Short abstract
Synucleins are small, soluble proteins expressed primarily in brain tissue, the peripheral nervous system, the retina and certain tumors. Their functions are not clear, although some data suggest a role in the regulation of membrane stability and/or turnover, and α-synuclein protein accumulates abnormally in several neurodegenerative illnesses.
Abstract
Synucleins are small, soluble proteins expressed primarily in neural tissue and in certain tumors. The family includes three known proteins: α-synuclein, β-synuclein, and γ-synuclein. All synucleins have in common a highly conserved α-helical lipid-binding motif with similarity to the class-A2 lipid-binding domains of the exchangeable apolipoproteins. Synuclein family members are not found outside vertebrates, although they have some conserved structural similarity with plant 'late-embryo-abundant' proteins. The α- and β-synuclein proteins are found primarily in brain tissue, where they are seen mainly in presynaptic terminals. The γ-synuclein protein is found primarily in the peripheral nervous system and retina, but its expression in breast tumors is a marker for tumor progression. Normal cellular functions have not been determined for any of the synuclein proteins, although some data suggest a role in the regulation of membrane stability and/or turnover. Mutations in α-synuclein are associated with rare familial cases of early-onset Parkinson's disease, and the protein accumulates abnormally in Parkinson's disease, Alzheimer's disease, and several other neurodegenerative illnesses. The current challenge is to understand the normal cellular function of these proteins and how they might contribute to the development of human disease.
Gene organization and evolutionary history
The synuclein family consists of three distinct genes, α-synuclein, β-synuclein, and γ-synuclein, which have so far been described only in vertebrates. Table 1 catalogs the unique members of the synuclein family that are currently listed in GenBank [1]; these 16 sequences encode the orthologs of each of the three synucleins in the species in which they have been described. The sequences are shown aligned in Figure 1a and their estimated relationships are indicated by the dendrogram in Figure 1b. The α-synuclein gene has been mapped to human chromosome 4q21.3-q22 [2], β-synuclein to human chromosome 5q35 [3], and γ-synuclein to human chromosome 10q23.2-q23.3 [4]. The α-synuclein gene is organized as 7 exons, 5 of which are protein-coding, while the β-synuclein gene has 6 exons (5 protein-coding) and the γ-synuclein gene has 5 exons (all protein-coding) (reviewed in [5]).
Table 1.
Summary of known synuclein family members
| Gene type | Species | Other names | OMIM accession number* | GenBank accession number† |
| α | Human | NACP | 163890 | 586087 |
| α | Rat | SYN1, SYN2, SYN3 (splice variants) | 9507125 | |
| α | Mouse | SYN2 (splice variant) | 6678047 | |
| α | Chicken | 7689260 | ||
| α | Canary | Synelfin | 2501104 | |
| β | Human | 602569 | 4507111 | |
| β | Bovine | PNP14 | 464424 | |
| β | Rat | PNP14 | 2501106 | |
| β | Mouse | 15809030 | ||
| β | Chicken | 7689264 | ||
| γ | Human | BCSG1, persyn | 602998 | 4507113 |
| γ | Rat | Sensory neuron synuclein | 13928954 | |
| γ | Mouse | Persyn | 6755592 | |
| γ | Chicken | Persyn | 7689262 | |
| γ | Bovine | Synoretin | 6942174 | |
| γ | Electric ray | Synuclein | 730882 |
*See OMIM [36]; †see GenBank [1].
Figure 1.
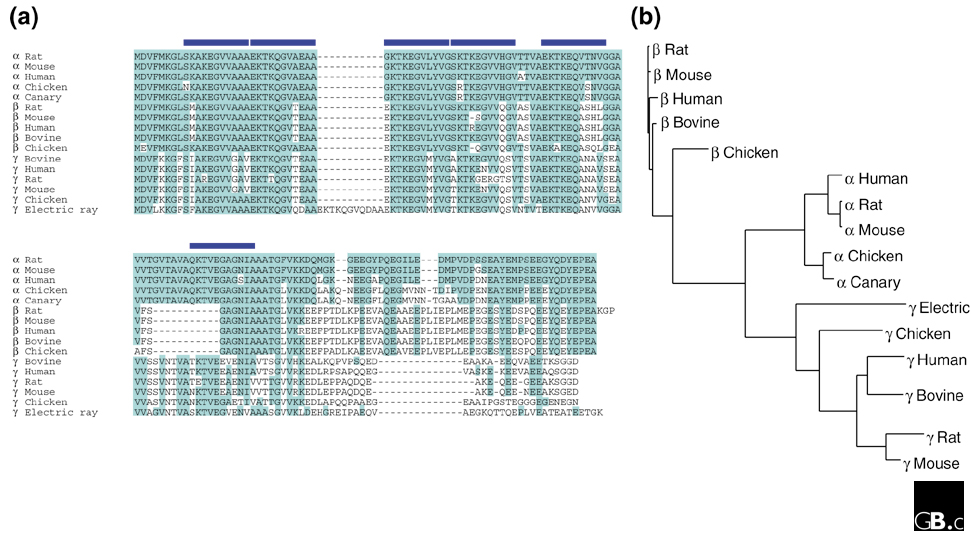
Alignment and relationships of the 16 known synuclein sequences. There are about 80 synuclein sequences in GenBank [1], which can be further sorted into 16 unique groups, each representing a single protein-coding sequence orthologous to one of the three synucleins (summarized in Table 1). (a) The resulting 16 synuclein sequences were aligned with the Multalin program [37]. Shading indicates identity with rat α-synuclein; blue bars represent the 11-residue repeats. (b) A dendrogram of synuclein relationships, generated with ClustalW [38] and displayed using TreeView [39].
Characteristic structural features
All synuclein protein sequences consist of a highly conserved amino-terminal domain that includes a variable number of 11-residue repeats and a less-conserved carboxy-terminal domain that includes a preponderance of acidic residues. The only significant divergences within the repeat domain are the deletion of 11 amino acids (residues 53-63) in all β-synucleins and the addition of a repeat after residue 32 in the γ-synuclein of the electric ray Torpedo californica (Figure 1a). The 11-mer repeats make up a conserved apolipoprotein-like class-A2 helix (Figure 2a,b), which mediates binding to phospholipid vesicles; lipid binding is accompanied by a large shift in protein secondary structure, from around 3% to over 70% α-helix [6].
Figure 2.
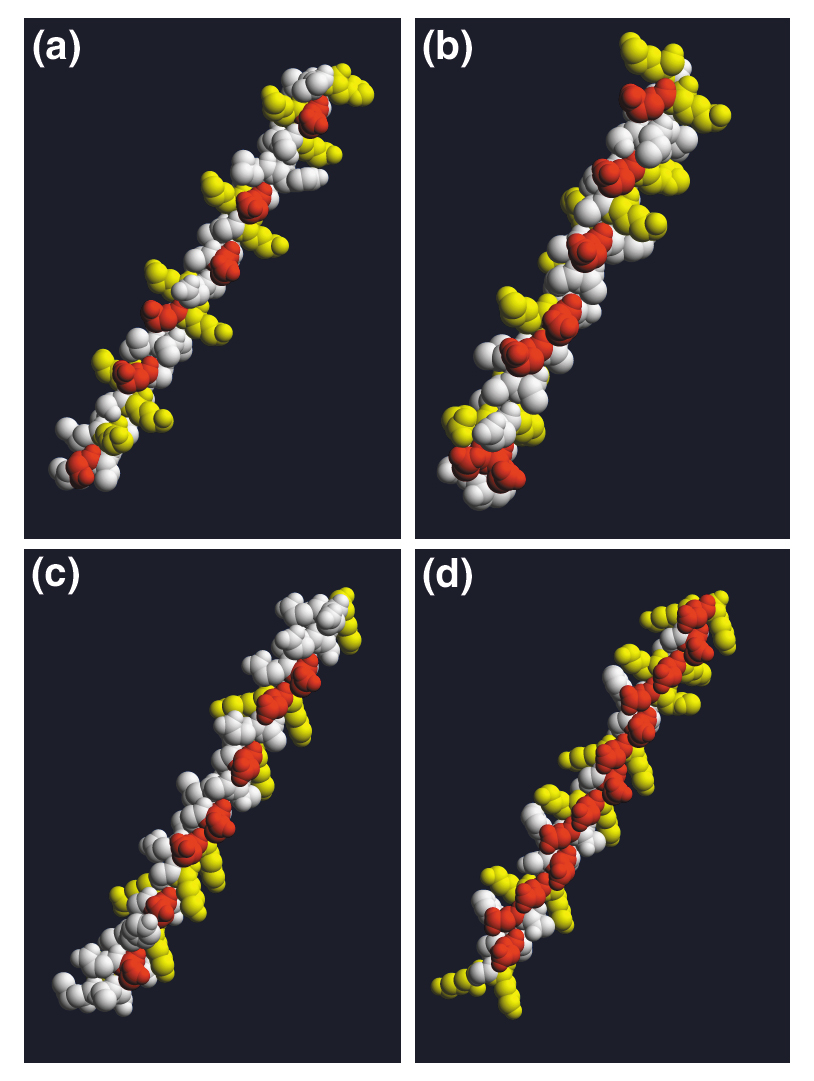
Comparison of the amphipathic α-helical domains of α-synuclein and related proteins. Sequences of interest were imported into Swiss-PdbViewer [40], assigned an ideal α-helical structure, and exported as .pdb files. Models were then formatted and exported with RASMol [41], and animations (available with the complete version of this article, online) were compiled with QuickTime Pro. (a) Human α-synuclein residues 1-50, modeled as an α-helix. The initial frame shown here shows just the hydrophilic face of the helix, with acidic residues confined to the center (red) and basic residues at each interface (yellow); the opposite hydrophobic face (shown in the animation online) contains only uncharged residues (white). (b) Human apolipoprotein AI residues 190-231; (c) Arabidopsis thaliana LEA76 residues 1-50; (d) C. elegans LEA residues 351-400; all are modeled as in (a).
Although no confirmed synuclein orthologs have been identified in non-vertebrates, a low-scoring BLAST 'hit' for similarity is obtained for LEA76, a plant protein belonging to the late embryo-abundant (LEA) group III protein family. Upon further examination, the sequence similarity is attributable to the presence of an 11-residue repeat encoding a similar class-A2 lipid-binding motif (Figure 2c). Like synucleins, LEA group III proteins are relatively unordered in solution; upon fast drying, however, they shift to a largely α-helical conformation [7], and are hypothesized to associate with and stabilize cellular membranes against desiccation stress. A Caenorhabditis elegans LEA homolog has been reported [8] that also shares this structural motif (Figure 2d). Thus, despite the low degree of primary sequence similarity, further scrutiny of the LEA proteins' potential functional relationships to the synucleins is warranted.
Localization and function
The first synuclein was identified in 1988 by Maroteaux et al. [9], who screened an expression library with an antiserum raised against purified cholinergic vesicles from the electric organ of the Pacific electric ray Torpedo californica. This initial cDNA clone (encoding electric-ray γ-synuclein; Table 1) was used to isolate a rat clone encoding a 140-amino-acid protein (rat α-synuclein, Table 1). The product of the β-synuclein gene was first isolated as a bovine brain-specific phosphoprotein (phosphoneuroprotein 14 kDa or PNP14), and its sequence was first described in 1993 [10].
The α- and β-synuclein proteins are predominately expressed in brain, particularly in the neocortex, hippocampus, striatum, thalamus, and cerebellum; protein immunoreactivity is enriched at presynaptic terminals [11,12]. Although their normal physiological functions are unknown, several lines of evidence suggest a role in membrane-associated processes at the presynaptic terminal: α-synuclein is specifically upregulated in a discrete population of presynaptic terminals of the songbird brain during a period of song-acquisition-related synaptic rearrangement [13]; α- and β-synuclein proteins were biochemically purified from bovine brain as constitutive inhibitors of phospholipase D2 [14], an enzyme that catalyzes the hydrolysis of phosphatidylcholine to phosphatidic acid and appears to play a role in cytoskeletal reorganization and/or endocytosis at the plasma membrane [15]; α-synuclein knockout mice have enhanced dopamine release at nigrostriatal terminals in response to paired electrical stimuli, suggesting that α-synuclein is an activity-dependent negative regulator of dopamine neurotransmission [16]; and, finally, depletion of α-synuclein from cultured primary hippocampal neurons by treatment with antisense oligonucleotides results in a decrease in the distal pool of presynaptic vesicles, as visualized by electron microscopy [17].
Mammalian γ-synuclein was first identified as breast cancer-specific gene 1 (BCSG1) in a high-throughput direct differential-cDNA-sequencing screen for markers of breast cancer [18]. The protein is expressed in the peripheral nervous system (in primary sensory neurons, sympathetic neurons, and motor neurons) [18] and is also detected in brain [19], ovarian tumors [20], and in the olfactory epithelium [21]. A sequence dubbed synoretin was independently isolated from ocular tissues in a screen for novel proteins regulating phototransduction and is now thought to represent the bovine ortholog of γ-synuclein [22]. The normal cellular function of γ-synuclein is likewise unknown, but exogenous expression of the protein increases the invasive and metastatic potential of breast tumors [23].
Synucleins in neurodegenerative disease
In 1993, Ueda et al. reported [24] that a short peptide (non-amyloid component, NAC) derived from purified amyloid plaques from the brains of people with Alzheimer's disease was derived from a larger precursor protein, non-amyloid component precursor (NACP), which is now known to be identical to human α-synuclein. Then, in 1997, Polymeropoulos and colleagues reported [25] that a missense mutation (A53T) in α-synuclein was genetically linked to early-onset, familial Parkinson's disease. Description of a second linked mutation (A30P) followed in 1998 [26]. Antibodies to α-synuclein protein were used to show that the protein accumulates in the ubiquitinated protein inclusions and dystrophic neurites (Lewy bodies and Lewy neurites) that are the hallmark pathological features of the disease; this localization was observed even in cases of sporadic Parkinson's disease, which are not associated with α-synuclein mutations [27]. Indeed, α-synuclein is the primary fibrillar component of Lewy bodies, and α-synuclein lesions are also observed in cases of dementia with Lewy bodies and multiple system atrophy, and in some cases of Alzheimer's disease, the parkinsonism-dementia complex of Guam, and Hallervorden-Spatz disease (reviewed in [28]). Although the accumulation of fibrillar α-synuclein in neurodegenerative disease can be interpreted as evidence that the fibrils are themselves toxic, an equally plausible view is that the fibrils are inert or even protective. Conway et al. [29] recently observed that the neurotransmitter dopamine forms adducts with α-synuclein that stabilize a protofibrillar form of the protein (at the expense of fibril formation); the authors proposed that the accumulation of toxic α-synuclein protofibrils could account for the selective loss of dopamine-containing neurons in Parkinson's disease.
The detection of α-synuclein in ubiquitinated inclusions raises the issue of whether α-synuclein is normally targeted for turnover by the ubiquitin-proteasome machinery. Although the evidence for α-synuclein turnover by the proteasome is equivocal [30,31,32], proteasomal inhibitors do not appear to cause accumulation of polyubiquitinated α-synuclein. The α-synuclein binding partner synphilin-1 was, however, recently shown to be ubiquitinated and targeted for proteasomal turnover by parkin, a ubiquitin ligase, mutation of which is itself a risk factor for familial Parkinson's disease. This may provide a common pathological mechanism linking familial mutations in α-synuclein and parkin via their common interactions with synphilin-1 [33].
The β- and γ-synuclein proteins are not found in Lewy bodies, but both are associated with hippocampal axon pathology in Parkinson's disease and dementia with Lewy bodies [34]. A change in the expression of γ-synuclein has also been specifically observed in the retina of patients with Alzheimer's disease [22].
Frontiers
The association of synucleins with human disease has focused a great deal of interest on this protein family. The question of what the synucleins do still remains, however. Most of the experimental evidence available with regard to function is gleaned from experiments with α-synuclein, but the conservation of an extended, class-A2 amphipathic α helix comprising more than half of each of the protein sequences indicates a common biochemical mechanism among all synucleins, probably involving lipid binding. Perrin et al. [35] report irreversible multimerization of α-, β-, and γ-synuclein upon exposure to polyunsaturated phospholipid species, cellular membrane components that are the most susceptible to oxidative damage. This specific interaction might serve to protect these vulnerable lipids from damage or to scavenge damaged lipids and target them for turnover. Paxinou et al. [32] also noted recently that α-synuclein is degraded, at least in part, by the lysosome, suggesting a role for α-synuclein in the autophagocytic clearance of damaged cellular organelles. Thus, it is tempting to speculate that the synucleins help to maintain cellular membrane integrity. But much work remains to be done to elucidate the normal cellular functions of these unusually conserved proteins and to determine how they contribute to diverse disease processes spanning neurodegenerative disease and cancer.
Additional data files
Additional data files available with the online version of this article include animated versions of Figure 2a, Figure 2b, Figure 2c and Figure 2d, which can be viewed with QuickTime Player.
Supplementary Material
Animated version of Figure 2a
Animated version of Figure 2b
Animated version of Figure 2c
Animated version of Figure 2d
Acknowledgments
Acknowledgements
The author is supported by NIH grant 1RO1 AG13762 from the National Institute on Aging.
References
- Searching GenBank http://www.ncbi.nlm.nih.gov/Genbank/GenbankSearch.html An annotated sequence database maintained by the National Institutes of Health.
- Chen X, de Silva HA, Pettenati MJ, Rao PN, St George-Hyslop P, Roses AD, Xia Y, Horsburgh K, Ueda K, Saitoh T. The human NACP/alpha-synuclein gene: chromosome assignment to 4q21.3-q22 and TaqI RFLP analysis. Genomics. 1995;26:425–427. doi: 10.1016/0888-7543(95)80237-g. Chromosomal mapping of the α-synuclein gene. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Divane A, Goedert M. Assignment of human alpha-synuclein (SNCA) and beta-synuclein (SNCB) genes to chromosomes 4q21 and 5q35. Genomics. 1995;27:379–381. doi: 10.1006/geno.1995.1063. Chromosomal mapping of the α- and β-synuclein genes. [DOI] [PubMed] [Google Scholar]
- Ninkina NN, Alimova-Kost MV, Paterson JW, Delaney L, Cohen BB, Imreh S, Gnuchev NV, Davies AM, Buchman VL. Organization, expression and polymorphism of the human persyn gene. Hum Mol Genet. 1998;7:1417–1424. doi: 10.1093/hmg/7.9.1417. Chromosomal mapping of the γ-synuclein gene. [DOI] [PubMed] [Google Scholar]
- Lavedan C. The synuclein family. Genome Res. 1998;8:871–880. doi: 10.1101/gr.8.9.871. A review of the common properties of the synuclein protein family, including gene organization, tissue distribution, and the relationship of protein family members to human disease. [DOI] [PubMed] [Google Scholar]
- Perrin RJ, Woods WS, Clayton DF, George JM. Interaction of human alpha-synuclein and Parkinson's disease variants with phospholipids. Structural analysis using site-directed mutagenesis. J Biol Chem. 2000;275:34393–34398. doi: 10.1074/jbc.M004851200. The α-helical lipid-binding domain of α-synuclein was mapped to exons 3, 4, and 5 using site directed mutagenesis. [DOI] [PubMed] [Google Scholar]
- Wolkers WF, McCready S, Brandt WF, Lindsey GG, Hoekstra FA. Isolation and characterization of a D-7 LEA protein from pollen that stabilizes glasses in vitro. Biochim Biophys Acta. 2001;1544:196–206. doi: 10.1016/S0167-4838(00)00220-X. The authors describe a shift in secondary structure from random coil to α helix by a plant LEA protein upon fast drying of the protein. The shift is thought to contribute to stabilization of the dehydrating cytoplasm. [DOI] [PubMed] [Google Scholar]
- Entrez Nucleotide entry for Caenorhabditis elegans LEA http://www.ncbi.nlm.nih.gov/entrez/viewer.cgi?val=AF016513.1 A C. elegans sequence with homology to plant late embryo abundant (LEA) proteins.
- Maroteaux L, Campanelli JT, Scheller RH. Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J Neurosci. 1988;8:2804–2815. doi: 10.1523/JNEUROSCI.08-08-02804.1988. The first synuclein family member was identified in the Pacific electric ray Torpedo californica. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajo S, Tsukada K, Omata K, Nakamura Y, Nakaya K. A new brain-specific 14-kDa protein is a phosphoprotein. Its complete amino acid sequence and evidence for phosphorylation. Eur J Biochem. 1993;217:1057–1063. doi: 10.1111/j.1432-1033.1993.tb18337.x. The first β-synuclein was directly isolated from bovine brain. [DOI] [PubMed] [Google Scholar]
- Iwai A, Masliah E, Yoshimoto M, Ge N, Flanagan L, de Silva HA, Kittel A, Saitoh T. The precursor protein of non-A beta component of Alzheimer's disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995;14:467–475. doi: 10.1016/0896-6273(95)90302-x. This study maps the distribution of α-synuclein protein in rat brain and demonstrates localization to presynaptic terminals. [DOI] [PubMed] [Google Scholar]
- Nakajo S, Shioda S, Nakai Y, Nakaya K. Localization of phosphoneuroprotein 14 (PNP 14) and its mRNA expression in rat brain determined by immunocytochemistry and in situ hybridization. Brain Res Mol Brain Res. 1994;27:81–86. doi: 10.1016/0169-328x(94)90187-2. The authors map the distribution of β-synuclein protein and mRNA in rat brain. [DOI] [PubMed] [Google Scholar]
- George JM, Jin H, Woods WS, Clayton DF. Characterization of a novel protein regulated during the critical period for song learning in the zebra finch. Neuron. 1995;15:361–372. doi: 10.1016/0896-6273(95)90040-3. Zebra finch α-synuclein protein is transiently expressed in a brain area associated with song acquisition during the critical period for song learning; expression declines concomitantly with song stabilization. [DOI] [PubMed] [Google Scholar]
- Jenco JM, Rawlingson A, Daniels B, Morris AJ. Regulation of phospholipase D2: selective inhibition of mammalian phospholipase D isoenzymes by alpha- and beta-synucleins. Biochemistry. 1998;37:4901–4909. doi: 10.1021/bi972776r. The activity of phospholipase D2 (PLD2) is thought to be constitutively repressed in vivo. Purification of an endogenous inhibitor of PLD2 yielded α- and β-synuclein proteins. [DOI] [PubMed] [Google Scholar]
- Colley WC, Sung TC, Roll R, Jenco J, Hammond SM, Altshuller Y, Bar-Sagi D, Morris AJ, Frohman MA. Phospholipase D2, a distinct phospholipase D isoform with novel regulatory properties that provokes cytoskeletal reorganization. Curr Biol. 1997;7:191–201. doi: 10.1016/s0960-9822(97)70090-3. Summarizes the enzymatic activity and potential physiological roles of PLD2. [DOI] [PubMed] [Google Scholar]
- Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, et al. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. doi: 10.1016/s0896-6273(00)80886-7. Electrophysiological characterization of α-synuclein null mice demonstrates altered dopamine release and a reduction in striatal dopamine. [DOI] [PubMed] [Google Scholar]
- Murphy DD, Rueter SM, Trojanowski JQ, Lee VM. Synucleins are developmentally expressed, and alpha-synuclein regulates the size of the presynaptic vesicular pool in primary hip-pocampal neurons. J Neurosci. 2000;20:3214–3220. doi: 10.1523/JNEUROSCI.20-09-03214.2000. Antisense depletion of α-synuclein from cultured primary hippocampal neurons causes a decline in a discrete population of presynaptic vesicles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Liu YE, Jia T, Wang M, Liu J, Xiao G, Joseph BK, Rosen C, Shi YE. Identification of a breast cancer-specific gene, BCSG1, by direct differential cDNA sequencing. Cancer Res. 1997;57:759–764. The first mammalian member of the γ-synuclein subfamily, breast-cancer-specific gene 1 (BCSG1), was identified in a screen for markers of breast cancer. [PubMed] [Google Scholar]
- Buchman VL, Adu J, Pinon LG, Ninkina NN, Davies AM. Persyn, a member of the synuclein family, has a distinct pattern of expression in the developing nervous system. J Neurosci. 1998;18:9335–9341. doi: 10.1523/JNEUROSCI.18-22-09335.1998. Mapping of the expression of γ-synuclein protein within the brain and peripheral nervous system. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavedan C, Leroy E, Dehejia A, Buchholtz S, Dutra A, Nussbaum RL, Polymeropoulos MH. Identification, localization and characterization of the human gamma-synuclein gene. Hum Genet. 1998;103:106–112. doi: 10.1007/s004390050792. Reports the organization and chromosomal location of the γ-synuclein gene and also maps the tissue distribution of the protein. [DOI] [PubMed] [Google Scholar]
- Duda JE, Shah U, Arnold SE, Lee VM, Trojanowski JQ. The expression of alpha-, beta-, and gamma-synucleins in olfactory mucosa from patients with and without neurodegenerative diseases. Exp Neurol. 1999;160:515–522. doi: 10.1006/exnr.1999.7228. Documentation of the expression of γ-synuclein protein in the olfactory epithelium. [DOI] [PubMed] [Google Scholar]
- Surguchov A, McMahan B, Masliah E, Surgucheva I. Synucleins in ocular tissues. J Neurosci Res. 2001;65:68–77. doi: 10.1002/jnr.1129. Mapping of the distributions of α-, β-, and γ-synuclein proteins in retina and optic nerve. [DOI] [PubMed] [Google Scholar]
- Jia T, Liu YE, Liu J, Shi YE. Stimulation of breast cancer invasion and metastasis by synuclein gamma. Cancer Res. 1999;59:742–747. Demonstrates that overexpression of γ-synuclein in breast-cancer cells increases motility and invasiveness in vitro and metastatic potential in vivo. [PubMed] [Google Scholar]
- Ueda K, Fukushima H, Masliah E, Xia Y, Iwai A, Yoshimoto M, Otero DA, Kondo J, Ihara Y, Saitoh T. Molecular cloning of cDNA encoding an unrecognized component of amyloid in Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:11282–11286. doi: 10.1073/pnas.90.23.11282. A fragment of α-synuclein was isolated from plaque material from patients with Alzheimer's disease. This is the first report of an association of α-synuclein with neurodegenerative disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. Identification of the first α-synuclein mutation linked to familial early-onset Parkinson's disease. [DOI] [PubMed] [Google Scholar]
- Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. Description of the second α-synuclein mutation linked to Parkinson's disease. [DOI] [PubMed] [Google Scholar]
- Irizarry MC, Growdon W, Gomez-Isla T, Newell K, George JM, Clayton DF, Hyman BT. Nigral and cortical Lewy bodies and dystrophic nigral neurites in Parkinson's disease and cortical Lewy body disease contain alpha-synuclein immunoreactivity. J Neuropathol Exp Neurol. 1998;57:334–337. doi: 10.1097/00005072-199804000-00005. Identification of α-synuclein as an excellent immunological marker for Lewy bodies and Lewy neurites. [DOI] [PubMed] [Google Scholar]
- Goedert M. Alpha-synuclein and neurodegenerative diseases. Nat Rev Neurosci. 2001;2:492–501. doi: 10.1038/35081564. A comprehensive review of the relationship between α-synuclein accumulation and human neurodegenerative disease. [DOI] [PubMed] [Google Scholar]
- Conway KA, Rochet JC, Bieganski RM, Lansbury PT. Kinetic stabilization of the α-synuclein protofibril by a dopamine-α-synuclein adduct. Science. 2001;294:1346–1349. doi: 10.1126/science.1063522. A screen for compounds inhibiting α-synuclein fibrilization yielded primarily catecholamines related to dopamine. [DOI] [PubMed] [Google Scholar]
- Bennett MC, Bishop JF, Leng Y, Chock PB, Chase TN, Mouradian MM. Degradation of alpha-synuclein by proteasome. J Biol Chem. 1999;274:33855–33858. doi: 10.1074/jbc.274.48.33855. The authors report that A53T α-synuclein has a longer half-life than wild-type α-synuclein in transfected SY5Y cells. Turnover of both isoforms was blocked by the selective proteasomal inhibitor beta-lactone. [DOI] [PubMed] [Google Scholar]
- Shimura H, Schlossmacher MG, Hattori N, Frosch MP, Trockenbacher A, Schneider R, Mizuno Y, Kosik KS, Selkoe DJ. Ubiquitination of a new form of alpha-synuclein by parkin from human brain: implications for Parkinson's disease. Science. 2001;293:263–269. doi: 10.1126/science.1060627. This paper reports the identification of a novel O-glycosylated form of α-synuclein (alphaSp22), which is a target for ubiquitination by parkin, an E3 ubiquitin ligase implicated in Parkinson's disease. [DOI] [PubMed] [Google Scholar]
- Paxinou E, Chen Q, Weisse M, Giasson BI, Norris EH, Rueter SM, Trojanowski JQ, Lee VM, Ischiropoulos H. Induction of alpha-synuclein aggregation by intracellular nitrative insult. J Neurosci. 2001;21:8053–8061. doi: 10.1523/JNEUROSCI.21-20-08053.2001. The authors conclude that inhibition of the proteasome in HEK-293 cells does not alter the steady-state levels of transfected α-synuclein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung KK, Zhang Y, Lim KL, Tanaka Y, Huang H, Gao J, Ross CA, Dawson VL, Dawson TM. Parkin ubiquitinates the alpha-synuclein-interacting protein, synphilin-1: implications for Lewy-body formation in Parkinson disease. Nat Med. 2001;7:1144–1150. doi: 10.1038/nm1001-1144. Demonstrates ubiquitination of the α-synuclein-associated protein synphilin by the E3 ubiquitin ligase parkin, and shows that mutations in parkin that are linked to Parkinson's disease disrupt its ability to ubiquitinate synphilin. [DOI] [PubMed] [Google Scholar]
- Galvin JE, Uryu K, Lee VM, Trojanowski JQ. Axon pathology in Parkinson's disease and Lewy body dementia hippocampus contains alpha-, beta-, and gamma-synuclein. Proc Natl Acad Sci USA. 1999;96:13450–13455. doi: 10.1073/pnas.96.23.13450. The authors report that β- and γ-synuclein, although not found in Lewy bodies, are associated with hippocampal axon pathology in both Parkinson's disease and dementia with Lewy bodies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin RJ, Woods WS, Clayton DF, George JM. Exposure to long-chain polyunsaturated fatty acids triggers rapid multimerization of synucleins. J Biol Chem. 2001;276:41958–41962. doi: 10.1074/jbc.M105022200. This paper reports rapid and irreversible multimerization of each of the synuclein family members when exposed to very long-chain polyunsaturated fatty acids such as the second-messenger arachidonic acid. [DOI] [PubMed] [Google Scholar]
- Online Mendelian Inheritance in Man (OMIM) http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=OMIM A catalog of human genes and genetic disorders at the National Center for Biotechnology Information.
- Corpet F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 1988;16:10881–10890. doi: 10.1093/nar/16.22.10881. The Multalin program is useful for creating multiple alignments and formatting them for display. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J, Higgins D, Gibson T. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. This program calculates the optimal alignment between multiple sequences; output can be as a multiple alignment or as a phylogenetic tree. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page RD. TreeView: an application to display phylogenetic trees on personal computers. Comput Appl Biosci. 1996;12:357–358. doi: 10.1093/bioinformatics/12.4.357. This application is useful for displaying the output of ClustalW. [DOI] [PubMed] [Google Scholar]
- Guex N, Peitsch MC. SWISS-MODEL and the Swiss-Pdb-Viewer: an environment for comparative protein modeling. Electrophoresis. 1997;18:2714–2723. doi: 10.1002/elps.1150181505. This program can be used to render molecular coordinates into three-dimensional images; it can also be used to assign an idealized secondary structure to a sequence and then generate the corresponding molecular coordinates. [DOI] [PubMed] [Google Scholar]
- Sayle RA, Milner-White EJ. RASMOL: biomolecular graphics for all. Trends Biochem Sci. 1995;20:374. doi: 10.1016/S0968-0004(00)89080-5. This program is useful for rendering three-dimensional images of molecules from their molecular coordinates. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Animated version of Figure 2a
Animated version of Figure 2b
Animated version of Figure 2c
Animated version of Figure 2d


