for degradation. When the putative ubiquitination sites within tBID were mutated, the nondegradable tBID induced stronger cytochrome c release [24]. Phosphorylation of BID by casein kinases I and II (CKI and CKII) has been very recently demonstrated to regulate its cleavage by caspase 8. A mutant form of BID, which could not be phosphorylated, was more easily cleaved by caspase 8 and was also more toxic to cells that the wild type form [25], suggesting that phosphorylation is inhibitory to caspase 8 cleavage.
Our results point to BID as a possible determinant for the choice of a Type I or II mechanism in response to death receptor-mediated signaling. In SW480 and HCT116 cells, the rate of BID cleavage appears to define the ability of the cells to activate mitochondrial cytochrome c release.
We suggest that different tissues have developed or optimized the use of the intrinsic or extrinsic pathway dependent on their normal functions. For example, liver cells are responsible for the detoxification of many chemicals that enter the blood, and many cytotoxic and genotoxic agents are known to activate the intrinsic pathway predominantly. Thymocytes or other cells responsible for immune responses are actually expected to signal to each other and to target cells through membrane-bound ligands, such as FAS-L or TRAIL, or secreted cytokines, and thus these cells have optimized the extrinsic apoptotic pathway. Based on this hypothesis, colon and kidney cells would be expected to be Type II cells also.
Knowing the mechanism by which death is propagated in different tissues would allow the design of better and targeted strategies for killing cancer cells and protecting normal cells. We have developed one such strategy for the protection of human liver [19]. We propose that treatment of Type I tumor cells with TRAIL in the presence of caspase 9 and/or caspase 2 inhibitors would allow the killing of the tumor cells while protecting the liver cells in vivo. TRAIL toxicity is an issue to be considered for the liver but not other primary human tissues, based on studies performed so far. A preliminary study by our group provides evidence that the proposed strategy is applicable to a second tissue, the esophagus (Kim KH, et al., submitted for publication). Primary human esophageal cells act in a Type II manner. When grown in a mixed population with Type I cancer cells and treated with TRAIL in the presence of a caspase 9 inhibitor, the cancer cells are killed, whereas the primary esophageal cells are protected. Our present studies provide further insights into the signaling pathways that govern cell death following TRAIL exposure.
Footnotes
Note added in proof:
Recently it has been reported that c-FLIP-L enhances pro-caspase 8 processing and promotes apoptosis when expressed at physiological levels. c-FLIP-L was shown to inhibit apoptosis only when expressed ectopically at high levels. (Chang, D.W. et al., 2002. EMBO J 21, 3704–14).
References
- 1.Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 2.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 3.Kataoka T, Schroter M, Hahne M, Schneider P, Irmler M, Thome M, Froelich CJ, Tschopp J. FLIP prevents apoptosis induced by death receptors but not by perforin/granzyme B, chemotherapeutic drugs, and gamma irradiation. J Immunol. 1998;161:3936–3942. (in process citation) [PubMed] [Google Scholar]
- 4.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 5.Bruey JM, Ducasse C, Bonniaud P, Ravagnan L, Susin SA, Diaz-Latoud C, Gurbuxani S, Arrigo AP, Kroemer G, Solary E, Garrido C. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol. 2000;2:645–652. doi: 10.1038/35023595. [DOI] [PubMed] [Google Scholar]
- 6.Pandey P, Saleh A, Nakazawa A, Kumar S, Srinivasula SM, Kumar V, Weichselbaum R, Nalin C, Alnemri ES, Kufe D, Kharbanda S. Negative regulation of cytochrome c-mediated oligomerization of Apaf-1 and activation of procaspase-9 by heat shock protein 90. EMBO J. 2000;19:4310–4322. doi: 10.1093/emboj/19.16.4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beere HM, Wolf BB, Cain K, Mosser DD, Mahboubi A, Kuwana T, Tailor P, Morimoto RI, Cohen GM, Green DR. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol. 2000;2:469–475. doi: 10.1038/35019501. [DOI] [PubMed] [Google Scholar]
- 8.Saleh A, Srinivasula SM, Balkir L, Robbins PD, Alnemri ES. Negative regulation of the Apaf-1 apoptosome by Hsp70. Nat Cell Biol. 2000;2:476–483. doi: 10.1038/35019510. [DOI] [PubMed] [Google Scholar]
- 9.Roy N, Deveraux QL, Takahashi R, Salvesen GS, Reed JC. The c-IAP-1 and c-IAP-2 proteins are direct inhibitors of specific caspases. EMBO J. 1997;16:6914–6925. doi: 10.1093/emboj/16.23.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deveraux QL, Reed JC. IAP family proteins — suppressors of apoptosis. Genes Dev. 1999;13:239–252. doi: 10.1101/gad.13.3.239. [DOI] [PubMed] [Google Scholar]
- 11.Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 12.Verhagen AM, Ekert PG, Pakusch M, Silke J, Connolly LM, Reid GE, Moritz RL, Simpson RJ, Vaux DL. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell. 2000;102:43–53. doi: 10.1016/s0092-8674(00)00009-x. [DOI] [PubMed] [Google Scholar]
- 13.Krammer PH. CD95's deadly mission in the immune system. Nature. 2000;407:789–795. doi: 10.1038/35037728. [DOI] [PubMed] [Google Scholar]
- 14.Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, Debatin KM, Krammer PH, Peter ME. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yin XM, Wang K, Gross A, Zhao Y, Zinkel S, Klocke B, Roth KA, Korsmeyer SJ. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature. 1999;400:886–891. doi: 10.1038/23730. [DOI] [PubMed] [Google Scholar]
- 16.Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA, Ulrich E, Waymire KG, Mahar P, Frauwirth K, Chen Y, Wei M, Eng VM, Adelman DM, Simon MC, Ma A, Golden JA, Evan G, Korsmeyer SJ, MacGregor GR, Thompson CB. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol Cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Honarpour N, Du C, Richardson JA, Hammer RE, Wang X, Herz J. Adult Apaf-1-deficient mice exhibit male infertility. Dev Biol. 2000;218:248–258. doi: 10.1006/dbio.1999.9585. [DOI] [PubMed] [Google Scholar]
- 18.Kuida K, Haydar TF, Kuan CY, Gu Y, Taya C, Karasuyama H, Su MS, Rakic P, Flavell RA. Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell. 1998;94:325–337. doi: 10.1016/s0092-8674(00)81476-2. [DOI] [PubMed] [Google Scholar]
- 19.Özören N, Kim K, Burns TF, Dicker DT, Moscioni AD, el Deiry WS. The caspase 9 inhibitor Z-LEHD-FMK protects human liver cells while permitting death of cancer cells exposed to tumor necrosis factor-related apoptosis-inducing ligand. Cancer Res. 2000;60:6259–6265. [PubMed] [Google Scholar]
- 20.Burns TF, El-Deiry WS. Identification of inhibitors of TRAIL-induced death (ITIDs) in the TRAIL sensitive colon carcinoma cell line, SW480, using a genetic approach. J Biol Chem. 2001;276:37879–37886. doi: 10.1074/jbc.M103516200. [DOI] [PubMed] [Google Scholar]
- 21.Sprick MR, Weigand MA, Reiser E, Rauch CT, Juo P, Blenis J, Krammer PH, Walczak H. FADD/MORT1 and caspase-8 are recruited to TRAIL receptors 1 and 2 and are essential for apoptosis mediated by TRAIL receptor 2. Immunity. 2000;12:599–609. doi: 10.1016/s1074-7613(00)80211-3. [DOI] [PubMed] [Google Scholar]
- 22.Kim K, Fisher MJ, Xu SQ, El-Deiry WS. Molecular determinants of response to TRAIL in killing of normal and cancer cells. Clin Cancer Res. 2000;6:335–346. [PubMed] [Google Scholar]
- 23.Zha J, Weiler S, Oh KJ, Wei MC, Korsmeyer SJ. Posttranslational N-myristoylation of BID as a molecular switch for targeting mitochondria and apoptosis. Science. 2000;290:1761–1765. doi: 10.1126/science.290.5497.1761. [DOI] [PubMed] [Google Scholar]
- 24.Breitschopf K, Zeiher AM, Dimmeler S. Ubiquitin-mediated degradation of the proapoptotic active form of bid. A functional consequence on apoptosis induction. J Biol Chem. 2000;275:21648–21652. doi: 10.1074/jbc.M001083200. [DOI] [PubMed] [Google Scholar]
- 25.Desagher S, Osen-Sand A, Montessuit S, Magnenat E, Vilbois F, Hochmann A, Journot L, Antonsson B, Martinou JC. Phosphorylation of bid by casein kinases I and II regulates its cleavage by caspase 8. Mol Cell. 2001;8:601–611. doi: 10.1016/s1097-2765(01)00335-5. [DOI] [PubMed] [Google Scholar]