Abstract
The microtubule motor cytoplasmic dynein performs multiple cellular functions; however, the regulation and targeting of the motor to different cargoes is not well understood. A biochemical interaction between the dynein intermediate chain subunit and the p150-Glued component of the dynein regulatory complex, dynactin, has supported the hypothesis that the intermediate chain is a key modulator of dynein attachment to cellular cargoes. In this report, we identify multiple intermediate chain polypeptides that cosediment with the 19S dynein complex and two differentially expressed transcripts derived from the single cytoplasmic dynein intermediate chain (Cdic) gene that differ in the 3′ untranslated region sequence. These results support previous observations of multiple Cdic gene products that may contribute to the specialization of dynein function. Most significantly, we provide genetic evidence that the interaction between the dynein intermediate chain and p150-Glued is functionally relevant. We use a genomic Cdic transgene to show that extra copies of the dynein intermediate chain gene act to suppress the rough eye phenotype of the mutant Glued1, a mutation in the p150-Glued subunit of dynactin. Furthermore, we show that the interaction between the dynein intermediate chain and p150-Glued is dependent on the dosage of the Cdic gene. This result suggests that the dynein intermediate chain may be a limiting component in the assembly of the dynein complex and that the regulation of the interaction between the dynein intermediate chain and dynactin is critical for dynein function.
INTRODUCTION
The intracellular trafficking of organelles and macromolecular complexes plays a major role in many cell and developmental processes. The directed transport of cellular components along polarized microtubule arrays uses two major classes of motor enzymes, the kinesins and the dyneins, which each move unidirectionally along the microtubule tracks. These motors are required for multiple cellular tasks, such as the transport and positioning of organelles and vesicles during interphase, the assembly of the mitotic spindle, and chromosome movement (reviewed by Karki and Holzbaur, 1999). Given these diverse functions, a major question that remains to be answered is how the association of motors with specific cargoes is regulated.
Mechanisms that account for the targeting of dyneins and kinesins may differ. For the kinesins, the multiple cellular functions are provided at least in part by multiple kinesin-related heavy chain polypeptides and associated light chains (reviewed by Goldstein, 1993; Moore and Endow, 1996). Sequence differences outside of the motor domain of the kinesin heavy chains contribute to the targeting of distinct kinesins to specific functions, either directly or by association with other proteins. For example, in Drosophila, the kinesin-like protein Nod contains a DNA-binding motif in the nonmotor domain that localizes it to chromosomes during female meiosis (Ashfar et al., 1995). The mechanisms that target the dyneins are less clear. Only three subfamilies of dynein heavy chains have been identified: the axonemal inner and outer arm dynein heavy chains and the cytoplasmic dynein heavy chains. The cytoplasmic dynein heavy chains include two isoforms, a ubiquitously expressed isoform (DHC 1a) and isoform DHC 1b, which appears to be more functionally restricted (Criswell et al., 1996; Vaisberg et al., 1996; Pazour et al., 1999; Porter et al., 1999). The heavy chains are the force-producing subunits that interact with microtubules and hydrolyze ATP. The intermediate, light-intermediate, and light chain subunits are located in a position to interact with other cellular components; their assembly and regulation may mediate the targeting of cytoplasmic dyneins to specific cargoes (see review by King, 2000).
The dynein intermediate chain may participate in the attachment of dynein to cellular cargoes by several different mechanisms. In flagellar axonemes, a direct attachment to cargo has been demonstrated for an axonemal dynein intermediate chain (IC) by chemical cross-linking of the Chlamydomonas IC78 to α-tubulin (King et al., 1991). Alternatively, the dynein IC may act indirectly through dynein light chain subunits to interact with other cellular proteins that modulate dynein function. For example, the light chain LC8 has been shown to bind the mammalian Bim protein, suggesting a role for dynein in the regulation of the apoptosis pathway (Puthalakath et al., 1999). Similarly, the 14-kDa light chain was found to bind rhodopsin in the mammalian eye and may function in turnover of photoreceptor membrane (Tai et al., 1999). Furthermore, in Drosophila, a family of at least five light chains related to the LC7 gene roadblock has been identified; these light chains are proposed to modulate specific dynein functions (Bowman et al., 1999). Because the light chains are thought to interact with the dynein ICs directly (Mitchell and Rosenbaum, 1986; King et al., 1991, 1996, 1998; King and Patel-King, 1995), a role for the IC may be the regulation and assembly of dynein complexes targeted for specific functions.
The dynein IC may also function through interaction with cargo adapters, such as the Glued (dynactin) complex. Dynactin was originally identified by its ability to stimulate dynein-mediated vesicle motility in vitro (Gill et al., 1991) and was subsequently shown to bind to dynein through the IC subunit (Karki and Holzbaur, 1995; Vaughan and Vallee, 1995). Dynactin is a large complex that is composed of eight or more subunits, including p150-Glued, Arp1 (centractin), and p50-dynamitin (reviewed by Holleran et al., 1998). Holleran et al. (1996) demonstrated an association between centractin and a Golgi-specific isoform of spectrin in cells overexpressing the centractin subunit as well as in rat brain cytosol. Together, these data support a model in which dynactin serves as an adapter molecule that binds the IC and links the dynein motor to vesicular cargo.
Previous work has provided evidence for multiple alternatively spliced dynein IC transcripts that may mediate the differential interaction of dynein and cytoplasmic cargoes. In Drosophila, the cytoplasmic dynein IC is encoded by a single X-linked gene that is expressed throughout development (Nurminsky et al., 1998a; this work). Nurminsky et al. (1998a) have provided evidence that the single Cdic (cytoplasmic dynein intermediate chain) gene gives rise to multiple alternatively spliced Cdic transcripts, some of which show a tissue-specific distribution. The reported splice variants differ only within small 5′ exons, and overexpression of Cdic–GFP constructs in transfected cells suggests that some of the variant IC isoforms may be targeted to different locations within the cell (Nurminsky et al., 1998a). Analyses of cytoplasmic dynein ICs in rat, human, and mouse have revealed two homologous genes in each species that display transcript variants resulting from the alternative splicing of 5′ exons (Paschal et al., 1992; Vaughan and Vallee, 1995; Pfister et al., 1996; Crackower et al., 1999). These data suggest that the dynein IC gene, and its variant products, may be important in the subcellular targeting of the dynein motor (Vaughan and Vallee, 1995; Pfister et al., 1996; Nurminsky et al., 1998a). In this report, we identify a triplet of IC polypeptides present in the dynein motor complex and a novel transcript variant. Furthermore, we provide new evidence for an in vivo interaction between the cytoplasmic dynein IC and the p150-Glued component of dynactin. We show that the interaction between p150-Glued and dynein is dependent on the dosage of the IC gene. This result raises the possibility that the IC subunit is a limiting component in the assembly and function of the dynein motor complex that could be subject to regulation by other interacting proteins.
MATERIALS AND METHODS
PCR and 3′ Rapid Amplification of cDNA Ends Analysis
Degenerate oligonucleotide primers were designed based on dynein IC sequences available from rat IC1 (X66845), Chlamydomonas IC70 (X55382), and two human expressed sequence tags (T06737 and T09431): primer 1 (sense): 5′-C-(G/A/T/C)-G-A-(A/G)-T-A-(T/C)-G-T-(G/A/T/C)-T-T-(T/C)-C-A-(T/C)-T-G-3′; primer 2 (antisense): 5′-A-C-(G/A/T/C)-A-C-(A/G)-A-A-(A/G)-T-T-(A/G)-T-T-(G/A/T/C)-A-C-(A/G)-T-C-3′. Primers were synthesized on an Applied Biosystems (Foster City, CA) 392 DNA synthesizer. Amplification reactions (50 μl) contained 2 μl of genomic DNA (∼50 ng), 0.2 mM dNTPs, 0.5 μM of each degenerate primer, buffer (10 mM Tris-HCl, pH 8.8, 50 mM KCl, 1.5 mM MgCl, 0.1% Triton X-100), and 1.5 U of Taq DNA polymerase (Promega, Madison, WI). Reactions underwent 35 cycles of amplification at 50°C for 2 min, 72°C for 3 min, and 95°C for 1 min. Amplification products were analyzed by running 8 μl on an agarose gel and staining with ethidium bromide. The resulting PCR product was gel purified and ligated into pBluescript II KS (Stratagene, La Jolla, CA) after the addition of EcoRI linkers. Plasmids containing inserts of the appropriate size were sequenced with the use of the vector primers.
Analysis of the 3′ end of the Cdic transcript was performed with the use of the 3′ rapid amplification of cDNA ends (RACE) kit (Life Technologies–BRL, Gaithersburg, MD) according to the supplier's instructions. Nested gene-specific primers for amplification of Cdic cDNAs were synthesized (Life Technologies–BRL custom primers): an outer primer: 5′-AAC-TCC-GAC-TAC-GTG-ATG-GAC-G-3′; and an inner primer: 5′-AAG-CTG-TAC-GTG-TAC-GAC-GTG-G-3′. One microgram of total RNA from Drosophila embryos, ovaries, or testes was used for first-strand cDNA synthesis with an oligo(dT) adapter primer. Two microliters of the target cDNA was amplified with the use of the outer gene–specific primer and the universal amplification primer supplied in the 3′ RACE kit. Nested PCR was performed on 1 μl of the primary amplification product with the use of the inner gene–specific primer and the universal amplification primer. Reactions underwent 30 cycles of amplification as described above. Amplification products were analyzed by Southern hybridization with a radiolabeled probe to the 3′ end of the Cdic cDNA. Products of the predicted size were gel purified and cloned into the pGEMT-easy vector (Promega) according to the supplier's instructions.
Isolation of cDNA Clones and DNA Sequencing and Analysis
A λ ZAP cDNA library constructed from Drosophila ovaries by random and oligo(dT) priming (Hazelrigg and Tu, 1994) was screened by hybridization with an IC PCR probe excised from a single subclone by digestion of the plasmid with restriction enzymes. The resulting DNA fragment was gel purified and radiolabeled with [32P]dATP with the use of random hexamers (Pharmacia LKB Biotechnology, Piscataway, NJ). Positive clones were identified after high-stringency hybridization and washing (described below), and plasmids were obtained by in vivo excision according to the supplier's instructions.
Both strands of the ovary cDNA were sequenced by a combination of the dideoxy chain termination method (Sanger et al., 1977) with the use of Sequenase 2.0 polymerase (United States Biochemical, Cleveland, OH) and automated cycle sequencing (Applied Biosystems 377 automated DNA sequencer, Perkin Elmer-Cetus, Norwalk, CT). A series of nested deletions was generated with the use of exonuclease III and S1 nuclease (Henikoff, 1987) and sequenced with the use of plasmid vector primers. Gaps in the sequence were filled in by synthesizing specific primers for sequencing from the full-length cDNA. Sequence was assembled with AssemblyLIGN (Oxford Molecular Group, Madison, WI) and analyzed with the use of GCG Wisconsin Package version 10.0 (Genetics Computer Group, Madison, WI) and MacVector version 6.0 (Oxford Molecular Group). Sequence data are available from EMBL/GenBank/DDBJ under accession number AF26337.
DNA and RNA Blot Analyses
Genomic DNA for Southern blot analysis was prepared as described previously (Rasmusson et al., 1994) from an isogenic Drosophila stock, iso-1 (Tamkun et al., 1991). Seven micrograms of DNA was digested with restriction enzymes, electrophoresed on a 0.8% agarose gel, and transferred to a nylon membrane (Zeta-Probe, Bio-Rad, Richmond, CA). Hybridizations to radiolabeled cDNA probes were carried out by standard methods (Sambrook et al., 1989). Final washes were done at high stringency (0.1× SSC, 0.1% SDS at 65°C). Isolation of total RNA from Oregon-R flies and subsequent blot analysis was done as described previously (Li et al., 1994). Twenty micrograms of total RNA was run on a 1.5% agarose, 9.25% formaldehyde denaturing gel and transferred, probed, and washed as for Southern blotting except that the final wash was done in 0.2× SSC, 0.1% SDS. A probe derived from the RP49 gene (Vaslet et al., 1980) was used to monitor loading and to verify the integrity of the RNA.
Protein Preparation
Microtubule-associated proteins (MAPs) were prepared from 0- to 20-h Drosophila embryos as described previously (Hays et al., 1994). Briefly, 12.5 ml of packed embryos were homogenized on ice in a Dounce homogenizer in 1.5 volumes of PMEG buffer (100 mM piperazine-N,N′-bis[2-ethanesulfonic acid], pH 6.9, 5 mM MgOAc, 5 mM EGTA, 0.1 mM EDTA, 0.5 mM DTT, 0.9 M glycerol) plus protease inhibitors (10 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μg/ml pepstatin, 0.1 μg/ml each of soybean trypsin inhibitor, n-tosyl l-arginine methylester, and benzamidine). A 125,000 × g extract was prepared, from which dynein was enriched by affinity with taxol-stabilized microtubules and released with 10 mM Mg-ATP.
For sucrose density gradient separation of proteins, frozen ATP-release fraction from embryo MAPs was thawed and clarified by centrifugation at 100,000 × g for 30 min at 4°C. MAPs (0.3 mg) in 550 μl volume were sedimented through an 11.2-ml 5–20% sucrose gradient prepared in PMEG buffer with protease inhibitors as described previously (Hays et al., 1994). The gradients were centrifuged at 230,000 × g for 15 h and collected into 0.5-ml fractions. Sedimentation standards were run in parallel on a separate gradient.
Immumoprecipitation experiments were carried out on extracts from hand-dissected ovaries. Homogenization, incubation, and wash steps were in 50 mM HEPES, pH 7.4, 150 mM KCl, 0.9 M glycerol, 0.5 mM DTT, and 0.1% Triton X-100 supplemented with protease inhibitors as described above plus 2 mM PMSF. mAbs to the rat cytoplasmic dynein IC (MAB 1618, Chemicon, Temecula, CA) or the Drosophila dynein heavy chain (P1H4; McGrail and Hays, 1997) were allowed to bind to protein A–Sepharose (Sigma-Aldrich, St. Louis, MO) and then incubated with equal amounts of ovary extract (0.6 mg of total protein in 400 μl) for 3 h at 4°C. Beads were washed three times, the last two in buffer lacking Triton X-100. Each pellet was eluted into 20 μl of SDS-PAGE sample buffer, and the entire volume was loaded onto a gel for blot analysis. Equal volumes of supernatants were analyzed by blot analysis (total protein, ∼25 μg).
Immunoblots and Immunolocalization
SDS-PAGE and immunoblotting were carried out as described by Laemmli (1970) and Towbin et al. (1979). Proteins were electrophoresed on 0.75-mm 7.5% slab gels prepared with a 1:100 ratio of bisacrylamide to total monomer. Gels were electroblotted to polyvinylidene difluoride membranes (Millipore, Burlington, MA). Blots were probed with mAbs to the rat cytoplasmic dynein IC (MAB 1618, Chemicon) or the Drosophila dynein heavy chain (P1H4). Proteins were detected with the use of alkaline phosphatase–linked secondary antibodies with a chemiluminescence detection system (Applied Biosystems), followed in some experiments by color development with the use of nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate substrate.
For immunofluorescence microscopy, ovaries were dissected from 2- to 4-d-old Oregon-R females and fixed and stained as described by McGrail and Hays (1997). The anti-IC mAb 1618 (Chemicon) was diluted 1:50. Egg chambers were examined on a Nikon (Garden City, NY) diaphot microscope with an MRC-600 confocal imaging system (Bio-Rad) with the use of a 60 × 1.4 planapochromat lens.
Construction of a Cdic Genomic Transgene
Cosmids containing genomic DNA from the cytological region 19B-C were provided by the European Drosophila Genome Project (Maduñeo et al., 1995). Two overlapping cosmids, 15E10 and 58G1, were used to assemble the 11-kilobase (kb) Cdic genomic transgene [called P(Cdic+)]. Briefly, a XbaI–SalI subclone of cosmid 58G1 was directionally cloned into the XbaI–XhoI-digested transformation vector pCaSpeR4 (Klemenz et al., 1987). This plasmid was opened by digestion with XbaI, and a 9-kb SpeI–XbaI fragment from cosmid 15E10 was inserted. The orientation of the insert and the integrity of the XbaI junction were verified by sequencing.
Germline transformants were made by microinjection of Drosophila Df (1)w-c embryos with the Cdic19C genomic transgene (60 μg/ml) and helper plasmid p-π 25.7wc (7.5 μg/ml) (Karess and Rubin, 1984) in injection buffer (10 mM Tris, pH 8.0, 1 mM EDTA). Multiple independent transformant lines were recovered and analyzed as described previously (Gepner et al., 1996).
β-Galactosidase Reporter Construction and Expression
An ∼2-kb BamHI–BglII fragment was isolated from a SpeI–XbaI subclone of genomic cosmid 15E10 in pBluescript II KS (Stratagene) and excised with the use of the BamHI site in the multiple cloning region of the vector. This fragment was ligated into the BamHI site of the P-element vector pCaSpeR-βgalAUG (Thummel et al., 1988). The orientation of the insert was determined by restriction mapping and verified by sequencing. Germline transformants of the reporter construct were made and analyzed as for the Cdic genomic transgene described above. β-Galactosidase activity during oogenesis was assayed by the protocol of Cheung et al. (1992) for transformant and control [Df (1)w-c] females as described previously (Li et al., 1994).
Fly Stocks and Genetic Analyses
The Glued mutant stock Glued1 Sb was provided by Dr. Douglas Kankel (Yale University, New Haven, CT) and has been described by Harte and Kankel (1982). The X chromosome–deficiency stock Df (1)mal 3 (breakpoints 19A1-2; 20E1-F) was obtained from the Bloomington Drosophila stock center (Bloomington, IN) and maintained in males with the duplication Dp (1;Y)mal 106 (breakpoints 1A1; B2 and 18F; 20F4). Progeny containing the Glued1 mutation were identified with the use of the dominant genetic marker Sb (Stubble, short blunt bristles), and the presence of the dynein IC transgene was determined with the use of the mini-w+ eye color marker. Progeny of the deficiency crosses were evaluated in females, which are heterozygous for the deficiency and do not carry the Y-linked duplication.
Scanning Electron Microscopy
Drosophila heads were dehydrated in an ethanol series as described by Carthew and Rubin (1990) and prepared for scanning electron microscopy by critical point drying and sputter coating with gold with the use of a Fullam sputter coat device (Ernest F. Fullam, Inc., Schenectady, NY). Images were recorded on type 55 Polaroid film (Technical Imaging Products, Cambridge, MA).
RESULTS
The Drosophila Cytoplasmic Dynein IC Is Present in the Dynein Motor Complex
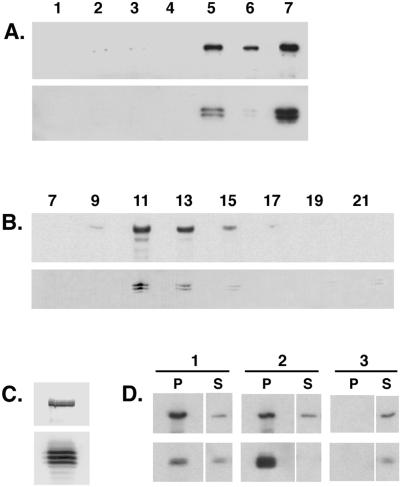
To establish the presence of a dynein IC subunit in the Drosophila dynein complex, a mAb against the rat cytoplasmic dynein IC (Dillman and Pfister, 1994) was used to probe an immunoblot of fractions from a preparation of MAPs from Drosophila embryos (Figure 1A, upper panel). The antibody recognizes a triplet of polypeptides of ∼74 kDa that are enriched in taxol-stabilized microtubule pellets prepared from embryo extracts (Figure 1A, lane 5). Similar to the dynein heavy chain, the IC polypeptides are released from microtubules in the presence of ATP (Figure 1A, lane 7) and migrate on sucrose density gradients as part of a 19S complex (Figure 1, B and C). These data further suggest that the differences between these IC polypeptides do not affect their assembly into the 19S complex. We also performed immunoprecipitation experiments with Drosophila ovary extracts and found that antibodies directed against the dynein heavy chain are able to precipitate dynein IC in a complex with the heavy chain subunit (Figure 1D). In reciprocal experiments, the IC antibody precipitates a complex that contains both the dynein heavy chain and IC polypeptides (Figure 1D). Comparison of the relative amounts of IC and heavy chain subunits in the resultant supernatants and pellets suggests that a free pool of IC and heavy chain subunits may exist in ovary extracts. Alternatively, this may reflect the partial disruption of the complex under the conditions used for our analyses (Figure 1D).
Figure 1.
The Drosophila cytoplasmic dynein IC associates with the dynein heavy chain. (A) The dynein IC is enriched in a standard preparation of MAPs from Drosophila embryos. Equal total protein from each step in the purification was analyzed by Western blotting with the use of an antibody that recognizes the cytoplasmic dynein heavy chain (upper panel) and an antibody that recognizes the dynein IC (lower panel). Lane 1, homogenate; lane 2, low-speed supernatant (57,000 × g); lane 3, high-speed supernatant (125,000 × g); lane 4, microtubule-depleted supernatant; lane 5, microtubule pellet; lane 6, Mg-ATP–extracted microtubule pellet; lane 7, Mg-ATP supernatant. (B) Embryo ATP MAPs were fractionated over a 5–20% sucrose density gradient and collected into 23 aliquots. Equal volumes of alternate fractions were analyzed by Western blotting. Upper panel, anti-dynein heavy chain; lower panel, anti-dynein IC. Fraction numbers are indicated above the appropriate lanes. Sedimentation standards were as follows: 19S, fraction 11; 11S, fraction 17; 2S, fraction 22. (C) The 19S dynein peak fraction from the sucrose gradient shown in B. The IC can be resolved as a triplet of polypeptides by one-dimensional PAGE. Upper panel, anti-dynein heavy chain; lower panel, anti-dynein IC. (D) Antibodies against the dynein heavy chain immunoprecipitate the dynein IC (column 1, P). Similarly, antibodies against the dynein IC immunoprecipitate the dynein heavy chain (column 2, P). No heavy chain or IC polypeptide is precipitated when beads alone are used (column 3). P, pellet; S, supernatant.
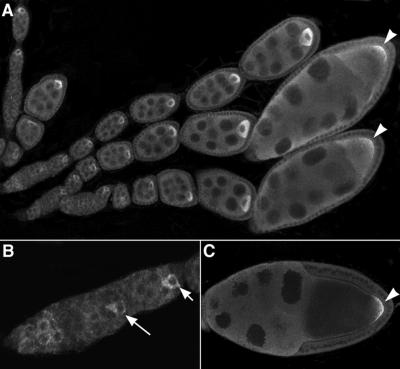
Our previous characterization of the distribution of the dynein heavy chain during Drosophila oogenesis has shown that the heavy chain polypeptide is enriched in the oocyte relative to the nurse cells in early-stage egg chambers and becomes localized to the posterior of the oocyte during later stages of development (Li et al., 1994). As shown in Figure 2, when the IC antibody is used to probe whole-mount preparations of Drosophila ovaries, the dynein IC polypeptide exhibits a distribution similar to that of the heavy chain polypeptide. The IC subunit accumulates in the presumptive oocyte in early egg chambers and at stage 9 becomes concentrated at the posterior of the oocyte. These results are consistent with the biochemical analyses and indicate that the IC is associated with the dynein heavy chain in situ.
Figure 2.
Localization of the dynein IC polypeptide during Drosophila oogenesis. Ovaries dissected from 2-d-old wild-type females were fixed and stained with a mAb to the rat cytoplasmic dynein IC. (A) The dynein IC is concentrated in the oocyte relative to the nurse cells in the developing egg chambers. In stage 9 and 10 egg chambers, the dynein IC is localized to the posterior of the oocyte (A and C, arrowheads). (B) The dynein IC is seen uniformly distributed in regions 1–2a of the germarium. The IC becomes enriched in the presumptive oocyte as early as region 2b in the germarium (large arrow).
The Drosophila Cytoplasmic Dynein IC Is Encoded by a Single-Copy Gene That Encodes Multiple Transcripts
As a first step in the genetic analysis of IC function, we cloned the cytoplasmic dynein IC gene in Drosophila. Degenerate oligonucleotide primers were designed based on dynein IC sequences from rat, Chlamydomonas, and a human expressed sequence tag. Amplification reactions were run with the use of fly genomic DNA as a template, and the predicted 250-base pair (bp) PCR product was recovered from an agarose gel, purified, and subcloned. Sequence analysis of six independent clones revealed the presence of identical 258-bp genomic inserts. The genomic IC PCR fragment was used subsequently to recover clones from a Drosophila ovarian cDNA library. Five overlapping clones were identified and characterized by restriction mapping and partial sequence analysis. One clone of 2.8 kb appeared to be full length and was chosen for further characterization. The coding sequence of the full-length ovary IC cDNA clone predicts a protein of 643 amino acids with a molecular mass of 71 kDa. The 169-bp 5′ untranslated region that lies upstream of the predicted translational start codon contains stop codons in all three reading frames. The predicted amino acid sequence of the Drosophila IC gene product is highly similar to cytoplasmic dynein IC sequences from other organisms and is considerably less similar to axonemal isoforms (Table 1). The sequences of numerous IC cDNA variants in Drosophila have been reported by Nurminsky et al. (1998a). Comparison of the sequence of the ovary cDNA with the sequences of these cDNAs shows it is identical to Cdic isoform 2a, which is constitutively expressed (Nurminsky et al., 1998a; accession number AF070692) and is not specific to the ovary.
Table 1.
Dynein intermediate chain sequence similarities with Drosophila Cdic19C
| Percent identity (% similarity) | Amino acid residues (range, 1–642) | |
|---|---|---|
| Cytoplasmic | ||
| Mouse IC2 | 56 (65) | 2–640 |
| Rat IC2a | 55 (65) | 4–640 |
| Rat IC1 | 51 (60) | 2–640 |
| Human IC1 | 51 (59) | 2–640 |
| Caenorhabditis elegans | 40 (50) | 3–641 |
| Dictyostelium | 43 (50) | 74–639 |
| Yeast | 30 (43) | 361–530 |
| Axonemal | ||
| Chlamydomonas IC78 | 28 (38) | 246–623 |
| Chlamydomonas IC70 | 28 (38) | 233–628 |
| Sea urchin IC2 | 25 (35) | 209–642 |
| Sea urchin IC3 | 25 (36) | 216–623 |
Amino acid sequence comparisons of the Drosophila dynein intermediate chain with both cytoplasmic and axonemal intermediate chains from other species. Sequences were aligned using the GCG program BESTFIT with the use of default parameters. Accession numbers are: Drosophila, AF263371, and Cdic2a, AF070692 (Nurminsky et al., 1998a); rat IC1, X66845 (Paschal et al., 1992); rat IC2a, U39044 (Vaughan and Vallee, 1995); mouse IC2, AF063231; human IC1, AF063228 (Crackower et al., 1999); C. elegans, cosmid CELC17H12, AF045642 (C. elegans Genome Sequencing Consortium, 1998); Dictyostelium, U25116 (Ma et al., 1999); yeast, U16820 (Geiser et al., 1997); Chlamydomonas IC78, U19120 (Wilkerson et al., 1995); Chlamydomonas IC70, X55382 (Mitchell and Kang, 1991); sea urchin IC2, D38538; sea urchin, IC3, D28863 (Ogawa et al., 1995).
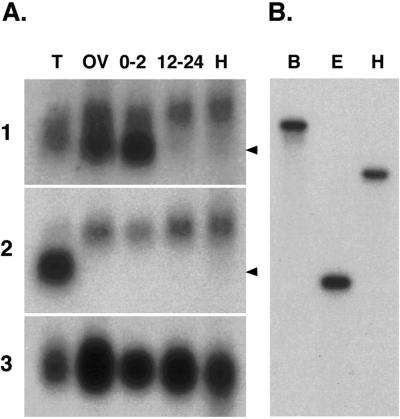
Using probes derived from the Drosophila Cdic cDNA, we identified Cdic transcripts of 2.8 and 2.4 kb that are differentially expressed (Figure 3A). The 2.8-kb transcript is expressed at relatively constant levels in all Drosophila tissues and stages examined and is recognized by overlapping cDNA probes that span the entire length of the 2.8-kb cDNA clone (our unpublished data). Two different 2.4-kb transcripts can be distinguished with the use of cDNA probes from coding and noncoding regions of the cDNA. Detection of one 2.4-kb transcript is limited to ovaries and early embryos, suggesting a maternal pattern of expression. The maternal 2.4-kb transcript is recognized by cDNA probes derived from the 5′ untranslated region (UTR) or the Cdic coding sequence (Figure 3A, panel 1) but is not recognized by a probe that contains only the 3′ UTR sequence of the 2.8-kb cDNA (Figure 3A, panel 2). This result suggests that the shorter 2.4-kb transcript in the ovary and early embryos differs from the 2.8-kb transcript in the length of the 3′ UTR. Consistent with this interpretation, both short and long polyadenylated 3′ cDNA fragments are amplified by 3′ RACE with the use of an oligo(dT) and a gene-specific primer at the 3′ end of the Cdic coding sequence (our unpublished data). The sequence of the shorter 3′ cDNA tail is fully contained within the longer sequence and ends ∼400 bp from the 3′ end of the long sequence. A second 2.4-kb transcript is detected in RNA from testis. The testis-specific transcript can be distinguished from the maternally expressed transcript because it is not recognized by the 5′ cDNA probe. A similar testis-specific dynein IC transcript that lacks the 5′ coding region has been described (Nurminsky et al., 1998a,b). Our results identify additional variation in Cdic transcripts that arises at the 3′ end of the molecule and complement previous demonstrations of alternative splicing of small 5′ exons (Vaughan and Vallee, 1995; Nurminsky et al., 1998a). Although our RNA blot analysis detects only two separate hybridization bands, the broadness of each band is consistent with previous reports of alternative splicing of small exon sequences within the N terminus (Vaughan and Vallee, 1995; Pfister et al., 1996; Nurminsky et al., 1998a).
Figure 3.
The Drosophila dynein IC is represented by a single gene that is expressed throughout development. (A) Northern analysis of Drosophila total RNA shows the presence of two transcripts. Blots of total RNA from various tissues were hybridized to probes derived from the 5′ end (panel 1) and the 3′ UTR (panel 2) of the Cdic cDNA. Transcripts of ∼2.4 and 2.8 kb are shown. T, testes; OV, ovaries; 0–2, 0- to 2-h embryos; 12–24, 12- to 24-h embryos; H, heads. Arrowheads indicate the mobility of the 2.4-kb RNA molecular size standard. Blots were rehybridized with RP49 as a loading control (panel 3). (B) Southern blots of Drosophila genomic DNA digested with different restriction enzymes and hybridized to the 5′ Cdic probe used in the Northern blot analysis. A single band is identified in each lane, showing that the dynein IC is represented by a single gene in the Drosophila genome. B, BamHI; E, EcoRI; H, HindIII.
To detect the presence of additional IC-related genes in the Drosophila genome, we used both Southern analysis and BLAST sequence analysis. Blots of Drosophila genomic DNA were hybridized with a Drosophila IC probe derived from the 5′ region of the cDNA that is divergent between cytoplasmic and axonemal IC sequences (Figure 4). Under both high- and low-stringency hybridization conditions, the 5′ probe identifies a single restriction enzyme fragment on genomic DNA blots (Figure 3B), suggesting the presence of a single cytoplasmic dynein IC gene in Drosophila. In situ hybridization to larval polytene chromosomes was conducted to identify the cytological location of the dynein IC gene. The IC PCR fragment hybridizes to a unique site at the proximal end of the X chromosome in region 19C (our unpublished data). During the course of our analysis, the presence of an annexin–dynein IC tandem repeat present in region 19F was reported (Benevolenskaya et al., 1995), and subsequently Nurminsky et al. (1998a) reported the presence of the adjacent single-copy Cdic gene. Sequence comparisons and a search of the recently completed Drosophila genomic sequence confirm that a single complete IC gene resides at the polytene chromosome region 19C. Our database searches identified three other sequences in the Drosophila genome (Adams et al., 2000) with high similarity to axonemal dynein ICs (Table 2). Two of these sequences, positioned at polytene positions 57B and 34BC, predict proteins that are most closely related to the Chlamydomonas IC78 and IC70, respectively (Mitchell and Kang, 1991; Wilkerson et al., 1995). Furthermore, these genomic sequences contain expressed sequence tags that are similar to the Chlamydomonas IC orthologues, suggesting that these regions are expressed. The third genomic region (62AB) shows the greatest similarity to Chlamydomonas IC78; however, no expressed sequence tag sequences were identified from this region. The completion of the fly genome sequence has revealed additional putative axonemal dynein IC homologues at polytene regions 7D, 61A, 66A, and 68C (Adams et al., 2000; Goldstein and Gunawardena, 2000). This information substantiates the finding that although multiple axonemal dynein IC genes are represented in the genome, the cytoplasmic dynein IC is encoded by a single gene in Drosophila.
Figure 4.
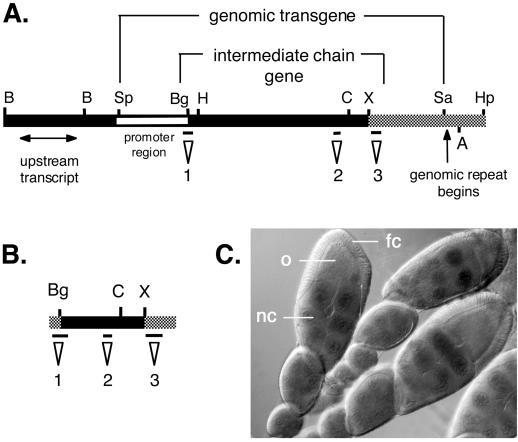
Molecular map of the Cdic genomic region 19C and cDNA. (A) Restriction map of the genomic cosmids used in construction of the Cdic genomic transgene and a diagram of the Cdic transcription unit showing the location of neighboring transcripts. The black bar indicates DNA from cosmid 15E10, and the gray bar indicates DNA from cosmid 58G1. The open bar indicates the region used for promoter fusion to β-galactosidase. The positions of the probes used for blot hybridizations are indicated: probe 1, 5′ cDNA; probe 2, PCR product; probe 3, 3′ UTR. (B) Restriction map of the IC cDNA showing the relative positions of the probes. The gray bar represents the 5′ and 3′ UTRs, and the black bar indicates the coding sequence. Regions 1, 2, and 3 are described in A. (C) Micrograph of ovaries from transformed females expressing the Cdic promoter fusion stained for β-galactosidase. LacZ staining is restricted to the nurse cell nuclei and is not seen in the oocyte or the somatic follicle cells surrounding the egg chamber. nc, nurse cell; o, oocyte; fc, follicle cell. B, BamHI; Sp, SpeI; Bg, BglII; H, HindIII; C, ClaI; X, XbaI; Sa, SalI; A, AatI; Hp, HpaII.
Table 2.
Dynein intermediate chain–related sequences from the Drosophila genomea
| Genomic region | Related IC | Blast probability |
|---|---|---|
| 57B6-13 (DS02397) | Chlamydomonas IC78 | 1.4 × 10−83 |
| Cdic19C | 4.1 × 10−8 | |
| Chlamydomonas IC70 | 3.3 × 10−7 | |
| 34B6-C2 (DS08787) | Chlamydomonas IC70 | 2.8 × 10−51 |
| Cdic19C | 1.3 × 10−6 | |
| 62A10-B5 (DS04848) | Chlamydomonas IC78 | 6.3 × 10−16 |
| Cdic19C | 0.0021 |
BLAST analysis of the Drosophila genome sequence from the Berkley Drosophila Genome Sequencing Project identifies three regions of the Drosophila genome with similarity to axonemal dynein intermediate chains (Chlamydomonas IC70 and IC78) as well as to the Drosophila cytoplasmic dynein intermediate chain gene at region 19C (Cdic19C). Amino acid sequences from the known intermediate chain genes were compared with the Drosophila genomic nucleotide sequence with the use of the program TBLASTN. BLAST probability represents the probability that the sequence similarity identified is due to chance.
The completion of the Drosophila genome sequence has identified additional related sequences that may be present at genomic regions 7D, 61A, 66A, and 68C (Adams et al., 2000; Goldstein and Gunawardena, 2000).
Construction of a Cdic Transgene
To pursue a functional analysis of the dynein IC, a genomic transgene was constructed. Cosmids in the cytological region of the Cdic gene were analyzed by DNA blot hybridization (Figure 4). Using probes derived from the 5′ and 3′ ends of the 2.8-kb Cdic cDNA, we identified two overlapping cosmids, 15E10 and 58G1, that span the complete dynein IC cDNA. These cosmids were further analyzed by a combination of restriction mapping and sequencing of selected subclones. Cosmid clone 15E10 ends less than 1 kb from the 3′ end of the Cdic cDNA. Cosmid 58G1 was determined to contain the full-length dynein IC gene and extends farther 3′ to include one or more copies of a flanking genomic rearrangement between the dynein IC gene and annexin X (Benevolenskaya et al., 1995).
The boundaries of the Cdic19C transcription unit were further defined by hybridizing blots of RNA from various Drosophila tissues with genomic DNA fragments extending 5′ and 3′ from the Cdic19C gene. We identified two transcription units that flank the Cdic19C gene. A 1.4-kb transcript expressed in embryos was identified ∼5 kb upstream of the Cdic19C cDNA (Figure 4A). Southern analysis and sequencing of genomic subclones 3′ to the Cdic19C transcription unit confirmed the presence of a previously identified genomic rearrangement containing tandem repeats of a 3′ fragment of the dynein IC gene fused to a truncation of the Drosophila annexin X gene (Benevolenskaya et al., 1995). The annexin–Cdic gene fusion borders the intact dynein IC gene (Nurminsky et al., 1998a). Genomic subclones from within the dynein IC transcription unit recognized only the 2.8- and 2.4-kb Cdic transcripts (our unpublished data).
An 11-kb genomic transgene containing only the Cdic19C transcription unit was constructed from subclones of cosmids 15E10 and 58G1. The transgene includes 2.0 kb of sequence 5′ to the predicted start of the coding sequence and extends 3′ to the beginning of the annexin–dynein repeat. The transgene was transformed into flies by P-element–mediated germline transformation. Twelve independent transformant lines were recovered that contain single transgene insertions, as determined by genomic DNA blot analysis with the use of probes for the Cdic19C gene. In parallel, 2.0 kb of genomic DNA upstream from the dynein IC gene was cloned separately into the transformation vector pCasPeRβgalAUG and shown to be sufficient for expression of the β-galactosidase reporter gene in transgenic flies (Figure 4C); we conclude that this 2-kb region contains a functional endogenous Cdic promoter.
The Cytoplasmic Dynein IC Interacts with p150-Glued In Vivo
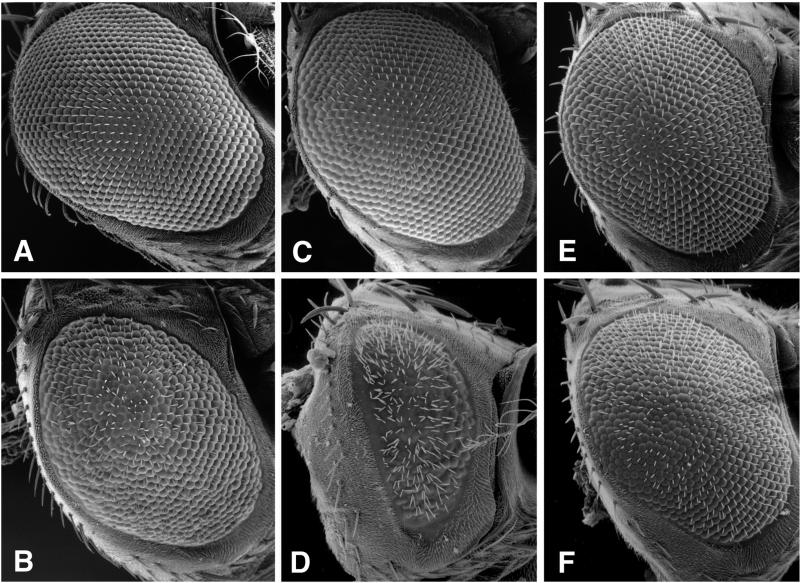
Biochemical experiments have shown a direct interaction between the cytoplasmic dynein IC and the p150-Glued subunit of dynactin (Karki and Holzbaur, 1995; Vaughan and Vallee, 1995). To determine whether this biochemical interaction is functionally relevant in vivo, we asked whether additional copies of the dynein IC could affect the dominant rough eye phenotype of Glued1, a mutation in the p150-Glued subunit of dynactin (Figure 5). The wild-type Drosophila eye is composed of ∼800 ommatidia organized in a highly ordered array (Figure 5A) (for a review of Drosophila eye development, see Dickson and Hafen, 1993). In flies containing the dominant mutation Glued1, the organization of the ommatidia is disrupted, causing a rough-eye phenotype (Figure 5B) (Harte and Kankel, 1982). To cross the Cdic transgene into the Glued1 background, males containing a single copy of the dynein IC transgene, P(Cdic+), were mated to Glued1 mutant virgin females (genotype w/w; Gl1 Sb/TM6B, D, Hu). When this additional copy of the dynein IC gene is introduced, the rough-eye phenotype of Glued1 is suppressed (Figure 5C). The degree of suppression was assessed visually by the extent of disruption of the ommatidial packing and by alteration in eye size. Nine independent P(Cdic+) transformant lines were tested against Glued1, and all suppressed the rough-eye phenotype, although because of position effects the extent of suppression varies. Generally, for an individual line, suppression was stronger in males than in females. Given that the Cdic19C locus is X-linked, this finding suggests that some element of dosage compensation is retained in the transgene. Moreover, we observed the level of suppression to be dependent on the dosage of the P(Cdic+) transgene. The Glued1 rough-eye phenotype was less severe in flies homozygous for the transgene than in flies heterozygous for the transgene or in flies hemizygous (i.e., containing only a single copy) for the endogenous Cdic gene (Figure 5).
Figure 5.
The dynein IC shows a dosage-sensitive interaction with the rough-eye phenotype of Glued1. Scanning electron micrographs of Drosophila eyes. (A) Wild-type eye: +/+. (B) The dominant mutation Glued1: Gl1/+. (C) A dynein IC transgene suppresses the rough-eye phenotype of Glued1: P(Cdic+)/+; Gl1/+. (D) A deficiency of the dynein IC locus dominantly enhances the Glued1 phenotype: Df (1)mal3/+; Gl1/+. (E) A duplication of the Cdic locus suppresses the Glued1 rough eye: Dp (1;Y)mal106/+; Gl1/+. (F) The dynein IC transgene reverses the genetic interaction between the deficiency and Glued1: Df (1)mal3/+; P(Cdic+)/+; Gl1/+ (compare with D).
To further investigate the functional significance of the dosage of the dynein IC gene on the behavior of the Glued1 mutation, we analyzed additional lesions that affect the Cdic gene. An X chromosome deficiency of region 19C [Df (1)mal3] was found to dominantly enhance the rough-eye phenotype of Glued1 mutant flies (Figure 5D). Conversely, a duplication that spans the 19C region [Dp (1;Y)mal 106] acts to suppress the Glued1 rough eye (Figure 5E). To show that the dynein IC gene was removed by the deficiency that interacts genetically with Glued1, we used restriction fragment-length polymorphisms and DNA blot analysis to distinguish the X chromosomes (our unpublished data). Results of the restriction fragment-length polymorphism analysis show that the deficiency that enhances the Glued1 rough eye removes the dynein IC gene. Accordingly, the duplication for region 19C that suppresses Glued1 includes the dynein IC gene.
To demonstrate the specificity of the interaction between Glued1 and the Cdic gene, we show that the Cdic19C transgene, P(Cdic+), acts to reverse the severe rough-eye phenotype seen in flies carrying the Glued1 mutation and the Cdic19C deficiency. Females of the genotype Df/+, P(Cdic+)/+, Glued1/+ (Figure 5F) have a rough-eye phenotype approximately equivalent to that of the Glued1 mutation alone (Figure 5B). This result shows that it is the reduction in dosage of the dynein IC gene and not some other gene under the deficiency that enhances the Glued1 rough eye. Together with the observations that an increased dosage of the Cdic19C gene acts to suppress the Glued1 eye phenotype, our results provide in vivo evidence that the dosage of the dynein IC gene can modulate the level of dynein function in the presence of the Glued1 gene product.
DISCUSSION
Our biochemical analyses of Drosophila cytoplasmic dynein show that the IC is a bona fide subunit in the dynein complex. The IC polypeptide cosediments in the 19S cytoplasmic dynein complex, associates with microtubules in an ATP-sensitive manner, and coimmunoprecipitates with the dynein heavy chain in cytoplasmic extracts. We show that at least three IC polypeptides can be distinguished by one-dimensional SDS-PAGE and that each is present in the 19S dynein complex. These multiple polypeptides are encoded by a single gene that is expressed throughout development. New functional evidence indicates that the interaction between dynein and dynactin is sensitive to the dosage of the dynein IC gene, Cdic19C. Our results extend previous in vitro biochemical studies and raise the possibility that dynein function may be regulated by the level of IC available for interaction with the p150-Glued subunit of dynactin or other adapter complexes.
As reported previously (Vaughan and Vallee, 1995; Nurminsky et al., 1998a), the pairwise comparisons of cytoplasmic IC sequences reveal several conserved domains of potential functional significance. At the N terminus, the first 50 amino acids of the IC polypeptides are predicted to form a coiled-coil structure. A second region of homology (amino acids 70–95) contains a serine-rich region that forms a putative region for phosphorylation (Vaughan and Vallee, 1995; Nurminsky et al., 1998a), and a third N-terminal region (amino acids 121–135; Drosophila sequence) contains a conserved motif of unknown function present only in cytoplasmic ICs (Nurminsky et al., 1998a). Across the C-terminal half of the polypeptide, cytoplasmic IC sequences also display high sequence similarity to axonemal IC sequences. This similarity is proposed to reflect the presence of multiple WD repeats that are characteristic of all known dynein ICs (Ogawa et al., 1995; Wilkerson et al., 1995; Nurminsky et al., 1998a; Yang and Sale, 1998). These observations are consistent with the model that the C-terminal domain in both axonemal and cytoplasmic ICs is important for interactions with the heavy chain subunit, whereas the divergent N-terminal region is important for isoform-specific interactions such as cargo binding (Paschal et al., 1992).
One mechanism that contributes to the divergence of N termini in cytoplasmic dynein IC polypeptides is the alternative splicing of IC transcripts. As suggested previously, such splice variants could provide a diversity in IC isoforms that contributes to the targeting of the dynein motor to specific cargoes and functions (Vaughan and Vallee, 1995; Pfister et al., 1996; Nurminsky et al., 1998a). Indeed, Nurminsky et al. (1998a) report that alternative splicing of small 5′ exons in the single Drosophila IC gene accounts for at least 10 distinct transcripts. Our data do not directly address this source of heterogeneity in IC transcripts, but the relatively broad banding of the IC transcripts on RNA blots is consistent with the presence of additional splice variants. Our analysis of Cdic cDNAs does provide evidence for at least two differentially expressed transcripts. The short, maternally expressed 2.4-kb transcript differs from the 2.8-kb ubiquitously expressed transcript only in the length of the 3′ UTR. Elements of 3′ UTRs are commonly involved in regulatory mechanisms used in early development (Seydoux, 1996; Wickens et al., 1997). How such 3′ UTRs contribute to the regulation of mRNA translation and/or the stability of dynein subunits and how these mechanisms might affect dynein function during development have not been investigated for any dynein subunit. Although the diversity in Cdic transcripts is intriguing as a regulatory mechanism for the functional specialization of cytoplasmic dynein, the physiological significance of these variations remains to be demonstrated.
Our genetic analysis of the dynein IC gene provides previously lacking evidence that the association between the dynein IC and p150-Glued polypeptides is functionally significant in Drosophila. Purified IC and p150-Glued have been shown to interact physically in rat and Xenopus extracts (Karki and Holzbaur, 1995; Vaughan and Vallee, 1995; Steffen et al., 1997) and when overexpressed in Dictyostelium (Ma et al., 1999). However, similar associations between the yeast homologues of IC and p150-Glued were not observed (Kahana et al., 1998). This and previous data showing that the dynein and dynactin complexes are separable by ion exchange chromatography (Schroer and Sheetz, 1991) suggest a low-affinity or regulated interaction between the dynein and dynactin complexes.
The transgenic expression of the Cdic19C transcription unit has shown that the interaction between IC and p150-Glued is sensitive to the dosage of the IC gene. Additional copies of the IC gene can suppress the dominant rough eye of Glued1, and removal of the Cdic19C locus by a deletion can enhance the Glued1 rough-eye phenotype. Previous molecular analysis of Glued1 has shown that the mutation results from the insertion of a transposon in the 3′ end of the Glued gene that creates a premature translation stop codon and the production of a truncated Glued polypeptide (Swaroop et al., 1985, 1987; McGrail et al., 1995). The truncated Glued polypeptide is unable to incorporate into a 20S complex like the wild-type dynactin complex (McGrail et al., 1995), but it retains the region that interacts with the dynein IC (Karki and Holzbaur, 1995; Vaughan and Vallee, 1995; Ma et al., 1999). The dosage-sensitive interaction between the dynein IC and Glued1 suggests that the dominant negative effects of Glued1 are mediated by titrating the level of dynein IC below a threshold required for normal eye development. The association of the dynein IC with a truncated p150-Glued polypeptide would have the effect of “uncoupling” dynein from its cargo. It remains possible that p150-Glued or dynactin may act in one or more pathways unrelated to dynein function. In this case, the truncated Glued polypeptide could “poison” another process, and the associated rough-eye phenotype might not reflect dynein dysfunction. This explanation seems unlikely for two reasons. First, recent analysis of IC truncations in Dictyostelium have shown that similar defects occur when the truncated IC is unable to associate with either the dynein heavy chain or the dynactin complex, suggesting that the association of dynein with dynactin is required for multiple cellular functions (Ma et al., 1999). Additionally, in previous work we have shown that specific alleles of the dynein heavy chain can act to enhance or suppress the Glued1 rough-eye phenotype (McGrail et al., 1995). Although the molecular nature of the lesions in the heavy chain mutants is not established, it is interesting to speculate that the Dhc alleles that act as dominant modifiers of the Glued1 phenotype may do so by altering the association between the dynein heavy chain and IC polypeptides. This prediction is also consistent with the observation that, in contrast to the activity of the Cdic19C transgene, additional copies of a wild-type dynein heavy chain transgene do not suppress the Glued1 phenotype.
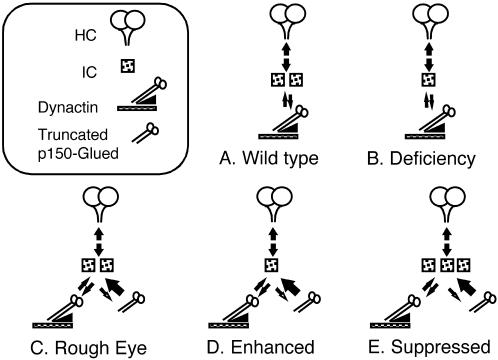
The demonstration of a dosage-sensitive interaction between Cdic and Glued emphasizes the possibility that this interaction is important for regulating dynein function. The Cdic19C locus is not haplo-insufficient, because flies with only a single wild-type copy of the gene exhibit no phenotype. Yet in the presence of two copies of the wild-type Cdic gene, the Glued1 mutant yields a rough-eye phenotype. To account for these observations, we infer that the interaction between the IC and the truncated p150-Glued polypeptide in some way prevents or diminishes the interaction between IC and wild-type p150-Glued. This could occur through the enhanced binding and sequestration of IC by the mutant p150-Glued polypeptide (Figure 6). Consequently, the level of IC subunit available for association with wild-type dynactin complex, or for assembly into the dynein motor complex, would be reduced and dynein function diminished. The increased affinity of a mutant protein for its substrate has been described for the actin-binding protein Sac6p (Sandrock et al., 1997).
Figure 6.
Model for the in vivo interaction between the dynein IC and Glued1. A cartoon showing a model for the dosage-sensitive effect of the dynein IC on the rough-eye phenotype of Glued1. (A) In the wild type, the dynein IC associates with the heavy chain subunit and mediates the interaction with the p150-Glued subunit of dynactin. (B) In the presence of a deficiency that removes one copy of the dynein IC gene, the level of dynein IC is reduced, but it is still present at a level sufficient to support the interaction with dynactin, and no phenotype is detected. (C) In the case of Glued1, association of the truncated p150-Glued subunit with the dynein IC is favored. The truncated Glued polypeptide binds to the dynein IC but is unable to assemble into the dynactin complex or associate with cargo. Because of a limited pool of the IC subunit, the level of dynein capable of transporting cargo is reduced below a threshold, resulting in a rough-eye phenotype. (D) In the presence of the Glued mutation, when the level of dynein IC is reduced by a deficiency, the level of dynein-mediated transport is further reduced, and the rough-eye phenotype is enhanced. (E) With the addition of a dynein IC transgene, the level of dynein able to associate with cargo is increased, and the rough-eye phenotype is suppressed.
In the case of the truncated Glued polypeptide, an enhanced binding to IC could reflect the loss of a regulatory site that modulates the association of the IC and Glued polypeptides. For example, in vertebrates, both the IC subunit and p150-Glued are phosphorylated (Dillman and Pfister, 1994; Pfister et al., 1996; Huang et al., 1999). In our analysis of Drosophila cytoplasmic IC, we observe a triplet of IC polypeptides of ∼74 kDa in ovary and embryo extracts that is consistent with the presence of phosphorylated variants of the cytoplasmic IC. Phosphorylation of the N-terminal serine residues in the p150-Glued polypeptide has been proposed to regulate the interaction of p150-Glued with microtubules or the dynein IC (Waterman-Storer et al., 1995; Farshori and Holzbaur, 1997). In Drosophila, we previously observed the presence of multiple p150-Glued polypeptides produced from a single transcript, suggesting posttranslational modification of the wild-type p150-Glued gene product. In contrast, the mutant Glued1 gene appears to produce a single truncated polypeptide (McGrail et al., 1995). Although the serine-rich cluster is retained within the truncated Glued polypeptide, an altered conformation of the mutant protein could modify the phosphorylation of these sites. Alternatively, C-terminal phosphorylation sites deleted from the truncated Glued polypeptide may be important for the regulation of interactions between the IC and Glued polypeptides. A better understanding of the nature of this interaction may provide insight into whether dynactin represents an adapter complex that couples dynein to specific functions or serves a more universal function in the regulation of dynein-based motility (King and Schroer, 2000).
The presence of multiple IC isoforms in human, mouse, rat, and Drosophila (Vaughan and Vallee, 1995; Pfister et al., 1996; Nurminsky et al., 1998a; Crackower et al. 1999) suggests that the IC may play a role in functional specification of the dynein complex; however, the association of different IC isoforms with distinct binding partners has not yet been demonstrated. With regard to the observed interaction between IC and p150-Glued, Vaughan and Vallee (1995) have provided preliminary evidence that the IC variants, IC-1A and IC-2B, can both bind the p150-Glued subunit of dynactin in vitro. At least in this one case, the domain involved in splicing appears to be separable from the binding of IC to dynactin. The dosage-sensitive interaction between the dynein IC and the p150-Glued subunit of dynactin suggests that the IC subunit may be a limiting component in the coupling of dynein to adapters and cellular cargoes. The observation that the dominant Glued1 phenotype is restricted to the eye indicates that IC levels are spatially and temporally regulated during development. For example, the IC polypeptide may be limiting during eye development such that the truncated Glued polypeptide significantly disrupts dynein function. In contrast, an excess pool of “free” IC may be maternally loaded into embryos and competitively block the effect of the Glued “poison” product in early development. Further genetic and biochemical analyses of the dynein IC may identify additional gene products that regulate the availability of the dynein IC for interaction with such adapters and so regulate dynein function. The genetic and molecular reagents developed in this study of the Drosophila dynein IC subunit will facilitate the investigation of such regulatory mechanisms in a developmental context.
ACKNOWLEDGMENTS
We thank Dr. Stanley Iyadurai for identifying the genetic interaction between the X-linked deficiency and duplication and Glued1 and Dr. Maura McGrail for assistance in immunohistochemistry and preparation of the figure of IC localization in the ovary. We also thank Dr. Mike O'Connor for critical reading of the manuscript. Parts of this work were completed by K.B. in partial fulfillment of the requirements for a Ph.D. (University of Minnesota). This work was supported by grants to T.H. from the National Institutes of Health (GM53695) and the American Heart Association. K.B. was supported in part by a research training grant from the National Science Foundation (DIR-91-11-44).
Abbreviations used:
- IC
intermediate chain
- MAPs
microtubule-associated proteins
REFERENCES
- Adams MD, Celniker SE, Holt RA, et al. The genome sequence of Drosophila melanogaster. Science. 2000;287:2185–2195. doi: 10.1126/science.287.5461.2185. [DOI] [PubMed] [Google Scholar]
- Ashfar K, Barton NR, Hawley RS, Goldstein LSB. DNA binding and meiotic chromosomal localization of the Drosophila Nod kinesin-like protein. Cell. 1995;81:129–138. doi: 10.1016/0092-8674(95)90377-1. [DOI] [PubMed] [Google Scholar]
- Benevolenskaya EV, Nurminsky DI, Gvozdev VA. Structure of the Drosophila melanogaster annexin X gene. DNA Cell Biol. 1995;14:349–357. doi: 10.1089/dna.1995.14.349. [DOI] [PubMed] [Google Scholar]
- Bowman AB, Patel-King RS, Benashski SE, McCaffery JM, Goldstein LSB, King S. Drosophila roadblock and Chlamydomonas LC7: a conserved family of dynein-associated proteins involved in axonal transport, flagellar motility and mitosis. J Cell Biol. 1999;146:165–179. [PMC free article] [PubMed] [Google Scholar]
- Carthew RW, Rubin GM. seven in absentia, a gene required for specification of R7 cell fate in the Drosophila eye. Cell. 1990;63:561–577. doi: 10.1016/0092-8674(90)90452-k. [DOI] [PubMed] [Google Scholar]
- C. elegans Genome Sequencing Consortium. Genome sequence of the nematode C. elegans: a platform for investigating biology. Science. 1998;282:2012–2018. doi: 10.1126/science.282.5396.2012. [DOI] [PubMed] [Google Scholar]
- Cheung HK, Serano TL, Cohen RS. Evidence for a highly selective RNA transport system and its role in establishing the dorsoventral axis of the Drosophila egg. Development. 1992;114:653–661. doi: 10.1242/dev.114.3.653. [DOI] [PubMed] [Google Scholar]
- Crackower MA, Sinasac DS, Xia J, Motoyama J, Prochazka M, Rommens JM, Scherer SW, Tsui L-C. Cloning and characterization of two cytoplasmic dynein intermediate chain genes in mouse and human. Genomics. 1999;55:257–267. doi: 10.1006/geno.1998.5665. [DOI] [PubMed] [Google Scholar]
- Criswell PS, Ostrowski LE, Asai DJ. A novel cytoplasmic dynein heavy chain: expression of DHC 1b in mammalian ciliated epithelial cells. J Cell Sci. 1996;109:1891–1898. doi: 10.1242/jcs.109.7.1891. [DOI] [PubMed] [Google Scholar]
- Dickson B, Hafen E. The genetic dissection of eye development. In: Bate M, Martinez-Arias A, editors. The Development of Drosophila melanogaster. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1993. pp. 1327–1362. [Google Scholar]
- Dillman JF, Pfister KK. Differential phosphorylation in vivo of cytoplasmic dynein associated with anterogradely moving organelles. J Cell Biol. 1994;127:1671–1681. doi: 10.1083/jcb.127.6.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farshori P, Holzbaur ELF. Dynactin phosphorylation is modulated in response to cellular effectors. Biochem Biophys Res Commun. 1997;232:810–816. doi: 10.1006/bbrc.1997.6379. [DOI] [PubMed] [Google Scholar]
- Gepner J, Li M-G, Ludmann S, Kortas C, Boylan K, Iyadurai S J, McGrail M, Hays TS. Cytoplasmic dynein function is essential in Drosophila melanogaster. Genetics. 1996;142:865–878. doi: 10.1093/genetics/142.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiser JR, Schott EJ, Kingsbury TJ, Cole NB, Totis LJ, Bhattacharayya G, He L, Hoyt MA. Saccharomyces cerevisiae genes required in the absence of the CIN8-encoded spindle motor act in functionally diverse mitotic pathways. Mol Biol Cell. 1997;8:1035–1050. doi: 10.1091/mbc.8.6.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill SR, Schroer TA, Szilak I, Steuer ER, Sheetz MP, Cleveland DW. Dynactin, a conserved, ubiquitously expressed component of an activator of vesicle motility mediated by cytoplasmic dynein. J Cell Biol. 1991;115:1639–1650. doi: 10.1083/jcb.115.6.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein LSB. With apologies to Scheherezade: tails of 1001 kinesin motors. Annu Rev Genet. 1993;27:319–351. doi: 10.1146/annurev.ge.27.120193.001535. [DOI] [PubMed] [Google Scholar]
- Goldstein LSB, Gunawardena S. Flying through the Drosophila genome. J Cell Biol. 2000;150:F63–F68. doi: 10.1083/jcb.150.2.f63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harte PJ, Kankel DR. Genetics 101, 477–501. 1982. Genetic analysis of mutations at the Glued locus and interacting loci in Drosophila melanogaster. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays TS, Porter ME, McGrail M, Grissom P, Gosch P, Fuller MT, McIntosh JR. J. Cell Sci. 107, 1557–1569. 1994. A cytoplasmic dynein motor in Drosophila: identification and localization during embryogenesis. [DOI] [PubMed] [Google Scholar]
- Hazelrigg T, Tu C. Sex-specific processing of the Drosophila exuperantia transcript is regulated in male germ cells by the tra-2 gene. Proc Natl Acad Sci USA. 1994;91:10752–10756. doi: 10.1073/pnas.91.22.10752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III in DNA sequence analysis. Methods Enzymol. 1987;155:156–165. doi: 10.1016/0076-6879(87)55014-5. [DOI] [PubMed] [Google Scholar]
- Holleran EA, Karki S, Holzbaur ELF. The role of the dynactin complex in intracellular motility. Int Rev Cytol. 1998;182:69–109. doi: 10.1016/s0074-7696(08)62168-3. [DOI] [PubMed] [Google Scholar]
- Holleran EA, Tokito MK, Karki S, Holzbaur ELF. Centractin (ARP1) associates with spectrin revealing a potential mechanism to link dynactin to intracellular organelles. J Cell Biol. 1996;135:1815–1829. doi: 10.1083/jcb.135.6.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C-YF, Chang C-PB, Huang C-L, Ferrell JE., Jr M phase phosphorylation of cytoplasmic dynein intermediate chain and p150Glued. J Biol Chem. 1999;274:14262–14269. doi: 10.1074/jbc.274.20.14262. [DOI] [PubMed] [Google Scholar]
- Kahana JA, Schlenstedt G, Evanchuk DM, Geiser JR, Hoyt MA, Silver PA. The yeast dynactin complex is involved in partitioning the mitotic spindle between the mother and daughter cells during anaphase B. Mol Biol Cell. 1998;9:1741–1756. doi: 10.1091/mbc.9.7.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karess RE, Rubin GM. Cell 38, 135–146. 1984. Analysis of P transposable element functions in Drosophila. [DOI] [PubMed] [Google Scholar]
- Karki S, Holzbaur ELF. Affinity chromatography demonstrates a direct binding between cytoplasmic dynein and the dynactin complex. J Biol Chem. 1995;270:28806–28811. doi: 10.1074/jbc.270.48.28806. [DOI] [PubMed] [Google Scholar]
- Karki S, Holzbaur ELF. Cytoplasmic dynein and dynactin in cell division and intracellular transport. Curr Opin Cell Biol. 1999;11:45–53. doi: 10.1016/s0955-0674(99)80006-4. [DOI] [PubMed] [Google Scholar]
- King SJ, Schroer TA. Dynactin increases the processivity of the cytoplasmic dynein motor. Nat Cell Biol. 2000;2:20–24. doi: 10.1038/71338. [DOI] [PubMed] [Google Scholar]
- King SM. The dynein microtubule motor. Biochim Biophys Acta. 2000;1496:60–75. doi: 10.1016/s0167-4889(00)00009-4. [DOI] [PubMed] [Google Scholar]
- King SM, Barbarese E, Dillman JF, III, Benashski SE, Do KT, Patel-King RS, Pfister KK. Cytoplasmic dynein contains a family of differentially expressed light chains. Biochemistry. 1998;37:15033–15041. doi: 10.1021/bi9810813. [DOI] [PubMed] [Google Scholar]
- King SM, Barbarese E, Dillman JF, III, Patel-King RS, Carson JH, Pfister KK. Brain cytoplasmic and flagellar outer arm dyneins share a highly conserved Mr 8,000 light chain. J Biol Chem. 1996;271:19358–19366. doi: 10.1074/jbc.271.32.19358. [DOI] [PubMed] [Google Scholar]
- King SM, Patel-King RS. The Mr = 8,000 and 11,000 outer arm dynein light chains from Chlamydomonas flagella have cytoplasmic homologues. J Biol Chem. 1995;270:11445–11452. doi: 10.1074/jbc.270.19.11445. [DOI] [PubMed] [Google Scholar]
- King SM, Wilkerson CG, Witman GB. The Mr 78,000 intermediate chain of Chlamydomonas outer arm dynein interacts with α-tubulin in situ. J Biol Chem. 1991;266:8401–8407. [PubMed] [Google Scholar]
- Klemenz R, Weber U, Gehring WJ. Nucleic Acids Res. 15, 3947–3959. 1987. The white gene as a marker in a new P-element vector for gene transfer in Drosophila. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of the structural protein during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li M-G, McGrail M, Serr M, Hays TS. Drosophila cytoplasmic dynein, a microtubule motor that is asymmetrically localized in the oocyte. J Cell Biol. 1994;126:1475–1494. doi: 10.1083/jcb.126.6.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Traviños-Lagos L, Gräf R, Chisholm RL. J. Cell Biol. 147, 1261–1273. 1999. Dynein intermediate chain mediated dynein-dynactin interaction is required for interphase microtubule organization and centrosome replication and separation in Dictyostelium. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maduñeo E, Papagiannakis G, Rimmington G, et al. A physical map to the X chromosome of Drosophila melanogaster: cosmid contigs and sequence tagged sites. Genetics. 1995;139:1631–1647. doi: 10.1093/genetics/139.4.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrail M, Gepner J, Silvanovich A, Ludmann S, Serr M, Hays TS. Regulation of cytoplasmic dynein function in vivo by the Drosophila Glued complex. J Cell Biol. 1995;131:411–425. doi: 10.1083/jcb.131.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrail M, Hays TS. Development 124, 2409–2419. 1997. The microtubule motor cytoplasmic dynein is required for spindle orientation during germline cell divisions and oocyte differentiation in Drosophila. [DOI] [PubMed] [Google Scholar]
- Mitchell DR, Kang Y. Identification of oda6 as a Chlamydomonas dynein mutant by rescue with the wild-type gene. J Cell Biol. 1991;113:835–842. doi: 10.1083/jcb.113.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DR, Rosenbaum JL. Protein-protein interactions in the 18S ATPase of Chlamydomonas outer dynein arms. Cell Motil Cytoskeleton. 1986;6:510–520. doi: 10.1002/cm.970060510. [DOI] [PubMed] [Google Scholar]
- Moore JD, Endow SA. Kinesin proteins: a phylum of motors for microtubule-based motility. BioEssays. 1996;18:207–209. doi: 10.1002/bies.950180308. [DOI] [PubMed] [Google Scholar]
- Nurminsky DI, Nurminskaya MV, Benevolenskaya EV, Shevelyov YY, Hartl DL, Gvozdev VA. Cytoplasmic dynein intermediate-chain isoforms with different targeting properties created by tissue-specific alternative splicing. Mol Cell Biol. 1998a;18:6816–6825. doi: 10.1128/mcb.18.11.6816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurminsky DI, Nurminskaya MV, DeAguiar D, Hartl DL. Nature 396, 572–575. 1998b. Selective sweep of a newly evolved sperm-specific gene in Drosophila. [DOI] [PubMed] [Google Scholar]
- Ogawa K, Kamiya R, Wilkerson CG, Witman GB. Interspecies conservation of outer arm dynein intermediate chain sequences defines two intermediate chain subclasses. Mol Biol Cell. 1995;6:685–696. doi: 10.1091/mbc.6.6.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschal BM, Mikami A, Pfister KK, Vallee RB. Homology of the 74-kD cytoplasmic dynein subunit with a flagellar dynein polypeptide suggests an intracellular targeting function. J Cell Biol. 1992;118:1133–1143. doi: 10.1083/jcb.118.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Dickert B, Witman GB. The DHC1b (DHC2) isoform of cytoplasmic dynein is required for flagellar assembly. J Cell Biol. 1999;144:473–481. doi: 10.1083/jcb.144.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister KK, Salata MW, Dillmann JF, III, Vaughan KT, Vallee RB, Torre E, Lye RJ. Differential expression and phosphorylation of the 74-kDa intermediate chains of cytoplasmic dynein in cultured neurons and glia. J Biol Chem. 1996;271:1687–1694. doi: 10.1074/jbc.271.3.1687. [DOI] [PubMed] [Google Scholar]
- Porter ME, Bower R, Knott JA, Byrd P, Dentler W. Cytoplasmic dynein heavy chain 1b is required for flagellar assembly in Chlamydomonas. Mol Biol Cell. 1999;10:693–712. doi: 10.1091/mbc.10.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthalakath H, Huang DC, O'Reilly LA, King SM, Strasser A. The proapoptotic activity of the Bcl-2 family member Bim is regulated by interaction with the dynein motor complex. Mol Cell. 1999;3:287–296. doi: 10.1016/s1097-2765(00)80456-6. [DOI] [PubMed] [Google Scholar]
- Rasmusson K, Serr M, Gepner J, Gibbons I, Hays TS. A family of dynein genes in Drosophila melanogaster. Mol Biol Cell. 1994;5:45–55. doi: 10.1091/mbc.5.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- Sandrock TM, O'Dell JL, Adams AE. Allele-specific suppression by formation of new protein-protein interactions in yeast. Genetics. 1997;147:1635–1642. doi: 10.1093/genetics/147.4.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F, Nicklen S, Coulson SR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroer TA, Sheetz MP. Two activators of microtubule-based vesicle transport. J Cell Biol. 1991;115:1309–1318. doi: 10.1083/jcb.115.5.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seydoux G. Mechanisms of translational control in early development. Curr Opin Genet Dev. 1996;6:555–561. doi: 10.1016/s0959-437x(96)80083-9. [DOI] [PubMed] [Google Scholar]
- Steffen W, Karki S, Vaughan KT, Vallee RB, Holzbaur ELF, Weiss DG, Kuznetsov SA. The involvement of the intermediate chain of cytoplasmic dynein in binding the motor complex to membranous organelles of Xenopus oocytes. Mol Biol Cell. 1997;8:2077–2088. doi: 10.1091/mbc.8.10.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaroop A, Paco-Larson ML, Garen A. Molecular genetics of a transposon-induced mutation in the Drosophila locus Glued. Proc Natl Acad Sci USA. 1985;82:1751–1755. doi: 10.1073/pnas.82.6.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaroop A, Swaroop M, Garen A. Sequence analysis of the complete cDNA and encoded polypeptide for the Glued gene of Drosophila melanogaster. Proc Natl Acad Sci USA. 1987;84:6501–6505. doi: 10.1073/pnas.84.18.6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai AW, Chuang J-Z, Bode C, Wolfrum U, Sung C-H. Rhodopsin's carboxy-terminal cytoplasmic tail acts as a membrane receptor for cytoplasmic dynein by binding to the dynein light chain Tctex-1. Cell. 1999;97:877–887. doi: 10.1016/s0092-8674(00)80800-4. [DOI] [PubMed] [Google Scholar]
- Tamkun JW, Kahn R, Kissinger AM, Brizuela BJ, Rulka C, Scott MP, Kennison JA. The arflike gene encodes an essential GTP-binding protein in Drosophila. Proc Natl Acad Sci USA. 1991;88:3120–3124. doi: 10.1073/pnas.88.8.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thummel CS, Boulet AM, Lipshitz HD. Vectors for Drosophila P-element-mediated transformation and tissue culture transfection. Gene. 1988;74:445–456. doi: 10.1016/0378-1119(88)90177-1. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4352. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaisberg EA, Grissom PM, McIntosh JR. Mammalian cells express three distinct dynein heavy chains that are localized to different cytoplasmic organelles. J Cell Biol. 1996;133:831–842. doi: 10.1083/jcb.133.4.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaslet CA, O'Connell P, Izquierdo M, Rosbash M. Isolation and mapping of a cloned ribosomal protein gene of Drosophila melanogaster. Nature. 1980;285:674–676. doi: 10.1038/285674a0. [DOI] [PubMed] [Google Scholar]
- Vaughan KT, Vallee RB. Cytoplasmic dynein binds dynactin through a direct interaction between the intermediate chains and p150Glued. J Cell Biol. 1995;131:1507–1516. doi: 10.1083/jcb.131.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman-Storer CM, Karki S, Holzbaur ELF. The p150Glued component of the dynactin complex binds to both microtubules and the actin-related protein centractin (Arp-1) Proc Natl Acad Sci USA. 1995;92:1634–1638. doi: 10.1073/pnas.92.5.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens M, Anderson P, Jackson RJ. Life and death in the cytoplasm: messages from the 3′ end. Curr Opin Genet Dev. 1997;7:220–232. doi: 10.1016/s0959-437x(97)80132-3. [DOI] [PubMed] [Google Scholar]
- Wilkerson CG, King SM, Koutoulis A, Pazour GJ, Witman GB. The Mr 78,000 intermediate chain of Chlamydomonas outer-arm dynein is a WD-repeat protein. J Cell Biol. 1995;129:169–178. doi: 10.1083/jcb.129.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Sale WS. The Mr 140,000 intermediate chain of Chlamydomonas flagellar inner arm dynein is a WD-repeat protein implicated in dynein arm anchoring. Mol Biol Cell. 1998;9:3335–3349. doi: 10.1091/mbc.9.12.3335. [DOI] [PMC free article] [PubMed] [Google Scholar]