Abstract
Tumor cell invasion and metastasis require precise coordination of adherence to extracellular matrix (ECM) and controlled degradation of its components. Invasive cells secrete proteolytic enzymes known as matrix metalloproteinases (MMPs) which degrade specific basement membrane molecules. Expression of these enzymes is regulated by multiple signaling mechanisms, including the mitogen-activated protein kinase (MAPK) pathway. One of the terminal effectors of this signaling cascade is jun N-terminal kinase 1 (JNK1) which phosphorylates the transcription factor c-jun, a component of the AP-1 complex. MMP-9 expression is regulated by two well-characterized AP-1 sites in the promoter of this gene. To determine how JNK1 activity regulated MMP-9 expression in human squamous cell carcinoma lines, we overexpressed this kinase in SCC25 cells. JNK1 overexpression induced MMP-9 protein levels and activity in this cell line. Elevated MMP-9 expression correlated with increased invasion of reconstituted basement membranes by JNK1-overexpressing clones. Site-directed mutagenesis of the MMP-9 promoter revealed that JNK1 cooperated with its transcription factor target c-jun to increase MMP-9 expression at the transcriptional level via the proximal AP-1 site. These results suggest that elevated JNK1 expression may contribute to increased MMP-9 activity and ECM invasion by tumor cells.
Keywords: invasion, kinase, AP-1, transcription, signal transduction
Introduction
The ability of tumor cells to invade surrounding tissue and vital structures is one of the most important features of the malignant phenotype. Degradation of the basement membrane and invasion of underlying connective tissue have long been the histologic criteria for diagnosis of carcinoma [1]. Invading tumor cells secrete proteolytic enzymes known as matrix metalloproteinases (MMP) to degrade extracellular matrix (ECM) [2]. The genes encoding MMPs are precisely regulated [3]. This regulation is provided, in part, by signals transmitted via the mitogen-activated protein kinase (MAPK) pathway.
The MAPK pathway consists of a series of protein kinases which are activated upon phosphorylation of specific amino acid residues in their regulatory domains [4]. The MAPK family can be divided into three branches: the extracellular signal-regulated kinases (ERK), jun N-terminal kinase 1 (JNK1), and p38 MAPK. ERKs have been shown to phosphorylate members of the ets transcription factor family [5]. The second MAPK branch, JNK1, phosphorylates c-jun on serine residues in its amino terminus, resulting in transcriptional activation via AP-1 sites in the promoters of target genes [6]. The third kinase, p38, phosphorylates ATF-2 and ets transcription factors [7]. Regulation of MMP-9 expression by MAPKs has been the subject of intense interest in recent years [8]. H-ras activation of MAPK signaling has been associated with metastatic behavior in cancer cells [9]. Growth factors have also been shown to activate MMP-9 gene expression via the MAPK pathway [10]. Inhibition of p38 kinase was shown to block induction of MMP-9 activity in cancer cell lines [11]. These studies demonstrate an important role for MAPK signaling in the regulation of MMP-9 expression.
At the transcriptional level, MMP-9 expression is regulated by binding of multiple factors to their response elements in the promoter of this gene. These include recognition sites for AP-1 which bind fos/jun family members and multiple PEA3 elements which are activated by ets transcription factors [12]. Cooperation among transcription factors in regulating MMP-9 promoter activity has been demonstrated in previous studies [13]. We have shown that conditional activation of a c-fos fusion protein inhibits the activity of the MMP-9 promoter and correlates with reduced tumor cell invasion [14]. In the present study, we wished to examine how activation of other components of AP-1, such as c-jun, regulated MMP-9 expression and tumor cell invasion. To accomplish this, we overexpressed the upstream activator of c-jun (JNK1) in the squamous cell carcinoma line, SCC25. JNK1 overexpression induced MMP-9 protein expression and activity. Elevated MMP-9 expression correlated with increased numbers of invasive cells in an in vitro assay. JNK1 cooperated with c-jun to induce MMP-9 promoter activity via the proximal AP-1 site. These results indicate that increased JNK1 expression may result in elevated MMP-9 activity and increased tumor cell invasion.
Materials and Methods
Cell Culture and Stable Transaction
The human squamous cell carcinoma cell line, SCC25, was purchased from the American Type Culture Collection and has been described previously [15]. Cells were cultured in Dulbecco's modified Eagle's medium (DMEM), 10% fetal bovine serum, 40 µg/ml gentamicin at 37°C in a humidified atmosphere of 5% CO2. SCC25 cells were stably transfected with 5 µg of JNK1 expression plasmid (constructed in pcDNA3 and kindly provided by Dr. Roger Davis) or blank vector (pcDNA3) using LipofectAMINE reagent according to manufacturer's recommendations (Life Technologies). Cells were selected in 400 µg/ml G418 for 14 days. Resistant clones were picked for expansion and characterization.
Western Blot
SCC25 cells were lysed in 1x Laemmli buffer and protein concentrations were determined by Bradford assay using a commercially available reagent (Bio-Rad). Seventy-five micrograms of total cellular lysate was subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) under denaturing and reducing conditions. Concentrated conditioned medium was diluted with 2x Laemmli buffer and separately subjected to SDS-PAGE. Separated proteins were electroblotted to polyvinylidene difluoride (PVDF) membranes according to manufacturer's recommendations (Bio-Rad). Blots were incubated with antihuman primary antibodies to JNK1 (Santa Cruz Biotechnology) or MMP-9 (Oncogene Science) for 16 hours at 4°C. After washing in Tris-buffered saline containing 0.1% Tween 20 (TBST, pH 7.5), blots were incubated with anti-IgG secondary antibodies conjugated to horseradish peroxidase for 30 minutes at room temperature. Following extensive washing in TBST, bands were visualized by the enhanced chemiluminescence method (Boehringer Mannheim). Blots were stripped and probed with anti-β-actin antibody (Santa Cruz Biotechnology) to verify equal amounts of protein in each lane. Blots were quantitated by laser densitometry.
Gelatin Zymography
Serum-free conditioned medium was collected from triplicate cultures of 5x105 G418 resistant SCC25 cells or JNK1-overexpressing clones. Media samples were subjected to gelatin zymography as described previously [16]. Unconditioned media were used as negative control. Protein concentrations were determined by Bradford assay. Conditioned media samples were separated on 10% SDS-PAGE gels containing 1 mg/ml gelatin under native conditions. Gels were washed in 2.5% Triton X-100 for 30 minutes at room temperature followed by incubation in 50 mM Tris-HCl (pH 7.5), 5 mM CaCl2 for 16 hours at 37°C. To verify the metalloproteinase activity of the gelatinolytic bands, 5 mM EDTA and 0.1 mM 1,10-phenanthroline were added to the incubation buffer of duplicate gels. To visualize gelatinolytic bands, gels were stained with Coomassie blue for 1 hour at room temperature followed by extensive washing in 20% methanol, 5% acetic acid.
Invasion Assays
G418-resistant SCC25 clones (5x104) or JNK1-expressing cells were plated in triplicate into Matrigel invasion chambers in six-well plates according to manufacturer's recommendations (Becton Dickinson). After 1 day, cells which had migrated through the reconstituted basement membrane were fixed in methanol, stained with hematoxylin, and counted.
Immunoprecipitation
4x106 SCC25 cells were lysed in 50 mM HEPES (pH 7.5), 150 mM NaCl, 1 mM EDTA, 2.5 mM EGTA, 1 mM DTT, 1% Nonidet P-40, 10% glycerol, 1 mM NaF, 0.1 mM sodium orthovanadate, and protease inhibitors for 30 minutes at 4°C. Lysates were centrifuged at 10,000g for 10 minutes and anti-human antibody to c-jun (Transduction Laboratories) was incubated with the supernatants for 1 hour at 4°C. Antigen-antibody complexes are precipitated with protein A/G agarose beads for 1 hour at 4°C. Immunoprecipitated proteins were washed three times with 1 ml lysis buffer. Samples were boiled in 1 x Laemmli buffer for 3 minutes, separated by SDS-PAGE, and blotted to PVDF membranes. To determine protein phosphorylation levels, blots were incubated with anti-phosphoserine antibody (Zymed) for 16 hours at 4°C. Blots were stripped and incubated with anti-c-jun antibody using the same conditions. Bands were visualized by the enhanced chemiluminescence method.
Electrophoretic Mobility Shift Assay
107 SCC25 nuclei were extracted in 20 mM HEPES (pH 7.9), 25% glycerol, 1.5 mM MgCl2, 1.2 M KCl, 0.2 mM EDTA, 0.2 mM PMSF, 0.5 mM DTT for 30 minutes at 4°C. Following centrifugation at 10,000g for 30 minutes at 4°C, the supernatant was removed and dialyzed against 20 mM HEPES (pH 7.9), 20% glycerol, 0.1 M KCl, 0.2 mM EDTA, 0.2 mM PMSF, 0.5 mM DTT for 1 hour at 4°C. Fifteen micrograms of dialyzed nuclear extract was incubated in binding reactions containing 2 µg poly(dl-dC)-poly(dl-dC) and 50,000 cpm 32P-labeled double-stranded oligonucleotide spanning the AP-1 site of the MMP-9 promoter from nucleotides -91 to -60. For binding competition analysis, 10- to 1000-fold molar excess of unlabeled AP-1 or mutated oligonucleotide was included in the reactions. To determine which AP-1 proteins were present in the shifted complexes, 1 µl of antihuman c-fos, Fra-2, c-jun, JunB, or JunD antibodies was included in the binding reactions. Reactions were incubated at room temperature for 15 minutes and subjected to native PAGE using 0.5x Tris-borate-EDTA running buffer. Gels were dried and exposed to Kodak XAR5 autoradiographic film for 16 hours at -80°C.
Transient Transfection and CAT Assay
5x105 SCC25 cells were plated in triplicate onto 35-mm tissue culture plastic dishes and transiently transfected with 5 µg of a CAT reporter construct driven by the MMP-9 promoter (constructed in pGEM4 and kindly provided by Dr. H. Sato [12]). Additional cultures were transfected with the same construct containing inactivating point mutations (5′-TGAGTtg-3′) in the AP-1 binding sites at the -79 or -533 bp [13]. Cultures were cotransfected with expression vectors for JNK1 and/or c-jun (kindly provided by Drs. Roger Davis and Tom Curran). The amount of DNA in each transfection was equalized with empty vector. Transfections were performed using LipofectAMINE reagent according to manufacturer's recommendations (Life Technologies). Two micrograms of β-galactosidase expression plasmid (VR-1412, Vical) was used to normalize for transfection efficiency. Each sample was divided equally for determination of CAT and β-galactosidase activities. CAT activity in each sample was determined by the liquid scintillation counting procedure using a commercially available kit (Promega). β-galactosidase activity was determined by luminometry using a commercially available kit (Tropix). CAT activity was normalized to levels of β-galactosidase in each sample. Results are relative to samples transfected with the wild-type MMP-9 construct and empty vector.
Results
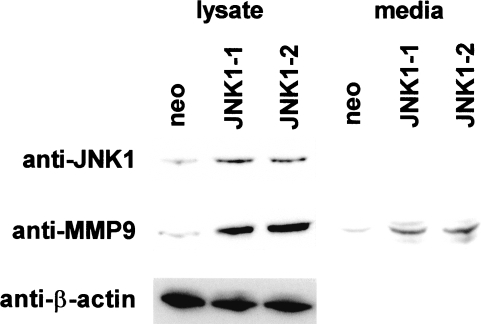
MAPK signaling via AP-1 proteins is an important regulator of MMP expression in many cell types [10]. To determine the role of JNK1 in regulating MMP-9 expression, we overexpressed this kinase in SCC25 cells. As shown in Figure 1, stable transfection of the JNK-1 vector resulted in a three-fold increase in expression of this protein compared to G418-resistant control clones as determined by laser densitometry. JNK1 clones showed a five-fold increase in MMP-9 protein expression compared to control cells. Increased amounts of MMP-9 protein were also detected in concentrated conditioned media samples by Western blotting. These data indicated that JNK1 overexpression can induce MMP-9 expression in SCC25 cells.
Figure 1.
JNK1 overexpression induces MMP-9 expression in SCC25 cells. The SCC25 line was stably transfected with human JNK1 expression vector as described in Materials and Methods section. Whole cell lysates from neomycin-resistant (neo) and JNK1-overexpressing clones (JNK1-1, -2) were subjected to Western blot using anti-JNK1 and anti-MMP-9 antibodies. Blots were stripped and probed with anti-β-actin antibody to verify equal amounts of protein in each lane. Concentrated conditioned media were also subjected to Western blotting to determine relative levels of secreted MMP-9. This experiment was performed three times with similar results. Representative blots are shown.
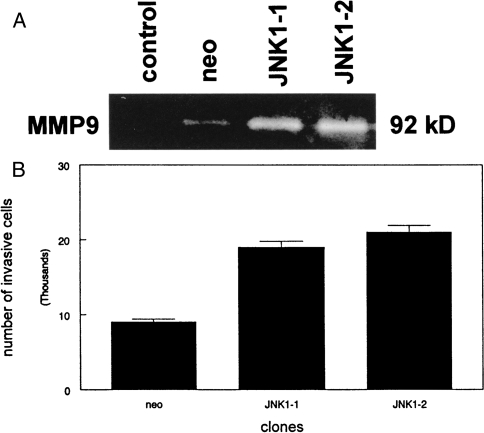
To determine if the additional MMP-9 protein expressed by the JNK1 clones was potentially active, we subjected conditioned media from these cells to gelatin zymography. As shown in Figure 2A, JNK1 clones showed a three-fold increase in MMP-9 activity when compared to control cells as determined by laser densitometry of negative photographic images of the gels. These data indicated that increased MMP-9 expression correlated with enzymatic activity in JNK1-overexpressing clones. To determine if elevated MMP-9 activity correlated with increased invasion of JNK1 cells, we plated these clones on reconstituted basement membranes in a modified Boyden chamber assay. As shown in Figure 2B, JNK1 overexpression resulted in a two-fold increase in the number of cells able to degrade and migrate through the semiporous Matrigel membrane (t-test, P<.05). These results indicate that JNK1 overexpression induces MMP-9 expression, activity, and the invasive phenotype of SCC25 cells.
Figure 2.
JNK1 overexpression induces MMP-9 activity and increased invasion of reconstituted basement membranes by SCC25 cells. (A) Serum-free conditioned medium from neomycin-resistant (neo) and JNK1-over-expressing clones (JNK1-1, -2) was subjected to gelatin zymography as described in Materials and Methods section. Unconditioned medium was used as a negative control. The 92-kDa gelatinolytic band representing MMP-9 is shown. This experiment was performed three times with similar results. A representative gel is shown. (B) 5x104 cells from neomycin-resistant (neo) or JNK1-overexpressing (JNK1-1, -2) clones were plated in modified Boyden chambers as described in Materials and Methods section. Invasive cells that migrated through the reconstituted basement membrane were fixed, stained, and counted. These experiments were performed three times with similar results. Error bars represent SEM.
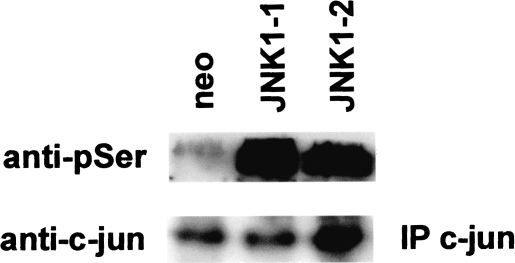
JNK1 phosphorylates the c-jun transcription factor, thereby increasing its ability to activate target gene expression [6]. To begin to determine the molecular basis for the effects of JNK1 on MMP-9 activity and the invasive phenotype of SCC25 cells, we examined c-jun phosphorylation in neomycin-resistant clones and JNK1 -overexpressing cells. As shown in Figure 3, c-jun phosphorylation was increased by 20-fold in JNK1-overexpressing clones compared to neomycin-resistant control cells. These results indicate that increased MMP-9 expression and invasion by JNK1-overexpressing clones correlate with hyperphosphorylation of c-jun.
Figure 3.
JNK1 overexpression results in hyperphosphorylation of c-jun on serine residues, c-jun protein was immunoprecipitated from cellular lysates of SCC25 neo or JNK1-overexpressing clones (JNK1-1, -2) and subjected to Western blotting as described in Materials and Methods section. Blots were incubated with anti-phosphoserine antibody (anti-pSer) to determine relative levels of c-jun phosphorylation on serine residues. Blots were stripped and incubated with anti-c-jun antibody to determine the amount of immunoprecipitated protein in each lane. These experiments were performed three times with similar results. Representative blots are shown.
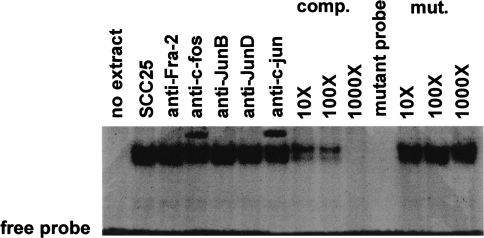
To determine if c-jun from SCC25 cells could bind to the -79 AP-1 site of the MMP-9 promoter, we performed electrophoretic mobility shift assays. As shown in Figure 4, nuclear extract from SCC25 cells produced an intense shifted complex when incubated with radiolabeled oligonucleotide corresponding to the -79 AP-1 site of the MMP-9 promoter. Inclusion of c-fos or c-jun antibodies in the binding reactions resulted in the formation of supershifted complexes. However, antibodies to other AP-1 proteins such as Fra-2, JunB, and JunD did not result in supershifted complexes. Unlabeled probe effectively competed for binding in these assays. Unlabeled mutant AP-1 probe did not compete for binding nor did this labeled oligonucleotide produce a shifted complex. No binding was observed in the absence of nuclear extract. We concluded that c-fos and c-jun could bind to the -79 AP-1 site of the MMP-9 promoter.
Figure 4.
c-fos and c-jun bind to the -79 AP-1 site of the MMP-9 promoter in SCC25 cells. Electrophoretic mobility shift assay was performed using nuclear extract from SCC25 cells and radiolabeled oligonucleotide probe containing the -79 AP-1 site of the MMP-9 promoter. To determine the identity of proteins in the shifted complexes, anti-human antibodies to Fra-2, c-fos, JunB, JunD, and c-jun were included in the binding reactions. To determine binding specificity, 10- to 1000-fold molar excess of unlabeled probe or mutant oligonucleotide was included in the reactions. No binding was observed in the absence of nuclear extract nor to the labeled mutant probe. The position of the free probe is shown. This experiment was performed three times with similar results. A representative gel is shown.
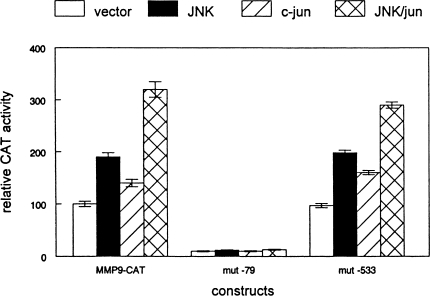
The MMP-9 promoter has two well-characterized recognition sites for AP-1 [10,12–14]. To determine if JNK1 and c-jun regulated MMP-9 expression at the transcriptional level, we examined the effects of JNK1 and c-jun overexpression on MMP-9 promoter activity. As shown in Figure 5, cotransfection of the MMP-9-CAT vector with the JNK1 expression plasmid resulted in a two-fold increase in reporter activity (t-test, P<.05). Transfection of the MMP-9-CAT vector with a c-jun expression plasmid resulted in a 50% increase in reporter activity. However, cotransfection of both JNK1 and c-jun plasmids with the MMP-9-CAT vector produced a more than three-fold increase in reporter activity. These results indicate that JNK1 and c-jun have combinatorial effects on MMP-9 promoter activity.
Figure 5.
JNK1 cooperates with c-jun to induce MMP-9 promoter activity via the proximal AP-1 site. The MMP9-CAT promoter construct was transiently transfected into SCC25 cells as described in Materials and Methods section. The same construct containing inactivating mutations in the AP-1 sites at -79 (mut -79) and -533 (mut -533) was transfected into duplicate cultures. JNK1 and/or c-jun expression vectors were cotransfected with the reporter construct. The amount of transfected plasmid DNA was equalized with blank vector. Relative CAT activity was normalized to β-galactosidase levels for each sample. Reporter gene activity is relative to that of cultures transfected with wild-type MMP-9 promoter construct and blank vector. These experiments were performed three times with similar results. Error bars indicate SEM.
To determine which AP-1 sites in the MMP-9 promoter mediate the effects of JNK1 and c-jun on expression of this gene, we transfected SCC25 cells with reporter vectors containing point mutations in these recognition sequences. Mutation of the AP-1 site at -79 bp completely abolished the effects of both JNK1 and c-jun on MMP-9 promoter activity (Figure 5). This mutation also reduced the basal activity of the MMP-9 promoter by 90%. However, mutation of the AP-1 site at -533 bp had no effect on JNK1 or c-jun regulation of the MMP-9 promoter. Cotransfection of JNK1 and/or c-jun expression plasmids with the MMP-9-CAT vector containing the -533 mutant AP-1 site produced the same combinatorial effects as on the wild-type construct. These results indicate that the effects of JNK1 and c-jun are mediated by the -79 AP-1 site in the MMP-9 promoter.
Discussion
The results of this study indicate that increased JNK1 expression can upregulate MMP-9 activity and the invasive phenotype of human cancer cells. These effects of JNK1 are mediated at the transcriptional level in conjunction with c-jun and the proximal AP-1 site of the MMP-9 promoter. This study provides an interesting correlation with previous work. Expression of a dominant negative c-jun has been shown to inhibit MMP-9 promoter activity [8]. Similarly, expression of a kinase-deficient JNK1 construct reduced the activity of the MMP-9 promoter. The expression of a kinase-deficient ERK1 had similar effects [8]. In a different cell line, the study found that JunD, rather than c-jun, bound to the -79 AP-1 site of the MMP-9 promoter. The SCC25 cells used in the present study express very low levels of JunD (our unpublished data), which likely accounts for these cell-specific differences. These studies point to an important role for MAPK signaling in transcriptional regulation of the MMP-9 promoter.
Previously, we demonstrated that a conditionally active c-fos could inhibit MMP-9 expression via the same AP-1 site at -79 bp in the promoter [14]. This transcriptional inhibition correlated with decreased MMP-9 activity and reduced numbers of invasive cells. In the present study, the -79-bp site mediated the basal activity of the promoter in SCC25 cells and transcriptional induction by c-jun while the -533-bp sequence had minimal effect. These results indicate that the -79-bp AP-1 site in the MMP-9 promoter is functionally dominant in SCC25 cells. Taken together, these studies demonstrate that opposing effects on MMP-9 expression can be mediated via the same recognition sequence in the promoter. This provides a potential mechanism for differential regulation of MMP-9 expression by diverse stimuli. Signals which activate c-fos expression would be predicted to inhibit MMP-9 expression. Conversely, those which activate JNK1 or c-jun may induce MMP-9 activity and, subsequently, cellular invasion. This model would also predict that malignant tumors which constitutively overexpress JNK1 would be more invasive than cancer cells containing normal levels of this enzyme. In support of this model, human squamous cell carcinoma lines which express constitutively active JNK1 have been characterized [14]. The results of these studies suggest that novel chemotherapeutic agents which inhibit JNK1 may be effective in preventing invasion and spread of cancer cells.
Previous studies have shown that MMP-9 expression is regulated by multiple signaling pathways. The MMP-9 promoter contains a recognition sequence for NF-κB at -600 bp [12]. Increased MMP-9 expression, as the result of phorbol ester or tumor necrosis factor treatment, was shown to be regulated by this site. In the present study, we did not find a linear relationship between MMP-9 induction and invasion, suggesting that additional factors also contribute to the invasive phenotype. The effects of JNK1 and c-jun overexpression on the regulation of the MMP-9 promoter provide an interesting contrast to an earlier study using dominant negative constructs [8]. However, both of these studies must be interpreted carefully when making predictions about how endogenous JNK1 and c-jun regulate MMP-9 gene expression.
Many interesting questions on how aberrant MAPK and AP-1 activities regulate MMP-9 expression in cancer cells must be answered. The identities of other fos/jun proteins which regulate MMP-9 expression in cancer cells remain to be determined. The role of altered MAPK signaling in regulating these transcription factors also must be elucidated. Determining the molecular mechanisms which regulate MMP-9 expression will lead to better understanding of the invasive phenotype of human cancer cells.
Acknowledgement(s)
We thank Dr. Roger Davis for the JNK1 expression vector, Dr. Tom Curran for the c-jun expression vector, and Dr. Hiroshi Sato for the MMP-9 reporter construct.
Abbreviations
- JNK1
jun N-terminal kinase 1
- MMP-9
matrix metalloproteinase 9
- AP-1
activator protein 1
- SCC
squamous cell carcinoma
- CAT
chloramphenicol acetyltransferase
- PVDF
polyvinylidene difluoride
- SDS
sodium dodecyl sulfate
Footnotes
This study was supported by National Institutes of Health grant DE10966 to D.L.C.
References
- 1.Liotta LA. Tumor invasion and metastases — role of the extracellular matrix. Cancer Res. 1986;46:1–7. [PubMed] [Google Scholar]
- 2.Kohn EC, Liotta LA. Molecular insights into cancer invasion: strategies for prevention and intervention. Cancer Res. 1995;55:1856–1862. [PubMed] [Google Scholar]
- 3.Witty JP, McDonnell S, Newell KJ, Cannon P, Navre M, Tressler RJ, Matrisian LM. Modulation of matrilysin levels in colon carcinoma cell lines affects tumorigenicity in vivo. Cancer Res. 1994;54:4805–4812. [PubMed] [Google Scholar]
- 4.Davis RJ. The mitogen-activated protein kinase signal transduction pathway. J Biol Chem. 1993;268:14553–14556. [PubMed] [Google Scholar]
- 5.Yang SH, Whitmarsh AJ, Davis RJ, Sharrocks AD. Differential targeting of MAP kinases to the ETS domain transcription factor Elk-1. EMBO J. 1998;17:1740–1749. doi: 10.1093/emboj/17.6.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Derijard B, Hibi M, Wu IH, Barrett T, Su B, Deng T, Karin M, Davis RJ. JNK1: a protein kinase stimulated by UV light and Ha-ras that binds and phosphorylates the c-jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 7.Meier R, Rouse J, Cuenda A, Nebreda AR, Cohen P. Cellular stresses and cytokines activate multiple mitogen-activated protein kinase kinase homologues in PC12 and KB cells. Eur J Biochem. 1997;236:796–805. doi: 10.1111/j.1432-1033.1996.00796.x. [DOI] [PubMed] [Google Scholar]
- 8.Gum R, Wang H, Lengyel E, Juarez J, Boyd D. Regulation of 92 kDa type IV collagenase expression by the jun amino terminal kinase and the extracellular signal regulated kinase-dependent signaling cascades. Oncogene. 1997;14:1481–1493. doi: 10.1038/sj.onc.1200973. [DOI] [PubMed] [Google Scholar]
- 9.Himelstein BP, Lee EJ, Sato H, Seiki M, Muschel RJ. Transcriptional activation of the matrix metalloproteinase 9 gene in an H-ras and v-myc transformed rat embryo cell line. Oncogene. 1997;14:1995–1998. doi: 10.1038/sj.onc.1201012. [DOI] [PubMed] [Google Scholar]
- 10.Gum R, Lengyel E, Juarez J, Chen JH, Sato H, Seiki M, Boyd D. Stimulation of 92 kDa gelatinase B promoter activity by ras is mitogen-activated protein kinase kinase 1 independent and requires multiple transcription factor binding sites including closely spaced PEA3/ets and AP-1 sequences. J Biol Chem. 1996;271:10672–10680. doi: 10.1074/jbc.271.18.10672. [DOI] [PubMed] [Google Scholar]
- 11.Simon C, Goepfert H, Boyd D. Inhibition of the p38 mitogen-activated protein kinase by SB203580 blocks PMA induced Mr 92,000 type IV collagenase secretion and in vitro invasion. Cancer Res. 1998;58:1135–1139. [PubMed] [Google Scholar]
- 12.Sato H, Seiki M. Regulatory mechanism of 92 kDa type IV collagenase gene expression which is associated with invasiveness of tumor cells. Oncogene. 1993;8:395–405. [PubMed] [Google Scholar]
- 13.Sato H, Kita M, Seiki M. v-src activates the expression of 92 kDa type IV collagenase gene through the AP-1 site and the GT box homologous to retinoblastoma control elements. J Biol Chem. 1993;268:23460–23468. [PubMed] [Google Scholar]
- 14.Crowe DL, Brown TN. Transcriptional inhibition of matrix metalloproteinase 9 (MMP-9) activity by a c-fos/estrogen receptor fusion protein is mediated by the proximal AP-1 site of the MMP-9 promoter and correlates with reduced tumor cell invasion. Neoplasia. 1999;1:368–372. doi: 10.1038/sj.neo.7900041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vo HP, Lee MK, Crowe DL. α2β1 integrin signaling via the mitogen-activated protein kinase (MAPK) pathway modulates retinoic acid (RA) dependent tumor cell invasion and transcriptional downregulation of matrix metalloproteinase 9 activity. Int J Oncol. 1998;13:1127–1134. doi: 10.3892/ijo.13.6.1127. [DOI] [PubMed] [Google Scholar]
- 16.Davies B, Miles DW, Happerfield LC, Naylor MS, Bobrow LG, Rubens RD, Balkwill FR. Activity of type IV collagenases in benign and malignant breast disease. Br J Cancer. 1993;67:1126–1131. doi: 10.1038/bjc.1993.207. [DOI] [PMC free article] [PubMed] [Google Scholar]