Abstract
We evaluated the role of COX-2 pathway in 35 head and neck cancers (HNCs) by analyzing COX-2 expression and prostaglandin E2 (PGE2) production in relation to tumor angiogenesis and lymph node metastasis. COX-2 activity was also correlated to vascular endothelial growth factor (VEGF) mRNA and protein expression. COX-2 mRNA and protein expression was higher in tumor samples than in normal mucosa. PGE2 levels were higher in the tumor front zone in comparison with tumor core and normal mucosa (P<.0001). Specimens from patients with lymph node metastasis exhibited higher COX-2 protein expression (P=.0074), PGE2 levels (P=.0011) and microvessel density (P<.0001) than specimens from patients without metastasis. A significant correlation between COX-2 and tumor vascularization (rs=0.450, P=.007) as well as between COX-2 and microvessel density with VEGF expression in tumor tissues was found (rs = 0.450, P=.007; rs=0.620, P=.0001, respectively). The induction of COX-2 mRNA and PGE2 synthesis by EGF and Escherichia coli lipopolysaccharide (LPS) in A-431 and SCC-9 cell lines, resulted in an increase in VEGF mRNA and protein production. Indomethacin and celecoxib reversed the EGF- and LPS-dependent COX-2, VEGF, and PGE2 increases. This study suggests a central role of COX-2 pathway in HNC angiogenesis by modulating VEGF production and indicates that COX-2 inhibitors may be useful in HNC treatment.
Keywords: head and neck cancer, COX-2, VEGF, angiogenesis
Introduction
Increased levels of prostaglandins have been detected in cancers of several anatomic sites, including those of the head and neck, and a role for these metabolites in tumor growth and metastasis has also been postulated [1–4]. Prostaglandins (PGs), particularly those of the E series, have been found to affect cell proliferation and host immune response, thus suggesting a promoting and/or facilitating action on tumor growth and dissemination [5–7].
Prostaglandin endoperoxide synthase 2 or COX-2 is a key enzyme in the production of prostaglandins. This enzyme has been recently reported to be upregulated in various malignancies, such as colon, lung, and breast cancers, and also in squamous cell carcinoma of the head and neck [8–11] and can be modulated by a variety of cytokines, hormones and tumor promoters, including benzo[a]pyrene, a well-known carcinogen involved in head and neck tumorigenesis [12,13].
Various epidemiological studies have revealed that the use of nonsteroidal anti-inflammatory drugs (NSAIDs) inhibiting COX activity can reduce the risk of colon cancer [14,15], and tumor regression has been induced by indomethacin in patients with breast cancer [16] and squamous cell carcinoma of the skin [17,18]. Preliminary results suggest that aspirin and indomethacin might have an analogous effect in delaying the growth of experimental head and neck tumors [19,20] as well as head and neck tumors in vivo [21].
The mechanism(s) by which NSAIDs inhibit tumor growth is not clearly understood, but it could involve blockage of COXs, which suppress eicosanoid production, especially PGs and might affect cell proliferation, apoptosis and immune response [8–10,22]. Recent evidence in colon cancer cells suggests that excessive PG production due to COX-2 overexpression plays a role in tumor growth and spread [23–25] also because of its ability to regulate angiogenesis by modulating production of several angiogenic factors including vascular endothelial growth factor (VEGF) [26].
Accordingly, we and others [2–4,11,27] reported that the levels of COX-2 products in head and neck cancer (HNC) are increased and seem to correlate with tumor stage and risk of lymph node metastasis; however, whether the increased PG production in tumors other than colon cancer is also due to enhanced COX-2 expression with potential effects on tumor angiogenesis needs to be determined.
Our present study focuses on the role of COX-2 and PGE2 expression in a series of 35 consecutive HNC patients. These results are then correlated with tumor angiogenesis (assessed by microvessel count) and with the expression of VEGF, a potent angiogenic factor. The potential role of COX-2 activity in controlling tumor angiogenesis possibly through VEGF regulation was also investigated by analyzing VEGF gene and protein expression in A-431 and SCC-9 epidermoid tumor cell lines.
Materials and Methods
Patients and Tissue Collection
We studied 35 consecutive HNC patients who underwent surgical treatment of the primary tumor and of the neck at the Institute of Otolaryngology Head and Neck Surgery, University of Florence, during the period from April 1998 through April 1999. Clinical, epidemiological, and histopathologic characteristics of these patients are shown in Table 1. All tumors were histologically confirmed to be squamous cell carcinomas and were graded as well-differentiated, moderately differentiated, and poorly differentiated. Among 35 cases, 16 (45.6%) had histologically confirmed lymph node metastasis (N+), whereas the remaining 17 (54.4%) patients had no clinical and histopathologic evidence of neck disease (N-).
Table 1.
Clinical, Epidemiological and Histologic Characteristics of 35 Head and Neck Cancer Patients
| Characteristics | No. (%) | ||
| Total no. of patients | 35 (100) | ||
| Age, yr | |||
| Mean 62.3 | |||
| Range 50–74 | |||
| Sex | Male | 31 (88.6) | |
| Female | 4 (11.4) | ||
| Tumor stage | |||
| I | 9 (25.7) | ||
| II | 11 (31.4) | ||
| III | 9 (25.7) | ||
| IV | 6 (17.2) | ||
| Tumor site | |||
| Larynx | 12 (34.3) | ||
| Oral cavity | 13 (37.1) | ||
| Oropharynx | 10 (28.6) | ||
| Tumor degree of differentiation | |||
| Well differentiated | 12 (34.3) | ||
| Moderately differentiated | 16 (45.7) | ||
| Poorly differentieted | 7 (20.0) | ||
| Lymph node metastasis | |||
| Yes | 16 (45.7) | ||
| No | 19 (54.3) | ||
Samples of the tumor core, the invasive edge of the tumor (tumor front), and microscopically healthy mucosa (control) were obtained from each surgical specimen. The portion of primary tumor was obtained by superficial biopsy of either the tumor bulk or the edge of the malignant ulcer for more infiltrative cancers. Samples of tissue identified as tumor edge were taken at the periphery of the tumor, whereas samples of the control mucosa were obtained from a macroscopically uninvolved area 2–5 cm away from the tumor. Samples of the tumor core were also obtained for the histopatologic and immunohistochemical assessment of each tumor.
Immunohistochemistry, Microvessel Density and Assessment of the Stains
Immunohistochemical studies were performed by the streptavidin-biotin technique (Dako, Milano, Italy) with diaminobenzidine as chromogen and hematoxylin as counterstain. To identify microvessels in formalin-fixed, paraffin-embedded tissue sections, we used a monoclonal antibody against CD31 (JC/70; Dako) diluted 1:20 in PBS. Incubation with the primary antibody was carried out for 30 minutes, after digestion of the tissue sections with protease XIV (Sigma, St. Louis, MO) for 3 minutes at 37°C. For COX-2 identification we employed a goat polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) at 1:30 dilution, with incubation lasting for 1 hour at 20°C. VEGF immunostaining was carried out using a monoclonal antibody (Neomarkers, Freemont, CA) at 1:30 dilution, following a microwave antigen retrieval protocol employing 10 mM Tris-HCI buffer (pH 10) and heating for 30 minutes at 300 W output. Negative controls were performed by substituting the primary antibody with nonimmune goat or mouse serum.
A semiquantitative system was employed to evaluate the level of COX-2 and VEGF expression: immunoreactivity was scored as either absent (-), low (+ , less than 20% of positive tumor cells), moderate (+ + , 20–50% of positive tumor cells), or diffuse (+ + + , more than 50% of positive tumor cells).
Microvessel density was determined according to the method described by Weidner et al. [28]. In the areas with the highest vascular density, identified at 40x magnification, single endothelial cells or groups of endothelial cells, with or without identifiable lumen, were counted at 200x magnification (0.73 mm2 per field), and results were expressed as the highest number of microvessels identified within a microscopic field.
Cell Line and Culture Conditions
Human epidermoid carcinoma A-431 cells were obtained from the American Type Culture Collection (Rockville, MD). Human laryngeal squamous cell carcinoma (SCC-9) cells were a kind gift of Prof. W. Issing (HNO Clinic, Munich, Germany). A-431 were maintained in Dulbecco's modified Eagle's medium (DMEM) (Biowhittaker, Belgium), supplemented with 10% fetal calf serum (FCS) (Hyclone Laboratories, Logan, UT). SCC-9 were maintained in RPMI/Ham's F12 (1:1) (Biowhittaker) supplemented with 10% FCS and 0.4 µg/ml hydrocortisone (Sigma).
A-431 cells are known to produce VEGF [26]; SCC-9 cells, tested in this laboratory for VEGF production, were found to behave in a similar way to A-431.
Western Blot Analysis of COX-2
A-431 and SCC-9 cells were seeded on a 6-cm Petri dish. When subconfluent, A-431 cells were washed twice with culture medium and incubated in 2 ml of DMEM without FCS for 24 hours. The medium was then removed and cells were incubated in 1.5 ml of DMEM without FCS for a further 3 hours. Following two washes with culture medium, subconfluent SCC-9 cells were subjected only to 3-hour serum starvation. Inhibitors (10 µM indomethacin, 10 µM celecoxib, 10 µM cycloheximide) were added 30 minutes before 100 ng/ml EGF and lipopolysaccharide (LPS) administration. After 24 hours of incubation, medium was collected for VEGF and PgE2 analysis, while cell layers were washed twice with phosphate-buffered saline (PBS), and collected by scraping. Cell pellets were resuspended in 50 µl of lysis buffer (1x PBS, 1% NP40, 0.5% sodium deoxycholate, 0.1% SDS, 10 mg/ml phenylmethylsulfonylfluoride, 10 µg/ml aprotinin and 1 mM sodium orthovanadate). Protein content of each sample was evaluated by the Bradford Coomassie assay reagent (Pierce, Rockford, IL). One hundred micrograms of each clarified cell lysate was denatured and fractionated by 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE). The proteins were transferred to nitrocellulose filters after electrophoresis, and the filters were probed with an antihuman COX-2 antibody (Santa Cruz Biotechnology) and developed by the enhanced chemiluminescence system (Amersham, Arlington Heights, IL).
For loading and transferring control, the same membrane used for anti-COX-2 immunoblot was stripped in stripping buffer (2% SDS, 50 mM Tris-HCI pH 6.5, 100 mM 2-mercaptoethanol, at 50°C for 30 minutes.) and reprobed with an anti-human actin polyclonal antibody (Santa Cruz Biotechnology).
Western Blot Analysis of VEGF
Incubation medium obtained from equal amounts of A-431 and SCC-9 cells as described for Western blot analysis of COX-2, was centrifuged to remove cell debris. Heparin-binding proteins were precipitated from the supernatant with 40 µl of heparin-sepharose (1:1 slurry, Pharmacia, Uppsala, Sweden) overnight at 4°C. Heparin-sepharose bound proteins were washed twice with the following buffer (20 mM Tris-HCI, pH 7.5, 300 mM NaCl, 200 µM phenylmethylsulfonyl fluride (PMSF), 1 mM dithiothreitol (DTT) and 1 µM leupeptin), extracted by boiling in 50 µl of Laemmli's sample buffer and separated on a 13% SDS-PAGE gel. After transfer to nitrocellulose filters, proteins were probed with an anti-human VEGF antibody (Santa Cruz Biotechnology) and the specific signal was revealed by the enhanced chemiluminescence system (Amersham).
The anti-VEGF immunoblot shows different positive bands. Accordingly, an altered and/or preferential VEGF isoform production is described for different tumor cell lines [29,30].
Northern Analysis of COX-2 and VEGF mRNA Expression
Total RNA was extracted by the guanidinium isothiocyanate method [31]. 20 µg of samples were separated by electrophoresis on 1% agarose gel containing 2.2 M formaldehyde and transferred to a nylon membrane (Hybond-N, Amersham). After 2 hours at 80°C, the resulting filters were prehybridized for 1–2 hours at 42°C in hybridization buffer containing 50% deionized formamide, 5x Denhardt's solution, 1x SSPE, 100 µ/ml denatured salmon sperm DNA for 18–24 hours at 42°C and washed essentially according to the manufacturer's instructions. As COX-2 and VEGF165 cDNA probes we used PCR product fragments obtained by amplification using the following primers: COX-2 FW: 5′-TCACAGGCTTCCATTGACCAG-3′, which gave rise to 644bp product; COX-2 RV: 5′-CGCAACAGGAGTACTGACTTC-3′; VEGF FW: 5′ CCGGTCGGGCCTCCGAAACCATGAACTTTCT-3′;VEGF RV: 5′-TCACCGCCTCGGCTTGTCACATCTGCAAGT-3′, which gave rise to a 590-bp product, which was then purified by QIAquick Gel Extraction kit (Qiagen). Each probe was labeled with 32PdCTP, using random priming reaction (Boehringer Mannheim, Mannheim, Germany).
We used the GAPDH probe obtained by a PCR using the primers: GAPDH FW: 5′-CCATGGAGAAGGCTGGGG-3′; GAPDH RV: 5′-CAAAGTTGTCATGGATGACC-3′ (196-bp PCR product) as an internal control to adjust for differences in the amount of RNA loaded in each lane. Then filters were exposed for autoradiography to Kodak Xar-5 film (Eastman Kodak, Rochester, NY) for 1 to 6 days at -80°C. The level of expression in cell line samples was assessed using GS-670 Imaging Densitometer (Bio-Rad, Hercules, CA). According to Pertschuk et al. [32], we considered the densitometric percentage of the autoradiographic signal. To exclude possible densitometric signal errors due to background and/or other specific phenomena, the intensity of mRNA expression was compared to that of a control gene (GAPDH).
PgE2 Measurement
Tissue fragments (normal control mucosa, tumor core and tumor edge sample tissues) were homogenized at 0–4°C in the presence of 10 µM indomethacin to prevent prostaglandin production during the procedure and then centrifuged at 600xg and the supernatants utilized for PgE2 determination. Five hundred microliters of supernatants of A-431 and SCC-9 cells, prepared as described for Western blot analysis of COX-2, was used for PgE2 determination by a specific radioimmunoassay [33]. Protein concentration in tumor samples and in the cells were determined according to standard procedures. Bovine serum albumin was used as the standard and the values of PgE2 were expressed as picograms per microgram (pg/µg) protein in the cells and micrograms per milligram (µg/mg) protein in the tissue samples.
Statistical Analysis
Statistical analysis was performed using the Stata Statistic Software (release 5.0; Stata Corporation, College Station, TX) and Number Cruncher Statistical System (version 5.03; J.L. Hintze, Kaysville, UT).
Data are reported as median and range for PGE2 values and microvessel density. Within different tissue samples, PGE2 levels were compared by using a paired-value Wilcoxon test. The Wilcoxon rank sum test for unpaired data was used to assess the differences between COX-2 and VEGF immunoreactivity in different tissues as well as for microvessel according to lymph node status.
The relationship between microvessel count and COX-2 and VEGF expression, and PgE2 levels are reported as Spearman product-moment correlation coefficients (rs). Moreover, differences between COX-2 and VEGF protein expression according to tumor stage and tumor grade were analyzed by using the Kruskal-Wallis test. A multivariate regression analysis was performed to explore the effects of several possible prognostic factors (age, sex, tumor grade, tumor stage, tumor site, COX-2 immunoreactivity, VEGF expression, and PgE2 levels) in predicting the extent of tumor vascularization.
All P values resulted from the use of two-sided statistical tests. P values less than 0.05 were considered to indicate statistically significant differences.
Results
COX-2 mRNA and Protein Expression in HNC: Correlation with Tumor Angiogenesis
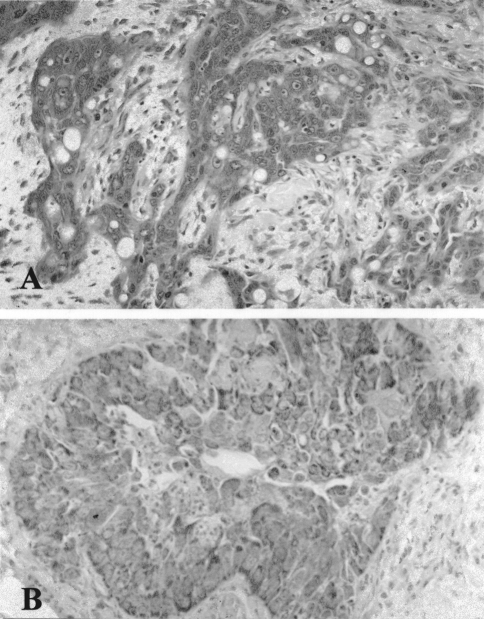
Immunohistochemical staining of formalin-fixed paraffin embedded tissue sections from 35 squamous cell carcinomas revealed that COX-2 was primarily localized in tumor cells, as well as in tumor-infiltrating inflammatory cells, endothelial cells, smooth muscle cells of blood vessel wall and striated muscle. Overall, 30 of 35 (86%) squamous cell carcinomas showed cytoplasmic immunoreactivity for COX-2 in neoplastic cells, 18 (51%) of which showed a moderate or diffuse immunostaining (Figure 1A). Tumors from patients with histologically confirmed cervical lymph node metastasis showed a significantly higher COX-2 immunoreactivity when compared with those without neck involvement (P=.0074). Moreover, COX-2 expression tended to be higher with increasing tumor stage and loss of differentiation (Kruskal-Wallis test, P=.01 and P=.06, respectively).
Figure 1.
Diffuse COX-2 (A) and VEGF (B) protein expression by neoplastic cells in a moderately differentiated squamous cell carcinoma of the oral cavity.
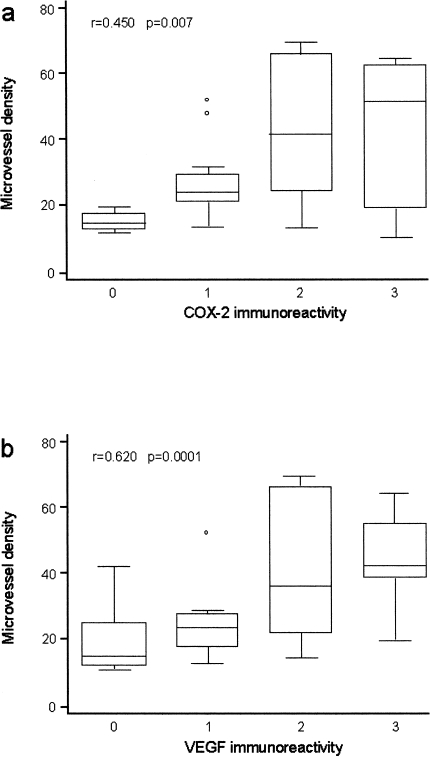
Microvessel count in cancers from different patients and also within different areas of the same carcinoma were heterogeneous, being higher at the invasive tumor edge. Microvessel count varied from 11 to 70 (median: 25; mean: 32; SD: 18.05). Microvessel density was significantly correlated with tumor grade, stage (Kruskal-Wallis test, P=.04 and P=.006, respectively), and with the presence of lymph node metastasis (unpaired value Wilcoxon test, P=.0001). When we correlated COX-2 protein expression and the extent of tumor vascularization, we observed the presence of increased angiogenesis in those cancers where COX-2 protein was overexpressed (>20% of tumor cells; rs=0.450, P=.007) (Figure 2A).
Figure 2.
Correlation between tumor angiogenesis (CD31 immunoreactivity, microvessel density) and COX-2 immunoreactivity (a) and VEGF immunoreactivity (b) in 19 N- and 16 N+ HNC patients.
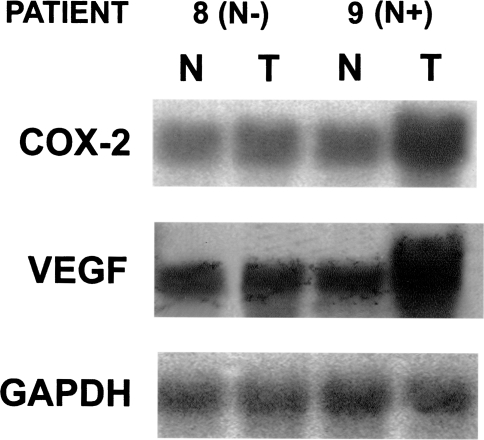
Similar results were obtained by Northern blot analysis of COX-2 mRNA expression in tissue samples from 12 HNC patients, including tumor tissues and normal control mucosa samples. Substantially higher levels of COX-2 mRNA were detected in cancer tissue in comparison with matched normal mucosa as well as in tumor tissues from patients with lymph node metastasis in comparison with HNC patients without neck involvement (Figure 3).
Figure 3.
Northern analysis of COX-2 and VEGF mRNA expression in two representative HNC patients (patient no. 8, N-; patient no. 9, N+).
PgE2 Production in Tumor Tissues and Matched Normal Tissues
The levels of PgE2 production were measured in the supernatants of tissue homogenates from the tumor core, the invasive edge of the tumor (tumor front) and normal mucosa in 35 patients who underwent surgery for HNC. In specimens from unaffected control mucosa, we detected a median baseline production of PgE2 of 1.04 (range, 0.42–3.70) µg/mg protein. In tumor tissue, PgE2 levels were elevated to 2.09 (range, 0.98–4.97) and 3.54 (range, 1.42–6.94) µg/mg protein in tumor core and tumor front area, respectively. These values were significantly higher than those from matched control mucosa (paired-value Wilcoxon test, P<.0001). Therefore, as previously reported [1,2], we found that the extent of PgE2 biosynthesis was significantly different according to the sampling site within the tumor mass. In fact the highest PgE2 levels were found in the supernatants of the specimens obtained from the invasive edge of the tumor.
We then analyzed PgE2 levels in tumor specimens from patients in the cohort according to tumor stage and metastasis. The levels of PgE2 from N+ patients were higher in supernatants from the specimens sampled at the periphery of the tumor when compared with those from patients without neck metastasis (unpaired value Wilcoxon test, P=.0011). Accordingly, specimens from the tumor front area had significantly higher PgE2 when disease was more advanced (Stage III–IV) than in early stage lesions (Stage I–II) (Kruskal-Wallis test, P=.019), as did poorly differentiated carcinomas in comparison with well-differentiated carcinomas (Kruskal-Wallis test, P=.04).
VEGF mRNA and Protein Expression in HNC: Correlation with Tumor Angiogenesis
Of the 35 carcinomas, 28 (80%) were positive for VEGF and the immunostaining was scored as moderate (20–50 of neoplastic cells) or diffuse (>50% of neoplastic cells) in 17 (48.6%) cases (Figure 1B). Immunoreactivity was also evidenced in tumor infiltrating inflammatory cells, endothelial cells and smooth muscle cells of vascular walls. Tissue samples from more advanced HNC (N +) patients showed a higher expression of VEGF protein in comparison with those from HNC patients without lymph node involvement (N-) (P=.014). Moreover, there was a close correlation between VEGF protein expression and microvessel density in our series (rs=0.619, P=.0001) (Figure 2B).
Correlation of Microvessel Density, VEGF Expression, COX-2 Expression, PgE2 Biosynthesis, Lymph Node Status, Tumor Grade, and Tumor Stage: Multivariate Analysis
According to our previous observations [31], we confirm that HNC in patients with lymph node metastasis (N+) showed a higher tumor vascularization when compared with tumor tissues from HNC patients without neck disease (N-) (P=.0001).
The analysis of VEGF expression by immunohistochemistry revealed that VEGF was clearly correlated with tumor angiogenesis in our series of HNC patients. An analogous result was found when we correlated COX-2 protein expression and the extent of tumor vascularization. Therefore, we correlated COX-2 expression and PGE2 biosynthesis with VEGF expression, showing that increased COX-2 pathway activity was associated with increased VEGF expression (rs=0.437, P=.009 and rs=0.498, P=.010, respectively), thus suggesting a possible regulation of VEGF expression by COX-2 pathway.
Overall, increased COX-2 expression and activity, particularly at the tumor front area, and VEGF expression were significantly correlated with tumor vascularization. Multivariate stepwise regression analysis confirmed that PgE2 levels in the supernatants of tumor core and tumor edge homogenates, and VEGF expression were the most important and independent predictors of tumor vascularization in our series (VEGF immunoreactivity: P=.001; PgE2 at the tumor core: P=.022; PgE2 at the tumor edge: P=.002).
VEGF mRNA and Protein Expression Is Correlated with COX-2 Activity in Squamous Cell Carcinoma Tumor Cell Lines (A-431 and SCC-9)
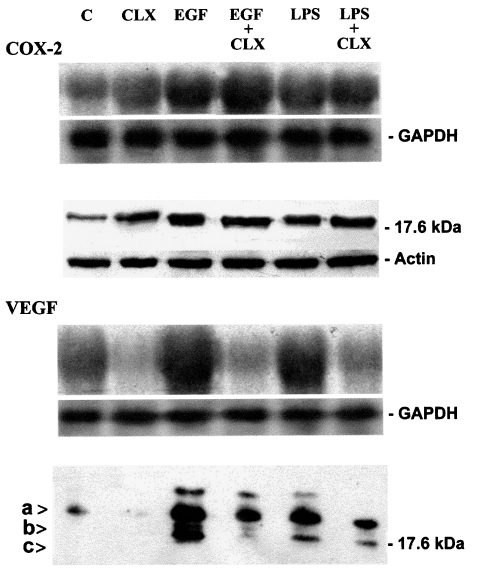
It is well known that HNC can produce partly well-characterized factors that may contribute to tumor angiogenesis and spreading, including VEGF, basic fibroblast growth factor (bFGF), interleukin 8 (IL-8), and nitric oxide (NO) [34]. On the basis of our findings suggesting a clear correlation between COX-2 activity, VEGF, and vascularization in 35 HNC tissue samples, we sought to explore the possible role of COX-2 in regulating VEGF production by squamous cell carcinomas in an in vitro model. We therefore measured the expression of VEGF mRNA and VEGF protein by Northern and immunoblot analyses under several conditions, in two different epidermoid tumor cell lines (A-431 and SCC-9). In both cell lines we determined the production of COX-2 and VEGF by detection of mRNA and proteins, as well as the levels of PgE2 in cell line supernatants (Table 2). Indomethacin (a COX inhibitor) slightly decreased VEGF production (data not shown), whereas celecoxib (a selective COX-2 inhibitor) determined a marked inhibition of VEGF protein expression (Figure 4). On the contrary, the same treatments increased COX-2 (Figure 4), according to the reported finding that COX inhibitors induce the expression of both COX-2 mRNA and protein [35] even if, as expected, PGE2 production was markedly reduced (Table 2).
Table 2.
Effect of COX-2 Inhibition on PGE2 Production (pg/µg Protein) Following 24 Hours EGF- and LPS-Stimulation of A-431 and SCC-9 Cell Supernatants
| Treatment | PGE2 Production | |
| A-431 Cell Line | SCC-9 Cell Line | |
| None | 17.1±4.3 | 24.9±4.3 |
| LPS (100 ng/ml) | 98±5.2* | 109.4±9.1* |
| EGF (100 ng/ml) | 78.5±6.2* | 94.4±10.2* |
| Indomethacin (10 µM) | 10.9±3.9 | 12.6 + 1.6 |
| Celecoxib (10 µM) | 5.4±2.7 | 10.6±2.0 |
| LPS+indomethacin | 20.3±5.3** | 19.6±6.0** |
| LPS+celecoxib | 10.2±1.9** | 18.8±5.8** |
| EGF+indomethacin | 24.3±4.3** | 24.6±3.0** |
| EGF+celecoxib | 26.8±2.9** | 37.8 ±1.8** |
The values are the mean±SEM of six experiments in triplicate.
Denotes statistically significant increase compared to unstimulated cells (P<.05, Wilcoxon rank sum test).
Denotes statistically significant inhibition compared to EGF- or LPS-treated cells (P<.05, Wilcoxon rank sum test).
Figure 4.
Analysis of mRNA (upper panels) and protein levels (lower panels) of COX-2 and VEGF in A-431 cell line. mRNA and proteins were prepared as described in the methods section. Cells were treated as described. Abbreviations: C=Control, untreated cells; CLX= celecoxib, EGF=epidermal growth factor, LPS = E coli lipopolysaccharide. The arrows indicate three forms of VEGF of apparently 23 kDa (arrow a), 22kDa (arrow b) and 18 kDa (arrow c). Densitometric analysis of Northern blot demonstrated an increase of VEGF expression following EGF (13-fold) and LPS (11-fold) treatments. Celecoxib treatment inhibited basal VEGF expression (to 0.5-fold), EGF- (to 0.6-fold) and LPS- (to 0.8-fold) induced VEGF increase. For densitometric analysis of Western blot, the signal intensity of the 23-kDa band of the control lane was normalized as 100%. An increase of VEGF was observed, both with EGF (15-fold) and LPS (10-fold) treatments. Celecoxib strongly inhibited the basal (to 0.2-fold), EGF- (to 7-fold) and LPS-induced (to 5-fold) VEGF increase. mRNA and protein analysis performed on the SCC-9 cell line confirmed data obtained from A431 cell line.
COX-2 overexpression induced by epidermal growth factor (EGF) and LPS treatment of tumor cells was associated with increased levels of VEGF mRNA and protein production (Figure 4). EGF- and LPS-dependent increase in VEGF was inhibited by indomethacin (data not shown) and celecoxib (Figure 4). Cycloheximide inhibited VEGF mRNA and protein accumulation, indicating that ongoing protein synthesis is required for EGF-dependent induction of VEGF (data not shown).
Table 2 shows that COX-2 activation following EGF and LPS treatments was confirmed by a strong increase in PgE2 levels in supernatants from both tumor cell lines. As expected, the PgE2 increase was inhibited by indomethacin and celecoxib treatments, in a way similar to VEGF behavior, as reported in Figure 4. These results suggest that COX-2 activity is involved in upregulation of VEGF mRNA and protein production through prostaglandin production.
Discussion
Angiogenesis is essential for tumor growth and spreading. Any significant increase in tumor mass must be preceded by an increase in the vascular supply to deliver nutrients and oxygen to the tumor. New tumor blood vessel growth appears to coincide with the development of tumor metastasis and bears adverse prognostic significance [28,36]. This process involves the proliferation of host endothelial cells that is likely to be regulated by several growth factors. Among the polypeptides known to be angiogenic, which stimulate the proliferation of endothelial cells in vitro and induce angiogenesis in vivo, VEGF is the first tumorsecreted factor discovered to be capable of increasing vascular permeability and of promoting endothelial cell proliferation and migration [37]. Recent reports indicate a correlation between VEGF expression and tumor angiogenesis and progression in several epithelial tumors, including HNC [38,39], even if the mechanism(s) of its upregulation in cancer cells are unknown.
In colon cancer cells, the down regulation of COX-2 expression by COX inhibitors associated with an evident antitumor and antiangiogenic effect [22,40] as well as the possible induction of VEGF expression by PgE1 and PgE2 in non neoplastic cell systems [41,42], suggest a possible role of COX-2 in regulating the production of VEGF.
More recently, it has been shown that selective or nonselective inhibition of cyclooxygenase pathways may inhibit tumor angiogenesis, reducing prostaglandin production by acting on several potential cell sources, i.e., tumor cells, endothelial cells, and stromal reactive cells [43,44].
In this study, we show that COX-2 is overexpressed in HNC tissues when compared to adjacent normal tissue. This increase in COX-2 mRNA and protein expression is associated with an increase in PgE2 production from tumor tissue, particularly at the tumor edge, and is significantly correlated with the occurrence of lymph node metastasis in HNC patients. The pattern and rate of COX-2 expression is comparable to that of colon cancer [45] and of adenocarcinoma of the lung [10], being detected in almost 80% of cancer tissues by immunohistochemical and immunoblot analyses. Conversely, the high rate of HNC expression of COX-2 protein is in contrast with that recently reported in squamous cell carcinomas of the lung and in breast cancer [9,10], suggesting a different biologic significance of COX-2 pathway in epithelial carcinogenesis.
In our series, COX-2 mRNA and protein expression parallels the observations that VEGF mRNA and protein are increased in tumor tissue and associated with increased tumor angiogenesis, as assessed by microvessel count. Statistical analysis shows a significant correlation between COX-2 activity and VEGF expression as well as between both COX-2 and VEGF expression with tumor angiogenesis and lymph node metastasis, suggesting that both COX-2 and VEGF are involved in the control of tumor angiogenesis and metastasis in HNC. Our data support the notion that the COX-2 pathway may be involved in the regulation of VEGF expression in HNC.
This hypothesis has been confirmed by in vitro studies, showing that in two epidermoid tumor cell lines (A-431 and SCC-9), both expressing VEGF mRNA and protein, COX-2 activation causes rapid induction of VEGF mRNA and VEGF production in tumor cells. The increase in VEGF production following COX-2 overexpression is also associated with an increase in PgE2 levels in culture supernatants, suggesting a possible direct VEGF induction by PGs. Because these effects can be reversed by inhibition of COX-2, following treatment with the COX inhibitor indomethacin and with the selective COX-2 inhibitor celecoxib, the production of prostaglandins seems to play an important role in regulating VEGF mRNA and VEGF protein release by A-431 and SCC-9 epidermoid cancer cells. The array of prostaglandins produced by A-431 and SCC-9 cells is currently under investigation; however, a central role in such a process seems to be linked to PgE2 production, one of the most abundant PGs found in HNC [1–4], which is markedly increased in more vascularized HNC in vivo as well as in supernatants from epidermoid cancer cells producing VEGF in vitro. These data support previous observations suggesting a potent angiogenic activity of prostaglandins of the E series in neoplastic and non neoplastic cell systems [22,41,42] and extend the knowledge about the role of COX-2 in human carcinogenesis.
In in vitro angiogenesis studies on COX-2 overexpressing colon cancer cells, Tsujii et al. [24] recently reported the rapid growth of COX-2 overexpressing cancer cells as xenografts in nude mice when compared with the poor growth of parental colon cancer cells that did not overproduce prostaglandins. In addition, the indomethacin treatment of xenografted nude mice inhibited tumor growth effectively, further confirming the central role of COX-2 activity in colon carcinogenesis. More recently, Masferrer et al. [40] provided evidence that the selective COX-2 inhibitor celecoxib is able to suppress growth of lung and colon tumors implanted into recipient mice, mainly because of a potent antiangiogenic activity. We are currently evaluating the effects of COX inhibitors on angiogenesis of several different epidermoid cell lines in these in vivo models; however, our preliminary results strongly suggest the possibility that the inhibition of COX activity in cancer cells could affect the growth and dissemination of epidermoid cancers because of in vivo antiangiogenic activity due to reduced VEGF production. In fact, previous studies on A-431 tumor cells demonstrate that VEGF inhibition alone, using a different therapeutic approach, such as VEGF-neutralizing antibodies, is sufficient to prevent tumor growth and tumor metastasis in animal models [46]. These data further support our findings about a clear correlation between VEGF protein expression and tumor stage and metastasis observed in our series of human HNCs.
The mechanism(s) responsible for COX-2 upregulation in HNC is currently under investigation in our laboratory, particularly in light of our recent data about a clear correlation between inducible nitric oxide synthase (iNOS) activity and angiogenesis [34]. Recent studies on non neoplastic [47–50] and neoplastic [22] cells seem to suggest a possible relationship between iNOS and COX-2 activities, which could both be upregulated in several human cancers. Moreover, the involvement of p53 tumor suppressor gene in the regulation of both VEGF and iNOS expression [51,52] raises the possibility that p53 gene mutations reported in more than 60% of HNCs [53] might result in increased NO production with possible upregulation of COX-2 activity, both conditions potentially capable of inducing a VEGF-increase in tumor mass. This hypothesis is currently under investigation in our laboratory [54].
In conclusion, further investigations will be required to determine both the exact role of COX-2 in HNC as well as the mechanism(s) by which COX-2 is involved in HNC vascularization and progression. Our study of the potential role of COX-2 in regulating VEGF production in epidermoid cancer cells raises the possibility that, analogously to colon cancer, COX inhibitors might have antiangiogenic and antitumor effects on cancers from other districts, such as those from the upper aereo-digestive tract, thus suggesting new therapeutic strategies for cancer treatment and prevention in humans.
Abbreviations
- COX-2
cyclooxygenase
- EGF
epidermal growth factor
- HNC
head and neck cancer
- LPS
lipopolysaccharide
- PG
prostaglandin
- VEGF
vascular endothelial growth factor
References
- 1.Pinto S, Gallo O, Di Laghi M, Gallina E, Paniccia R, Abbate R. Prostaglandins in squamous cell carcinoma of the larynx: tumor and peritumor synthesis. Prostaglandins, Leukotrienes Essent Fatty Acids. 1990;39:53–57. doi: 10.1016/0952-3278(90)90172-h. [DOI] [PubMed] [Google Scholar]
- 2.Pinto S, Gori L, Gallo O, Boccuzzi S, Paniccia R, Abbate R. Increased thromboxane A2 production at primary tumor site in metastasizing squamous cell carcinoma of the larynx. Prostaglandins, Leukotrienes Essent Fatty Acids. 1993;49:527–530. doi: 10.1016/0952-3278(93)90042-u. [DOI] [PubMed] [Google Scholar]
- 3.Snyderman CH, Milanovich M, Wagner RL, Johnson J. Prognostic significance of prostaglandin E2 production in fresh tissues of head and neck cancer patients. Head Neck. 1995;17:108–113. doi: 10.1002/hed.2880170206. [DOI] [PubMed] [Google Scholar]
- 4.Ondrey FG. Arachidonic acid metabolism: a primer for head and neck surgeons. Head Neck. 1998;20:334–349. doi: 10.1002/(sici)1097-0347(199807)20:4<334::aid-hed9>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 5.Young M. Eicosanoids and the immunology of cancer. Cancer Metastasis Rev. 1994;13:337–348. doi: 10.1007/BF00666103. [DOI] [PubMed] [Google Scholar]
- 6.Fulton AM. Effects of indomethacin on the growth of cultured mammary tumors. Int J Cancer. 1984;33:375–379. doi: 10.1002/ijc.2910330316. [DOI] [PubMed] [Google Scholar]
- 7.Goodwin JS. Prostaglandins and host defense in cancer. Med Clin North Am. 1981;65:829–844. doi: 10.1016/s0025-7125(16)31500-0. [DOI] [PubMed] [Google Scholar]
- 8.Chan G, Boyle JO, Yang EK, Zhang F, Sacks PG, Shah JP, Edelstein D, Soslow RA, Koki AT, Woerner BM, Masferrer JL, Dannenberg AJ. Cyclooxygenase-2 expression is up-regulated in squamous cell carcinoma of the head and neck. Cancer Res. 1999;59:991–994. [PubMed] [Google Scholar]
- 9.Fujita T, Matsui M, Takaku K, Uetake H, Ichicawa W, Taketo MM, Sugihara K. Size- and invasion-dependent increase in cyclooxygenase 2 levels in human colorectal carcinomas. Cancer Res. 1998;58:4823–4826. [PubMed] [Google Scholar]
- 10.Hwang D, Scollard D, Byrne J, Levine E. Expression of cyclooxygenase-1 and cyclooxygenase-2 in human breast cancer. J Natl Cancer Inst. 1998;90:455–460. doi: 10.1093/jnci/90.6.455. [DOI] [PubMed] [Google Scholar]
- 11.Hida T, Yatabe Y, Achiwa H, Muramatsu H, Kozaki K, Nakamura S, Ogawa M, Mitsudomi T, Sugiura T, Takahashi T. Increased expression of cyclooxygenase 2 occurs frequently in human lung cancers, specifically in adenocarcinomas. Cancer Res. 1998;58:3761–3764. [PubMed] [Google Scholar]
- 12.Du Bois RN, Awad J, Morrow J, Roberts JI, Bishop PR. Regulation of eicosanoid production and mitogenesis in rat intestinal epithelial cells by transforming growth factor-alpha and phorbol ester. J Clin Invest. 1994;93:493–498. doi: 10.1172/JCI116998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelley DJ, Mestre JR, Subbaramaiah K, Sacks PG, Schantz SP, Tanabe T, Inoue H, Ramonetti JT, Dannenberg AJ. Benzo[a]pyrene up-regulates cyclooxygenase-2 gene expression in oral epithelial cells. Carcinogenesis. 1997;18:795–799. doi: 10.1093/carcin/18.4.795. [DOI] [PubMed] [Google Scholar]
- 14.Smalley W, Du Bois RN. Colorectal cancer and nonsteroidal anti-inflammatory drugs. Adv Pharmacol. 1997;39:1–20. doi: 10.1016/s1054-3589(08)60067-8. [DOI] [PubMed] [Google Scholar]
- 15.Sheng H, Shao J, Kirkland SC, Isakson P, Coffey RJ, Morrow J, Beauchamp RD, DuBois RN. Inhibition of human colon cancer cell growth by selective inhibition of cyclooxygenase-2. J Clin Invest. 1997;9:2254–2259. doi: 10.1172/JCI119400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stoll BA. Indomethacin in breast cancer. Lancet. 1973;2:384. doi: 10.1016/s0140-6736(73)93234-0. [DOI] [PubMed] [Google Scholar]
- 17.Al-Salem T, Ali ZS, Qassab M. Skin cancers in xeroderma pigmentosum: response to indomethacin and steroids. Lancet. 1980;2:264–265. doi: 10.1016/s0140-6736(80)90154-3. [DOI] [PubMed] [Google Scholar]
- 18.Fischer SM, Lo HH, Gordon GB, Seibert K, Kelloff G, Lubet RA, Conti CJ. Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, and indomethacin against ultraviolet light-induced skin carcinogenesis. Mol Carcinog. 1999;25:231–240. [PubMed] [Google Scholar]
- 19.Ondrey FG, Juhn SK, Adams GL. Inhibition of head and neck tumor cell growth with arachidonic acid metabolism inhibition. Laryngoscope. 1996;106:129–134. doi: 10.1097/00005537-199602000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Scioscia KA, Snyderman CH, Ruege R, Reddy J, D'amico F, Comsa S, Collins B. Role of arachidonic acid metabolites in tumor growth inhibition by nonsteroidal antiinflammatory drugs. Am J Otolaryngol. 1997;18:1–8. doi: 10.1016/s0196-0709(97)90041-7. [DOI] [PubMed] [Google Scholar]
- 21.Panje WR. Regression of head and neck carcinoma with a prostaglandin-synthesis inhibitor. Arch Otolaryngol. 1981;107:658–663. doi: 10.1001/archotol.1981.00790470006003. [DOI] [PubMed] [Google Scholar]
- 22.Sawaoka H, Kawano S, Tsuji S, Tsuji M, Gunawan ES, Takei Y, Nagano K, Hori M. Cyclooxygenase-2 inhibitors suppress the growth of gastric cancer xenografts via induction of apoptosis in nude mice. Am J Physiol. 1998;247:G1061–G1067. doi: 10.1152/ajpgi.1998.274.6.G1061. [DOI] [PubMed] [Google Scholar]
- 23.Tsujii M, Du Bois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostagladin endoperoxide synthase 2. Cell. 1995;83:493–501. doi: 10.1016/0092-8674(95)90127-2. [DOI] [PubMed] [Google Scholar]
- 24.Tsujii M, Kawano S, Du Bois RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci USA. 1997;94:3336–3340. doi: 10.1073/pnas.94.7.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, Du Bois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705–716. doi: 10.1016/s0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]
- 26.Myoken Y, Kayada Y, Okamoto T, Kan M, Sato GH, Sato JD. Vascular endothelial cell growth factor (VEGF) produced by A-431 human epidermoid carcinoma cells and identification of VEGF membrane binding sites. Proc Natl Acad Sci USA. 1991;88:5819–5823. doi: 10.1073/pnas.88.13.5819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zimmermann KC, Sarbia M, Weber A-A, Borchard F, Gabbert HE, Schrör K. Cyclooxygenase-2 expression in human esophageal carcinoma. Cancer Res. 1999;59:198–204. [PubMed] [Google Scholar]
- 28.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis. Correlation in invasive breast carcinoma. New Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 29.Chin K, Kurashima Y, Ogura T, Tajiri H, Yoshida S, Esumi H. Induction of vascular endothelial growth factor by nitric oxide in human glioblastoma and hepatocellular carcinoma cells. Oncogene. 1997;15:437–442. doi: 10.1038/sj.onc.1201201. [DOI] [PubMed] [Google Scholar]
- 30.Tomisawa T, Tokunaga T, Oshika Y, Tsuchida T, Fukushima Y, Sato H, Kijima H, Yamazaki H, Ueyama Y, Nakamura M. Expression pattern of vascular endothelial growth factor isoform is closely correlated with tumor stage and vascularization in renal cell carcinoma. Eur J Cancer. 1999;35:133–137. doi: 10.1016/s0959-8049(98)00278-0. [DOI] [PubMed] [Google Scholar]
- 31.Chirgwin JM, Przybyla AE, MacDonald RJ, Rutter WJ. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979;18:5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- 32.Pertschuck LP, Feldman JG, Kim DS, Nayeri K, Eisenberg KB, Carter AC, Thelmo WT, Rhong ZT, Benn P, Grossman A. Steroid hormone receptor immunohistochemistry and amplification of c-myc protooncogene. Relationship to disease-free survival in breast cancer. Cancer. 1993;71:162–171. doi: 10.1002/1097-0142(19930101)71:1<162::aid-cncr2820710126>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 33.Gentilini P, Laffi G, Meacci E, La Villa G, Cominelli F, Pinzani M, Buzzelli G. Effects of OKY 046, a thromboxane-synthase inhibitor, on renal function in nonazotemic cirrhotic patients with ascites. Gastroenterology. 1988;94:1470–1477. doi: 10.1016/0016-5085(88)90688-9. [DOI] [PubMed] [Google Scholar]
- 34.Gallo O, Masini E, Morbidelli L, Franchi A, Fini Storchi I, Vergari WA, Ziche M. Angiogenesis and tumor progression are under the control of nitric oxide in head and neck cancer. J Natl Cancer Inst. 1998;90:587–596. doi: 10.1093/jnci/90.8.587. [DOI] [PubMed] [Google Scholar]
- 35.Lu X, Xie W, Reed D, Bradshaw WS, Simmons DL. Nonsteroidal antiinflammatory drugs cause apoptosis and induce cyclooxygenases in chicken embryo fibroblasts. Proc Natl Acad Sci USA. 1995;92:7961–7965. doi: 10.1073/pnas.92.17.7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Folkman J, Shing Y. Angiogenesis. J Biol Chem. 1992;267:10931–10934. [PubMed] [Google Scholar]
- 37.Leung DW, Cachianes G, Kuang DW, Goeddel DW, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1991;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 38.Inoue K, Ozeki Y, Saganuma T, Sugiura Y, Tanaka S. Vascular endothelial growth factor expression in primary esophageal squamous cell carcinoma. Cancer. 1997;79:206–213. doi: 10.1002/(sici)1097-0142(19970115)79:2<206::aid-cncr2>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 39.Salven P, Heikkila P, Anttonen A, Kajanti M, Joensuu H. Vascular endothelial growth factor in squamous cell head and neck carcinoma: expression and prognostic significance. Mod Pathol. 1997;10:1128–1133. [PubMed] [Google Scholar]
- 40.Masferrer JL, Leahy KM, Koki AT, Zweifel BS, Settle SL, Woerner BM, Edwards DA, Flickinger AG, Moore RJ, Seibert K. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000;60:1306–1311. [PubMed] [Google Scholar]
- 41.Harada S-H, Nagy JA, Sullivan KA, Thomas KA, Endo N, Rodan GA, Rodan SB. Induction of vascular endothelial growth factor expression of prostaglandin E2 and E1 in osteoblasts. J Clin Invest. 1994;93:2490–2496. doi: 10.1172/JCI117258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Form DM, Auerbach R. PGE2 and angiogenesis. Proc Soc Exp Biol Med. 1983;172:214–218. doi: 10.3181/00379727-172-41548. [DOI] [PubMed] [Google Scholar]
- 43.Jones MK, Wang H, Peskar BM, Levin E, Itani RM, Sarfeh IJ, Tarnawski AS. Inhibition of angiogenesis by nonsteroidal anti-inflammatory drugs: insight into mechanisms and implications for cancer growth and ulcer healing. Nat Med. 1999;5:1418–1423. doi: 10.1038/70995. [DOI] [PubMed] [Google Scholar]
- 44.Williams CS, Tsujii M, Reese J, Dey SK, DuBois RN. Host cyclooxygenase-2 modulates carcinoma growth. J Clin Invest. 2000;105:1589–1594. doi: 10.1172/JCI9621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sano H, Kawahito Y, Wilder R, Hashiramoto A, Mukai S, Asai K, Kimura S, Kato H, Kondo M, Hla T. Expression of cyclooxygenase-1 and -2 in human colorectal cancer. Cancer Res. 1995;55:3785–3789. [PubMed] [Google Scholar]
- 46.Melnyk O, Shuman MA, Kim J. Vascular endothelial growth factor promotes tumor dissemination by a mechanism distinct from its effect on primary tumor growth. Cancer Res. 1996;56:921–924. [PubMed] [Google Scholar]
- 47.Salvemini D, Seibert K, Masferrer JL, Misko TP, Currie MG, Needleman P. Endogenous nitric oxide enhances prostaglandin production in a model of renal inflammation. J Clin Invest. 1994;93:1940–1947. doi: 10.1172/JCI117185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salvemini D, Settle SL, Masferrer JL, Seibert K, Currie MG, Needleman P. Regulation of prostaglandin production by nitric oxide; an in vivo analysis. Br J Pharmacol. 1995;114:1171–1178. doi: 10.1111/j.1476-5381.1995.tb13330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suh N, Honda T, Finlay HJ, Barchowsky A, Williams C, Benoit NE, Quiao-wen X, Nathan C, Gribble GW, Sporn MB. Novel triterpenoids suppress inducible nitric oxide synthase (iNOS) and inducible cyclooxygenase (COX-2) in mouse macrophages. Cancer Res. 1998;58:717–723. [PubMed] [Google Scholar]
- 50.Watkins DN, Garlepp MJ, Thompson PJ. Regulation of the inducible cyclo-oxygenase pathway in human cultered airway epithelial (A549) cells by nitric oxide. Br J Pharmacol. 1997;121:1482–1488. doi: 10.1038/sj.bjp.0701283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ambs S, Merriam WG, Mofolusara O, Ogunfusika MO, Bennet WP, Ishibe N, Hussian P, Tzeng EE, Geller D, Billiar TR, Harris CC. p53 and vascular endothelial growth factor regulate tumor growth of NOS2-expressing human carcinoma cells. Nat Med. 1998;12:1371–1376. doi: 10.1038/3957. [DOI] [PubMed] [Google Scholar]
- 52.Ambs S, Ogunfusika MO, Merriam WG, Bennet WP, Billiar TR, Harris CC. Up-regulation of inducible nitric oxide synthase expression in cancer-prone p53 knockout mice. Proc Natl Acad Sci USA. 1998;95:8823–8828. doi: 10.1073/pnas.95.15.8823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gallo O, Chiarelli I, Bianchi S, Calzolari A, Simonetti L, Porfirio B. Loss of the p53 mutation after irradiation is associated with increased aggressiveness in recurred head and neck cancer. Clin Cancer Res. 1996;2:1577–1583. [PubMed] [Google Scholar]
- 54.Chiarugi V, Magnelli L, Gallo O. iNOS and p53 as play-makers of tumor angiogenesis. Int J Mol Med. 1998;2:715–719. doi: 10.3892/ijmm.2.6.715. [DOI] [PubMed] [Google Scholar]